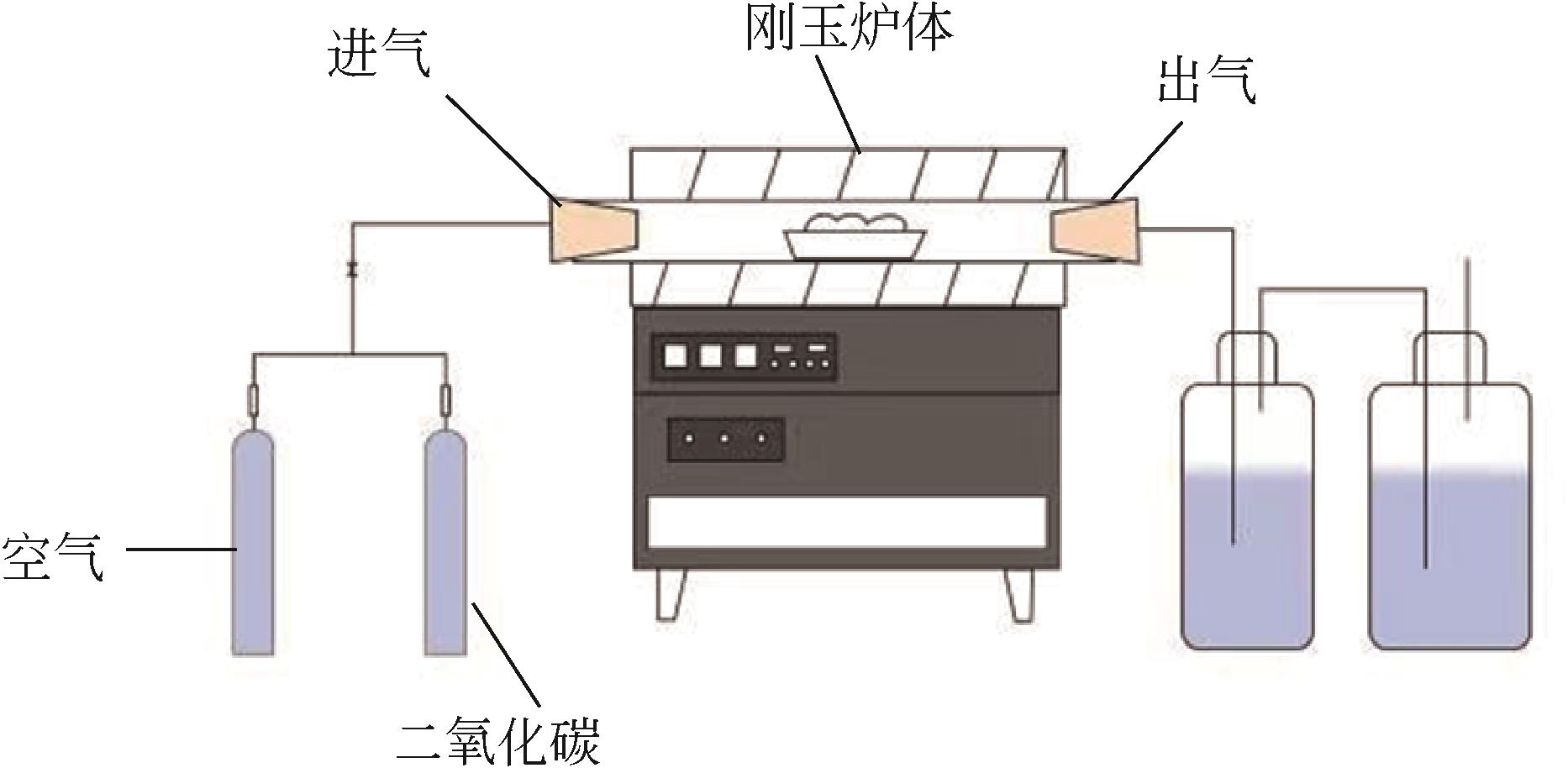
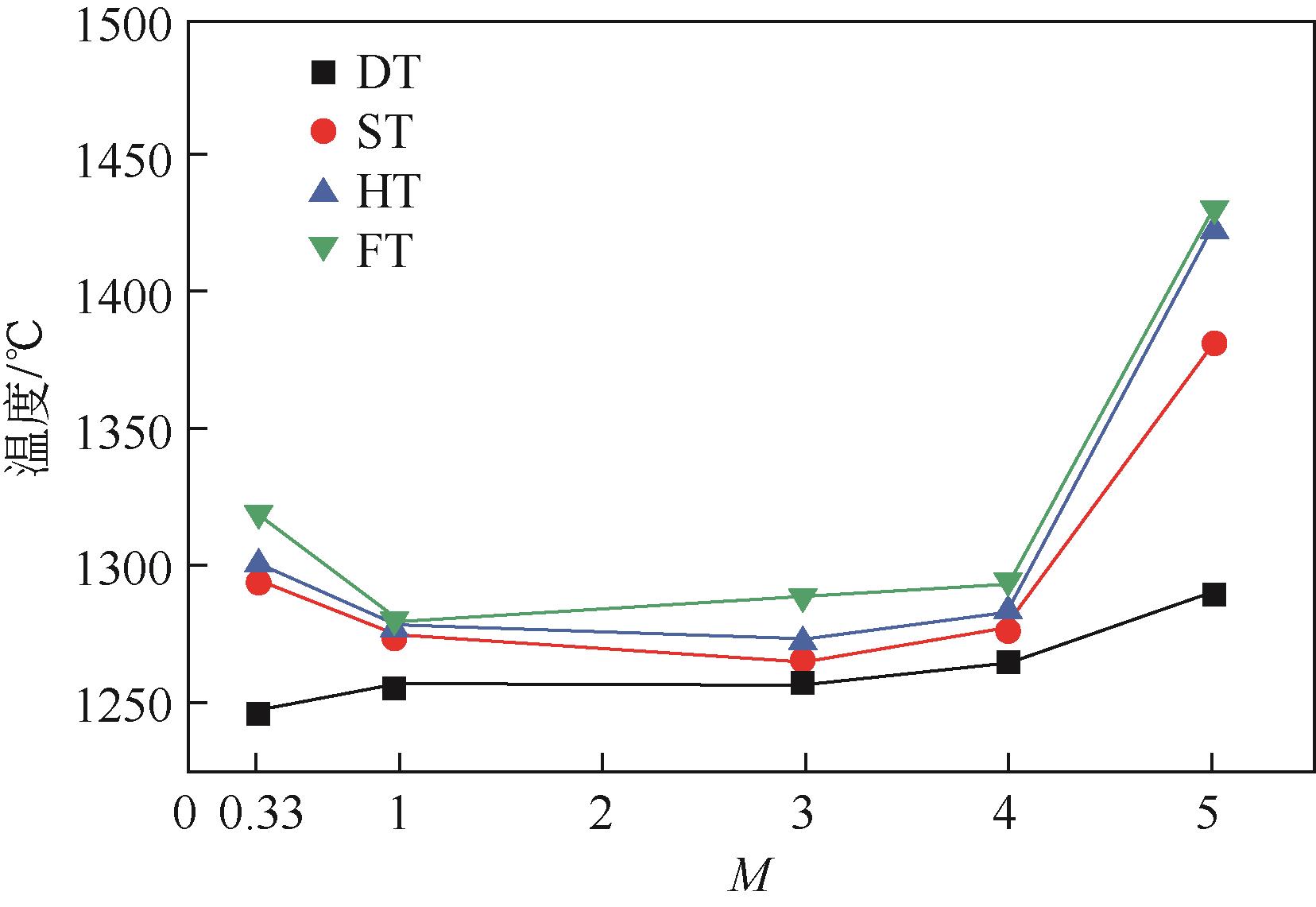
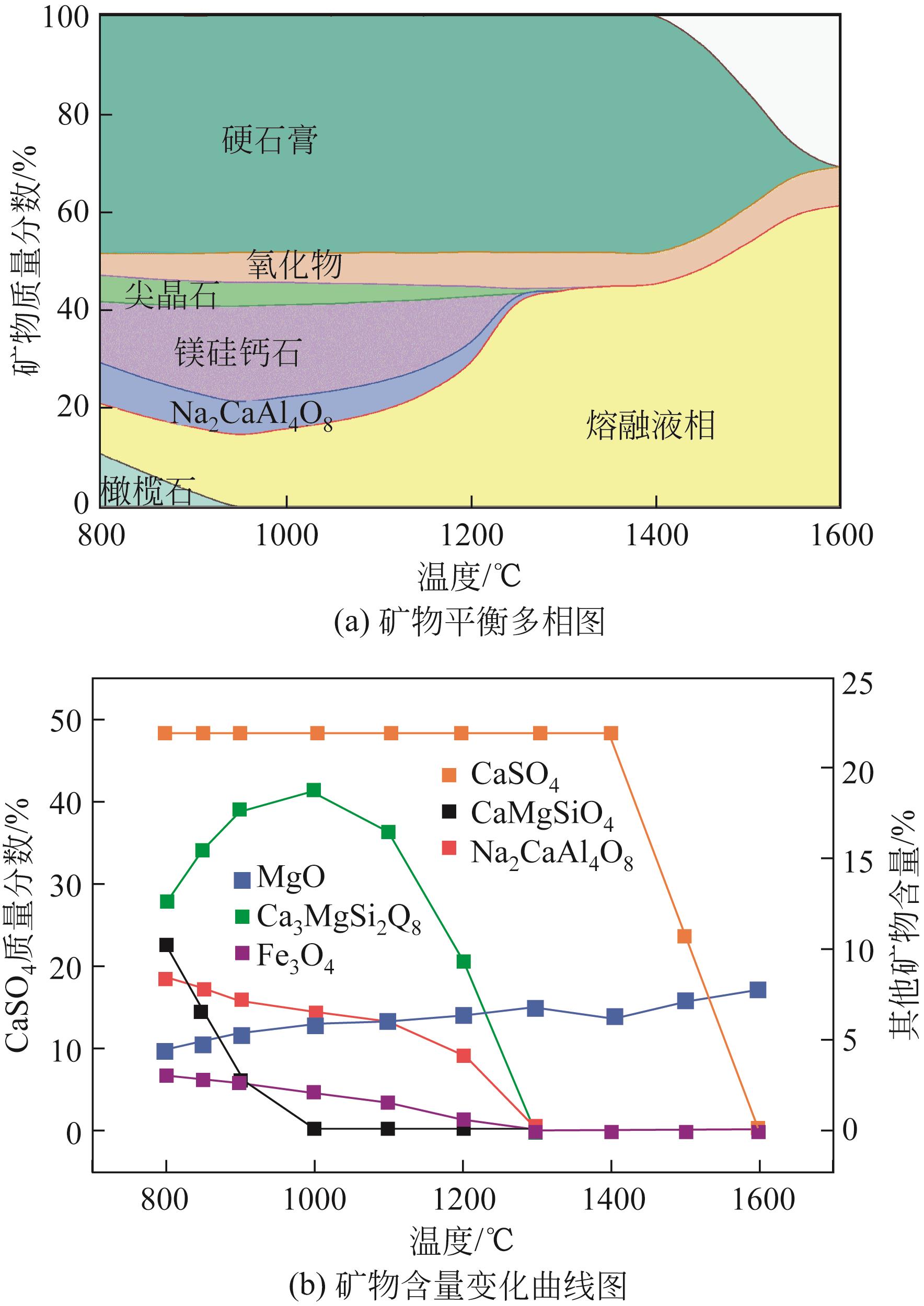
| 1 |
FISHER-VANDEN K. Energy in China: understanding past trends and future directions[J]. International Review of Environmental and Resource Economics, 2009, 3(3): 217-244.
|
| 2 |
QI X B, SONG G L, SONG W J, et al. Influence of sodium-based materials on the slagging characteristics of Zhundong coal[J]. Journal of the Energy Institute, 2017, 90(6): 914-922.
|
| 3 |
ZHANG Y F, ZHAO J, WEI X L, et al. Effects of alkali metals on the formation of particulate matter and adsorption of floating beads during Zhundong coal combustion[J]. Energy & Fuels, 2019, 33(6): 5422-5429.
|
| 4 |
HUANG Q, ZHANG Y Y, YAO Q, et al. Mineral manipulation of Zhundong lignite towards fouling mitigation in a down-fired combustor[J]. Fuel, 2018, 232: 519-529.
|
| 5 |
WANG X B, XU Z X, WEI B, et al. The ash deposition mechanism in boilers burning Zhundong coal with high contents of sodium and calcium: a study from ash evaporating to condensing[J]. Applied Thermal Engineering, 2015, 80: 150-159.
|
| 6 |
HUANG Q, LI S Q, LI G D, et al. Mechanisms on the size partitioning of sodium in particulate matter from pulverized coal combustion[J]. Combustion and Flame, 2017, 182: 313-323.
|
| 7 |
ZHU C, TU H, BAI Y, et al. Evaluation of slagging and fouling characteristics during Zhundong coal co-firing with a Si/Al dominated low rank coal[J]. Fuel, 2019, 254: 115730.
|
| 8 |
LIU Y Q, CHENG L M, JI J Q, et al. Ash deposition behavior in co-combusting high-alkali coal and bituminous coal in a circulating fluidized bed[J]. Applied Thermal Engineering, 2019, 149: 520-527.
|
| 9 |
QI X B, SONG G L, SONG W J, et al. Effects of wall temperature on slagging and ash deposition of Zhundong coal during circulating fluidized bed gasification[J]. Applied Thermal Engineering, 2016, 106: 1127-1135.
|
| 10 |
XIONG Z, GAO Y X, LI X, et al. A novel CO2-water leaching method for AAEM removal from coal: suppression of PM formation and release during Zhundong coal combustion[J]. Fuel, 2020, 271: 117689.
|
| 11 |
YANG H R, JIN J, LIU D Y, et al. The influence of vermiculite on the ash deposition formation process of Zhundong coal[J]. Fuel, 2018, 230: 89-97.
|
| 12 |
NIU Y Q, GONG Y H, ZHANG X, et al. Effects of leaching and additives on the ash fusion characteristics of high-Na/Ca Zhundong coal[J]. Journal of the Energy Institute, 2019, 92(4): 1115-1122.
|
| 13 |
NEL M V, STRYDOM C A, SCHOBERT H H, et al. Reducing atmosphere ash fusion temperatures of a mixture of coal-associated minerals—The effect of inorganic additives and ashing temperature[J]. Fuel Processing Technology, 2014, 124: 78-86.
|
| 14 |
VASSILEV S V, KITANO K, TAKEDA S, et al. Influence of mineral and chemical composition of coal ashes on their fusibility[J]. Fuel Processing Technology, 1995, 45(1): 27-51.
|
| 15 |
ZHANG G J, REINMÖLLER M, KLINGER M, et al. Ash melting behavior and slag infiltration into alumina refractory simulating co-gasification of coal and biomass[J]. Fuel, 2015, 139: 457-465.
|
| 16 |
XU L L, LIU J, KANG Y, et al. Safely burning high alkali coal with kaolin additive in a pulverized fuel boiler[J]. Energy & Fuels, 2014, 28(9): 5640-5648.
|
| 17 |
WEI B, WANG X B, TAN H Z, et al. Effect of silicon-aluminum additives on ash fusion and ash mineral conversion of Xinjiang high-sodium coal[J]. Fuel, 2016, 181: 1224-1229.
|
| 18 |
DAI B Q, WU X J, DE GIROLAMO A, et al. Inhibition of lignite ash slagging and fouling upon the use of a silica-based additive in an industrial pulverised coal-fired boiler. Part 1. Changes on the properties of ash deposits along the furnace[J]. Fuel, 2015, 139: 720-732.
|
| 19 |
FAN Y Q, LYU Q G, ZHU Z P, et al. The impact of additives upon the slagging and fouling during Zhundong coal gasification[J]. Journal of the Energy Institute, 2020, 93(4): 1651-1665.
|
| 20 |
WANG Y Z, JIN J, LIU D Y, et al. Understanding ash deposition for Zhundong coal combustion in 330MW utility boiler: focusing on surface temperature effects[J]. Fuel, 2018, 216: 697-706.
|
| 21 |
MA J C, TIAN X, WANG C Q, et al. Fate of fuel‑nitrogen during in situ gasification chemical looping combustion of coal[J]. Fuel Processing Technology, 2021, 215: 106710.
|
| 22 |
ZHANG Y J, NIU S L, HAN K H, et al. Synthesis of the SrO-CaO-Al2O3 trimetallic oxide catalyst for transesterification to produce biodiesel[J]. Renewable Energy, 2021, 168: 981-990.
|
| 23 |
CHEN X D, KONG L X, BAI J, et al. Effect of Na2O on mineral transformation of coal ash under high temperature gasification condition[J]. Journal of Fuel Chemistry and Technology, 2016, 44(3): 263-272.
|
| 24 |
ZHANG H X, GUO X W, ZHU Z P. Effect of temperature on gasification performance and sodium transformation of Zhundong coal[J]. Fuel, 2017, 189: 301-311.
|
| 25 |
LI X M, ZHI L F, SHI W J, et al. Effect of K2O/Na2O on fusion behavior of coal ash with high silicon and aluminum level[J]. Fuel, 2020, 265: 116964.
|
| 26 |
WU X J, ZHANG X, DAI B Q, et al. Ash deposition behaviours upon the combustion of low-rank coal blends in a 3MWth pilot-scale pulverised coal-fired furnace[J]. Fuel Processing Technology, 2016, 152: 176-182.
|
| 27 |
WANG C A, ZHAO L, SUN R J, et al. Effects of silicon-aluminum additives on ash mineralogy, morphology, and transformation of sodium, calcium, and iron during oxy-fuel combustion of Zhundong high-alkali coal[J]. International Journal of Greenhouse Gas Control, 2019, 91: 102832.
|
| 28 |
ZHAO Y L, ZHANG Y M, BAO S X, et al. Effect of stone coal chemical composition on sintering behavior during roasting[J]. Industrial & Engineering Chemistry Research, 2014, 53(1): 157-163.
|
| 29 |
YAO Y X, JIN J, LIU D Y, et al. Evaluation of vermiculite in reducing ash deposition during the combustion of high-calcium and high-sodium Zhundong coal in a drop-tube furnace[J]. Energy & Fuels, 2016, 30(4): 3488-3494.
|
| 30 |
WANG Y, WANG D X, DONG C Q, et al. The behaviour and reactions of sodium containing minerals in ash melting process[J]. Journal of the Energy Institute, 2017, 90(2): 167-173.
|
| 31 |
SHI W J, KONG L X, BAI J, et al. Effect of CaO/Fe2O3 on fusion behaviors of coal ash at high temperatures[J]. Fuel Processing Technology, 2018, 181: 18-24.
|
| 32 |
SONG G L, YANG S B, SONG W J, et al. Release and transformation behaviors of sodium during combustion of high alkali residual carbon[J]. Applied Thermal Engineering, 2017, 122: 285-296.
|
 ), 金晶1,2(
), 金晶1,2( ), 张云鹏1,2, 刘薄鉴治1,2, 梁诗雨1,2, 翟中媛3
), 张云鹏1,2, 刘薄鉴治1,2, 梁诗雨1,2, 翟中媛3
 ), JIN Jing1,2(
), JIN Jing1,2( ), ZHANG Yunpeng1,2, LIU Bojianzhi1,2, LIANG Shiyu1,2, ZHAI Zhongyuan3
), ZHANG Yunpeng1,2, LIU Bojianzhi1,2, LIANG Shiyu1,2, ZHAI Zhongyuan3