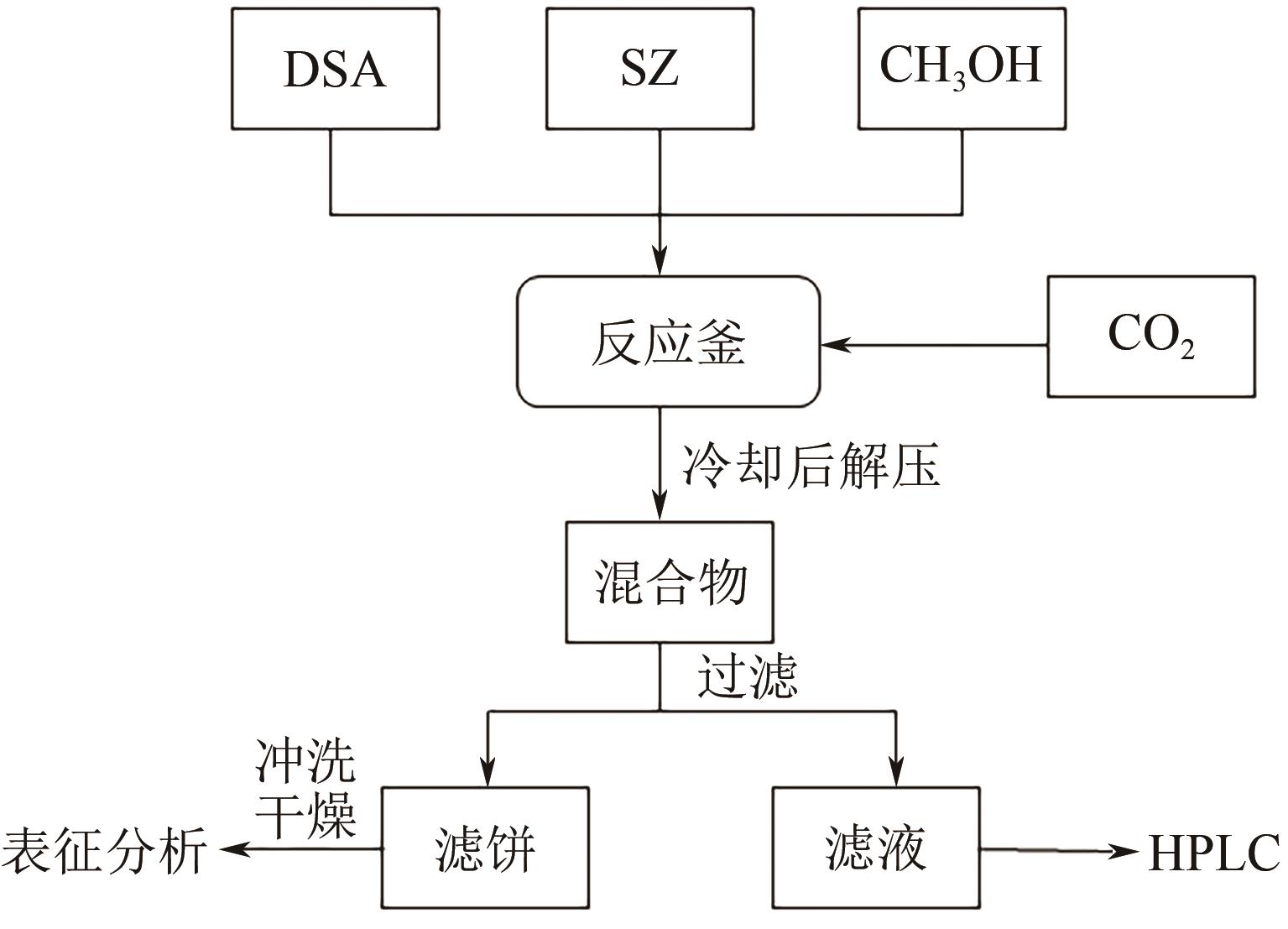
化工进展 ›› 2023, Vol. 42 ›› Issue (10): 5213-5222.DOI: 10.16085/j.issn.1000-6613.2022-2139
固体超强酸SO42-/ZrO2催化生物基丁二酸盐直接酯化
- 太原理工大学化学工程与技术学院,山西 太原 030024
-
收稿日期:2022-11-18修回日期:2023-03-24出版日期:2023-10-15发布日期:2023-11-11 -
通讯作者:王玉高 -
作者简介:尹科科(1997—),男,硕士研究生,研究方向为羧酸盐直接酯化。E-mail:1229801267@qq.com。 -
基金资助:山西省基础研究计划(202203021221069);国家自然科学基金青年基金(21706172)
Direction esterification of bio-based succinate catalyzed by solid super acid SO42-/ZrO2
YIN Keke( ), WANG Yugao(
), WANG Yugao( ), GU Bao, SHEN Jun
), GU Bao, SHEN Jun
- College of Chemical Engineering and Technology, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
-
Received:2022-11-18Revised:2023-03-24Online:2023-10-15Published:2023-11-11 -
Contact:WANG Yugao
摘要:
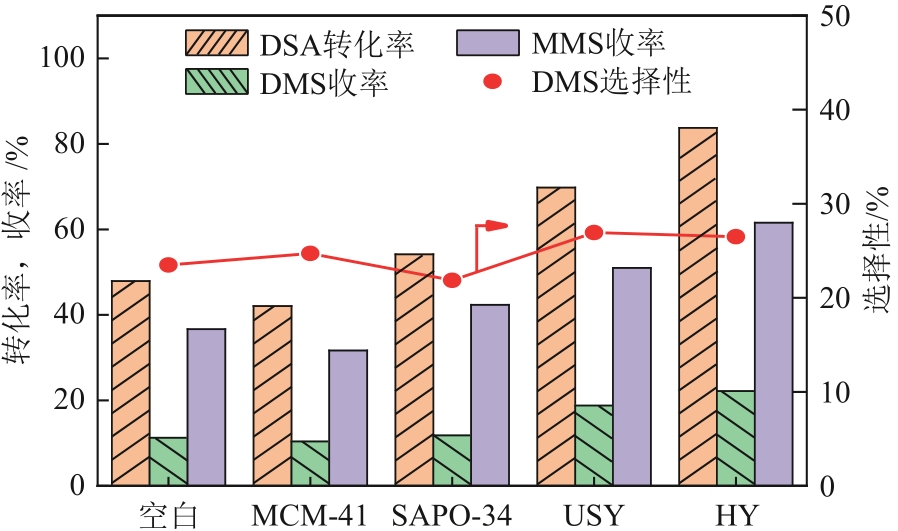
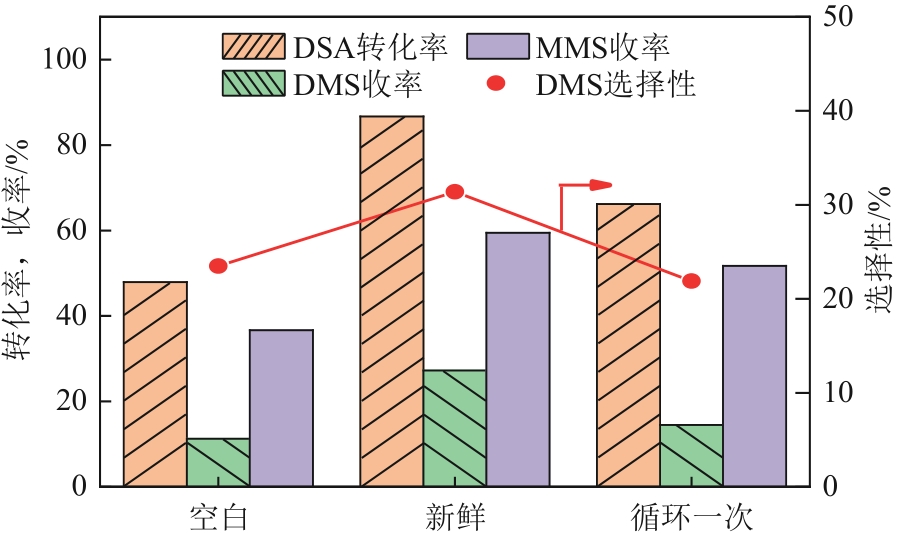
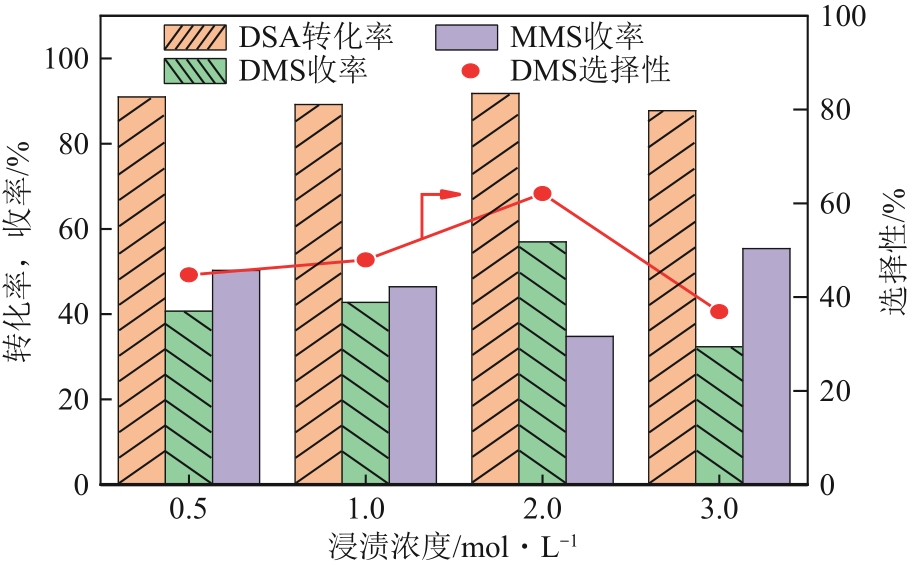
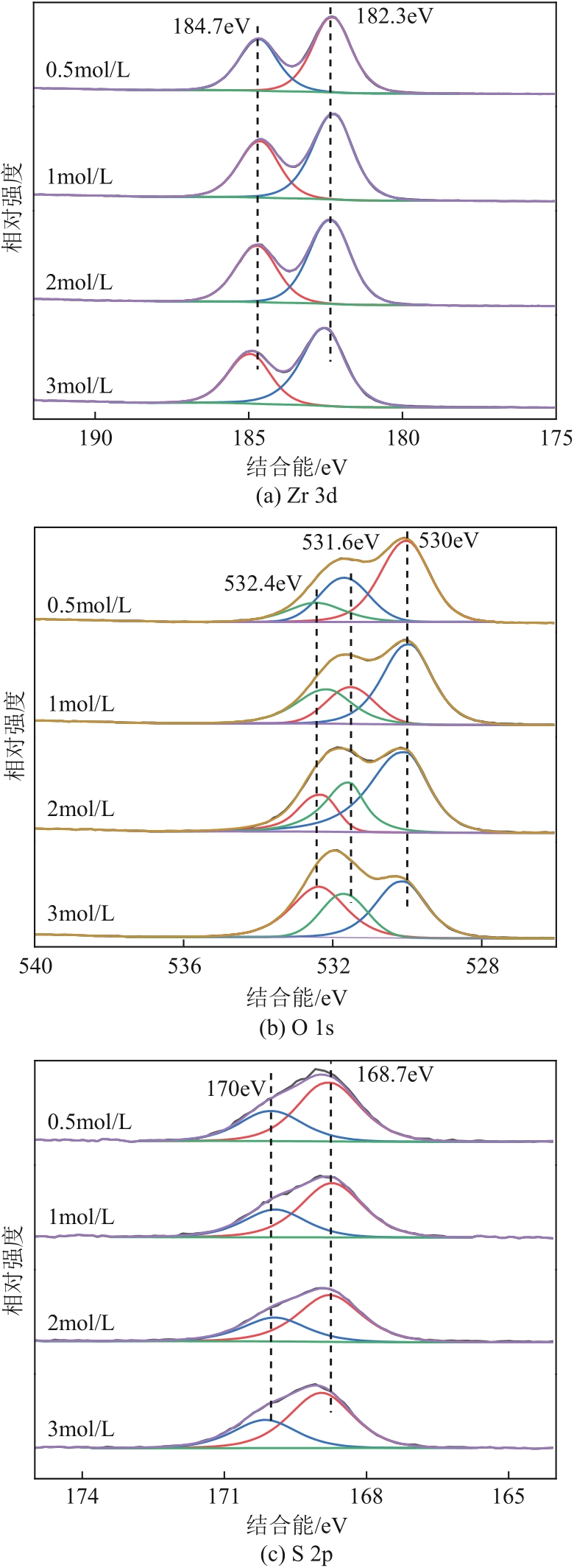
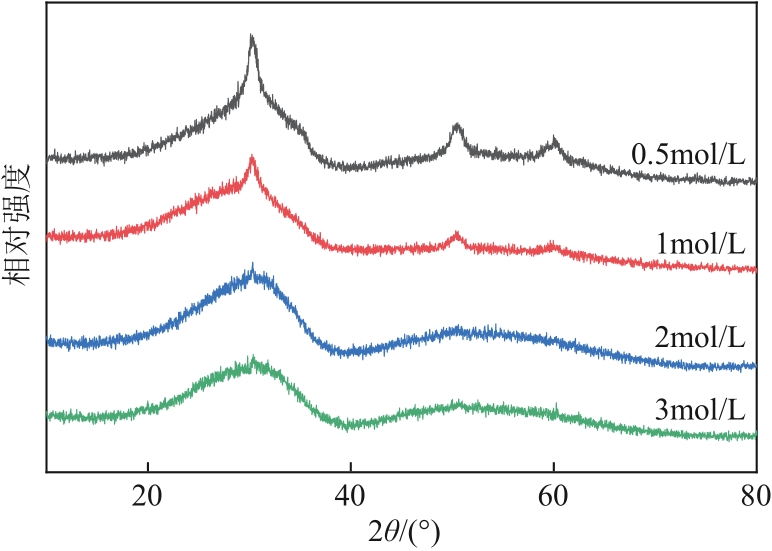
生物发酵法制备丁二酸因制备原料廉价易得、制备工艺对环境影响较小等优点,在替代石油基原料生产丁二酸方面受到广泛关注,但生物发酵生产的丁二酸主要以丁二酸盐形式存在。因此,常需要使用无机酸将丁二酸盐进行酸化处理。以CH3OH和CO2为酯化试剂,可实现丁二酸二钠的直接酯化,获取丁二酸二甲酯,有效地避免了无机酸的使用,且生成的碳酸盐既可固定二氧化碳,也可用于生物发酵液pH的调节。本文在此基础上系统考察了不同分子筛对此反应的催化效果,针对分子筛容易失活和酸量较低的缺点,将分子筛替换为固体超强酸SO42-/ZrO2,对该反应体系进行进一步改进。采用X射线衍射仪、比表面积及孔隙度分析仪、热重分析仪、X射线光电子能谱仪、程序升温化学吸附仪等探究了焙烧温度和浸渍液浓度对SO42-/ZrO2催化丁二酸二钠直接酯化制备丁二酸二甲酯的影响以及催化剂循环稳定性,结果表明在H2SO4浸渍浓度为2mol/L和焙烧温度为550℃的制备条件下所得SO42-/ZrO2催化剂具有较优的催化活性,丁二酸二甲酯的收率和选择性分别为89.25%和93.56%,此外,催化剂不经处理连续使用5次依旧维持较高催化活性,表明其具有较好的稳定性。
中图分类号:
引用本文
尹科科, 王玉高, 谷豹, 申峻. 固体超强酸SO42-/ZrO2催化生物基丁二酸盐直接酯化[J]. 化工进展, 2023, 42(10): 5213-5222.
YIN Keke, WANG Yugao, GU Bao, SHEN Jun. Direction esterification of bio-based succinate catalyzed by solid super acid SO42-/ZrO2[J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5213-5222.
| 类型 | Si/Al | 比表面积/m2∙g-1 | 孔径/nm |
|---|---|---|---|
| MCM-41 | 全硅 | ≥1000 | 4 |
| USY | 5 | ≥805 | 3.8 |
| SAPO-34 | 0.25 | ≥550 | 0.4 |
| HY | ≥2.7 | ≥650 | 0.74 |
表1 实验中涉及的几种分子筛的参数
| 类型 | Si/Al | 比表面积/m2∙g-1 | 孔径/nm |
|---|---|---|---|
| MCM-41 | 全硅 | ≥1000 | 4 |
| USY | 5 | ≥805 | 3.8 |
| SAPO-34 | 0.25 | ≥550 | 0.4 |
| HY | ≥2.7 | ≥650 | 0.74 |
| 浸渍浓度/mol·L-1 | C | O | S | Zr |
|---|---|---|---|---|
| 0.5 | 15.92 | 56.63 | 5.83 | 21.62 |
| 1 | 16.73 | 56.53 | 6.12 | 20.61 |
| 2 | 26.33 | 49.93 | 6.47 | 17.27 |
| 3 | 18.58 | 56.08 | 8.86 | 16.48 |
表2 不同H2SO4浸渍浓度所得SZ表面组分的元素组成(物质的量分数)
| 浸渍浓度/mol·L-1 | C | O | S | Zr |
|---|---|---|---|---|
| 0.5 | 15.92 | 56.63 | 5.83 | 21.62 |
| 1 | 16.73 | 56.53 | 6.12 | 20.61 |
| 2 | 26.33 | 49.93 | 6.47 | 17.27 |
| 3 | 18.58 | 56.08 | 8.86 | 16.48 |
| 浸渍浓度/mol·L-1 | 比表面积/m2·g-1 | 孔径/nm | 孔容/cm3·g-1 |
|---|---|---|---|
| 2 | 86.83 | 7.93 | 0.17 |
| 3 | 60.43 | 7.08 | 0.11 |
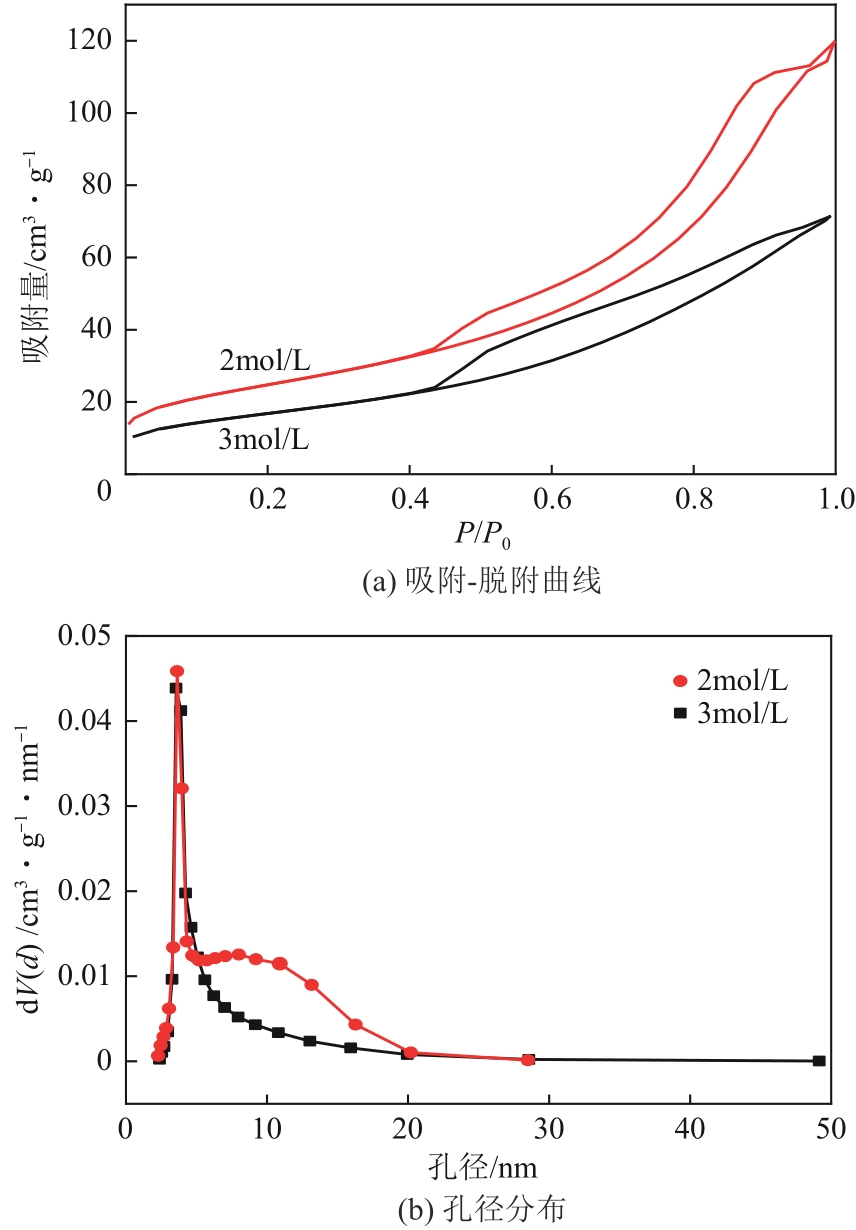
表3 不同H2SO4浸渍浓度所得SZ的比表面积、孔径和孔容
| 浸渍浓度/mol·L-1 | 比表面积/m2·g-1 | 孔径/nm | 孔容/cm3·g-1 |
|---|---|---|---|
| 2 | 86.83 | 7.93 | 0.17 |
| 3 | 60.43 | 7.08 | 0.11 |

图13 反应条件对DSA直接酯化的影响[(a)的反应条件为:反应温度160℃、反应时间2h、CO2初始压力1MPa、DSA初始添加量1mmol;(b)的反应条件为:反应温度160℃、CO2初始压力1MPa、DSA初始添加量1mmol、催化剂添加量0.2g;(c)的反应条件为:反应时间8h、CO2初始压力1MPa、DSA初始添加量1mmol、催化剂添加量0.2g]
| 样品 | C | O | S | Zr |
|---|---|---|---|---|
| SZ-未使用 | 26.33 | 49.93 | 6.47 | 17.27 |
| SZ-使用后 | 27.73 | 50.08 | 2.69 | 19.5 |
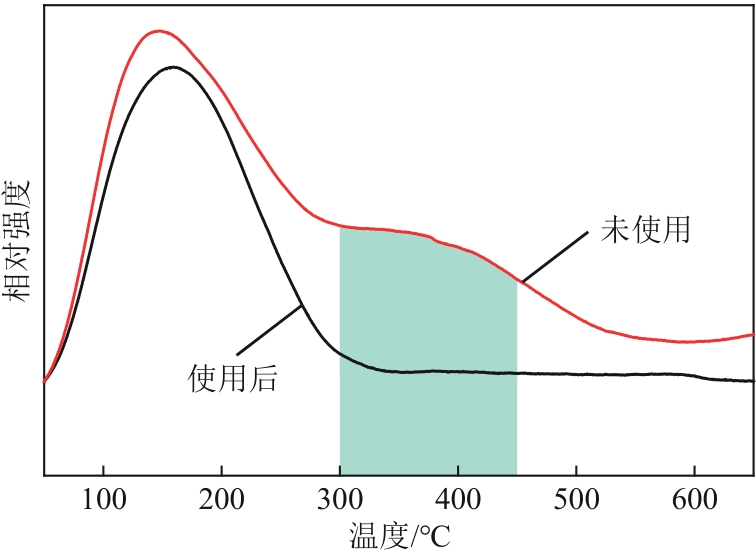
表4 反应前后SZ表面组分的元素组成(物质的量分数)
| 样品 | C | O | S | Zr |
|---|---|---|---|---|
| SZ-未使用 | 26.33 | 49.93 | 6.47 | 17.27 |
| SZ-使用后 | 27.73 | 50.08 | 2.69 | 19.5 |
| 1 | 张耀, 邱晓曼, 陈程鹏, 等. 生物法制造丁二酸研究进展[J]. 化工学报, 2020, 71(5): 1964-1975. |
| ZHANG Yao, QIU Xiaoman, CHEN Chengpeng, et al. Recent progress in microbial production of succinic acid[J]. CIESC Journal, 2020, 71(5): 1964-1975. | |
| 2 | HE Liangtu, LIU Lei, HUANG Yuzhang, et al. One-pot synthesis of dimethyl succinate from d-fructose using Amberlyst-70 catalyst[J]. Molecular Catalysis, 2021, 508: 111584. |
| 3 | 夏榆, 姚勇波, 姚菊明, 等. PBS/天然高分子复合材料的现状及进展[J]. 塑料, 2022, 51(2): 85-88, 100. |
| XIA Yu, YAO Yongbo, YAO Juming, et al. Current status and development of PBS/natural polymer composite[J]. Plastics, 2022, 51(2): 85-88, 100. | |
| 4 | 李求进, 梁伯润. 聚乙二醇丁二酸酯/酚醛树脂共混物的结晶行为[J]. 合成技术及应用, 2006, 21(1): 1-3. |
| LI Qiujin, LIANG Borun. Study on crystallization behavior of PESu/phenolic blends[J]. Synthetic Technology and Application, 2006, 21(1): 1-3. | |
| 5 | WAN Xinyan, REN Dezhang, LIU Yunjie, et al. Facile synthesis of dimethyl succinate via esterification of succinic anhydride over ZnO in methanol[J]. ACS Sustainable Chemistry and Engineering, 2018, 6(3): 2969-2975. |
| 6 | 姚伶俐, 潘海峰, 田文娟, 等. 代谢工程改造大肠杆菌合成丁二酸及发酵罐放大工艺[J]. 微生物学通报, 2018, 45(12): 2541-2551. |
| YAO Lingli, PAN Haifeng, TIAN Wenjuan, et al. Production of succinate by a metabolic engineered Escherichia coli and its scale-up process in fermentor[J]. Microbiology China, 2018, 45(12): 2541-2551. | |
| 7 | OKINO Shohei, NOBURYU Ryoji, SUDA Masako, et al. An efficient succinic acid production process in a metabolically engineered Corynebacterium glutamicum strain[J]. Applied Microbiology and Biotechnology, 2008, 81(3): 459-464. |
| 8 | SONG Hyohak, LEE Sang Yup. Production of succinic acid by bacterial fermentation[J]. Enzyme and Microbial Technology, 2006, 39(3): 352-361. |
| 9 | ORJUELA Alvaro, YANEZ Abraham J, PEEREBOOM Lars, et al. A novel process for recovery of fermentation-derived succinic acid[J]. Separation and Purification Technology, 2011, 83: 31-37. |
| 10 | ORJUELA Alvaro, KOLAH Aspi, HONG Xi, et al. Diethyl succinate synthesis by reactive distillation[J]. Separation and Purification Technology, 2012, 88: 151-162. |
| 11 | CABRERA-RODRÍGUEZ Carlos I, VAN DER WIELEN Luuk A M, STRAATHOF Adrie J J. Separation and catalysis of carboxylates: Byproduct reduction during the alkylation with dimethyl carbonate[J]. Industrial & Engineering Chemistry Research, 2015, 54(44): 10964-10973. |
| 12 | LÓPEZ-GARZÓN Camilo S, OTTENS Marcel, VAN DER WIELEN Luuk A M, et al. Direct downstream catalysis: From succinate to its diethyl ester without intermediate acidification[J]. Chemical Engineering Journal, 2012, 200/201/202: 637-644. |
| 13 | LÓPEZ-GARZÓN Camilo S, VAN DER WIELEN Luuk A M, STRAATHOF Adrie J J. Green upgrading of succinate using dimethyl carbonate for a better integration with fermentative production[J]. Chemical Engineering Journal, 2014, 235: 52-60. |
| 14 | LóPEZ-GARZóN Camilo S, VAN DER WIELEN Luuk A M, STRAATHOF Adrie J J. Ester production from bio-based dicarboxylates via direct downstream catalysis: Succinate and 2, 5-furandicarboxylate dimethyl esters[J]. RSC Advances, 2016, 6(5): 3823-3829. |
| 15 | CABRERA-RODRÃGUEZ Carlos I, PALTRINIERI Laura, DE SMET Louis C P M, et al. Recovery and esterification of aqueous carboxylates by using CO2-expanded alcohols with anion exchange[J]. Green Chemistry, 2017, 19(3): 729-738. |
| 16 | BARVE Prashant P, KAMBLE Sanjay P, JOSHI Jyeshtharaj B, et al. Preparation of pure methyl esters from corresponding alkali metal salts of carboxylic acids using carbon dioxide and methanol[J]. Industrial & Engineering Chemistry Research, 2012, 51(4): 1498-1505. |
| 17 | 谷豹. 丁二酸钠直接酯化及固体酸催化酯化制备丁二酸二甲酯[D]. 太原: 太原理工大学, 2022. |
| GU Bao. Preparation of dimethyl succinate by direct esterification of sodium succinate and esterification catalyzed by solid acid[D]. Taiyuan: Taiyuan University of Technology, 2022. | |
| 18 | 薛淼, 朱美华, 钟彩君, 等. ZSM-5分子筛膜在乙酸异戊酯酯化反应中的应用[J]. 膜科学与技术, 2018, 38(4): 107-112. |
| XUE Miao, ZHU Meihua, ZHONG Caijun, et al. Application of ZSM-5 zeolite membrane for esterification of isoamyl acetate[J]. Membrane Science and Technology, 2018, 38(4): 107-112. | |
| 19 | 李三喜, 徐妍如, 王松. SO4 2-/TiO2-HZSM-5固体超强酸催化剂的制备及酯化性能[J]. 化工进展, 2015, 34(3): 745-750. |
| LI Sanxi, XU Yanru, WANG Song. Preparation of solid superacid SO4 2-/TiO2-HZSM-5 catalyst and its catalytic performance in esterification[J]. Chemical Industry and Engineering Progress, 2015, 34(3): 745-750. | |
| 20 | WANG Shan, PU Jianglong, WU Jiaqin, et al. SO4 2-/ZrO2 as a solid acid for the esterification of palmitic acid with methanol: Effects of the calcination time and recycle method[J]. ACS Omega, 2020, 5(46): 30139-30147. |
| 21 | WANG Anqi, WANG Junxia, WANG Hui, et al. Synthesis of SO4 2-/TiO2-ZnAl2O4 composite solid acids as the esterification catalysts[J]. RSC Advances, 2017, 7(23): 14224-14232. |
| 22 | WANG Pengzhao, YUE Yuanyuan, WANG Tinghai, et al. Alkane isomerization over sulfated zirconia solid acid system[J]. International Journal of Energy Research, 2020, 44(5): 3270-3294. |
| 23 | IGLESIAS Jose, MELERO Juan A, MORALES Gabriel, et al. Dehydration of xylose to furfural in alcohol media in the presence of solid acid catalysts[J]. ChemCatChem, 2016, 8(12): 2089-2099. |
| 24 | THIMMARAJU N, MOHAMED SHAMSHUDDIN S Z, PRATAP S R, et al. Effective synthesis of novel O-acetylated compounds over ZrO2-Al2O3 solid acid[J]. Arabian Journal of Chemistry, 2019, 12(8): 1860-1869. |
| 25 | 胡亚飞, 蔡晶, 李小森. 环戊烷-甲烷水合物生成过程的温度特性[J]. 化工进展, 2016, 35(5): 1418-1427. |
| HU Yafei, CAI Jing, LI Xiaosen. System temperature properties in the process of the cyclopentane-methane binary hydrates formation[J]. Chemical Industry and Engineering Progress, 2016, 35(5): 1418-1427. | |
| 26 | 赵培瑞, 李娜, 陈茜文, 等. 改性固体酸SO4 2-/ZrO2催化合成没食子酸甲酯的研究[J]. 中南林业科技大学学报, 2017, 37(6): 114-118. |
| ZHAO Peirui, LI Na, CHEN Qianwen, et al. Study on synthesis of methyl gallate catalyzed by modified solid superacid SO4 2-/ZrO2 [J]. Journal of Central South University of Forestry & Technology, 2017, 37(6): 114-118. | |
| 27 | 张萍, 李露, 于凤丽, 等. SO4 2-/ZrO2的制备工艺对催化橡胶籽油裂解油酯化的影响[J]. 燃料化学学报, 2013, 41(11): 1322-1327. |
| ZHANG Ping, LI Lu, YU Fengli, et al. Preparation of SO4 2-/ZrO2 catalyst and its performance in the esterification of pyrolytic rubber seed oil[J]. Journal of Fuel Chemistry and Technology, 2013, 41(11): 1322-1327. | |
| 28 | ZHANG Hongwei, YANG Weijia, ROSLAN Irwan Iskandar, et al. A combo Zr-HY and Al-HY zeolite catalysts for the one-pot cascade transformation of biomass-derived furfural to γ-valerolactone[J]. Journal of Catalysis, 2019, 375: 56-67. |
| 29 | SONG Hua, CUI Xuehan, JIANG Bolong, et al. Preparation of SO4 2-/ZrO2 solid superacid and oxidative desulfurization using K2FeO4 [J].Research on Chemical Intermediates, 2015, 41(1): 365-382. |
| 30 | WARD D A, KO E I. One-step synthesis and characterization of zirconia-sulfate aerogels as solid superacids[J]. Journal of Catalysis, 1994, 150(1): 18-33. |
| 31 | 张亚平, 黎汉生, 甄彬, 等. SO4 2-/TiO2-SiO2固体超强酸的制备、表征及其性能[J]. 化工进展, 2011, 30(S1): 155-158. |
| ZHANG Yaping, LI Hansheng, ZHEN Bin, et al. Preparation, characterization and catalytic properties of SO4 2-/TiO2-SiO2 solid superacid[J]. Chemical Industry and Engineering Progress, 2011, 30(S1): 155-158. | |
| 32 | ARDIZZONE S, BIANCHI C L, GRASSI E. The role of the oxide precursor on the features of sulphated zirconia[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1998, 135(1/2/3): 41-51. |
| 33 | KUMARI Latha, LI W Z, XU J M, et al. Controlled hydrothermal synthesis of zirconium oxide nanostructures and their optical properties[J]. Crystal Growth & Design, 2009, 9(9): 3874-3880. |
| 34 | BEDILO Alexander F, KIM Vladimir I, VOLODIN Alexander M. Effect of light on reactions over sulfated zirconia[J]. Journal of Catalysis, 1998, 176(2): 294-304. |
| 35 | WANG Anqi, WANG Junxia, LU Can, et al. Esterification for biofuel synthesis over an eco-friendly and efficient kaolinite-supported SO4 2-/ZnAl2O4 macroporous solid acid catalyst[J]. Fuel, 2018, 234: 430-440. |
| 36 | 马惠琴, 王卫, 马媛媛. La改性固体超强酸S2O8 2-/ZrO2-Al2O3的制备及催化性能研究[J]. 材料导报, 2014, 28(6): 48-52, 64. |
| MA Huiqin, WANG Wei, MA Yuanyuan. Preparation and catalytic properties study of solid superacid catalyst S2O8 2-/ZrO2-Al2O3 modified by lanthanum[J]. Materials Review, 2014, 28(6): 48-52, 64. | |
| 37 | 金顶峰, 王新庆, 金红晓, 等. 硫酸化介孔氧化锆固体超强酸的制备和应用研究[J]. 材料工程, 2008, 36(10): 223-227. |
| JIN Dingfeng, WANG Xinqing, JIN Hongxiao, et al. Synthesis and performance of sulfated mesoporous zorcina solid superacid[J]. Journal of Materials Engineering, 2008, 36(10): 223-227. | |
| 38 | 赵燕, 郑琦宏, 于洁. SO4 2-/TiO2型光催化材料的制备与表征[J]. 材料导报, 2015, 29(S1): 313-315, 322. |
| ZHAO Yan, ZHENG Qihong, YU Jie. Preparation and characterization of SO4 2-/TiO2 photocatalyst[J]. Materials Review, 2015, 29(S1): 313-315, 322. | |
| 39 | 戎梅竹, 申延明, 刘宏伟, 等. SO4 2-/ZrO2固体超强酸的制备及表征[J]. 化工科技, 2006, 14(4): 34-37. |
| RONG Meizhu, SHEN Yanming, LIU Hongwei, et al. Preparation and characterization of superacid SO4 2-/ZrO2 [J]. Science & Technology in Chemical Industry, 2006, 14(4): 34-37. | |
| 40 | 郑云天, 位艳宾, 徐庆茹, 等. SO4 2-/ZrO2固体超强酸的制备及催化性能[J]. 化工技术与开发, 2016, 45(7): 7-10. |
| ZHENG Yuntian, WEI Yanbin, XU Qingru, et al. Preparation and catalytic properties of SO4 2-/ZrO2 solid superacid[J]. Technology & Development of Chemical Industry, 2016, 45(7): 7-10. | |
| 41 | 王晋东, 李文志, 杜志杰, 等. Ni-S2O8 2-/TiO2固体超强酸催化解聚木质素的研究[J]. 太阳能学报, 2017, 38(4): 867-873. |
| WANG Jindong, LI Wenzhi, DU Zhi jie, et al. Study of lignin catalytic depolymerization by solid superacid Ni-S2O8 2-/TiO2 [J]. Acta Energiae Solaris Sinica, 2017, 38(4): 867-873. | |
| 42 | SHI Xuejun, WU Yulong, LI Panpan, et al. Catalytic conversion of xylose to furfural over the solid acid SO4 2-/ZrO2-Al2O3/SBA-15 catalysts[J]. Carbohydrate Research, 2011, 346(4): 480-487. |
| [1] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [2] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [3] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [4] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [5] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [6] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [7] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [8] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [9] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [10] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [11] | 赵景超, 谭明. 表面活性剂对电渗析减量化工业含盐废水的影响[J]. 化工进展, 2023, 42(S1): 529-535. |
| [12] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [13] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [14] | 王谨航, 何勇, 史伶俐, 龙臻, 梁德青. 气体水合物阻聚剂研究进展[J]. 化工进展, 2023, 42(9): 4587-4602. |
| [15] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||