化工进展 ›› 2022, Vol. 41 ›› Issue (1): 277-285.DOI: 10.16085/j.issn.1000-6613.2021-0180
磁性石墨烯复合材料制备与应用研究进展
- 天津大学化工学院,天津 300350
-
收稿日期:2021-01-26修回日期:2021-04-26出版日期:2022-01-05发布日期:2022-01-24 -
通讯作者:袁才登 -
作者简介:耿佳琦(1995—),女,硕士研究生,研究方向为磁性石墨烯。E-mail:gengjiaqi@tju.edu.cn 。
Progress in preparation and application of magnetic graphene composites
GENG Jiaqi( ), MEN Yuanli, LIU Chen, YUAN Caideng(
), MEN Yuanli, LIU Chen, YUAN Caideng( )
)
- School of Chemical Engineering and Technology, Tianjin University, Tianjin 300350, China
-
Received:2021-01-26Revised:2021-04-26Online:2022-01-05Published:2022-01-24 -
Contact:YUAN Caideng
摘要:
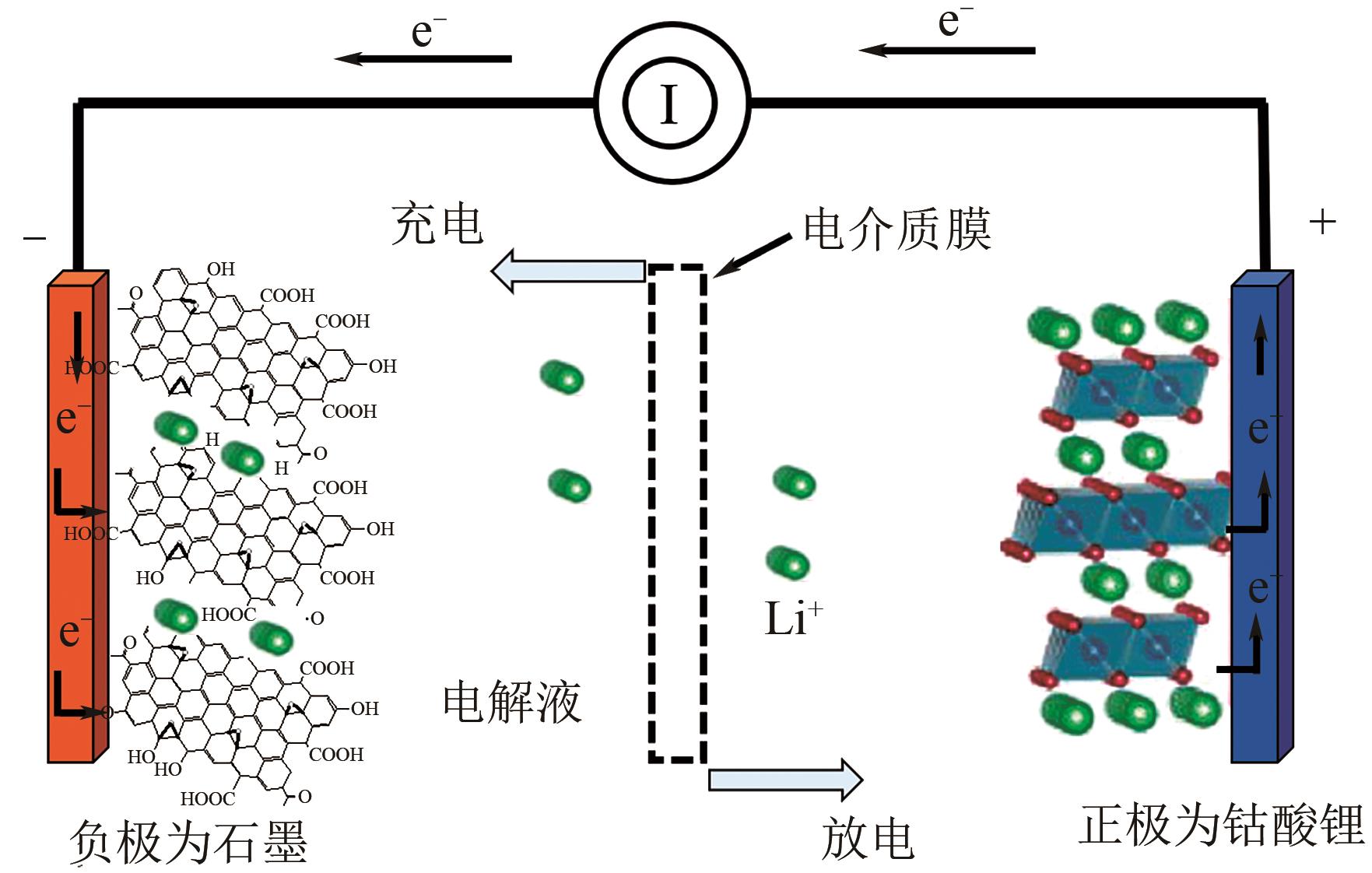
石墨烯具备多种优异的性能,但容易通过π-π堆积和范德华力作用产生聚集,重新堆叠成石墨。为了改善石墨烯的堆叠问题,提高石墨烯材料的应用性,越来越多的研究者将石墨烯及其衍生物和磁性纳米粒子复合,制备综合性能更优的新型材料。本文结合近年来国内外研究报道,总结了磁性石墨烯纳米复合材料的制备方法(水热/溶剂热、化学接枝法、微波辅助法等),概述了磁性石墨烯复合材料在环境样品分离富集、催化、涂层耐腐蚀性、吸波材料及能源等方面的应用,指出了目前磁性石墨烯复合材料研究中存在的一些问题,例如磁性颗粒容易发生团聚、生物安全性有待验证、氧化石墨烯的还原导致其表面吸附位点减少等。目前(氧化)石墨烯的制备工艺正在得到改善,而未来最重要的发展方向是加强对磁性石墨烯的表面改性,从而可使其表面具有更丰富的吸附位点,同时也可使石墨烯表面的磁性纳米粒子的形态及分布更均匀,更有利于稳定发挥磁性石墨烯的功能性。
中图分类号:
引用本文
耿佳琦, 门园丽, 刘晨, 袁才登. 磁性石墨烯复合材料制备与应用研究进展[J]. 化工进展, 2022, 41(1): 277-285.
GENG Jiaqi, MEN Yuanli, LIU Chen, YUAN Caideng. Progress in preparation and application of magnetic graphene composites[J]. Chemical Industry and Engineering Progress, 2022, 41(1): 277-285.
| 制备方法 | 方法简介 | 优点 | 缺点 | 实例 |
|---|---|---|---|---|
| 直接磁化 | 在超声或振荡条件下直接通过物理吸附到石墨烯上 | 操作简单 | 不稳定、不能多次重复利用 | 采用静电自组装制备Fe3O4/GO纳米复合材料[ |
| 水热法 | 以水为反应介质,在一定温度和压强条件下进行化学反应 | 污染小;纯度高、晶粒发育好、粒度分布窄、可控性好;团聚程度轻 | 无法观察晶体生长和材料合成的过程;温压控制较严格 | 采用水热法合成纳米杂化物MGZ@SiO,用作降解水中有机污染物的催化剂[ |
| 溶剂热法 | 以有机溶剂为介质,在一定温度和压强条件下进行化学反应 | 绿色环保、成本低;磁性颗粒分散均匀,防止石墨烯团聚 | 温压控制严格;不太适合批量生产 | 利用溶剂法制备成磁性材料Fe3O4@N-rGO,用于提取饮料中的双酚内分泌干扰物[ |
| 化学共沉淀法 | 磁力搅拌或超声辅助下,在石墨烯片层表面直接形成磁性纳米颗粒的沉积 | 可通过调节实验条件调节所生成复合物的形态、颗粒大小 | 磁性颗粒在石墨烯表面分散不均匀,有团聚的可能 | 通过化学共沉淀法制备磁性吸附剂,用于去除黄曲霉毒素[ |
| 化学接枝法 | 先对磁性纳米粒子和石墨烯进行改性,再利用偶联剂和交联剂将二者通过化学键形式复合在一起 | 磁性石墨烯的稳定性较好 | 工艺复杂、产率低 | 将乙二胺接枝到磁性氧化石墨烯,制备DEA-GO@Fe3O4复合材料,研究多种因素对去除重金属废水中Cr(Ⅵ)的影响[ |
| 微波辅助法 | 微波辐射可以为化学反应提供直接能量,加速化学反应进行 | 制备时间短;纳米颗粒细小、形状规则且分布均匀 | 对设备要求较高,存在安全隐患 | 通过微波辐射制备了Pd-CoFe2O4-GE三元复合纳米片,研究其对硼氢化钠还原4-硝基苯酚的催化性能[ |
| 溶胶-凝胶法 | 以磁性金属盐或醇盐为前体,水解生成的活性单体吸附在石墨烯表面并聚合形成溶胶,进而形成凝胶 | 操作简单、设备低廉;磁性颗粒都键合在石墨烯的表面 | 工艺时间较长;原料较昂贵;所得到半成品制品容易产生开裂 | 采用溶胶-凝胶法制备rGO/TiO2复合材料,并研究该材料的光催化性能[ |
表1 磁性石墨烯常用制备方法对比
| 制备方法 | 方法简介 | 优点 | 缺点 | 实例 |
|---|---|---|---|---|
| 直接磁化 | 在超声或振荡条件下直接通过物理吸附到石墨烯上 | 操作简单 | 不稳定、不能多次重复利用 | 采用静电自组装制备Fe3O4/GO纳米复合材料[ |
| 水热法 | 以水为反应介质,在一定温度和压强条件下进行化学反应 | 污染小;纯度高、晶粒发育好、粒度分布窄、可控性好;团聚程度轻 | 无法观察晶体生长和材料合成的过程;温压控制较严格 | 采用水热法合成纳米杂化物MGZ@SiO,用作降解水中有机污染物的催化剂[ |
| 溶剂热法 | 以有机溶剂为介质,在一定温度和压强条件下进行化学反应 | 绿色环保、成本低;磁性颗粒分散均匀,防止石墨烯团聚 | 温压控制严格;不太适合批量生产 | 利用溶剂法制备成磁性材料Fe3O4@N-rGO,用于提取饮料中的双酚内分泌干扰物[ |
| 化学共沉淀法 | 磁力搅拌或超声辅助下,在石墨烯片层表面直接形成磁性纳米颗粒的沉积 | 可通过调节实验条件调节所生成复合物的形态、颗粒大小 | 磁性颗粒在石墨烯表面分散不均匀,有团聚的可能 | 通过化学共沉淀法制备磁性吸附剂,用于去除黄曲霉毒素[ |
| 化学接枝法 | 先对磁性纳米粒子和石墨烯进行改性,再利用偶联剂和交联剂将二者通过化学键形式复合在一起 | 磁性石墨烯的稳定性较好 | 工艺复杂、产率低 | 将乙二胺接枝到磁性氧化石墨烯,制备DEA-GO@Fe3O4复合材料,研究多种因素对去除重金属废水中Cr(Ⅵ)的影响[ |
| 微波辅助法 | 微波辐射可以为化学反应提供直接能量,加速化学反应进行 | 制备时间短;纳米颗粒细小、形状规则且分布均匀 | 对设备要求较高,存在安全隐患 | 通过微波辐射制备了Pd-CoFe2O4-GE三元复合纳米片,研究其对硼氢化钠还原4-硝基苯酚的催化性能[ |
| 溶胶-凝胶法 | 以磁性金属盐或醇盐为前体,水解生成的活性单体吸附在石墨烯表面并聚合形成溶胶,进而形成凝胶 | 操作简单、设备低廉;磁性颗粒都键合在石墨烯的表面 | 工艺时间较长;原料较昂贵;所得到半成品制品容易产生开裂 | 采用溶胶-凝胶法制备rGO/TiO2复合材料,并研究该材料的光催化性能[ |
| 1 | GE S G, LAN F F, YU F, et al. Applications of graphene and related nanomaterials in analytical chemistry[J]. New Journal of Chemistry, 2015, 39(4): 2380-2395. |
| 2 | AKBARZADEH A, SAMIEI M, DAVARAN S. Magnetic nanoparticles: preparation, physical properties, and applications in biomedicine[J]. Nanoscale Research Letters, 2012, 7(1): 144. |
| 3 | HAN Q, WANG Z H, XIA J F, et al. Facile and tunable fabrication of Fe3O4/graphene oxide nanocomposites and their application in the magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples[J]. Talanta, 2012, 101: 388-395. |
| 4 | AERRROB Y, CHO J Y, JANG W K, et al. Enhanced sonocatalytic degradation of organic dyes from aqueous solutions by novel synthesis of mesoporous Fe3O4-graphene/ZnO@SiO2 nanocomposites[J]. Ultrasonics Sonochemistry, 2018, 41: 267-278. |
| 5 | LI N, CHEN J, SHI Y P. Magnetic nitrogen-doped reduced graphene oxide as a novel magnetic solid-phase extraction adsorbent for the separation of bisphenol endocrine disruptors in carbonated beverages[J]. Talanta, 2019, 201: 194-203. |
| 6 | JI J M, XIE W L. Detoxification of Aflatoxin B1 by magnetic graphene composite adsorbents from contaminated oils[J]. Journal of Hazardous Materials, 2020, 381: 120915. |
| 7 | HU X J, XU J W, WU C Y, et al. Ethylenediamine grafted to graphene oxide@Fe3O4 for chromium(Ⅵ) decontamination: performance, modelling, and fractional factorial design[J]. PLos One, 2017, 12(10): e0187166. |
| 8 | LU X F, YANG L, BIAN X J, et al. Rapid, microwave-assisted, and one-pot synthesis of magnetic palladium-CoFe2O4-graphene composite nanosheets and their applications as recyclable catalysts[J]. Particle & Particle Systems Characterization, 2014, 31(2): 245-251. |
| 9 | 任建, 李光照, 韩锐, 等. 溶胶-凝胶法原位制备还原氧化石墨烯/二氧化钛复合材料及光催化性能[J].功能材料, 2019, 50(7): 7185-7190, 7198. |
| REN J, LI G Z, HAN R, et al. In-situ preparation of reduced graphene oxide/titanium dioxide composites by sol-gel method and their photocatalytic properties[J]. Journal of Functional Materials, 2019, 50(7): 7185-7190, 7198. | |
| 10 | ŠAFAŘÍKOVÁ M, ŠAFAŘÍK I. Magnetic solid-phase extraction[J]. Journal of Magnetism and Magnetic Materials, 1999, 194(1): 108-112. |
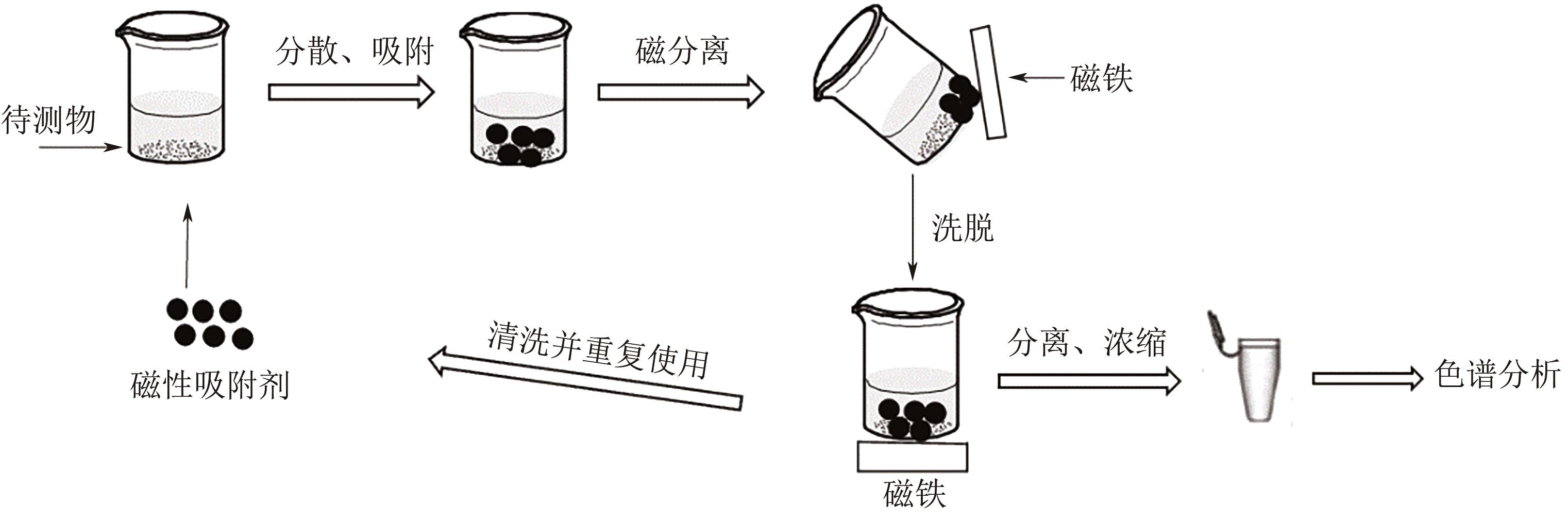
| 11 | 丁青青, 张文敏, 张兰. 磁性纳米材料在样品前处理中的应用进展与展望[J]. 色谱, 2020, 38(1): 14-21. |
| DING Q Q, ZHANG W M, ZHANG L. Advances in magnetic nanomaterials for sample pretreatment and future prospects[J]. Chinese Journal of Chromatography, 2020, 38(1): 14-21. | |
| 12 | 孙爱娟, 方芬. 磁性固相萃取吸附剂的分类及其应用自动化[J]. 材料导报, 2014, 28(7): 72-77. |
| SUN A J, FANG F. Classification of magnetic solid-phase extraction sorbent and its application automation[J]. Materials Review, 2014, 28(7): 72-77. | |
| 13 | MAHPISHANIAN S, SERESHTI H, BAGHDADI M. Superparamagnetic core-shells anchored onto graphene oxide grafted with phenylethyl amine as a nano-adsorbent for extraction and enrichment of organophosphorus pesticides from fruit, vegetable and water samples[J]. Journal of Chromatography A, 2015, 1406: 48-58. |
| 14 | WANG Y K, OU Y H, XIE S Y, et al. Magnetic graphene solid-phase extraction for the determination of 47 kinds of non-steroidal anti-inflammatory drug residues in animal food with liquid chromatography tandem mass spectrometry[J]. Food Analytical Methods, 2019, 12(6): 1346-1368.[LinkOut] |
| 15 | ZOU S J, CHEN Y F, ZHANG Y, et al. A hybrid sorbent of α-iron oxide/reduced graphene oxide: studies for adsorptive removal of tetracycline antibiotics[J]. Journal of Alloys and Compounds, 2021, 836: 158475. |
| 16 | CAO X J, CHEN J Y, YE X M, et al. Ultrasound-assisted magnetic SPE based on Fe3O4-grafted graphene for the determination of polychlorinated biphenyls in water samples[J]. Journal of Separation Science, 2013, 36(21/22): 3579-3585. |
| 17 | PINSRITHONG S, BUNKOED O. Hierarchical porous nanostructured polypyrrole-coated hydrogel beads containing reduced graphene oxide and magnetite nanoparticles for extraction of phthalates in bottled drinks[J]. Journal of Chromatography A, 2018, 1570: 19-27. |
| 18 | 杨成雄, 严秀平. 金属-有机骨架ZIF-8@Fe3O4复合物的制备及其用于磁固相萃取对水中内分泌干扰物的测定[J]. 分析测试学报, 2019, 38(5): 563-568. |
| YANG C X, YAN X P. Fabrication of a metal-organic frameworks ZIF-8@Fe3O4 composite and its magnetic solid phase extraction of endocrine disrupting chemicals in water[J]. Journal of Instrumental Analysis, 2019, 38(5): 563-568. | |
| 19 | SUN W Y, WU H M, XU Z W, et al. Adsorption of heavy metal ions by carbon-nanofibers-blended carbon nanotubes[J]. Chemistry Select, 2018, 3(44): 12410-12414. |
| 20 | USMAN T M, SU X T, ZHAO M Q, et al. Preparation of hydroxypropyl-cyclodextrin-graphene/Fe3O4 and its adsorption properties for heavy metals[J]. Surfaces and Interfaces, 2019, 16: 43-49. |
| 21 | SUO L Z, DONG X Y, GAO X, et al. Silica-coated magnetic graphene oxide nanocomposite based magnetic solid phase extraction of trace amounts of heavy metals in water samples prior to determination by inductively coupled plasma mass spectrometry[J]. Microchemical Journal, 2019, 149: 1-10. |
| 22 | CHANG L, PU Y P, JING P, et al. Magnetic core-shell MnFe2O4@TiO2 nanoparticles decorated on reduced graphene oxide as a novel adsorbent for the removal of ciprofloxacin and Cu(Ⅱ) from water[J]. Applied Surface Science, 2021, 541: 148400. |
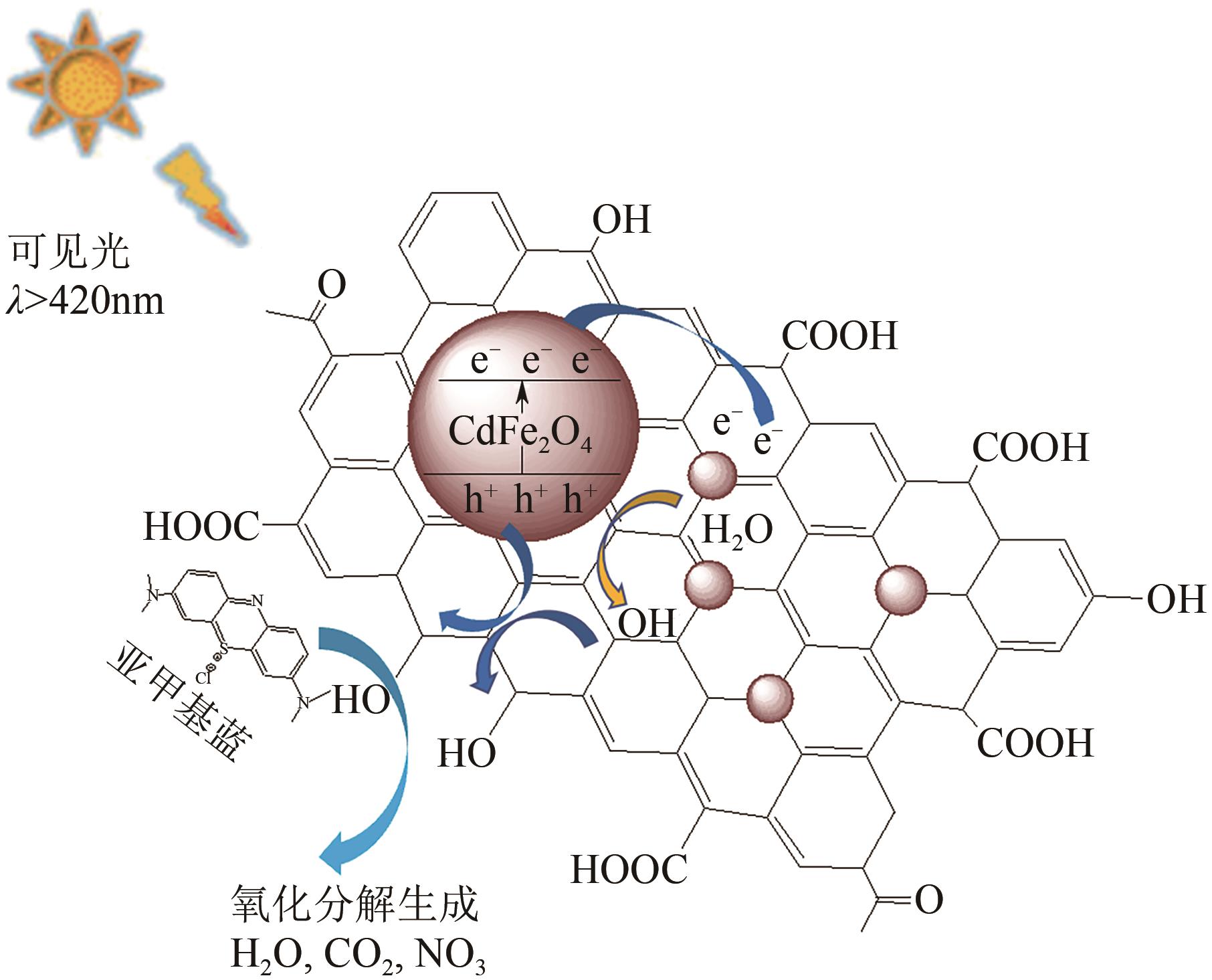
| 23 | ZHANG D F, WANG Q, WANG L, et al. Magnetically separable CdFe2O4/graphene catalyst and its enhanced photocatalytic properties[J]. Journal of Materials Chemistry A, 2015, 3(7): 3576-3585. |
| 24 | SHEORAN A, KAUR J, KAUR P, et al. Graphene based magnetic nanohybrids as promising catalysts for the green synthesis of β-amino alcohol derivatives[J]. Journal of Molecular Structure, 2020, 1204: 127522. |
| 25 | 杜晨辉, 周宇辰, 张卫红, 等. 磁性氧化石墨烯负载离子液体催化碳酸二甲酯的合成[J]. 现代化工, 2019, 39(9): 141-146. |
| DU C H, ZHOU Y C, ZHANG W H, et al. Synthesis of dimethyl carbonate catalyzed by magnetic graphene oxide supported ionic liquid[J]. Modern Chemical Industry, 2019, 39(9): 141-146. | |
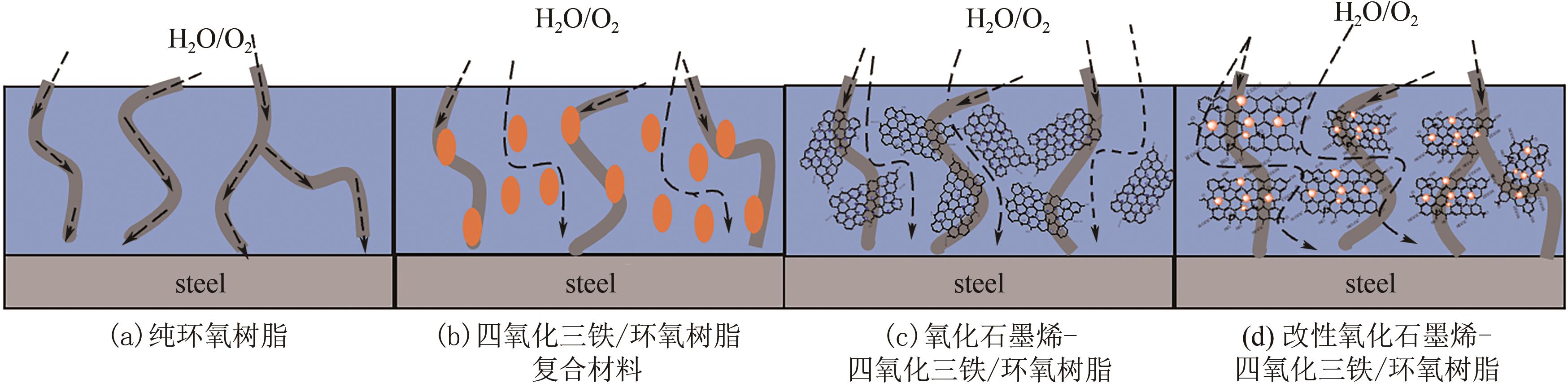
| 26 | ZHAN Y Q, ZHANG J M, WAN X Y, et al. Epoxy composites coating with Fe3O4 decorated graphene oxide: modified bioinspired surface chemistry, synergistic effect and improved anti-corrosion performance[J]. Applied Surface Science, 2018, 436: 756-767. |
| 27 | CHHETRI S, GHOSH S, SAMANTA P, et al. Effect of Fe3O4-decorated N-doped reduced graphene oxide nanohybrid on the anticorrosion performance of epoxy composite coating[J]. Chemistry Select, 2019, 4(46): 13446-13454. |
| 28 | 康帅, 乔士亚, 胡祖明, 等. 石墨烯基吸波材料的研究进展[J]. 中国材料进展, 2020, 39(1): 64-69. |
| KANG S, QIAO S Y, HU Z M, et al. Advances in graphene-based microwave absorption materials[J]. Materials China, 2020, 39(1): 64-69. | |
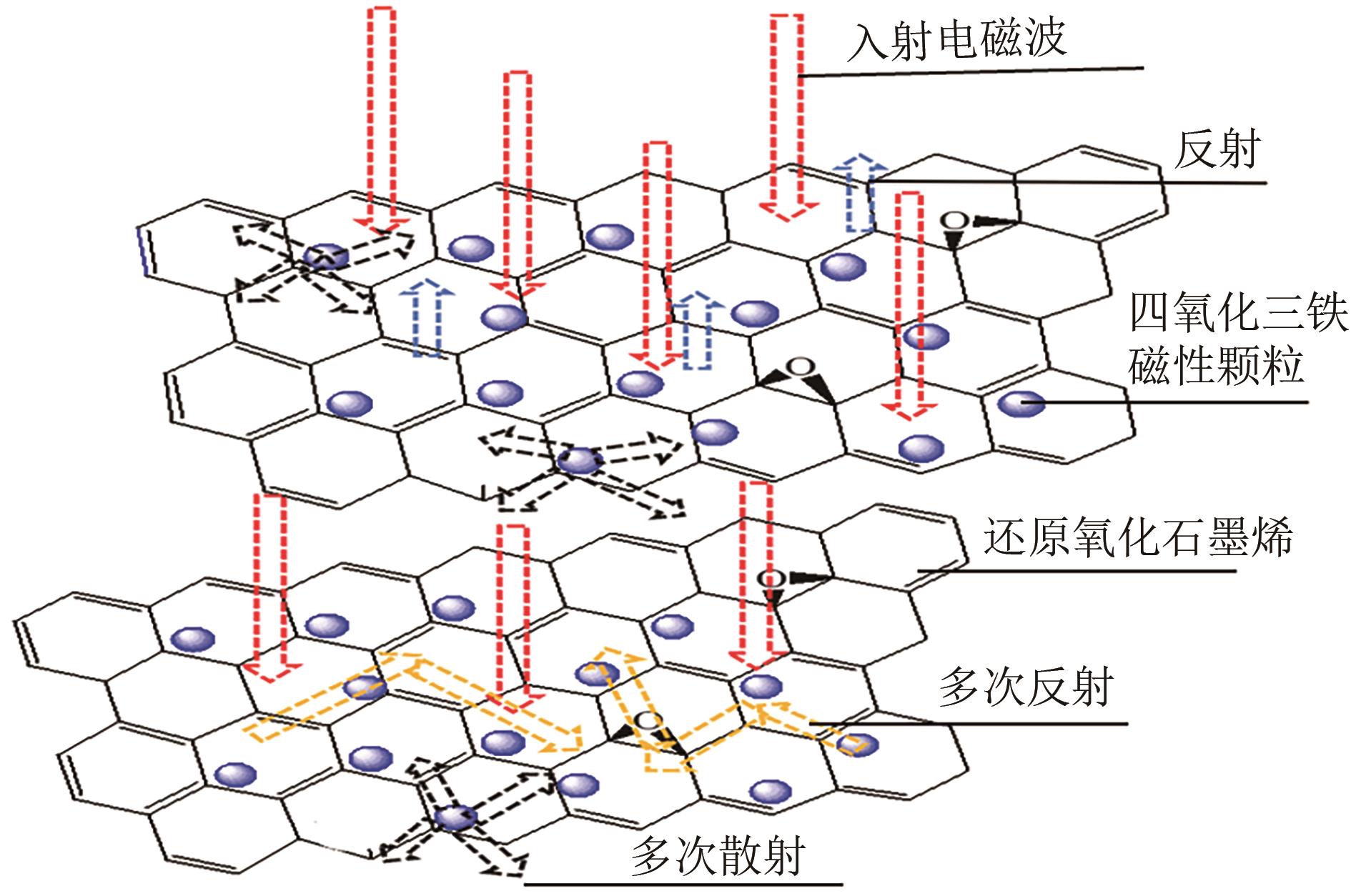
| 29 | BAI X, ZHAI Y H, ZHANG Y. Green approach to prepare graphene-based composites with high microwave absorption capacity[J]. The Journal of Physical Chemistry C, 2011, 115(23): 11673-11677. |
| 30 | SUN X, HE J P, LI G X, et al. Laminated magnetic graphene with enhanced electromagnetic wave absorption properties[J]. Journal of Materials Chemistry C, 2012, 1(4): 765-777. |
| 31 | LIU X D, HUANG Y, ZHANG N, et al. Fabrication of carbon-doped ZnCo2O4 yolk-shell microspheres compounded with magnetic graphene for enhanced electromagnetic wave absorption performance[J]. Ceramics International, 2019, 45(16): 19720-19729. |
| 32 | CROGUENNEC L, PALACIN M R. Recent achievements on inorganic electrode materials for lithium-ion batteries[J]. Journal of the American Chemical Society, 2015, 137(9): 3140-3156. |
| 33 | 李培. 石墨烯/四氧化三铁复合材料的制备及在锂离子电池中的应用研究[D]. 天津: 天津大学, 2014. |
| LI P. The preparation of graphene/Fe3O4 composite and its application in anode materials for lithium ion batteries[D]. Tianjin: Tianjin University, 2014. | |
| 34 | LI H P, WANG J Y,LI Y, et al. Hierarchical sandwiched Fe3O4@C/graphene composite as anode material for lithium-ion batteries[J]. Journal of Electroanalytical Chemistry, 2019, 847: 113240. |
| 35 | WU Q C, JIANG R L, LIU H W. Carbon layer encapsulated Fe3O4@reduced graphene oxide lithium battery anodes with long cycle performance[J]. Ceramics International, 2020, 46(8): 12732-12739. |
| 36 | LI Y, XU W K, ZHAO X R, et al. Electrochemical sensors based on molecularly imprinted polymers on Fe3O4/graphene modified by gold nanoparticles for highly selective and sensitive detection of trace ractopamine in water[J]. The Analyst, 2018, 143(21): 5094-5102. |
| 37 | FARANI M R, KHADIV-PARSI P, RIAZI G H, et al. PEGylation of graphene/iron oxide nanocomposite: assessment of release of doxorubicin, magnetically targeted drug delivery and photothermal therapy[J]. Applied Nanoscience, 2020, 10(4): 1205-1217. |
| 38 | LIANG W T, HUANG Y, LU D T, et al. β-cyclodextrin-hyaluronic acid polymer functionalized magnetic graphene oxide nanocomposites for targeted photo-chemotherapy of tumor cells[J]. Polymers, 2019, 11(1): 133. |
| 39 | 贾纬民. 一种持压连续、微波膨化制备石墨烯的方法及设备: CN201510091213.4[P]. 2017-03-15. |
| JIA W M. Method and device for preparing graphene by holding pressure continuous microwave puffing: CN201510091213.4[P]. 2017-03-15. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [6] | 胡喜, 王明珊, 李恩智, 黄思鸣, 陈俊臣, 郭秉淑, 于博, 马志远, 李星. 二硫化钨复合材料制备与储钠性能研究进展[J]. 化工进展, 2023, 42(S1): 344-355. |
| [7] | 王正坤, 黎四芳. 双子表面活性剂癸炔二醇的绿色合成[J]. 化工进展, 2023, 42(S1): 400-410. |
| [8] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [9] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [10] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [11] | 许友好, 王维, 鲁波娜, 徐惠, 何鸣元. 中国炼油创新技术MIP的开发策略及启示[J]. 化工进展, 2023, 42(9): 4465-4470. |
| [12] | 耿源泽, 周俊虎, 张天佑, 朱晓宇, 杨卫娟. 部分填充床燃烧器中庚烷均相/异相耦合燃烧[J]. 化工进展, 2023, 42(9): 4514-4521. |
| [13] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [14] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [15] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||