化工进展 ›› 2021, Vol. 40 ›› Issue (12): 6846-6858.DOI: 10.16085/j.issn.1000-6613.2021-0106
生物电化学技术降解疏水性新兴污染物的研究进展
张钤1( ), 崔敏华1,2,3(
), 崔敏华1,2,3( ), 陈蕾1, 吴平1, 刘和1,2,3(
), 陈蕾1, 吴平1, 刘和1,2,3( )
)
- 1.江南大学环境与土木工程学院,江苏 无锡 214122
2.江苏省厌氧生物技术重点实验室,江苏 无锡 214122
3.江苏省水处理技术与材料协同创新中心,江苏 苏州 215009
-
收稿日期:2021-01-15修回日期:2021-03-01出版日期:2021-12-05发布日期:2021-12-21 -
通讯作者:崔敏华,刘和 -
作者简介:张钤(1996—),女,硕士研究生。E-mail:zq1what@163.com 。 -
基金资助:国家自然科学基金(52000088);江苏省自然科学基金(BK20180633);中国博士后科学基金(2020M671379)
A critical review of bioelectrochemical system in the degradation of hydrophobic emerging contaminants
ZHANG Qian1( ), CUI Minhua1,2,3(
), CUI Minhua1,2,3( ), CHEN Lei1, WU Ping1, LIU He1,2,3(
), CHEN Lei1, WU Ping1, LIU He1,2,3( )
)
- 1.College of Environment and Civil Engineering, Jiangnan University, Wuxi 214122, Jiangsu, China
2.Jiangsu Key Laboratory of Anaerobic Biotechnology, Wuxi 214122, Jiangsu, China
3.Jiangsu Collaborative Innovation Center of Water Treatment Technology and Material, Suzhou 215009, Jiangsu, China
-
Received:2021-01-15Revised:2021-03-01Online:2021-12-05Published:2021-12-21 -
Contact:CUI Minhua,LIU He
摘要:
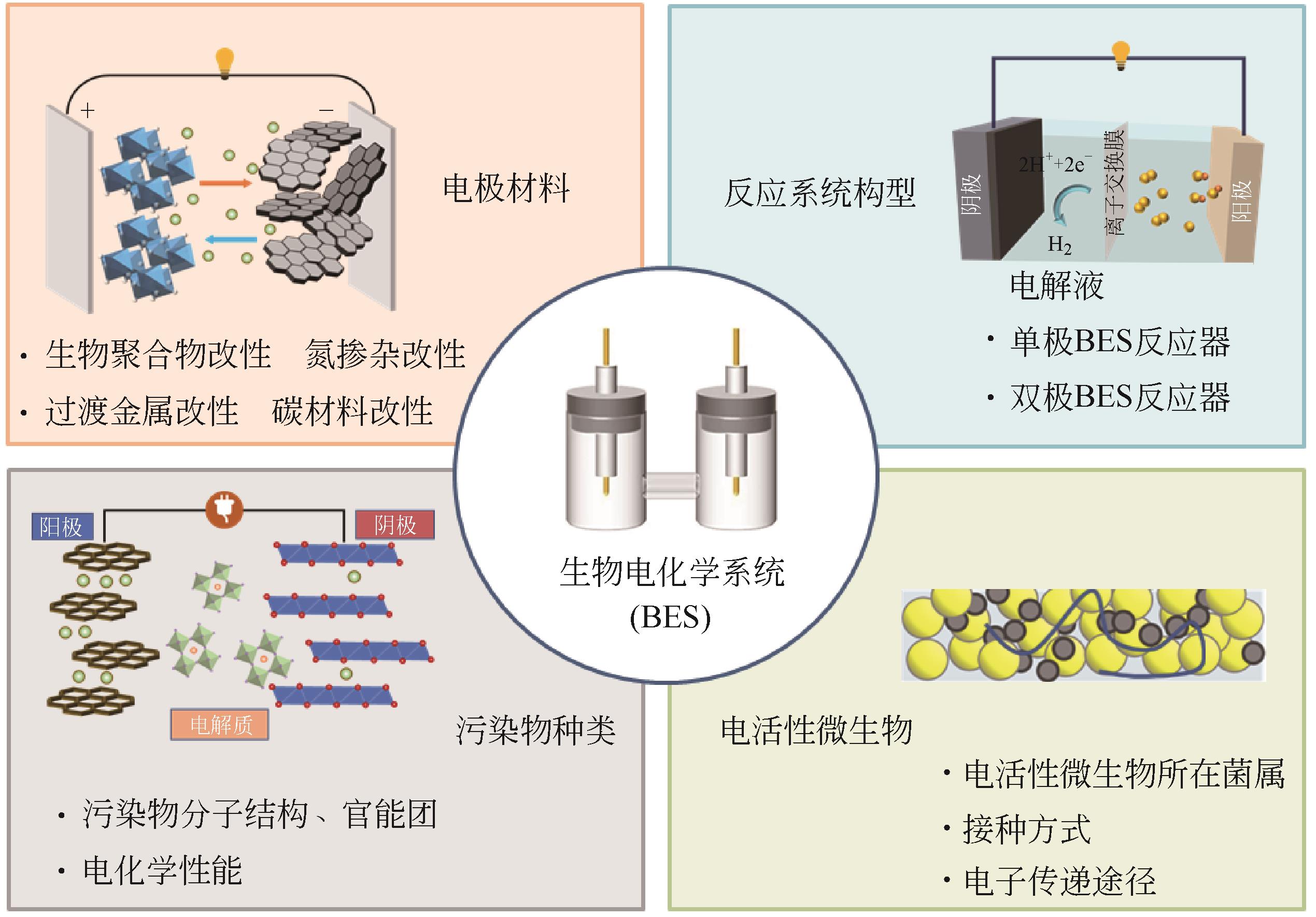
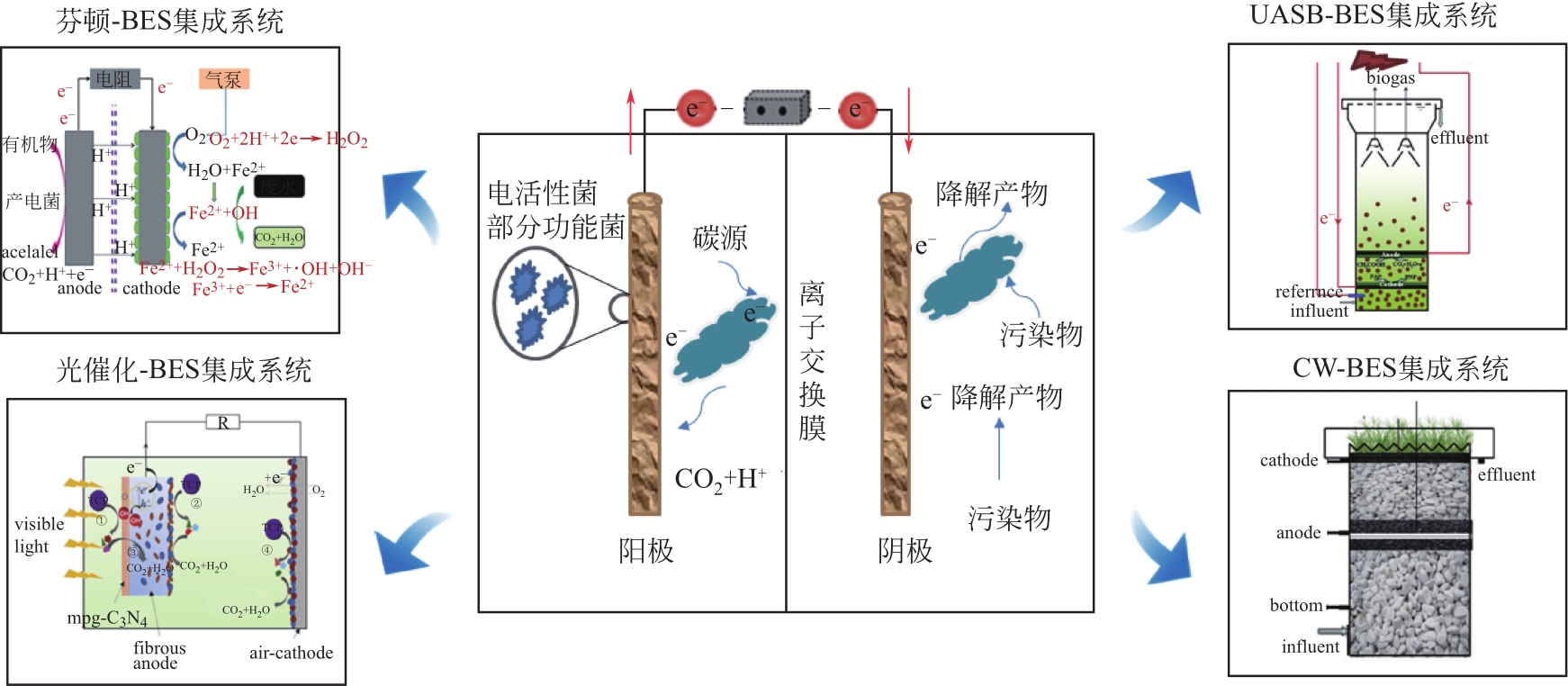
疏水性新兴污染物(hydrophobic emerging contaminants,HECs)具有环境危害大、分布范围广和处理难度高等特点,利用生物电化学系统(bioelectrochemical system,BES)实现HECs的降解和脱毒是当前研究热点。本文综述了BES降解转化HECs的研究现状,分析了影响BES去除HECs效果的关键因素,着重介绍了BES降解转化不同类型HECs(包括药物类、个人护理品类、卤代烃类和抗生素及抗性基因类)的效能,然后回顾了BES与其他技术(传统厌氧工艺、芬顿、复合湿地及光催化等)结合协同降解HECs的最新进展,最后在功能电极材料设计研发、HECs降解去除与安全转化的理论研究及工程化应用等方面进行了总结和展望,以期对该领域的研究人员提供一定的理论参考和技术支持,从而推进生物电化学技术在疏水性新兴污染物降解领域的应用和发展。
中图分类号:
引用本文
张钤, 崔敏华, 陈蕾, 吴平, 刘和. 生物电化学技术降解疏水性新兴污染物的研究进展[J]. 化工进展, 2021, 40(12): 6846-6858.
ZHANG Qian, CUI Minhua, CHEN Lei, WU Ping, LIU He. A critical review of bioelectrochemical system in the degradation of hydrophobic emerging contaminants[J]. Chemical Industry and Engineering Progress, 2021, 40(12): 6846-6858.
| 处理方法 | 具体工艺/试剂 | 污染物种类 | 去除效果 | 优点 | 缺点 | 参考 文献 |
|---|---|---|---|---|---|---|
| 好氧生物降解 | 传统活性污泥法 | 佳乐麝香 | 73%~96% | 降解、矿化程度高;成本低; 降解产物毒性一般小于母体污染物 | 降解周期长;可降解污染物种类少、具有局限性 | [ |
| 膜生物反应器 | 双氯酚酸 | 60%~80% | ||||
| 水解酸化-生物处理 | 环丙沙星 | 90% | [ | |||
| 厌氧生物降解 | 污泥厌氧消化 | 萘普生 | 80% | |||
| 麝香 | 50%~90% | [ | ||||
| 布洛芬 | 30%~60% | |||||
| 卡马西平 | 无明显降解 | |||||
| 化学氧化法 | β-FeOOH/Al2O3 | 布洛芬 | 100% | 降解/矿化程度高、效果稳定、反应过程容易控制 | 基建/运行成本高,二次污染重;中间降解产物生态毒性不稳定 | [ |
| β-FeOOH/Al2O3 | 环丙沙星 | 100% | [ | |||
| 臭氧催化氧化 | Mg3Fe0.5Al | 双氯酚酸 | 73% | [ | ||
| 芬顿法 | FeS2 | 三氯生 | 90% | [ | ||
| Fe3O4 | 卡马西平 布洛芬 | 86.26% 83.29% | [ | |||
| Fe3O4 | 萘啶酸 | 60% | [ | |||
| 电离辐射 | 60Co(1.1KGy) | 布洛芬 | 100% | [ | ||
| 60Co(2.0KGy) | 双氯酚酸 | 100% | [ | |||
| 60Co(5.0KGy) | 氯贝酸 | 100% | [ | |||
| 60Co(20Gy) | 4-壬基酚 | 100% | [ | |||
| 吸附法 | 颗粒活性炭 | 双氯酚酸、三氯生 布洛芬 | 10%~68% | 系统简单,不形成有毒中间产物;处理方法成本基建及设备投资少,稳定性高,能耗低 | 污染物没有进一步降解转化,仍存在介质中 | [ |
| 粉状活性炭(PAC) | 卡马西平 | 60% | ||||
| AmberliteXAD-4 | 双氯酚酸 | 92.80% | ||||
| 磁性树脂W150 | 呋喃西林 | 180mg·g-1 | ||||
| 矿物 | 双氯酚酸 | 37.5%~95.9% | ||||
| 膜分离法 | 微滤-颗粒活性炭-纳滤 | 疏水性PPCPs | 45%~80% | 操作简单,污染物去除率高,占用空间小 | 易造成膜孔隙堵塞污染 | [ |
| 超滤-纳滤 | 磺胺甲 双氯芬酸 | 70% |
表1 常见HECs处理工艺效果以及优缺点比较
| 处理方法 | 具体工艺/试剂 | 污染物种类 | 去除效果 | 优点 | 缺点 | 参考 文献 |
|---|---|---|---|---|---|---|
| 好氧生物降解 | 传统活性污泥法 | 佳乐麝香 | 73%~96% | 降解、矿化程度高;成本低; 降解产物毒性一般小于母体污染物 | 降解周期长;可降解污染物种类少、具有局限性 | [ |
| 膜生物反应器 | 双氯酚酸 | 60%~80% | ||||
| 水解酸化-生物处理 | 环丙沙星 | 90% | [ | |||
| 厌氧生物降解 | 污泥厌氧消化 | 萘普生 | 80% | |||
| 麝香 | 50%~90% | [ | ||||
| 布洛芬 | 30%~60% | |||||
| 卡马西平 | 无明显降解 | |||||
| 化学氧化法 | β-FeOOH/Al2O3 | 布洛芬 | 100% | 降解/矿化程度高、效果稳定、反应过程容易控制 | 基建/运行成本高,二次污染重;中间降解产物生态毒性不稳定 | [ |
| β-FeOOH/Al2O3 | 环丙沙星 | 100% | [ | |||
| 臭氧催化氧化 | Mg3Fe0.5Al | 双氯酚酸 | 73% | [ | ||
| 芬顿法 | FeS2 | 三氯生 | 90% | [ | ||
| Fe3O4 | 卡马西平 布洛芬 | 86.26% 83.29% | [ | |||
| Fe3O4 | 萘啶酸 | 60% | [ | |||
| 电离辐射 | 60Co(1.1KGy) | 布洛芬 | 100% | [ | ||
| 60Co(2.0KGy) | 双氯酚酸 | 100% | [ | |||
| 60Co(5.0KGy) | 氯贝酸 | 100% | [ | |||
| 60Co(20Gy) | 4-壬基酚 | 100% | [ | |||
| 吸附法 | 颗粒活性炭 | 双氯酚酸、三氯生 布洛芬 | 10%~68% | 系统简单,不形成有毒中间产物;处理方法成本基建及设备投资少,稳定性高,能耗低 | 污染物没有进一步降解转化,仍存在介质中 | [ |
| 粉状活性炭(PAC) | 卡马西平 | 60% | ||||
| AmberliteXAD-4 | 双氯酚酸 | 92.80% | ||||
| 磁性树脂W150 | 呋喃西林 | 180mg·g-1 | ||||
| 矿物 | 双氯酚酸 | 37.5%~95.9% | ||||
| 膜分离法 | 微滤-颗粒活性炭-纳滤 | 疏水性PPCPs | 45%~80% | 操作简单,污染物去除率高,占用空间小 | 易造成膜孔隙堵塞污染 | [ |
| 超滤-纳滤 | 磺胺甲 双氯芬酸 | 70% |
| 分类 | 名称 | 分子式 | 辛醇-水分配系数 lgKow | 分子量 | 结构式 | 浓度水平/ng·L-1 | 国家/地区 |
|---|---|---|---|---|---|---|---|
药品及 个人护理用品 | 阿奇霉素① | C38H72N2O12 | 4.02 | 749 |  | 47~82 | 加拿大[ |
| 克拉霉素① | C38H69NO13 | 3.16 | 748 |  | 89~231 | 加拿大[ | |
| 双氯芬酸② | C14H10Cl2O2 | 4.51 | 296 |  | 147~320 | 上海[ | |
| 氯贝酸① | C10H11ClO3 | 2.57 | 214 |  | 7.4~51.3 | 上海[ | |
| 布洛芬② | C13H18O2 | 3.97 | 206 |  | 480 | 意大利[ | |
| 卡马西平② | C15H12N2O | 2.47 | 236 |  | 0.1~8.5 44.6~118 | 北京[ 长江流域 | |
| 三氯生② | C12H7Cl3O2 | 4.71 | 289 |  | 5.74~105 | 东江[ | |
| 三氯卡班② | C13H9ClN2O | 4.90 | 315 |  | 4.11~71.8 | 东江[ | |
| 萘普生② | C14H14O3 | 3.18 | 230 |  | 6.32~145 | 美国[ | |
| 苯扎贝特 | C19H20ClNO4 | 4.25 | 362 |  | 72.1 | 美国[ | |
| 氯霉素② | C11H12Cl2N2O5 | 1.14 | 323 |  | 93~1178 | 河北[ | |
| 吲哚美辛① | C19H16ClNO4 | 4.27 | 358 |  | 83~979 | 长江 流域[ | |
| 内分泌干扰物 | 邻苯二甲酸二(2-乙基)己酯 | C24H38O4 | 7.60 | 391 |  | 61 | 全国 平均值[ |
| 酞酸二丁酯 | C16H22O4 | 4.50 | 278 |  | 284 | 长江 上游[ | |
| 双酚A② | C15H16O2 | 3.32 | 228 |  | 26.2 | 黄河口[ | |
| 壬基酚 | C15H24O | 5.99 | 220 |  | 60~730 | 全国[ |
表2 常见HECs基本信息及浓度分布
| 分类 | 名称 | 分子式 | 辛醇-水分配系数 lgKow | 分子量 | 结构式 | 浓度水平/ng·L-1 | 国家/地区 |
|---|---|---|---|---|---|---|---|
药品及 个人护理用品 | 阿奇霉素① | C38H72N2O12 | 4.02 | 749 |  | 47~82 | 加拿大[ |
| 克拉霉素① | C38H69NO13 | 3.16 | 748 |  | 89~231 | 加拿大[ | |
| 双氯芬酸② | C14H10Cl2O2 | 4.51 | 296 |  | 147~320 | 上海[ | |
| 氯贝酸① | C10H11ClO3 | 2.57 | 214 |  | 7.4~51.3 | 上海[ | |
| 布洛芬② | C13H18O2 | 3.97 | 206 |  | 480 | 意大利[ | |
| 卡马西平② | C15H12N2O | 2.47 | 236 |  | 0.1~8.5 44.6~118 | 北京[ 长江流域 | |
| 三氯生② | C12H7Cl3O2 | 4.71 | 289 |  | 5.74~105 | 东江[ | |
| 三氯卡班② | C13H9ClN2O | 4.90 | 315 |  | 4.11~71.8 | 东江[ | |
| 萘普生② | C14H14O3 | 3.18 | 230 |  | 6.32~145 | 美国[ | |
| 苯扎贝特 | C19H20ClNO4 | 4.25 | 362 |  | 72.1 | 美国[ | |
| 氯霉素② | C11H12Cl2N2O5 | 1.14 | 323 |  | 93~1178 | 河北[ | |
| 吲哚美辛① | C19H16ClNO4 | 4.27 | 358 |  | 83~979 | 长江 流域[ | |
| 内分泌干扰物 | 邻苯二甲酸二(2-乙基)己酯 | C24H38O4 | 7.60 | 391 |  | 61 | 全国 平均值[ |
| 酞酸二丁酯 | C16H22O4 | 4.50 | 278 |  | 284 | 长江 上游[ | |
| 双酚A② | C15H16O2 | 3.32 | 228 |  | 26.2 | 黄河口[ | |
| 壬基酚 | C15H24O | 5.99 | 220 |  | 60~730 | 全国[ |
| 污染物种类 | 污染物名称 | 污染物浓度 | 反应装置 类型 | 输出电压 (MFC) | 外加电压(MEC) | 电极材料 | 降解 周期 | 降解 效率/% | 参考 文献 |
|---|---|---|---|---|---|---|---|---|---|
疏水性药品 及个人护理 用品 | 三氯生 | 10mg·L-1 | AD-MFC | (420±40)mV | — | 碳毡(9×9cm2) | 8天 | 100 | [ |
| 三氯生 | 1mg·L-1 | MFC | 60mV | — | 碳刷(5.9×6.9cm2) | 48h | 94 | [ | |
| 氯霉素 | 50mg·L-1 | MFC | >50mV | — | 碳毡(3×3cm2) | 12h | 84 | [ | |
| 氯霉素 | 20mg·L-1 | 电辅助厌氧系统 | — | 0~2V | 石墨板 | 1天 | 53~88 | [ | |
| 双氯芬酸 | 10mg·L-1 | CF-MFC | 0.51V | Ru/Fe-碳毡 | 2天 | 76 | [ | ||
卤代烃类 化合物 | 对氯硝基苯 | 40~120mg·L-1 | BES | — | 0.2~0.8V | 碳刷(3×3cm2) | 55h | >80 | [ |
| 二氯硝基苯 | 100mg·L-1 | MEC | — | 1.5V | 碳毡(200×70mm2) | 24h | 91 | [ | |
| 多氯联苯 | (20.2±4.0)mg·kg-1 | BES | — | 1.5、2.2、3.0V | 碳毡 | 24h | 40~60 | [ | |
| 2,4-二氯酚 | 100mg·L-1 | BES | — | 保持恒电流密度50mA/cm2 | Pd/MWNTs气体 扩散电极 | 2h | >79 | [ | |
| 对氟硝基苯 | 0.4mmol·L-1 | BES | — | 0~1.4V | 石墨电极 | 10h | 0~100 | [ | |
| 对硝基酚 | 0.93~6.77mol·m-3·d-1 | UASB–BES | — | 1.2V | 碳毡(5×3cm2) | 9h | 100 | [ |
表3 BES去除典型HECs的参数设置及降解效率
| 污染物种类 | 污染物名称 | 污染物浓度 | 反应装置 类型 | 输出电压 (MFC) | 外加电压(MEC) | 电极材料 | 降解 周期 | 降解 效率/% | 参考 文献 |
|---|---|---|---|---|---|---|---|---|---|
疏水性药品 及个人护理 用品 | 三氯生 | 10mg·L-1 | AD-MFC | (420±40)mV | — | 碳毡(9×9cm2) | 8天 | 100 | [ |
| 三氯生 | 1mg·L-1 | MFC | 60mV | — | 碳刷(5.9×6.9cm2) | 48h | 94 | [ | |
| 氯霉素 | 50mg·L-1 | MFC | >50mV | — | 碳毡(3×3cm2) | 12h | 84 | [ | |
| 氯霉素 | 20mg·L-1 | 电辅助厌氧系统 | — | 0~2V | 石墨板 | 1天 | 53~88 | [ | |
| 双氯芬酸 | 10mg·L-1 | CF-MFC | 0.51V | Ru/Fe-碳毡 | 2天 | 76 | [ | ||
卤代烃类 化合物 | 对氯硝基苯 | 40~120mg·L-1 | BES | — | 0.2~0.8V | 碳刷(3×3cm2) | 55h | >80 | [ |
| 二氯硝基苯 | 100mg·L-1 | MEC | — | 1.5V | 碳毡(200×70mm2) | 24h | 91 | [ | |
| 多氯联苯 | (20.2±4.0)mg·kg-1 | BES | — | 1.5、2.2、3.0V | 碳毡 | 24h | 40~60 | [ | |
| 2,4-二氯酚 | 100mg·L-1 | BES | — | 保持恒电流密度50mA/cm2 | Pd/MWNTs气体 扩散电极 | 2h | >79 | [ | |
| 对氟硝基苯 | 0.4mmol·L-1 | BES | — | 0~1.4V | 石墨电极 | 10h | 0~100 | [ | |
| 对硝基酚 | 0.93~6.77mol·m-3·d-1 | UASB–BES | — | 1.2V | 碳毡(5×3cm2) | 9h | 100 | [ |
| 1 | WURZER C, MAŠEK O. Feedstock doping using iron rich waste increases the pyrolysis gas yield and adsorption performance of magnetic biochar for emerging contaminants[J]. Bioresource Technology, 2021, 321: 124473. |
| 2 | AZAROFF A, DE MONPERRUS M, MIOSSEC C, et al. Microbial degradation of hydrophobic emerging contaminants from marine sediment slurries (Capbreton Canyon) to pure bacterial strain[J]. Journal of Hazardous Materials, 2021, 402: 123477. |
| 3 | NOUTSOPOULOS C, CHARALAMBOUS V, KOUMAKI E. Evaluating the fate of emerging contaminants in wastewater treatment plants through plant-wide mathematical modelling[J]. Environmental Processes, 2020, 7(4): 1065-1094. |
| 4 | GHERGHEL A, TEODOSIU C, NOTARNICOLA M, et al. Sustainable design of large wastewater treatment plants considering multi-criteria decision analysis and stakeholders’ involvement[J]. Journal of Environmental Management, 2020, 261: 110158. |
| 5 | WESTERHOFF P, YOON Y, SNYDER S, et al. Fate of endocrine-disruptor, pharmaceutical, and personal care product chemicals during simulated drinking water treatment processes[J]. Environmental Science & Technology, 2005, 39(17): 6649-6663. |
| 6 | 张盼伟. 海河流域典型水体中PPCPs的环境行为及潜在风险研究[D]. 北京: 中国水利水电科学研究院, 2018. |
| ZHANG Panwei. Environmental behavior and pollution characteristics of pharmaceuticals and personal care products, and their associated environmental risks in typical water-body from Haihe river basin, China[D]. Beijing: China Institute of Water Resources and Hydropower Research, 2018. | |
| 7 | SCHAIDER L A, RUDEL R A, ACKERMAN J M, et al. Pharmaceuticals, perfluorosurfactants, and other organic wastewater compounds in public drinking water wells in a shallow sand and gravel aquifer[J]. Science of the Total Environment, 2014, 468/469: 384-393. |
| 8 | SHARMA B M, BEČANOVÁ J, SCHERINGER M, et al. Health and ecological risk assessment of emerging contaminants (pharmaceuticals, personal care products, and artificial sweeteners) in surface and groundwater (drinking water) in the Ganges River Basin, India[J]. Science of the Total Environment, 2019, 646: 1459-1467. |
| 9 | KANDA R, GRIFFIN P, JAMES H A, et al. Pharmaceutical and personal care products in sewage treatment works[J]. Journal of Environmental Monitoring, 2003, 5(5): 823-830. |
| 10 | 时红蕾, 王晓昌, 李倩. 人粪便好氧堆肥过程中典型抗生素的消减特性[J]. 环境科学, 2018, 39(7): 3434-3442. |
| SHI Honglei, WANG Xiaochang, LI Qian. Removal of typical antibiotics during aerobic composting of human feces[J]. Environmental Science, 2018, 39(7): 3434-3442. | |
| 11 | WANG J L, CHEN L J, SHI H C, et al. Microbial degradation of phthalic acid esters under anaerobic digestion of sludge[J]. Chemosphere, 2000, 41(8): 1245-1248. |
| 12 | YANG L, HU C, NIE Y L, et al. Surface acidity and reactivity of β-FeOOH/Al2O3 for pharmaceuticals degradation with ozone: In situ ATR-FTIR studies[J]. Applied Catalysis B: Environmental, 2010, 97(3/4): 340-346. |
| 13 | ATANDA L A, BALASAMY R J, KHURSHID A, et al. Ethylbenzene dehydrogenation over Mg3Fe0.5-xCoxAl0.5 catalysts derived from hydrotalcites: comparison with Mg3Fe0.5-yNiyAl0.5 catalysts[J]. Applied Catalysis A: General, 2011, 396(1/2): 107-115. |
| 14 | SI X R, HU Z F, DING D, et al. Effects of effluent organic matters on endocrine disrupting chemical removal by ultrafiltration and ozonation in synthetic secondary effluent[J]. Journal of Environmental Sciences, 2019, 76: 57-64. |
| 15 | 冯勇, 吴德礼, 马鲁铭. 黄铁矿催化H2O2氧化降解水中三氯生[J]. 环境工程学报, 2012, 6(10): 3433-3437. |
| FENG Yong, WU Deli, MA Luming. Catalytic oxidation of triclosan in water by pyrite and hydrogen peroxide[J]. Chinese Journal of Environmental Engineering, 2012, 6(10): 3433-3437. | |
| 16 | LAERA G, CASSANO D, LOPEZ A, et al. Removal of organics and degradation products from industrial wastewater by a membrane bioreactor integrated with ozone or UV/H2O2 treatment[J]. Environmental Science & Technology, 2012, 46(2): 1010-1018. |
| 17 | TANG X, WU Q Y, YANG Y, et al. Genotoxicity removal of reclaimed water during ozonation[J]. Journal of Environmental Sciences, 2014, 26(6): 1243-1248. |
| 18 | SUN S P, ZENG X, LEMLEY A T. Nano-magnetite catalyzed heterogeneous Fenton-like degradation of emerging contaminants carbamazepine and ibuprofen in aqueous suspensions and montmorillonite clay slurries at neutral pH[J]. Journal of Molecular Catalysis A: Chemical, 2013, 371: 94-103. |
| 19 | ARDO S G, NÉLIEU S, ONA-NGUEMA G, et al. Oxidative degradation of nalidixic acid by nano-magnetite via Fe2+/O2- Mediated reactions[J]. Environmental Science & Technology, 2015, 49(7): 4506-4514. |
| 20 | ZHENG B G, ZHENG Z, ZHANG J B, et al. Degradation of the emerging contaminant ibuprofen in aqueous solution by gamma irradiation[J]. Desalination, 2011, 276(1/2/3): 379-385. |
| 21 | HOMLOK R, TAKÁCS E, WOJNÁROVITS L. Elimination of diclofenac from water using irradiation technology[J]. Chemosphere, 2011, 85(4): 603-608. |
| 22 | CSAY T, RÁCZ G, SALIK Á, et al. Reactions of clofibric acid with oxidative and reductive radicals—products, mechanisms, efficiency and toxic effects[J]. Radiation Physics and Chemistry, 2014, 102: 72-78. |
| 23 | 王月, 熊振湖, 周建国. 杯[4]芳烃修饰Amberlite XAD-4树脂去除水中双氯芬酸[J]. 中国环境科学, 2012, 32(1): 81-88. |
| WANG Yue, XIONG Zhenhu, ZHOU Jianguo. Removal of diclofenac on calyx [4] arene based Amberlite XAD-4 resin from aqueous solutions[J]. China Environmental Science, 2012, 32(1): 81-88. | |
| 24 | WANG W, MA Y, ZHOU Q, et al. Preparation of a permanent magnetic hypercrosslinked resin and assessment of its ability to remove organic micropollutants from drinking water[J]. Frontiers of Environmental Science & Engineering, 2015, 9(1): 96-104. |
| 25 | KIMURA A, TAGUCHI M, OHTANI Y, et al. Decomposition of p-nonylphenols in water and elimination of their estrogen activities by 60Co γ-ray irradiation[J]. Radiation Physics and Chemistry, 2006, 75(1): 61-69. |
| 26 | 张轩. 活性炭纤维阴极增强电化学—高锰酸盐体系(E-ACF-PM)降解水中双氯芬酸的研究[D]. 重庆: 重庆大学, 2018. |
| ZHANG Xuan. Research on activated carbon fiber (ACF) cathode enhanced degradation of diclofenac from aqueous solution in coupling electrolysis with permanganate[D]. Chongqing: Chongqing University, 2018. | |
| 27 | ACERO J L, BENITEZ F J, TEVA F, et al. Retention of emerging micropollutants from UP water and a municipal secondary effluent by ultrafiltration and nanofiltration[J]. Chemical Engineering Journal, 2010, 163(3): 264-272. |
| 28 | MU Y, ROZENDAL R A, RABAEY K, et al. Nitrobenzene removal in bioelectrochemical systems[J]. Environmental Science & Technology, 2009, 43(22): 8690-8695. |
| 29 | XU Y F, GE Z P, ZHANG X Q, et al. Validation of effective roles of non-electroactive microbes on recalcitrant contaminant degradation in bioelectrochemical systems[J]. Environmental Pollution, 2019, 249: 794-800. |
| 30 | 王欣宇, 邢德峰, 任南琪. 碳源对生物阴极降解对硝基苯酚效能及生物阴极群落结构分析[J]. 环境科学学报, 2020, 40(10): 3703-3709. |
| WANG Xinyu, XING Defeng, REN Nanqi. Effect of carbon source on PNP degradation and biocathode community structure in bioelectrochemical system[J]. Acta Scientiae Circumstantiae, 2020, 40(10): 3703-3709. | |
| 31 | CUI M H, CUI D, GAO L, et al. Evaluation of anaerobic sludge volume for improving azo dye decolorization in a hybrid anaerobic reactor with built-in bioelectrochemical system[J]. Chemosphere, 2017, 169: 18-22. |
| 32 | CUI M H, CUI D, GAO L, et al. Efficient azo dye decolorization in a continuous stirred tank reactor (CSTR) with built-in bioelectrochemical system[J]. Bioresource Technology, 2016, 218: 1307-1311. |
| 33 | HASSAN H, JIN B, DONNER E, et al. Microbial community and bioelectrochemical activities in MFC for degrading phenol and producing electricity: microbial consortia could make differences[J]. Chemical Engineering Journal, 2018, 332: 647-657. |
| 34 | MIRAN W, NAWAZ M, JANG J, et al. Chlorinated phenol treatment and in situ hydrogen peroxide production in a sulfate-reducing bacteria enriched bioelectrochemical system[J]. Water Research, 2017, 117: 198-206. |
| 35 | CAI P J, XIAO X, HE Y R, et al. Involvement of c-type cytochrome CymA in the electron transfer of anaerobic nitrobenzene reduction by Shewanella oneidensis MR-1[J]. Biochemical Engineering Journal, 2012, 68: 227-230. |
| 36 | MALVANKAR N S, TUOMINEN M T, LOVLEY D R. Lack of cytochrome involvement in long-range electron transport through conductive biofilms and nanowires of Geobactersulfurreducens[J]. Energy & Environmental Science, 2012, 5(9): 8651-8659. |
| 37 | BRUTINEL E D, GRALNICK J A. Shuttling happens: soluble flavin mediators of extracellular electron transfer in Shewanella[J]. Applied Microbiology and Biotechnology, 2012, 93(1): 41-48. |
| 38 | WU D, SUN F Q, ZHOU Y. Degradation of chloramphenicol with novel metal foam electrodes in bioelectrochemical systems[J]. Electrochimica Acta, 2017, 240: 136-145. |
| 39 | MIAO X S, BISHAY F, CHEN M, et al. Occurrence of antimicrobials in the final effluents of wastewater treatment plants in Canada[J]. Environmental Science &Technology, 2004, 38(13): 3533-3541. |
| 40 | TOPP E, RENAUD J, SUMARAH M, et al. Reduced persistence of the macrolide antibiotics erythromycin, clarithromycin and azithromycin in agricultural soil following several years of exposure in the field[J]. Science of the Total Environment, 2016, 562: 136-144. |
| 41 | 宋昭. 三维电极电催化降解双氯芬酸研究[D]. 哈尔滨: 哈尔滨工业大学, 2017. |
| SONG Zhao. Degradation of diclofenac in wastewater by three-dimensional electrocatalytic system[D]. Harbin: Harbin Institute of Technology, 2017. | |
| 42 | 滑熠龙, 周雪飞, 陈家斌, 等. 环境中布洛芬和氯贝酸的赋存及在SND工艺中的去除[J]. 水处理技术, 2013, 39(7): 35-38. |
| HUA Yilong, ZHOU Xuefei, CHEN Jiabin, et al. Existence of ibuprofen and clofibric acid in environment and biodegradation in SND system[J]. Technology of Water Treatment, 2013, 39(7): 35-38. | |
| 43 | 高品. 典型抗生素和抗药性基因在污水处理系统中的归趋及迁移分布规律[D]. 上海: 东华大学, 2011. |
| GAO Pin. Fate, transport and distribution of typical antibiotics and antibiotic resistance genes in a sewage treatment system[D]. Shanghai: Donghua University, 2011. | |
| 44 | 柳王荣. 典型杀生剂在污水处理厂与受纳水环境中的分布、归趋及生态风险研究[D]. 广州: 中国科学院大学(中国科学院广州地球化学研究所), 2016. |
| LIU Wangrong. Occurrence, fate and ecological risk of biocides in the wastewater treatment plants and receiving aquatic environment[D]. Guangzhou: University of Chinese Academy of Sciences, 2016. | |
| 45 | FERGUSON P J, BERNOT M J, DOLL J C, et al. Detection of pharmaceuticals and personal care products (PPCPs) in near-shore habitats of southern Lake Michigan[J]. Science of the Total Environment, 2013, 458/459/460: 187-196. |
| 46 | BOYD G R, PALMERI J M, ZHANG S Y, et al. Pharmaceuticals and personal care products (PPCPs) and endocrine disrupting chemicals (EDCs) in stormwater canals and Bayou St. John in New Orleans, Louisiana, USA[J]. Science of the Total Environment, 2004, 333(1/2/3): 137-148. |
| 47 | 邹荣婕, 邓旭修, 王斌, 等. 黄河调水调沙对黄河口海域双酚A的影响[J]. 生态学报, 2015, 35(2): 263-269. |
| ZOU Rongjie, DENG Xuxiu, WANG Bin, et al. Effect of water-sediment Flushing events on bisphenol A contamination in the Yellow River estuary[J]. Acta Ecologica Sinica, 2015, 35(2): 263-269. | |
| 48 | 陈慰双. 我国水环境中壬基酚的污染现状及生态风险评估[D]. 青岛: 中国海洋大学, 2013. |
| CHEN Weishuang. The current pollution status and ecological risk assessment of nonylphenol in domestic water environment[D]. Qingdao: Ocean University of China, 2013. | |
| 49 | LIU Z D, LI H R. Effects of bio- and abio-factors on electricity production in a mediatorless microbial fuel cell[J]. Biochemical Engineering Journal, 2007, 36(3): 209-214. |
| 50 | 董跃. 微生物电化学中试系统构型设计、能耗分析及处理效能评价[D]. 哈尔滨: 哈尔滨工业大学, 2018. |
| DONG Yue. Configuration design, energy analysis and treatment efficiency evaluation of pilot-scale microbial electrochemical system[D]. Harbin: Harbin Institute of Technology, 2018. | |
| 51 | TAHIR K, MIRAN W, NAWAZ M, et al. Investigating the role of anodic potential in the biodegradation of carbamazepine in bioelectrochemical systems[J]. Science of the Total Environment, 2019, 688: 56-64. |
| 52 | 蔚清玲. 废水中几种典型污染组分的微生物电化学处理过程及性能研究[D]. 扬州: 扬州大学, 2019. |
| YU Qingling. Microbial electrochemical treatment process and performance of several typical pollutant components in wastewater[D]. Yangzhou: Yangzhou University, 2019. | |
| 53 | 许明熠. 耦合厌氧氨氧化技术的生物电化学系统同步脱氮产电机理研究及功能菌群落分析[D]. 广州: 华南理工大学, 2017. |
| XU Mingyi. Study on reactor’s performance and bacterial colony analysis of integrated bioelectrochemical-anammox system for simultaneous nitrogen treatment and bioenergy production[D]. Guangzhou: South China University of Technology, 2017. | |
| 54 | WANG L, LIU Y L, WANG C, et al. Anoxic biodegradation of triclosan and the removal of its antimicrobial effect in microbial fuel cells[J]. Journal of Hazardous Materials, 2018, 344: 669-678. |
| 55 | XU W L, JIN B, ZHOU S F, et al. Triclosan removal in microbial fuel cell: the contribution of adsorption and bioelectricity generation[J]. Energies, 2020, 13(3): 761. |
| 56 | ZHANG Q H, ZHANG Y Y, LI D P. Cometabolic degradation of chloramphenicol via a meta-cleavage pathway in a microbial fuel cell and its microbial community[J]. Bioresource Technology, 2017, 229: 104-110. |
| 57 | GUO N, MA X F, REN S J, et al. Mechanisms of metabolic performance enhancement during electrically assisted anaerobic treatment of chloramphenicol wastewater[J]. Water Research, 2019, 156: 199-207. |
| 58 | QIU B, HU Y Y, LIANG C, et al. Enhanced degradation of diclofenac with Ru/Fe modified anode microbial fuel cell: kinetics, pathways and mechanisms[J]. Bioresource Technology, 2020, 300: 122703. |
| 59 | 王思祺. 微生物电化学技术处理磺胺甲恶唑和萘普生共存污染[D]. 兰州: 西北师范大学, 2020. |
| WANG Siqi. Treatment of coexistence pollution sulfamethoxazole and naproxen by microbial electrochemical technology[D]. Lanzhou: Northwest Normal University, 2020. | |
| 60 | PENG X H, PAN X H, WANG X, et al. Accelerated removal of high concentration p-chloronitrobenzene using bioelectrocatalysis process and its microbial communities analysis[J]. Bioresource Technology, 2018, 249: 844-850. |
| 61 | LIU Y, WANG C, ZHANG K J, et al. Rapid degradation of 2, 4-dichloronitrobenzene in single-chamber microbial electrolysis cell with pre-acclimated bioanode: a comprehensive assessment[J]. Science of the Total Environment, 2020, 724: 138053. |
| 62 | CHUN C L, PAYNE R B, SOWERS K R, et al. Electrical stimulation of microbial PCB degradation in sediment[J]. Water Research, 2013, 47(1): 141-152. |
| 63 | 王辉, 王建龙. Pd/C气体扩散电极用于电化学降解4-氯酚的研究[J]. 环境科学学报, 2007, 27(10): 1593-1598. |
| WANG Hui, WANG Jianlong. Electrochemical degradation of 4-chlorophenol using a Pd/C gas diffusion electrode[J]. Acta Scientiae Circumstantiae, 2007, 27(10): 1593-1598. | |
| 64 | FENG H J, ZHANG X Q, LIANG Y X, et al. Enhanced removal of p-fluoronitrobenzene using bioelectrochemical system[J]. Water Research, 2014, 60: 54-63. |
| 65 | HAY A G, DEES P M, SAYLER G S. Growth of a bacterial consortium on triclosan[J]. FEMS Microbiology Ecology, 2001, 36(2/3): 105-112. |
| 66 | GAO Y P, JI Y M, LI G Y, et al. Mechanism, kinetics and toxicity assessment of OH-initiated transformation of triclosan in aquatic environments[J]. Water Research, 2014, 49: 360-370. |
| 67 | WU D, SUN F Q, CHUA F J D, et al. Enhanced power generation in microbial fuel cell by an agonist of electroactive biofilm - sulfamethoxazole[J]. Chemical Engineering Journal, 2020, 384: 123238. |
| 68 | SONG H L, LI H, ZHANG S, et al. Fate of sulfadiazine and its corresponding resistance genes in up-flow microbial fuel cell coupled constructed wetlands: effects of circuit operation mode and hydraulic retention time[J]. Chemical Engineering Journal, 2018, 350: 920-929. |
| 69 | YUAN H Y, MILLER J H, ABU-REESH I M, et al. Effects of electron acceptors on removal of antibiotic resistant Escherichia coli, resistance genes and class 1 integrons under anaerobic conditions[J]. Science of the Total Environment, 2016, 569/570: 1587-1594. |
| 70 | SHEN J Y, XU X P, JIANG X B, et al. Coupling of a bioelectrochemical system for p-nitrophenol removal in an upflow anaerobic sludge blanket reactor[J]. Water Research, 2014, 67: 11-18. |
| 71 | JIANG X B, SHEN J Y, HAN Y, et al. Efficient nitro reduction and dechlorination of 2, 4-dinitrochlorobenzene through the integration of bioelectrochemical system into upflow anaerobic sludge blanket: a comprehensive study[J]. Water Research, 2016, 88: 257-265. |
| 72 | 魏祥甲. Pd/MWNTs气体扩散电极制备及降解氯酚类有机物研究[D]. 北京: 北京林业大学, 2012. |
| WEI Xiangjia. Preparation of Pd/MWNTs gas diffusion electrodes and the degradation of chlorophenols[D]. Beijing: Beijing Forestry University, 2012. | |
| 73 | WEN Q, YANG T, WANG S Y, et al. Dechlorination of 4-chlorophenol to phenol in bioelectrochemical systems[J]. Journal of Hazardous Materials, 2013, 244/245: 743-749. |
| 74 | 萨一德. 四溴双酚A(TBBPA)在升流式MFC生物滤池中降解特性研究[D]. 哈尔滨: 哈尔滨工业大学, 2019. |
| ISLAM A S. Study on the degradation of tetrabromobisphenol A(TBBPA) in an up-flow MFC-AF coupling system[D]. Harbin: Harbin Institute of Technology, 2019. | |
| 75 | WU H M, ZHANG J, NGO H H, et al. A review on the sustainability of constructed wetlands for wastewater treatment: design and operation[J]. Bioresource Technology, 2015, 175: 594-601. |
| 76 | 陈桐清. 产电型人工湿地对典型PPCPs的去除作用及机理解析[D]. 南京: 东南大学, 2018. |
| CHEN Tongqing. Removal efficiency and mechanismof typical PPCPs in microbial fuel cell coupled with constructed wetlands[D]. Nanjing: Southeast University, 2018. | |
| 77 | LI H, ZHANG S, YANG X L, et al. Enhanced degradation of bisphenol A and ibuprofen by an up-flow microbial fuel cell-coupled constructed wetland and analysis of bacterial community structure[J]. Chemosphere, 2019, 217: 599-608. |
| 78 | LI H, CAI Y, GU Z L, et al. Accumulation of sulfonamide resistance genes and bacterial community function prediction in microbial fuel cell-constructed wetland treating pharmaceutical wastewater[J]. Chemosphere, 2020, 248: 126014. |
| 79 | WANG Y H, FENG C J, LI Y, et al. Enhancement of emerging contaminants removal using Fenton reaction driven by H2O2-producing microbial fuel cells[J]. Chemical Engineering Journal, 2017, 307: 679-686. |
| 80 | WANG X X, HU J P, CHEN Q, et al. Synergic degradation of 2, 4, 6-trichlorophenol in microbial fuel cells with intimately coupled photocatalytic-electrogenic anode[J]. Water Research, 2019, 156: 125-135. |
| [1] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [2] | 杨建平. 降低HPPO装置反应系统原料消耗的PSE[J]. 化工进展, 2023, 42(S1): 21-32. |
| [3] | 王福安. 300kt/a环氧丙烷工艺反应器降耗减排分析[J]. 化工进展, 2023, 42(S1): 213-218. |
| [4] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [5] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [6] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [7] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [8] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [9] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [10] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [11] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [12] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [13] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [14] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [15] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 唑、
唑、