化工进展 ›› 2021, Vol. 40 ›› Issue (3): 1653-1666.DOI: 10.16085/j.issn.1000-6613.2020-0883
基于碳基催化剂活化过二硫酸盐降解有机污染物的研究进展
孙金龙1( ), 张宇1, 刘福跃1, 田浩然1, 刘崎峰1,2(
), 张宇1, 刘福跃1, 田浩然1, 刘崎峰1,2( )
)
- 1.内蒙古大学生态与环境学院,内蒙古 呼和浩特 010021
2.内蒙古自治区煤化工废水处理与资源化工程技术 研究中心,内蒙古 呼和浩特 010021
-
收稿日期:2020-05-22出版日期:2021-03-05发布日期:2021-03-17 -
通讯作者:刘崎峰 -
作者简介:孙金龙(1995—),男,硕士研究生,研究方向为水污染控制与处理技术。E-mail:18332723925@163.com 。 -
基金资助:内蒙古自然基金面上项目(2017MS0522);内蒙古“一湖两海”水污染控制与综合治理关键技术研发与集成示范(ZDZX2018054);国家自然科学基金地区基金(51860854);内蒙古自治区科技创新引导(KCBJ2018005)
Research progress in degradation of organic pollutants by activation of persulfates with carbon-based catalysts
SUN Jinlong1( ), ZHANG Yu1, LIU Fuyue1, TIAN Haoran1, LIU Qifeng1,2(
), ZHANG Yu1, LIU Fuyue1, TIAN Haoran1, LIU Qifeng1,2( )
)
- 1.School of Ecology and Environment, Inner Mongolia University, Hohhot 010021, Inner Mongolia, China
2.Inner Mongolia Coal Chemical Wastewater Treatment and Resource Utilization Engineering Technology Research Center, Hohhot 010021, Inner Mongolia, China
-
Received:2020-05-22Online:2021-03-05Published:2021-03-17 -
Contact:LIU Qifeng
摘要:
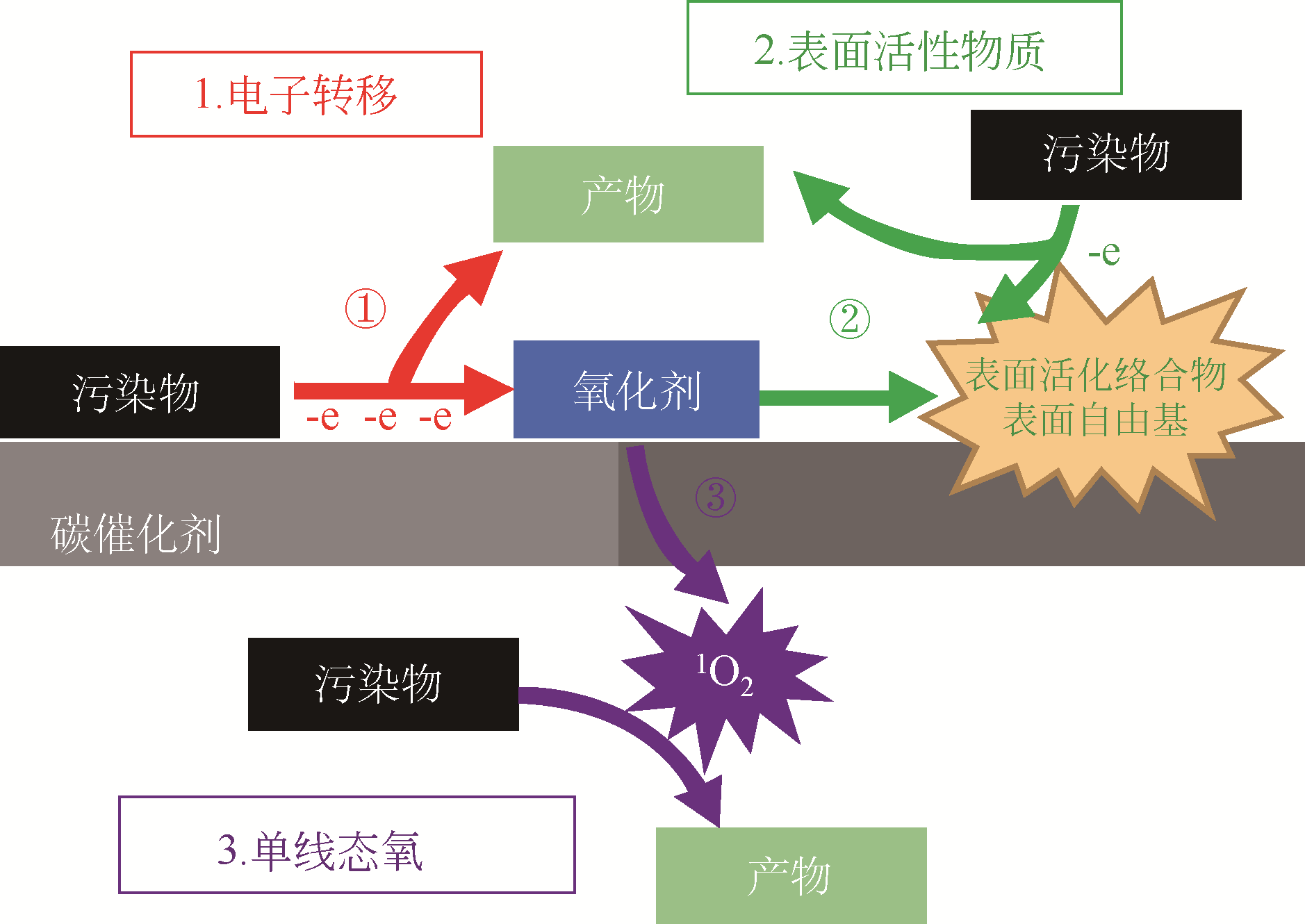
碳基催化剂作为一种绿色催化材料,可以有效防止有毒金属离子的浸出和二次污染。本文首先对碳基催化剂活化过二硫酸盐(PDS)降解有机污染物存在的三种反应机制进行了具体的阐述,对自由基机制和非自由基机制的优缺点进行了对比和讨论,对使用不同碳基材料(包括活性炭、石墨烯、碳纳米管、中孔炭、纳米金刚石、生物炭)作为催化剂活化过二硫酸盐降解有机污染物的研究进展进行了梳理,比较了不同碳基催化剂对有机污染物的选择性和降解效果,并对各种碳基材料存在的问题进行了总结;然后探讨了碳基催化剂掺杂改性对催化活性的影响及其机理,针对碳基催化剂存在稳定性和重复利用性差的问题介绍了几种碳催化剂再生方法,最后对碳催化剂用于活化PDS降解实际有机废水的前景做出了展望。
中图分类号:
引用本文
孙金龙, 张宇, 刘福跃, 田浩然, 刘崎峰. 基于碳基催化剂活化过二硫酸盐降解有机污染物的研究进展[J]. 化工进展, 2021, 40(3): 1653-1666.
SUN Jinlong, ZHANG Yu, LIU Fuyue, TIAN Haoran, LIU Qifeng. Research progress in degradation of organic pollutants by activation of persulfates with carbon-based catalysts[J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1653-1666.
| 催化剂 | 催化剂来源 | 目标污染物 | 反应机制 | 催化活性评价 | 参考文献 |
|---|---|---|---|---|---|
| 活性炭 | 商业购买 | 对氯苯胺(PCA) | 非自由基机制 | 在0.5mmol·L-1 PCA,2.5mmol·L-1 PDS,5g·L-1 AC和 pH=7.0的条件下,120min内PDS/AC系统的PCA降解效率为98.03%,TOC去除率约41% 在30min内实现PCA快速脱氯,并且在初始pH 3~9时PCA去除无明显影响 | [ |
| 煤质活性炭、木质活性炭、椰壳活性炭 | 商业购买 | 偶氮染料橙黄G | 非自由基机制 | 3个体系对染料的去除率在120min内均可达到97%以上 活性炭/过硫酸盐体系对1~100mmol·L-1氯化钠有很强的耐受作用,适用于高盐废水中有机污染物的去除 | [ |
| 表面改性活性炭ACNH | 硝酸氧化联合高温处理 | 苯酚 | 非自由基机制 | 苯酚浓度80mg·L-1,活性炭投加量0.4g·L-1,PDS与苯酚摩尔比3∶1,初始pH=6.6,ACNH/PS体系反应2h苯酚的降解率达到100% 改性后苯酚的降解速率是未改性的5倍,ACNH的pH适用范围宽 | [ |
| 原始石墨烯、氧化石墨烯、纳米石墨粉末 | 商业购买 | 对羟基苯甲酸酯(PP) | 自由基机制 | 在pH=9,PP浓度1mg·L-1,催化剂量500mg·L-1,PDS投加量20mg·L-1,原始石墨烯,纳米石墨粉末在20min内PP降解率超过95%,GO在20min内PP降解约为70% | [ |
| 还原氧化石墨烯rGO-900 | 改良Hummers法 | 苯酚、2,4-二氯苯酚、 邻苯二酚、1,4-二羟基苯、亚甲基蓝 | 自由基机制 | 催化剂投加量0.2g·L-1,6.5mmol·L-1 PDS,苯酚浓度20μg·g-1,30min内可使苯酚完全降解。催化活性:rGO-900>CMK-8>SWCNT>g-C3N4>C60 对其他有机污染物(2,4-二氯苯酚,邻苯二酚和 1,4-二羟基苯,亚甲基蓝)均表现出极大的催化氧化效率 | [ |
| 多壁碳纳米管 | 商业购买 | 2,4-二氯苯酚、邻苯二甲酸二甲酯、双酚A、邻苯二甲酸二乙酯、己烯雌酚 | 非自由基机制 | 催化剂量0.1g·L-1,PDS和2,4-DCP量为0.031mmol·L-1,在30min时PDS/CNTs系统中2,4-DCP的降解率高达95.9%,实现了40%的矿化 对酚类化合物产生选择性降解,BPA、DES、苯酚和 2,4-DCP 30min内得到有效降解 | [ |
| 单壁碳纳米管SWCNT多壁碳纳米管MWCNT | 商业购买 | 苯酚、对乙酰氨基酚、 三氯苯酚、磺胺甲 唑、 普萘洛尔、卡马西平、 4-氯苯酚、双酚A、苯甲酸、硝基苯、糠醇 唑、 普萘洛尔、卡马西平、 4-氯苯酚、双酚A、苯甲酸、硝基苯、糠醇 | 非自由基机制 | 催化剂量0.1g·L-1,PDS量为1mmol·L-1,苯酚为0.1mmol·L-1,MWCNT/PDS和SWCNT/PDS系统在20min内完全降解苯酚,其他碳材料,如活性炭和石墨,在PDS存在下对苯酚的降解无效 酚类化合物和某些药物(即卡马西平、普萘洛尔、磺胺甲 | [ |
| 中孔炭CMK-3和CMK-8 | 二氧化硅作为硬模板合成 | 苯酚 | 自由基机制 非自由基机制 | 在催化剂投加量0.2g·L-1,苯酚浓度为20mg·L-1,PDS投加量为6.5mmol·L-1条件下,CMK-3和CMK-8在20min和45min内实现了完全的苯酚氧化,分别具有0.209min-1和0.104min-1的高速率常数 中孔炭比均相Ag+/PS和Fe2+/PS具有更好的去除苯酚的性能 | [ |
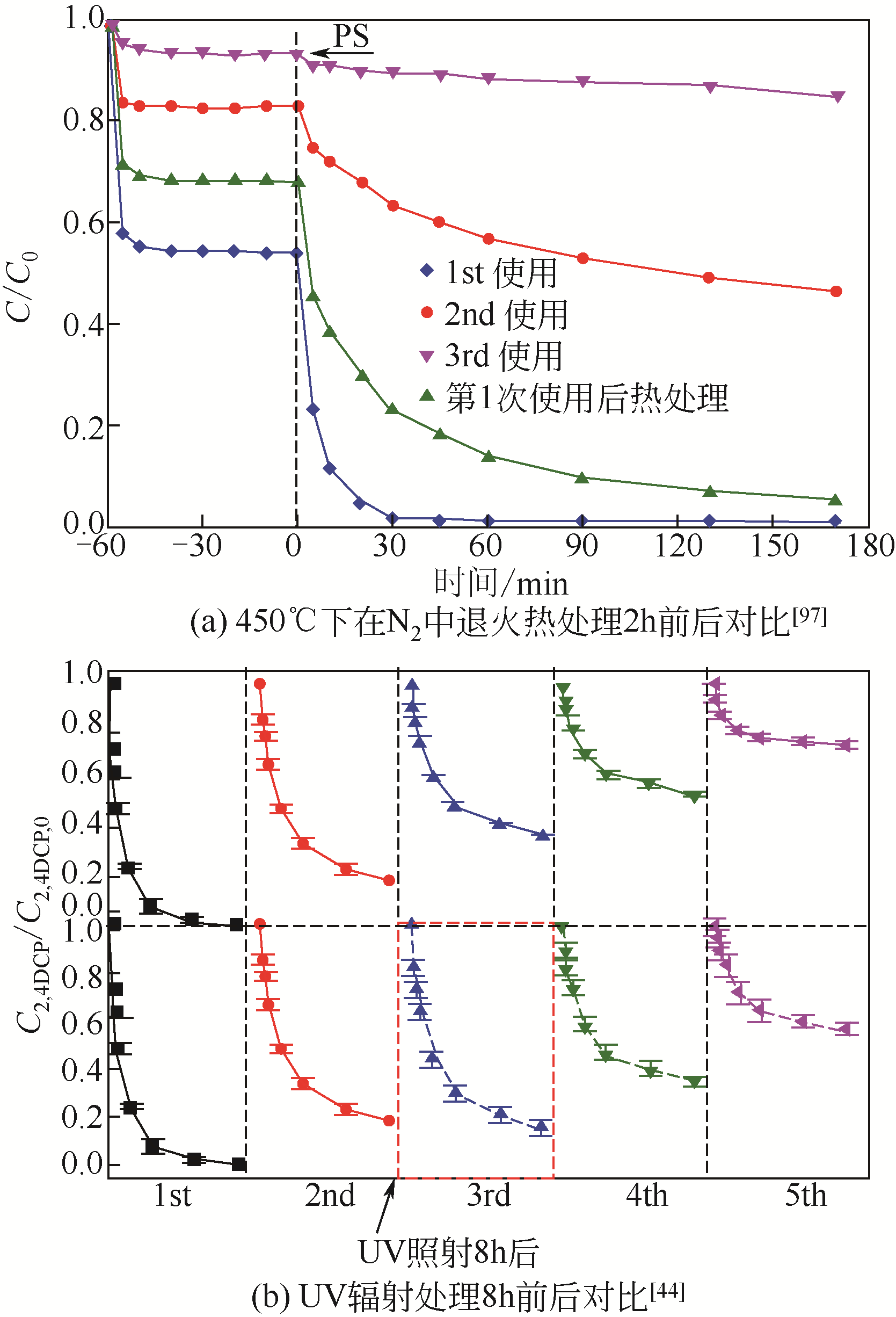
| 中孔炭CMK-3 | 浸渍法 | 2,4-二氯苯酚 | 自由基机制 非自由基机制 | 2,4-DCP浓度为200mg·L-1,催化剂用量和PDS用量的分别为0.2μg·L-1和2μg·L-1条件下,20min内去除率为90% 与其他碳基催化剂相比,如碳纳米管、立方有序介孔碳(CMK-8)、活性炭、还原氧化石墨烯(rGO),CMK-3稳定性更好且与纳米金刚石(AND)相似,具有更好的可重复使用性 | [ |
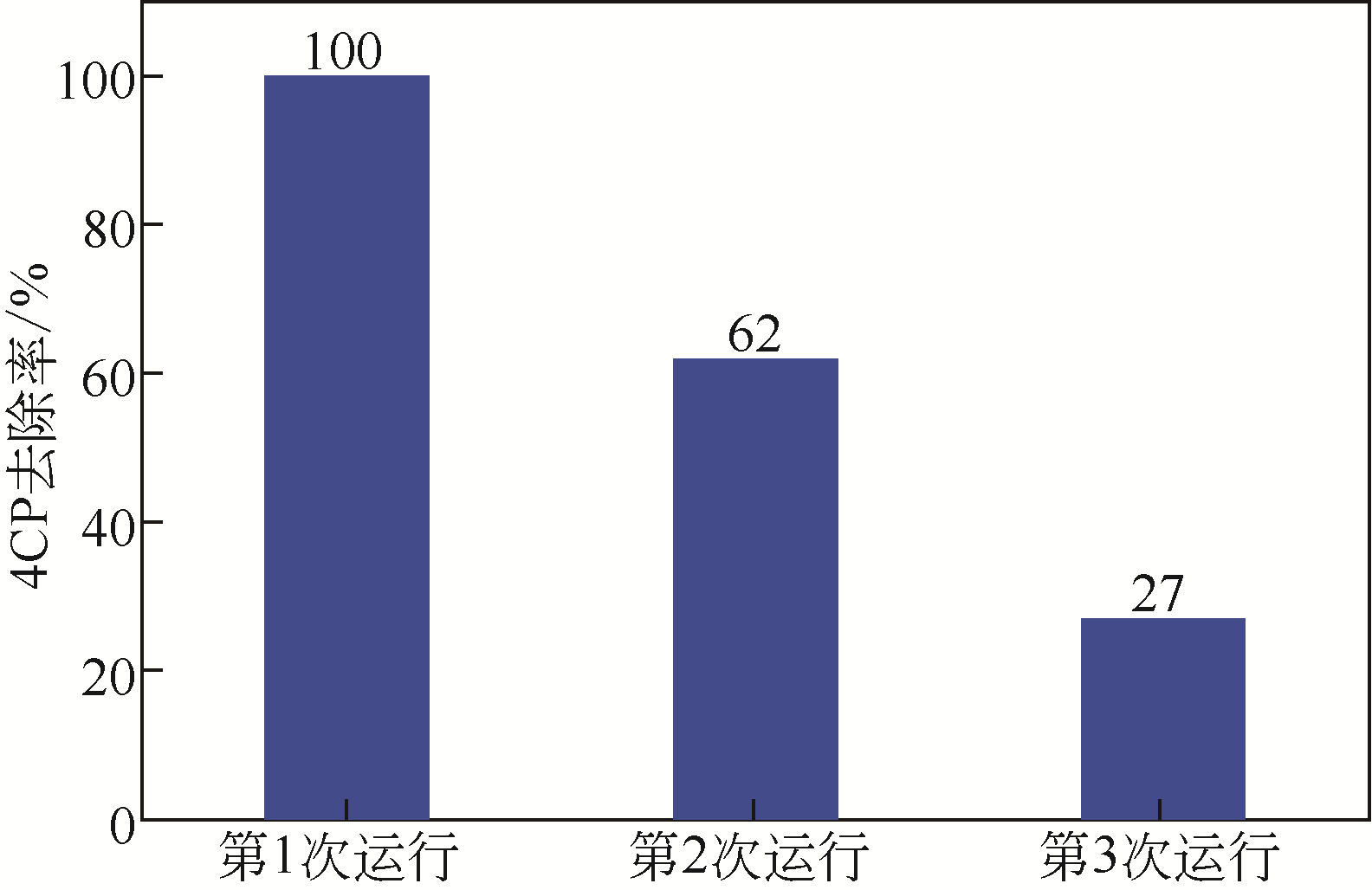
| 退火纳米金刚石(ANDs) | N2下于600~1000℃退火 | 苯酚 | 自由基机制 | 在催化剂量为0.2g·L-1,PDS投加量为6.5mmol·L-1,苯酚浓度20mg·L-1条件下,苯酚在120min实现完全氧化 AND-1000表现出优异的可重复使用性,第3次回收催化剂在相同反应时间仍可达到89.0%的苯酚去除效率 | [ |
石墨化纳米金刚石 G-ND | 在600~1200℃的温度下对NDs进行退火 | 苯酚、苯胺、雷尼替丁、双酚-A、对乙酰氨基酚、磺胺甲基 唑、卡马西平、苯甲酸 唑、卡马西平、苯甲酸 | 非自由基机制 | 污染物浓度0.01mmol·L-1,催化剂量为0.1g·L-1,PDS投加量为1mmol·L-1条件下,10min内苯酚实现完全去除 G-ND在PDS活化方面优于石墨、石墨烯、富勒烯和碳纳米管 G-ND/PDS显示出对酚类化合物和一些药物的选择性反应性,对乙酰氨基酚、苯胺、双酚A、磺胺甲 | [ |
| 废麦芽根生物炭 | 900℃热解 | 磺胺甲 唑(SMX) 唑(SMX) | 自由基机制 非自由基机制 | 在600mg·L-1 PDS和500mg·L-1 BC存在下,250μg·L-1 SMX在30min后完全去除 与纯水的运行相比,瓶装水和二级处理废水的实验表明水基质对降解的影响很小或没有影响 | [ |
| 木基生物炭 | 400~700℃的不同温度热解 | 氯贝特酸(CA) | 自由基机制 非自由基机制 | 在10mmol·L-1 PDS和0.5g·L-1 BC700的条件下,60min实现了97.8%的CA去除 BC700/PDS工艺去除的CA远远高于均相的Fe2+/PDS和UV/PDS工艺 在pH从4.0至9.0时显示出略微的下降,在pH=11.0时,CA去除率显著下降至22.8% | [ |
表1 各种碳催化剂的催化剂来源、目标污染物及催化活性评价
| 催化剂 | 催化剂来源 | 目标污染物 | 反应机制 | 催化活性评价 | 参考文献 |
|---|---|---|---|---|---|
| 活性炭 | 商业购买 | 对氯苯胺(PCA) | 非自由基机制 | 在0.5mmol·L-1 PCA,2.5mmol·L-1 PDS,5g·L-1 AC和 pH=7.0的条件下,120min内PDS/AC系统的PCA降解效率为98.03%,TOC去除率约41% 在30min内实现PCA快速脱氯,并且在初始pH 3~9时PCA去除无明显影响 | [ |
| 煤质活性炭、木质活性炭、椰壳活性炭 | 商业购买 | 偶氮染料橙黄G | 非自由基机制 | 3个体系对染料的去除率在120min内均可达到97%以上 活性炭/过硫酸盐体系对1~100mmol·L-1氯化钠有很强的耐受作用,适用于高盐废水中有机污染物的去除 | [ |
| 表面改性活性炭ACNH | 硝酸氧化联合高温处理 | 苯酚 | 非自由基机制 | 苯酚浓度80mg·L-1,活性炭投加量0.4g·L-1,PDS与苯酚摩尔比3∶1,初始pH=6.6,ACNH/PS体系反应2h苯酚的降解率达到100% 改性后苯酚的降解速率是未改性的5倍,ACNH的pH适用范围宽 | [ |
| 原始石墨烯、氧化石墨烯、纳米石墨粉末 | 商业购买 | 对羟基苯甲酸酯(PP) | 自由基机制 | 在pH=9,PP浓度1mg·L-1,催化剂量500mg·L-1,PDS投加量20mg·L-1,原始石墨烯,纳米石墨粉末在20min内PP降解率超过95%,GO在20min内PP降解约为70% | [ |
| 还原氧化石墨烯rGO-900 | 改良Hummers法 | 苯酚、2,4-二氯苯酚、 邻苯二酚、1,4-二羟基苯、亚甲基蓝 | 自由基机制 | 催化剂投加量0.2g·L-1,6.5mmol·L-1 PDS,苯酚浓度20μg·g-1,30min内可使苯酚完全降解。催化活性:rGO-900>CMK-8>SWCNT>g-C3N4>C60 对其他有机污染物(2,4-二氯苯酚,邻苯二酚和 1,4-二羟基苯,亚甲基蓝)均表现出极大的催化氧化效率 | [ |
| 多壁碳纳米管 | 商业购买 | 2,4-二氯苯酚、邻苯二甲酸二甲酯、双酚A、邻苯二甲酸二乙酯、己烯雌酚 | 非自由基机制 | 催化剂量0.1g·L-1,PDS和2,4-DCP量为0.031mmol·L-1,在30min时PDS/CNTs系统中2,4-DCP的降解率高达95.9%,实现了40%的矿化 对酚类化合物产生选择性降解,BPA、DES、苯酚和 2,4-DCP 30min内得到有效降解 | [ |
| 单壁碳纳米管SWCNT多壁碳纳米管MWCNT | 商业购买 | 苯酚、对乙酰氨基酚、 三氯苯酚、磺胺甲 唑、 普萘洛尔、卡马西平、 4-氯苯酚、双酚A、苯甲酸、硝基苯、糠醇 唑、 普萘洛尔、卡马西平、 4-氯苯酚、双酚A、苯甲酸、硝基苯、糠醇 | 非自由基机制 | 催化剂量0.1g·L-1,PDS量为1mmol·L-1,苯酚为0.1mmol·L-1,MWCNT/PDS和SWCNT/PDS系统在20min内完全降解苯酚,其他碳材料,如活性炭和石墨,在PDS存在下对苯酚的降解无效 酚类化合物和某些药物(即卡马西平、普萘洛尔、磺胺甲 | [ |
| 中孔炭CMK-3和CMK-8 | 二氧化硅作为硬模板合成 | 苯酚 | 自由基机制 非自由基机制 | 在催化剂投加量0.2g·L-1,苯酚浓度为20mg·L-1,PDS投加量为6.5mmol·L-1条件下,CMK-3和CMK-8在20min和45min内实现了完全的苯酚氧化,分别具有0.209min-1和0.104min-1的高速率常数 中孔炭比均相Ag+/PS和Fe2+/PS具有更好的去除苯酚的性能 | [ |
| 中孔炭CMK-3 | 浸渍法 | 2,4-二氯苯酚 | 自由基机制 非自由基机制 | 2,4-DCP浓度为200mg·L-1,催化剂用量和PDS用量的分别为0.2μg·L-1和2μg·L-1条件下,20min内去除率为90% 与其他碳基催化剂相比,如碳纳米管、立方有序介孔碳(CMK-8)、活性炭、还原氧化石墨烯(rGO),CMK-3稳定性更好且与纳米金刚石(AND)相似,具有更好的可重复使用性 | [ |
| 退火纳米金刚石(ANDs) | N2下于600~1000℃退火 | 苯酚 | 自由基机制 | 在催化剂量为0.2g·L-1,PDS投加量为6.5mmol·L-1,苯酚浓度20mg·L-1条件下,苯酚在120min实现完全氧化 AND-1000表现出优异的可重复使用性,第3次回收催化剂在相同反应时间仍可达到89.0%的苯酚去除效率 | [ |
石墨化纳米金刚石 G-ND | 在600~1200℃的温度下对NDs进行退火 | 苯酚、苯胺、雷尼替丁、双酚-A、对乙酰氨基酚、磺胺甲基 唑、卡马西平、苯甲酸 唑、卡马西平、苯甲酸 | 非自由基机制 | 污染物浓度0.01mmol·L-1,催化剂量为0.1g·L-1,PDS投加量为1mmol·L-1条件下,10min内苯酚实现完全去除 G-ND在PDS活化方面优于石墨、石墨烯、富勒烯和碳纳米管 G-ND/PDS显示出对酚类化合物和一些药物的选择性反应性,对乙酰氨基酚、苯胺、双酚A、磺胺甲 | [ |
| 废麦芽根生物炭 | 900℃热解 | 磺胺甲 唑(SMX) 唑(SMX) | 自由基机制 非自由基机制 | 在600mg·L-1 PDS和500mg·L-1 BC存在下,250μg·L-1 SMX在30min后完全去除 与纯水的运行相比,瓶装水和二级处理废水的实验表明水基质对降解的影响很小或没有影响 | [ |
| 木基生物炭 | 400~700℃的不同温度热解 | 氯贝特酸(CA) | 自由基机制 非自由基机制 | 在10mmol·L-1 PDS和0.5g·L-1 BC700的条件下,60min实现了97.8%的CA去除 BC700/PDS工艺去除的CA远远高于均相的Fe2+/PDS和UV/PDS工艺 在pH从4.0至9.0时显示出略微的下降,在pH=11.0时,CA去除率显著下降至22.8% | [ |
| 1 | 吴晨炜, 王震, 代海波, 等. 过硫酸盐高级氧化技术在工业废水处理中的应用[J]. 四川化工, 2018, 21(5): 50-53. |
| WU Chenwei, WANG Zhen, DAI Haibo, et al. Application of persulfate advanced oxidation technology in industrial wastewater treatment[J]. Sichuan Chemical Industry, 2018, 21(5): 50-53. | |
| 24 | LIU Lei, ZHU Yunpei, SU Ming, et al. Metal-free carbonaceous materials as promising heterogeneous catalysts[J]. ChemCatChem, 2015, 7(18): 2765-2787. |
| 25 | 白静, 扶咏梅, 王文琪, 等. 非均相催化过硫酸盐氧化技术研究进展[J]. 化工环保, 2019, 39(3): 247-254. |
| 2 | 吴光锐, 王德军, 王永剑, 等. 过一硫酸盐的活化及其降解水中有机污染物机理的研究进展[J]. 化工环保, 2018, 38(5): 23-31. |
| WU Guangrui, WANG Dejun, WANG Yongjian, et al. Research progresses on activation of peroxymonosulfate and its degradation mechanism to organic pollutants in aqueous solutions[J]. Environmental Protection of Chemical Industry, 2018, 38(5): 23-31. | |
| 3 | 赵文莉, 王广智, 弋凡, 等. 过硫酸盐活化技术的研究进展[J]. 现代化工, 2018, 38(7): 53-56. |
| 25 | BAI Jing, FU Yongmei, WANG Wenqi. et al. Research progresses on heterogeneous catalytic persulfate oxidation technology[J]. Environmental Protection of Chemical Industry, 2019, 39(3): 247-254. |
| 26 | DUAN Xiaoguang, SUN Hongqi, TADE M, et al. Metal-free activation of persulfate by cubic mesoporous carbons for catalytic oxidation via radical and nonradical processes[J]. Catalysis Today, 2018, 307: 140-146. |
| 3 | ZHAO Wenli, WANG Guangzhi, YI Fan, et al. Research progress on persulfate activation technology[J]. Modern Chemical Industry, 2018, 38(7): 53-56. |
| 4 | 冯雪梅, 卫新来, 陈俊, 等. 高级氧化技术在废水处理中的应用进展[J]. 应用化工, 2020, 49(4): 993-996, 1001. |
| 27 | SUN Hongqi, LIU Shizhen, ZHOU Guanliang, et al. Reduced graphene oxide for catalytic oxidation of aqueous organic pollutants[J]. ACS Applied Materials & Interfaces, 2012, 4(10): 5466-5471. |
| 28 | BEKRIS L, ZACHARIAS F, GEORGE T, et al. Graphene: a new activator of sodium persulfate for the advanced oxidation of parabens in water[J]. Water Research, 2017, 126: 111-121. |
| 4 | FENG Xuemei, WEI Xinlai, CHEN Jun, et al. Progress in the application of advanced oxidation technology in wastewater treatment[J]. Applied Chemical Industry, 2020, 49(4): 993-996, 1001. |
| 5 | 肖羽堂, 吴晓慧, 王冠平, 等. 垃圾渗滤液高级氧化及其组合工艺深度处理研究进展[J]. 水处理技术, 2020, 46(2): 8-12. |
| XIAO Yutang, WU Xiaohui, WANG Guanping, et al. Research progress of advanced oxidation processes and its combined processes for advanced treatment of landfill leachate[J]. Technology of Water Treatment, 2020, 46(2): 8-12. | |
| 6 | HAN Meina, DUAN Xiaoguang, CAO Guoliang, et al. Graphitic nitride-catalyzed advanced oxidation processes (AOPs) for landfill leachate treatment: a mini review[J]. Process Safety and Environmental Protection, 2020, 139: 230-240. |
| 7 | 吴丹, 苏冰琴, 宋秀兰, 等. 紫外光激活过硫酸盐处理含吡啶有机废水的研究[J]. 工业水处理, 2018, 38(12): 31-34. |
| WU Dan, SU Bingqin, SONG Xiulan, et al. Research on the treatment of organic wastewater containing pyridine by ultraviolet light-activated persulfate process[J]. Industrial Water Treatment, 2018, 38(12): 31-34. | |
| 8 | MA Zhifei, YANG Yu, JIANG Yonghai, et al. Enhanced degradation of 2,4-dinitrotoluene in groundwater by persulfate activated using iron-carbon micro-electrolysis[J]. Chemical Engineering Journal, 2017, 311: 183-190. |
| 9 | AO Xiuwei, LIU Wenjun. Degradation of sulfamethoxazole by medium pressure UV and oxidants: peroxymonosulfate, persulfate, and hydrogen peroxide[J]. Chemical Engineering Journal, 2017, 313: 629-637. |
| 10 | 王杰, 曹世玮, 周南. 硫酸根自由基高级氧化技术处理苯胺废水的试验研究[J]. 化工技术与开发, 2018, 47(9): 58-62. |
| WANG Jie, CAO Shiwei, ZHOU Nan. Experimental study on treatment of aniline wastewater by advanced oxidation with sulfate radical[J]. Technology Development of Chemical Industry, 2018, 47(9): 58-62. | |
| 11 | 唐海, 沙俊鹏, 金磊, 等. 基于硫酸根自由基氧化深度处理焦化尾水的研究[J]. 工业水处理, 2015, 35(6): 58-92. |
| TANG Hai, SHA Junpeng, JIN Lei, et al. Research of the advanced treatment of coking biologically treated effluent based on sulfate radicals oxidation[J]. Industrial Water Treatment, 2015, 35(6): 58-92. | |
| 12 | WANG Dayang, WANG Mingming, ZHANG Xuezhen, et al. The performance of a sulfate-radical mediated advanced oxidation process in the degradation of organic matter from secondary effluents[J]. Environmental Science: Water Research & Technology, 2018, 4(6): 773-782. |
| 13 | WANG Jianlong, WANG Shizong. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants[J]. Chemical Engineering Journal, 2018, 334: 1502-1517. |
| 14 | XIAO Ruiyang, LUO Zonghao, WEI Zongsu, et al. Activation of peroxymonosulfate/persulfate by nanomaterials for sulfate radical-based advanced oxidation technologies[J]. Current Opinion in Chemical Engineering, 2018, 19: 51-58. |
| 15 | ZHANG Botao, ZHANG Yang, TENG Yanguo, et al. Sulfate radical and its application in decontamination technologies[J]. Critical Reviews in Environmental Science and Technology, 2014, 45(16): 1756-1800. |
| 16 | PEDROSA M, DRAZIC G, TAVARES P B, et al. Metal-free graphene-based catalytic membrane for degradation of organic contaminants by persulfate activation[J]. Chemical Engineering Journal, 2019, 369: 223-232. |
| 17 | ZHAO Qingxia, MAO Qiming, ZHOU Yaoyu, et al. Metal-free carbon materials-catalyzed sulfate radical-based advanced oxidation processes: a review on heterogeneous catalysts and applications[J]. Chemosphere, 2017, 189: 224-238. |
| 18 | FARSHID G, MAHSA M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: review[J]. Chemical Engineering Journal, 2017, 310: 41-62. |
| 19 | MATZEK L W, CARTER K E. Activated persulfate for organic chemical degradation: a review[J]. Chemosphere, 2016, 151: 178-188. |
| 20 | Wen-Da OH, DONG Zhili, Teik-Thye LIM. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: current development, challenges and prospects[J]. Applied Catalysis B: Environmental, 2016, 194: 169-201. |
| 21 | YANG Shiying, XIAO Tuo, ZHANG Jun, et al. Activated carbon fiber as heterogeneous catalyst of peroxymonosulfate activation for efficient degradation of Acid Orange 7 in aqueous solution[J]. Separation and Purification Technology, 2015, 143: 19-26. |
| 22 | QI Wei, SU Dangsheng. Metal-free carbon catalysts for oxidative dehydrogenation reactions[J]. ACS Catalysis, 2014, 4(9): 3212-3218. |
| 23 | DUAN Xiaoguang, AO Zhimin, LI Degang, et al. Surface-tailored nanodiamonds as excellent metal-free catalysts for organic oxidation[J]. Carbon, 2016, 103: 404-411. |
| 29 | DUAN Xiaoguang, INDRAWIRAWAN S, KANG Jian, et al. Synergy of carbocatalytic and heat activation of persulfate for evolution of reactive radicals toward metal-free oxidation[J]. Catalysis Today, 2019, 355: 319-324. |
| 30 | WU Yao, GUO Jing, HAN Yijie, et al. Insights into the mechanism of persulfate activated by rice straw biochar for the degradation of aniline[J]. Chemosphere, 2018, 200: 373-379. |
| 31 | GUAN Chaoting, JIANG Jin, LUO Congwei, et al. Oxidation of bromophenols by carbon nanotube activated peroxymonosulfate (PMS) and formation of brominated products: comparison to peroxydisulfate (PDS)[J]. Chemical Engineering Journal, 2018, 337: 40-50. |
| 32 | GUAN Chaoting, JIANG Jin, PANG Suyan, et al. Oxidation kinetics of bromophenols by nonradical activation of peroxydisulfate in the presence of carbon nanotube and formation of brominated polymeric products[J]. Environmental Science & Technology, 2017, 51(18): 10718-10728. |
| 33 | TANG Lin, LIU Yani, WANG Jiajia, et al. Enhanced activation process of persulfate by mesoporous carbon for degradation of aqueous organic pollutants: electron transfer mechanism[J]. Applied Catalysis B: Environmental, 2018, 231: 1-10. |
| 34 | ZHU Shishu, HUANG Xiaochen, MA Fang, et al. Catalytic removal of aqueous contaminants on N-doped graphitic biochars: inherent roles of adsorption and nonradical mechanisms[J]. Environmental Science & Technology, 2018, 52(15): 8649-8658. |
| 35 | DUAN Xiaoguang, SUN Hongqi, KANG Jian, et al. Insights into heterogeneous catalysis of persulfate activation on dimensional-structured nanocarbons[J]. ACS Catalysis, 2015, 5(8): 4629-4636. |
| 36 | DUAN Xiaoguang, SU Chao, ZHOU Li, et al. Surface controlled generation of reactive radicals from persulfate by carbocatalysis on nanodiamonds[J]. Applied Catalysis B: Environmental, 2016, 194: 7-15. |
| 37 | DUAN Xiaoguang, SUN Hongqi, SHAO Zongping, et al. Nonradical reactions in environmental remediation processes: uncertainty and challenges[J]. Applied Catalysis B: Environmental, 2018, 224: 973-982. |
| 38 | CHEN Xiao, Wen-Da OH, Teik-Thye LIM. Graphene- and CNTs-based carbocatalysts in persulfates activation: material design and catalytic mechanisms[J]. Chemical Engineering Journal, 2018, 354: 941-976. |
| 39 | Hongshin LEE, Hye Jin LEE, JEONG Joonseon, et al. Activation of persulfates by carbon nanotubes: oxidation of organic compounds by nonradical mechanism[J]. Chemical Engineering Journal, 2015, 266: 28-33. |
| 40 | FOROUZESH M, EBADI A, AGHAEINEJAD-M A. Degradation of metronidazole antibiotic in aqueous medium using activated carbon as a persulfate activator[J]. Separation and Purification Technology, 2019, 210: 145-151. |
| 41 | WANG Xiaobo, QIN Yanlei, ZHU Lihua, et al. Nitrogen-doped reduced graphene oxide as a bifunctional material for removing bisphenols: synergistic effect between adsorption and catalysis[J]. Environmental Science & Technology, 2015, 49(11): 6855-6864. |
| 42 | Hongshin LEE, KIM Hyoung Il, WEON Seunghyun, et al. Activation of persulfates by graphitized nanodiamonds for removal of organic compounds[J]. Environmental Science & Technology, 2016, 50(18): 10134-10142. |
| 43 | 杨峰. 羰基化纳米金刚石活化过硫酸盐降解对氯苯酚的研究[D]. 哈尔滨: 哈尔滨工业大学, 2017. |
| YANG Feng. Activation of peroxymonosulfate by carbonylation nanodiamonds for elimination of p-chlorophenol[D]. Harbin: Harbin Institute of Technology, 2017. | |
| 44 | CHENG Xin, GUO Hongguang, ZHANG Yongli, et al. Insights into the mechanism of nonradical reactions of persulfate activated by carbon nanotubes: activation performance and structure-function relationship[J]. Water Research, 2019, 157: 406-414. |
| 45 | CHENG Xin, GUO Hongguang, ZHANG Yongli, et al. Non-photochemical production of singlet oxygen via activation of persulfate by carbon nanotubes[J]. Water Research, 2017, 113: 80-88. |
| 46 | ANFAR Z, FAKIR A A E, AHSAINE H A, et al. Nitrogen doped graphitic porous carbon from almond shells as an efficient persulfate activator for organic compound degradation[J]. New Journal of Chemistry, 2020, 44(22): 9391-9401. |
| 47 | 刘慧英, 张秀钦, 方艺梅, 等. T型石墨烯及其衍生物的结构与电子特性[J]. 物理学报, 2017, 66(16): 186-195. |
| LIU Huiying, ZHANG Xiuqin, FANG Yimei, et al. Structural and electronic properties of T-graphene and its derivatives[J]. Acta Physica Sinica, 2017, 66(16): 186-195. | |
| 48 | HUANG Fei, LIU Hongyang, SU Dangsheng. Graphitized nanocarbon-supported metal catalysts: synthesis, properties, and applications in heterogeneous catalysis[J]. Science China Materials, 2017, 60(12): 1149-1167. |
| 49 | 王斓懿, 于学华, 赵震. 无机多孔材料的合成及其在环境催化领域的应用[J]. 物理化学学报, 2017(12): 52-69. |
| WANG Lanyi, YU Xuehua, ZHAO Zhen. Synthesis of inorganic porous materials and their applications in the field of environmental catalysis[J]. Acta Physico-Chimica Sinica, 2017(12): 52-69. | |
| 50 | INDRAWIRAWAN S, SUN Hongqi, DUAN Xiaoguang, et al. Nanocarbons in different structural dimensions (0-3D) for phenol adsorption and metal-free catalytic oxidation[J]. Applied Catalysis B: Environmental, 2015, 179: 352-362. |
| 51 | JAIN A, BALASUBRAMANIAN R, SRINIVASAN M P. Hydrothermal conversion of biomass waste to activated carbon with high porosity: a review[J]. Chemical Engineering Journal, 2016, 283: 789-805. |
| 52 | YAO Chenhui, ZHANG Yongqing, DU Meimei, et al. Insights into the mechanism of non-radical activation of persulfate via activated carbon for the degradation of p-chloroaniline[J]. Chemical Engineering Journal, 2019, 362: 262-268. |
| 53 | 史宸菲, 薛瑞杰, 李雨濛, 等. 不同活性炭活化过硫酸盐的效能及机理的规律研究[J]. 环境科学学报, 2018, 38(4): 1501-1508. |
| SHI Chenfei, XUE Ruijie, LI Yumeng, et al. Efficiency and mechanism of persulfate activation using different activated carbons[J]. Acta Scientiae Circumstantiae, 2018, 38(4): 1501-1508. | |
| 54 | 刘子乐, 曾泽泉, 杨洁杨, 等. 表面改性活性炭活化过硫酸盐降解苯酚[J]. 高等学校化学学报, 2017, 38(7): 1241-1248. |
| LIU Zile, ZENG Zequan, YANG Jieyang, et al. Degradation of phenol with persulfate activated by surface modified activated carbon[J]. Chemical Journal of Chinese Universities, 2017, 38(7): 1241-1248. | |
| 55 | DUAN Xiaoguang, AO Zhimin, SUN Hongqi, et al. Nitrogen-doped graphene for generation and evolution of reactive radicals by metal-free catalysis[J]. ACS Applied Materials & Interfaces, 2015, 7(7): 4169-4178. |
| 56 | OLMEZ-HANCI T, ARELAN-ALATON I, GURMEN S, et al. Oxidative degradation of Bisphenol A by carbocatalytic activation of persulfate and peroxymonosulfate with reduced graphene oxide[J]. Journal of Hazardous Materials, 2018, 360: 141-149. |
| 57 | FENG Leiyu, QIN Zhiyi, HUANG Yujun, et al. Boron-, sulfur-, and phosphorus-doped graphene for environmental applications[J]. Science of the Total Environment, 2019, 698: 134239. |
| 58 | WANG Changlong, ASTRUC D. Recent developments of metallic nanoparticle-graphene nanocatalysts[J]. Progress in Materials Science, 2018, 94: 306-383. |
| 59 | QU Yu, ULLAH H, ABUDUWAILI A, et al. Graphene-covered sandwich nanostructure for enhanced light absorption[J]. Optical Materials, 2019, 96: 109316. |
| 60 | SARKAR B, MANDL S C, TSANG Yiu Fai, et al. Designer carbon nanotubes for contaminant removal in water and wastewater: a critical review[J]. Science of the Total Environment, 2018, 612: 561-581. |
| 61 | YI Huan, HUANG Danlian, QIN Lei, et al. Selective prepared carbon nanomaterials for advanced photocatalytic application in environmental pollutant treatment and hydrogen production[J]. Applied Catalysis B: Environmental, 2018, 239: 408-424. |
| 62 | LIU Fengling, GUO Zhaobing, LING Hugeng, et al. Effect of pore structure on the adsorption of aqueous dyes to ordered mesoporous carbons[J]. Microporous and Mesoporous Materials, 2016, 227: 104-111. |
| 63 | XIN Wang, SONG Yonghui. Mesoporous carbons: recent advances in synthesis and typical applications[J]. RSC Advances, 2015, 5(101): 83239-83285. |
| 64 | LIANG Yen Nan, Wen-Da OH, LI Yongmei, et al. Nanocarbons as platforms for developing novel catalytic composites: overview and prospects[J]. Applied Catalysis A: General, 2018, 562: 94-105. |
| 65 | NI Chuyi, HUANG Junlong, XIE Xintong, et al. Simple fabrication of zirconium and nitrogen co-doped ordered mesoporous carbon for enhanced adsorption performance towards polar pollutants[J]. Analytica Chimica Acta, 2019, 1070: 43-50. |
| 66 | PASTRANA-MARTINEZ LM, MORALES-TORRES S, CARABINEIRO S A C, et al. Photocatalytic activity of functionalized nanodiamond-TiO2 composites towards water pollutants degradation under UV/Vis irradiation[J]. Applied Surface Science, 2018, 458: 839-848. |
| 67 | LIN Yangming, SUN Xiaoyan, SU Dangsheng, et al. Catalysis by hybrid sp2/sp3 nanodiamonds and their role in the design of advanced nanocarbon materials[J]. Chemical Society Reviews, 2018, 47(22): 8438-8473. |
| 68 | LIU Yuefeng, BA H, LUO Jingjie, et al. Structure-performance relationship of nanodiamonds @ nitrogen-doped mesoporous carbon in the direct dehydrogenation of ethylbenzene[J]. Catalysis Today, 2018, 301: 38-47. |
| 69 | DUAN Xiaoguang, SUN Hongqi, WANG Shaobin. Metal-free carbocatalysis in advanced oxidation reactions[J]. Accounts of Chemical Research, 2018, 51(3): 678-687. |
| 70 | DUAN Xiaoguang, AO Zhimin, ZHANG Huayang, et al. Nanodiamonds in sp2/sp3 configuration for radical to nonradical oxidation: core-shell layer dependence[J]. Applied Catalysis B: Environmental, 2018, 222: 176-181. |
| 71 | LIN Yangming, SU Dangsheng. Fabrication of nitrogen-modified annealed nanodiamond with improved catalytic activity[J]. ACS Nano, 2014, 8(8): 7823-7833. |
| 72 | BY E. Biochar: production, characterization, and applications[J]. ACS Applied Materials & Interfaces, 2015, 5(21): 10920-10925. |
| 73 | OUYANG Da, CHEN Yun, YAN Jingchun, et al. Activation mechanism of peroxymonosulfate by biochar for catalytic degradation of 1,4-dioxane: important role of biochar defect structures[J]. Chemical Engineering Journal, 2019, 370: 614-624. |
| 74 | XIONG Xinni, YU I K M, CAO Leichang, et al. A review of biochar-based catalysts for chemical synthesis, biofuel production, and pollution control[J]. Bioresource Technology, 2017, 246: 254-270. |
| 75 | WANG Rongzhong. HUANG Danlian, LIU Yunguo,et al. Recent advances in biochar-based catalysts: properties, applications and mechanisms for pollution remediation[J]. Chemical Engineering Journal, 2019, 371: 380-403. |
| 76 | CAO Xuefei, SUN Shaoni, SUN Runcang. Application of biochar-based catalysts in biomass upgrading: a review[J]. RSC Advance, 2017, 7(77): 48793-48805. |
| 77 | KEMMOU L, FRONTISTIS Z, VAKROS J, et al. Degradation of antibiotic sulfamethoxazole by biochar-activated persulfate: factors affecting the activation and degradation processes[J]. Catalysis Today, 2018, 313: 128-133. |
| 78 | ZHU Kangmeng, WANG Xisong, GENG Mengzi, et al. Catalytic oxidation of clofibric acid by peroxydisulfate activated with wood-based biochar: effect of biochar pyrolysis temperature, performance and mechanism[J]. Chemical Engineering Journal, 2019, 374: 1253-1263. |
| 79 | LENG Lijian, HUANG Huajun. An overview of the effect of pyrolysis process parameters on biochar stability[J]. Bioresource Technology, 2018, 270: 627-642. |
| 80 | QIN Yaxin, LI Guiying, GAO Yanpeng, et al. Persistent free radicals in carbon-based materials on transformation of refractory organic contaminants (ROCs) in water: a critical review[J]. Water Research, 2018, 137: 130-143. |
| 81 | LIU Zheng, ZHANG Longhai, SHENG Lizhi, et al. Edge-nitrogen-rich carbon dots pillared graphene blocks with ultrahigh volumetric/gravimetric capacities and ultralong life for sodium-ion storage[J]. Advanced Energy Materials, 2018, 8(30): 1802042. |
| 82 | ZHANG Lipeng, XIA Zhenhai. Mechanisms of oxygen reduction reaction on nitrogen-doped graphene for fuel cells[J]. The Journal of Physical Chemistry C, 2011, 115(22): 11170-11176. |
| 83 | ZHENG Wan, XIAO Xin, CHEN Baoliang. A nonradical reaction-dominated phenol degradation with peroxydisulfate catalyzed by nitrogen-doped graphene[J]. Science of the Total Environment, 2019, 667: 287-296. |
| 84 | PENG Wenchao, LIU Shizhen, SUN Hongqi, et al. Synthesis of porous reduced graphene oxide as metal-free carbon for adsorption and catalytic oxidation of organics in water[J]. Journal of Materials Chemistry A, 2013, 1(19): 5854-5859. |
| 85 | DUAN Xiaoguang, SUN H Q, KANG J, et al. Insights into N-doping in single-walled carbon nanotubes for enhanced activation of superoxides: a mechanistic study[J]. Chemical Communications, 2015, 51(83): 15249-15252. |
| 86 | Shih-Hsin HO, CHEN Yi-di, RUI Ruixiang, et al. N-doped graphitic biochars from C-phycocyanin extracted Spirulina residue for catalytic persulfate activation toward nonradical disinfection and organic oxidation[J]. Water Research, 2019, 159: 77-86. |
| 87 | GUO Yaoping, ZENG Zequan, LI Yulin, et al. In-situ sulfur-doped carbon as a metal-free catalyst for persulfate activated oxidation of aqueous organics[J]. Catalysis Today, 2018, 307: 12-19. |
| 88 | ZHENG Bo, WANG Jiaxin, PAN Zhaorui, et al. An efficient metal-free catalyst derived from waste lotus seedpod for oxygen reduction reaction[J]. Journal of Porous Materials, 2020, 27(3): 637-646. |
| 89 | HAN Lei, CUI Xingyu, LIU Yanyan, et al. Nitrogen and phosphorus modification to enhance the catalytic activity of biomass-derived carbon toward the oxygen reduction reaction[J]. Sustainable Energy & Fuels, 2020, 4(6): 2707-2717. |
| 90 | KONG Xiangkai, CHEN Changle, CHEN Qianwang. Doped graphene for metal-free catalysis[J]. Chemical Society Reviews, 2014, 43(8): 2841-2857. |
| 91 | WANG Qiang, LI Lei, LUO Li, et al. Activation of persulfate with dual-doped reduced graphene oxide for degradation of alkylphenols[J]. Chemical Engineering Journal, 2019, 376: 120891. |
| 92 | BAG S, MONDAL B, DAS A, et al. Nitrogen and sulfur dual-doped reduced graphene oxide: synergistic effect of dopants towards oxygen reduction reaction[J]. Electrochimica Acta, 2015, 163: 16-23. |
| 93 | CHENG Xin, GUO Hongguang, ZHANG Yongli, et al. Oxidation of 2,4-dichlorophenol by non-radical mechanism using persulfate activated by Fe/S modified carbon nanotubes[J]. Journal of Colloid and Interface Science, 2016, 469: 277-286. |
| 94 | YU Jiangfang, TANG Lin, PANG Ya, et al. Magnetic nitrogen-doped sludge-derived biochar catalysts for persulfate activation: internal electron transfer mechanism[J]. Chemical Engineering Journal, 2019, 364: 146-159. |
| 95 | RUSSO A V, DE ANGELIS L E, JACOBO S E. Adsorption with catalytic oxidation in a recirculating bed reactor for contaminated groundwater[J]. Journal of Water Process Engineering, 2018, 23: 129-133. |
| 96 | AHMAD A, GU Xiaogang, LI Li, et al. Effects of pH and anions on the generation of reactive oxygen species (ROS) in nZVI-rGO-activated persulfate system[J]. Water, Air & Soil Pollution, 2015, 226(11): 369. |
| 97 | GUO Yaoping, ZENG Zequan, LIU Yongjin, et al. One-pot synthesis of sulfur doped activated carbon as a superior metal-free catalyst for the adsorption and catalytic oxidation of aqueous organics[J]. Journal of Materials Chemistry A, 2018, 6(9): 4055-4067. |
| [1] | 宋亚丽, 李紫燕, 杨彩荣, 黄龙, 张宏忠. 非金属元素掺杂石墨相氮化碳光催化材料的研究进展[J]. 化工进展, 2023, 42(10): 5299-5309. |
| [2] | 许泽涛, 曹怡婷, 王俏, 王志红. 固相钴基催化剂活化过一硫酸盐在水处理中的研究进展[J]. 化工进展, 2022, 41(2): 730-739. |
| [3] | 吕孝琦, 李洪, 赵振宇, 李鑫钢, 高鑫, 范晓雷. 微波与碳基催化剂协同促进果糖制5-羟甲基糠醛[J]. 化工进展, 2022, 41(2): 637-647. |
| [4] | 孔维杰, 杨春亮, 卜婷婷, 周金波. 碳基硼基催化体系丙烷氧化脱氢催化剂的研究进展[J]. 化工进展, 2021, 40(S1): 223-230. |
| [5] | 张英杰, 朱子翼, 董鹏, 赵少博, 章艳佳, 杨成云, 杨城沣, 韦克毅, 李雪. 钠离子电池碳基负极材料的研究进展[J]. 化工进展, 2017, 36(11): 4106-4115. |
| [6] | 叶永清,肖新颜,万彩霞. 锂离子电池正极材料LiNi1/3Co1/3Mn1/3O2研究进展 [J]. 化工进展, 2009, 28(7): 1192-. |
| [7] | 韦正乐;黄碧纯;叶代启;徐雪梅. 烟气NOx低温选择性催化还原催化剂研究进展 [J]. 化工进展, 2007, 26(3): 325-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||


 唑和对乙酰氨基酚)在CNT/PDS系统中可实现有效降解,硝基苯、苯甲酸和糠醇的化合物对降解具有抗性
唑和对乙酰氨基酚)在CNT/PDS系统中可实现有效降解,硝基苯、苯甲酸和糠醇的化合物对降解具有抗性 唑的化合物显示出相对较高的降解效率,而苯甲酸对降解有一定的抵抗力
唑的化合物显示出相对较高的降解效率,而苯甲酸对降解有一定的抵抗力