化工进展 ›› 2021, Vol. 40 ›› Issue (S2): 315-321.DOI: 10.16085/j.issn.1000-6613.2021-0962
CO2在ZIF-8/乙二醇-2-甲基咪唑浆液中溶解能力理论分析
- 1.油气藏地质及开发工程国家重点实验室,西南石油大学,四川 成都 610500
2.胜利油田鲁明油气勘探开发 有限公司,山东 东营 257000
-
收稿日期:2021-05-06修回日期:2021-07-06出版日期:2021-11-12发布日期:2021-11-12 -
通讯作者:刘煌 -
作者简介:姚德松(1999—),男,硕士研究生,研究方向为油气藏开发。E-mail:1938765506@qq.com 。 -
基金资助:国家自然科学基金(21606184);四川省科技计划(2021YFQ0044)
Theoretical analysis of CO2 dissolution ability in ZIF-8/glycol-2-methylimidazole slurry
YAO Desong1( ), LIU Huang1(
), LIU Huang1( ), CHEN Li2, LI Ruijing1, WANG Jian1
), CHEN Li2, LI Ruijing1, WANG Jian1
- 1.State Key Lab of Oil and Gas Reservoir Geology and Exploitation (Southwest Petroleum University), Chengdu 610500, Sichuan, China
2.Shengli Oilfield Luming Oil-Gas Exploration & Development Limited Company, Dongying 257000, Shandong, China
-
Received:2021-05-06Revised:2021-07-06Online:2021-11-12Published:2021-11-12 -
Contact:LIU Huang
摘要:
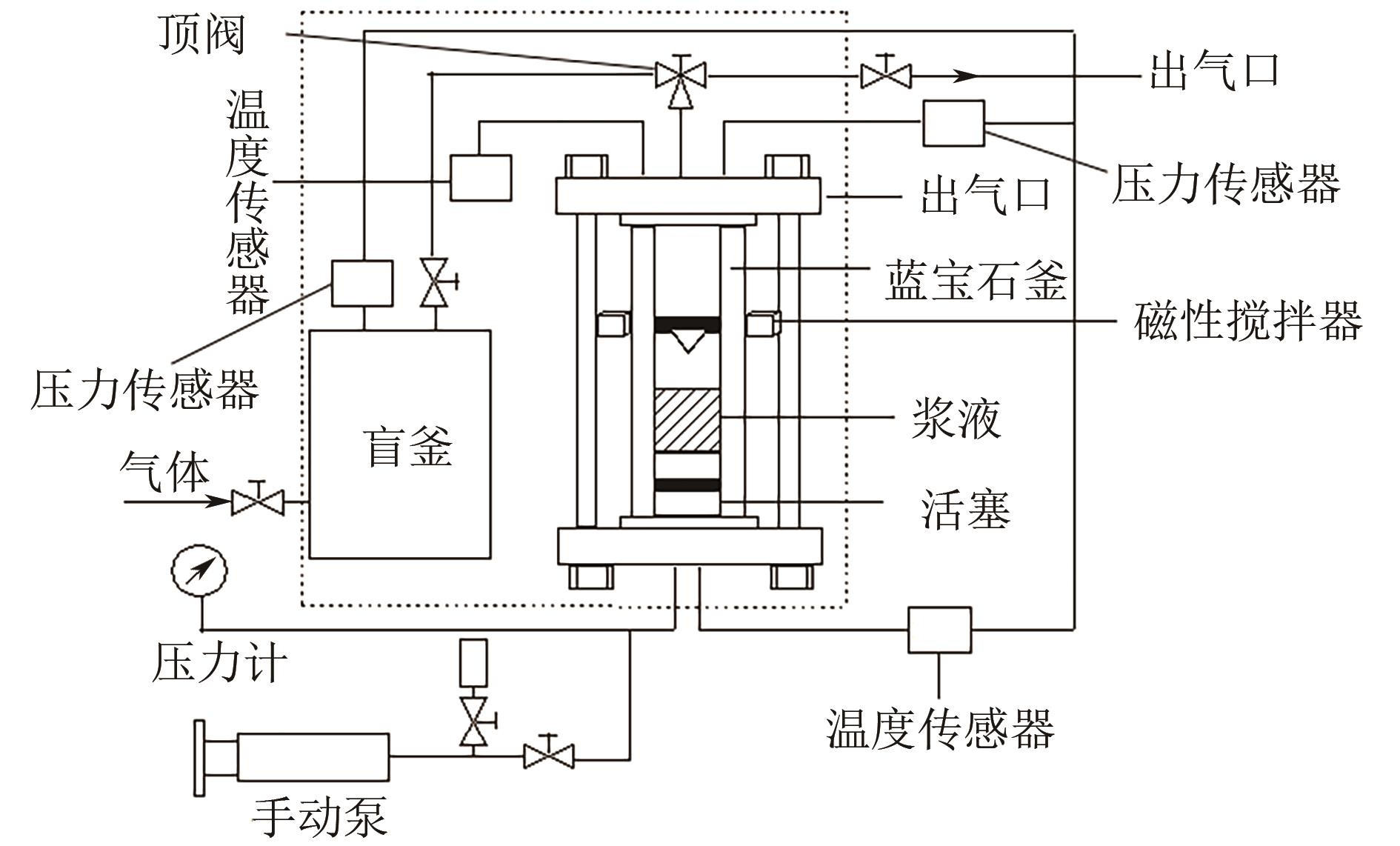
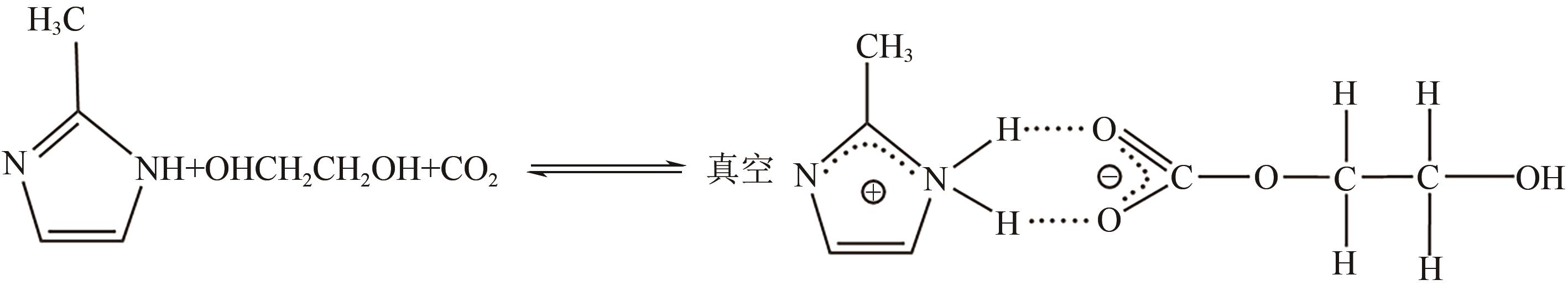
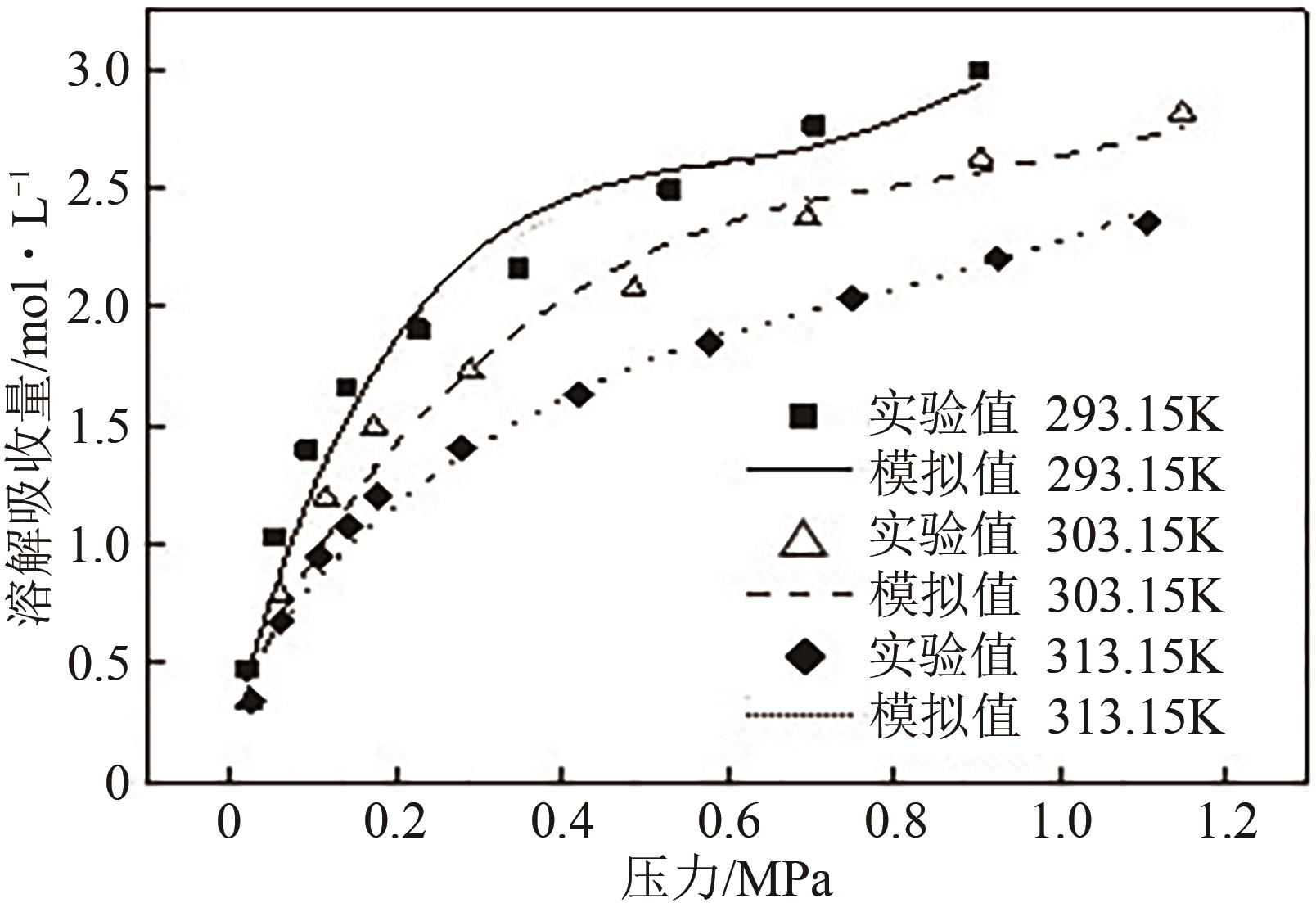
有效降低CO2排量实现碳中和是当前研究热点之一。ZIF-8/乙二醇-2-甲基咪唑浆液被发现不仅能高效地捕集CO2,同时能利用浆液良好的流动性实现一个碳捕集-浆液流动再生-浆液再利用的连续碳捕集过程。为了有效地掌握纯CO2气体在ZIF-8/乙二醇-2-甲基咪唑浆液中的溶解能力,本文首先测定了293.15K、303.15K和313.15K下CO2在干ZIF-8上的吸附量和在2-甲基咪唑-乙二醇溶液中的溶解性;然后基于Langmuir方程和CO2溶解机理进一步建立了相应吸附量和溶解度计算数学模型;最后综合考虑ZIF-8/乙二醇-2-甲基咪唑浆液中乙二醇和ZIF-8之间的共存特征和相互影响,建立了CO2在目标浆液中溶解吸收量计算数学关联式。所得研究结果对浆液法CO2技术的推广和后续流程模拟具有良好的指导作用和理论意义。
中图分类号:
引用本文
姚德松, 刘煌, 陈莉, 李瑞景, 王舰. CO2在ZIF-8/乙二醇-2-甲基咪唑浆液中溶解能力理论分析[J]. 化工进展, 2021, 40(S2): 315-321.
YAO Desong, LIU Huang, CHEN Li, LI Ruijing, WANG Jian. Theoretical analysis of CO2 dissolution ability in ZIF-8/glycol-2-methylimidazole slurry[J]. Chemical Industry and Engineering Progress, 2021, 40(S2): 315-321.
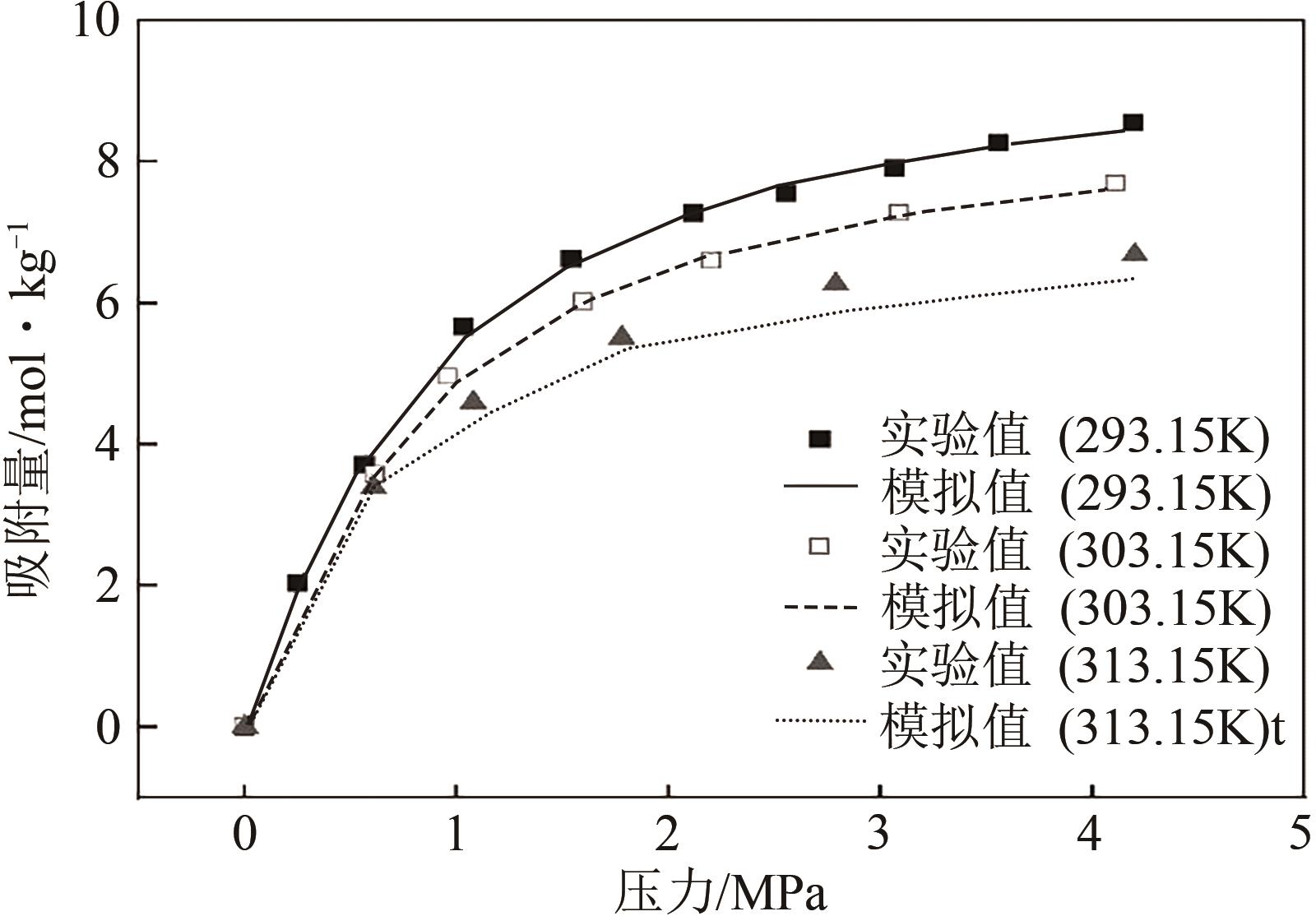
| 293.15K | 303.15K | 313.15K | |||
|---|---|---|---|---|---|
| 压力 /MPa | 吸附量 /mol·kg-1 | 压力 /MPa | 吸附量 /mol·kg-1 | 压力 /MPa | 吸附量 /mol·kg-1 |
| 0 | 0 | 0 | 0 | 0 | 0 |
| 0.25 | 2.028 | 0.617 | 3.575 | 0.61 | 3.389 |
| 0.567 | 3.711 | 0.959 | 4.956 | 1.08 | 4.588 |
| 1.031 | 5.659 | 1.599 | 6.018 | 1.78 | 5.510 |
| 1.541 | 6.627 | 2.202 | 6.592 | 2.79 | 6.267 |
| 2.115 | 7.274 | 3.092 | 7.273 | 4.2 | 6.681 |
| 2.552 | 7.552 | 4.11 | 7.691 | ||
| 3.066 | 7.913 | ||||
| 3.556 | 8.277 | ||||
| 4.192 | 8.564 | ||||
表1 CO2在干ZIF-8材料上吸附实验结果
| 293.15K | 303.15K | 313.15K | |||
|---|---|---|---|---|---|
| 压力 /MPa | 吸附量 /mol·kg-1 | 压力 /MPa | 吸附量 /mol·kg-1 | 压力 /MPa | 吸附量 /mol·kg-1 |
| 0 | 0 | 0 | 0 | 0 | 0 |
| 0.25 | 2.028 | 0.617 | 3.575 | 0.61 | 3.389 |
| 0.567 | 3.711 | 0.959 | 4.956 | 1.08 | 4.588 |
| 1.031 | 5.659 | 1.599 | 6.018 | 1.78 | 5.510 |
| 1.541 | 6.627 | 2.202 | 6.592 | 2.79 | 6.267 |
| 2.115 | 7.274 | 3.092 | 7.273 | 4.2 | 6.681 |
| 2.552 | 7.552 | 4.11 | 7.691 | ||
| 3.066 | 7.913 | ||||
| 3.556 | 8.277 | ||||
| 4.192 | 8.564 | ||||
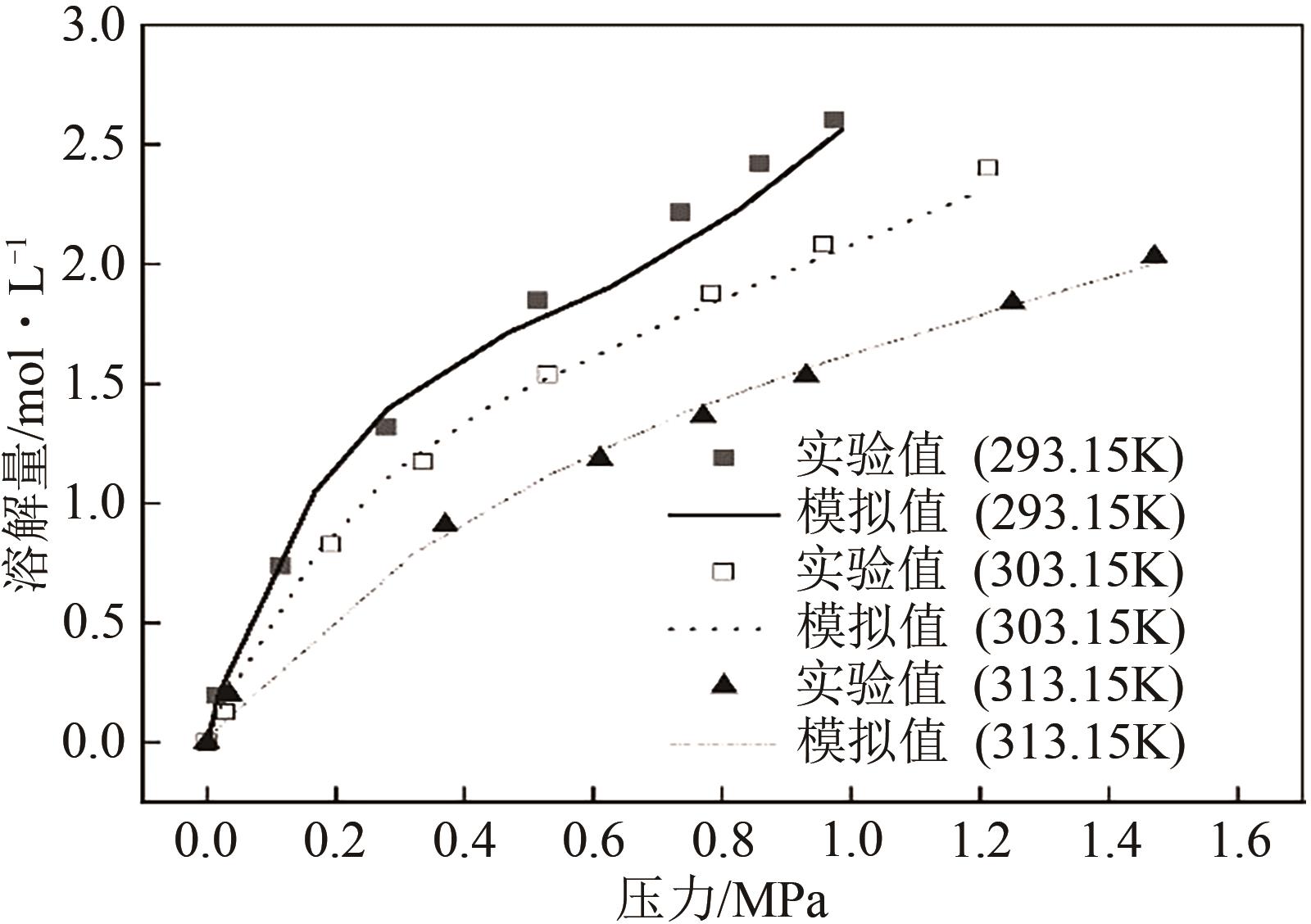
| 293.15K | 303.15K | 313.15K | |||
|---|---|---|---|---|---|
| 压力 /MPa | 溶解度 /mol·L-1 | 压力 /MPa | 溶解度 /mol·L-1 | 压力 /MPa | 溶解度 /mol·L-1 |
| 0 | 0 | 0 | 0 | 0 | 0 |
| 0.0153 | 0.195 | 0.0289 | 0.126 | 0.035 | 0.201 |
| 0.114 | 0.738 | 0.193 | 0.826 | 0.37 | 0.907 |
| 0.279 | 1.319 | 0.335 | 1.172 | 0.61 | 1.185 |
| 0.513 | 1.851 | 0.529 | 1.537 | 0.77 | 1.365 |
| 0.735 | 2.217 | 0.783 | 1.877 | 0.93 | 1.534 |
| 0.857 | 2.422 | 0.957 | 2.082 | 1.25 | 1.837 |
| 0.974 | 2.607 | 1.213 | 2.401 | 1.47 | 2.031 |
表2 CO2在2-甲基咪唑-乙二醇溶液中溶解实验结果
| 293.15K | 303.15K | 313.15K | |||
|---|---|---|---|---|---|
| 压力 /MPa | 溶解度 /mol·L-1 | 压力 /MPa | 溶解度 /mol·L-1 | 压力 /MPa | 溶解度 /mol·L-1 |
| 0 | 0 | 0 | 0 | 0 | 0 |
| 0.0153 | 0.195 | 0.0289 | 0.126 | 0.035 | 0.201 |
| 0.114 | 0.738 | 0.193 | 0.826 | 0.37 | 0.907 |
| 0.279 | 1.319 | 0.335 | 1.172 | 0.61 | 1.185 |
| 0.513 | 1.851 | 0.529 | 1.537 | 0.77 | 1.365 |
| 0.735 | 2.217 | 0.783 | 1.877 | 0.93 | 1.534 |
| 0.857 | 2.422 | 0.957 | 2.082 | 1.25 | 1.837 |
| 0.974 | 2.607 | 1.213 | 2.401 | 1.47 | 2.031 |
| 温度/K | 饱和吸附量A | 系数1 | 系数2 |
|---|---|---|---|
| 293.15 | 9.7966 | 1.2403 | 1.1435 |
| 303.15 | 8.7831 | 1.2650 | 1.1801 |
| 313.15 | 7.7411 | 1.3359 | 1.0952 |
表3 CO2吸附模型参数汇总
| 温度/K | 饱和吸附量A | 系数1 | 系数2 |
|---|---|---|---|
| 293.15 | 9.7966 | 1.2403 | 1.1435 |
| 303.15 | 8.7831 | 1.2650 | 1.1801 |
| 313.15 | 7.7411 | 1.3359 | 1.0952 |
| 温度/K | 生成焓(Hm)/kJ·mol-1 | 平衡常数(km) |
|---|---|---|
| 293.15 | 3.8441 | 0.1340 |
| 303.15 | 5.3812 | 0.1139 |
| 313.15 | 8.1756 | 0.0765 |
表4 参数生成焓和平衡常数数值
| 温度/K | 生成焓(Hm)/kJ·mol-1 | 平衡常数(km) |
|---|---|---|
| 293.15 | 3.8441 | 0.1340 |
| 303.15 | 5.3812 | 0.1139 |
| 313.15 | 8.1756 | 0.0765 |
| 系数 | 293.15K | 303.15K | 313.15K |
|---|---|---|---|
| x1 | 4.0171 | 1.6542 | -1.1366 |
| x2 | -88.0456 | -68.0804 | -65.2812 |
| x3 | -1.0840 | -1.7063 | -3.0891 |
| x4 | 0.4112 | -0.1267 | 0.2531 |
| x5 | 4.4929 | 3.7818 | 4.2774 |
| x6 | 2.2519 | 2.8605 | 4.5994 |
表5 系数x1~x6数值
| 系数 | 293.15K | 303.15K | 313.15K |
|---|---|---|---|
| x1 | 4.0171 | 1.6542 | -1.1366 |
| x2 | -88.0456 | -68.0804 | -65.2812 |
| x3 | -1.0840 | -1.7063 | -3.0891 |
| x4 | 0.4112 | -0.1267 | 0.2531 |
| x5 | 4.4929 | 3.7818 | 4.2774 |
| x6 | 2.2519 | 2.8605 | 4.5994 |
| 1 | 崔国凯, 吕书贞, 王键吉. 功能化离子液体在二氧化碳吸收分离中的应用[J].化工学报,2020,71(1):16-25. |
| CUI G K, LYU S Z, WANG J J. Functional ionic liquids for carbon dioxide capture and separation[J]. CIESC Journal, 2020, 71(1): 16-25. | |
| 2 | 亓士超, 朱蓉蓉, 刘昕,等.乙二胺不同掺杂模式下多孔有机聚合物对CO2的吸附[J]. 化工学报, 2020, 71(4): 1666-1675. |
| QI S C, ZHU R R, LIU X, et al. CO2 adsorption over porous organic polymers with different doping modes of ethanediamine[J]. CIESC Journal, 2020, 71(4): 1666-1675. | |
| 3 | 吴彬, 黄坤荣, 刘子健.化学吸收法捕集二氧化碳研究进展[J]. 广州化工, 2017, 45(11): 11-14. |
| WU B, HUANG K R, LIU Z J. Research progress on carbon dioxide capture by chemical absorption[J]. Guangzhou Chemical Industry, 2017, 45(11): 11-14. | |
| 4 | 安山龙, 侯天锐, 臧欣怡, 等. 燃煤烟气CO2化学吸收剂研究进展[J]. 广州化工, 2019, 47(3): 19-21. |
| AN S L, HOU T R, ZANG X Y, et al. Research progress on chemical absorbents for coal-fired flue gas CO2[J]. Guangzhou Chemical Industry, 2019, 47(3): 19-21. | |
| 5 | 许咪咪, 王淑娟. 液-液相变溶剂捕集CO2技术研究进展[J]. 化工学报, 2018, 69(5): 1809-1818. |
| XU M M, WANG S J. Research progress in CO2 capture technology using liquid-liquid biphasic solvents[J]. CIESC Journal, 2018, 69(5): 1809-1818. | |
| 6 | 王建行, 赵颖颖, 李佳慧, 等. 二氧化碳的捕集、固定与利用的研究进展[J]. 无机盐工业, 2020, 52(4): 12-17. |
| WANG J H, ZHAO Y Y, LI J H, et al. Research progress of carbon dioxide capture, fixation and utilization[J]. Inorganic Chemicals Industry, 2020, 52(4): 12-17. | |
| 7 | 樊佑坤, 陈彤, 王公应. 二碳酸酯的二氧化碳吸收性能研究[J]. 化学研究与应用, 2020, 32(12): 2218-2223. |
| FAN Y K, CHEN T, WANG G Y. A study on carbon dioxide absorption by di-carbonates[J]. Chemical Research and Application, 2020, 32(12): 2218-2223. | |
| 8 | 谭方园, 李康康, 于海, 等. 金属离子促进乙醇胺捕集二氧化碳研究[J].化工学报,2021,72(2): 1026-1035. |
| TAN F Y, LI K K, YU H, et al. Metal ions mediated amine-based post-combustion CO2 capture[J]. CIESC Journal, 2021, 72(2): 1026-1035. | |
| 9 | JUNG W, LEE J S, YOON H, et al. Water membrane for carbon dioxide separation[J]. Separation and Purification Technology, 2018, 203: 268-273. |
| 10 | 张卫风, 李娟, 王秋华. Ca(OH)2解吸并固定混合吸收液中CO2的实验研究[J]. 环境科学学报, 2020, 40(4): 1436-1442. |
| ZHANG W F, LI J, WANG Q H. Experimental study on desorption and mineralization of CO2 in amine-rich solution[J]. Acta Scientiae Circumstantiae, 2020, 40(4): 1436-1442. | |
| 11 | 何凯武, 唐思扬, 刘长军, 等. 有机胺功能化介孔固体吸附剂吸附分离CO2性能研究[J]. 化工学报, 2018, 69(9): 3887-3895. |
| HE K W, TANG S Y, LIU C J, et al. Performance of amine functionalized mesoporous adsorbents for CO2 adsorption[J]. CIESC Journal,2018,69(9):3887-3895. | |
| 12 | 王芃, 胡云峰. CO2分离方法的研究进展[J]. 化工科技, 2019, 27(2): 62-65. |
| WANG P, HU Y F. Advances in carbon dioxide separation[J]. Science & Technology in Chemical Industry, 2019, 27(2): 62-65. | |
| 13 | ALIVIA M, JUDE A O, AMIRA A, et al. Review of post-combustion carbon dioxide capture technologies using activated carbon[J]. Journal of Environmental Sciences, 2019, 83(9): 46-63. |
| 14 | 张宇航,李伟,马春慧,等.多孔炭材料吸附CO2研究进展[J]. 林产化学与工业, 2021, 41(1): 107-122. |
| ZHANG Y H, LI W, MA C H, et al. Progress of research on CO2 adsorption by porous carbon materials[J]. Chemistry and Industry of Forest Products, 2021, 41(1): 107-122. | |
| 15 | 靖宇,韦力,王运东.吸附法捕集二氧化碳吸附剂的研究进展[J]. 化工进展, 2011, 30(S2): 133-138. |
| JING Y, WEI L, WANG Y D. The advances of adsorbents in the field of CO2 capture[J]. Chemical Industry and Engineering Progress, 2011, 30(S2): 133-138. | |
| 16 | 徐纯刚, 李小森, 陈朝阳. 水合物法分离二氧化碳的研究现状[J]. 化工进展, 2011, 30(4): 701-708. |
| XU C G, LI X S, CHEN Z Y. Research on hydrate-based carbon dioxide separation[J]. Chemical Industry and Engineering Progress, 2011, 30(4): 701-708. | |
| 17 | 樊栓狮, 尤莎莉, 郎雪梅, 等. 笼型水合物膜分离和捕获二氧化碳研究进展[J]. 化工进展, 2020, 39(4): 1211-1218. |
| FAN S S, YOU S, LANG X M, et al. Separation and capture carbon dioxide by clathrate-hydrate membranes:a review[J]. Chemical Industry and Engineering Progress, 2020, 39(4): 1211-1218. | |
| 18 | 谢文俊,李小森,邹颖楠, 等.含环戊烷体系中二氧化碳水合物形成分解热特性[J]. 化工进展, 2020, 39(1): 129-136. |
| XIE W J, LI X S, ZOU Y N, et al. Characteristics of carbon dioxide hydrate formation and decomposition with the system of cyclopentane[J]. Chemical Industry and Engineering Progress, 2020, 39(1): 129-136. | |
| 19 | 李琦, 蔡博峰, 陈帆, 等. 二氧化碳地质封存的环境风险评价方法研究综述[J]. 环境工程, 2019, 37(2): 13-21. |
| LI Q, CAI B F, CHEN F, et al. Review of environmental risk assessment methods for carbon dioxide geological storage[J]. Environmental Engineering, 2019, 37(2): 13-21. | |
| 20 | 孙玉景, 周立发, 李越. CO2海洋封存的发展现状[J]. 地质科技情报, 2018, 37(4): 212-218. |
| SUN Y J, ZHOU L F, LI Y. Development status of CO2 marine sequestration[J]. Geological Science and Technology Information, 2018, 37(4): 212-218. | |
| 21 | 杨红, 赵习森, 康宇龙,等. 鄂尔多斯盆地CO2地质封存适宜性与潜力评价[J]. 气候变化研究进展, 2019, 15(1): 95-102. |
| YANG H, ZHAO X S, KANG Y L, et al. Evaluation on geological sequestration suitability and potential of CO2 in Ordos Basin[J]. Climate Change Research, 2019, 15(1): 95-102. | |
| 22 | 王建秀,吴远斌,于海鹏. 二氧化碳封存技术研究进展[J]. 地下空间与工程学报, 2013, 9(1): 81-90. |
| WANG J B, WU Y B, YU H P. Review of the technology for sequestration of carbon dioxide[J]. Chinese Journal of Underground Space and Engineering, 2013,9(1):81-90. | |
| 23 | 陈兵,肖红亮,李景明, 等.二氧化碳捕集、利用与封存研究进展[J].应用化工, 2018, 47(3): 589-592. |
| CHEN B, XIAO H L, LI J M, et al. Advances in research on carbon capture, utilization and storage[J]. Applied Chemical Industry, 2018, 47(3): 589-592. | |
| 24 | 郭平, 许清华, 孙振,等. 天然气藏CO2驱及地质埋存技术研究进展[J]. 岩性油气藏, 2016, 28(3): 6-11. |
| GUO P, XU Q H, SUN Z, et al. Research progress of CO2 flooding and geological storage in gas reservoirs[J]. Lithologic Reservoirs, 2016, 28(3): 6-11. | |
| 25 | 张忠林, 王伟, 赵习森, 等. 注CO2对延长化子坪原油物性的影响[J]. 油田化学, 2019, 36(4): 646-650. |
| ZHANG Z L, WANG W, ZHAO X S, et al. Effect of CO2 injection on physical properties of huaziping crude oil in Yanchang oilfiled[J]. Oilfield Chemistry, 2019, 36(4): 646-650. | |
| 26 | 郭茂雷, 黄春霞, 董小刚, 等. 延长油田致密砂岩油藏CO2驱油机理研究[J]. 石油与天然气化工, 2018, 47(2): 75-79, 88. |
| GUO M L, HUANG C X, DONG X G, et al. CO2 EOR mechanism of tight sandstone reservoir in Yanchang oilfield[J]. Chemical Engineering of Oil & Gas, 2018, 47(2): 75-79, 88. | |
| 27 | 曾亮, 罗四维, 李繁星,等. 化学链技术及其在化石能源转化与二氧化碳捕集领域的应用[J]. 中国科学: 化学, 2012, 42(3): 260-281. |
| ZENG L, LUO S W, LI F X, et al. Chemical looping technology and its applications in fossil fuel conversion and CO2 capture[J]. Scientia Sinica (Chimica), 2012, 42(3): 260-281. | |
| 28 | LIU H, LIU B, LIN L C, et al. A hybrid absorption-adsorption method to efficiently capture carbon[J]. Nature Communications, 2014, 5: 5147-5153. |
| 29 | 刘煌, 吴雨晴, 陈光进,等. 油水乳液分离沼气实验研究[J]. 化工学报, 2014, 65(5):1743-1749. |
| LIU H, WU Y Q, CHEN G J, et al. Separation of biogas with W/O emulsion[J]. CIESC Journal, 2014, 65(5): 1743-1749. | |
| 30 | LI J, LIU B, ZHANG X R, et al. Hydrogen bond networks of glycol molecules on ZIF-8 surfaces as semipermeable films for efficient carbon capture[J]. The Journal of Physical Chemistry C, 2017, 121(45): 25347-25352. |
| [1] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [2] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [3] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [4] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [5] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [6] | 徐晨阳, 都健, 张磊. 基于图神经网络的化学反应优劣评价[J]. 化工进展, 2023, 42(S1): 205-212. |
| [7] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [8] | 陈林, 徐培渊, 张晓慧, 陈杰, 徐振军, 陈嘉祥, 密晓光, 冯永昌, 梅德清. 液化天然气绕管式换热器壳侧混合工质流动及传热特性[J]. 化工进展, 2023, 42(9): 4496-4503. |
| [9] | 张帆, 陶少辉, 陈玉石, 项曙光. 基于改进恒热传输模型的精馏模拟初始化[J]. 化工进展, 2023, 42(9): 4550-4558. |
| [10] | 王琦, 寇丽红, 王冠宇, 王吉坤, 刘敏, 李兰廷, 王昊. 焦化废水生物出水中可溶解性有机物的分子识别[J]. 化工进展, 2023, 42(9): 4984-4993. |
| [11] | 张智琛, 朱云峰, 成卫戍, 马守涛, 姜杰, 孙冰, 周子辰, 徐伟. 高压聚乙烯失控分解研究进展:反应机理、引发体系与模型[J]. 化工进展, 2023, 42(8): 3979-3989. |
| [12] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [13] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| [14] | 黄玉飞, 李子怡, 黄杨强, 金波, 罗潇, 梁志武. 光催化CO2和CH4重整催化剂研究进展[J]. 化工进展, 2023, 42(8): 4247-4263. |
| [15] | 张振, 李丹, 陈辰, 吴菁岚, 应汉杰, 乔浩. 吸附树脂对唾液酸的分离纯化[J]. 化工进展, 2023, 42(8): 4153-4158. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||