化工进展 ›› 2021, Vol. 40 ›› Issue (12): 6859-6875.DOI: 10.16085/j.issn.1000-6613.2021-0092
超薄二维多孔纳米片在水处理中的研究进展
黄从新1,2( ), 王顺藤1,2, 范宇莹2,3, 简美鹏4,5(
), 王顺藤1,2, 范宇莹2,3, 简美鹏4,5( ), 唐朝春1, 刘锐平2,6(
), 唐朝春1, 刘锐平2,6( )
)
- 1.华东交通大学土木建筑学院,江西 南昌 330013
2.中国科学院生态环境研究中心,中国科学院饮用水科学与技术重点实验室,北京 100085
3.东北师范大学环境工程系,吉林 长春 130117
4.广东工业大学材料与能源学院,广东 广州 510006
5.蒙纳士大学化学工程系,澳大利亚 维多利亚州 3800
6.清华大学环境学院,清华大学水质与水生态研究中心,北京 100084
-
收稿日期:2021-01-14修回日期:2021-03-07出版日期:2021-12-05发布日期:2021-12-21 -
通讯作者:简美鹏,刘锐平 -
作者简介:黄从新(1997—),男,硕士研究生,研究方向为水处理理论与技术。E-mail:huangcongxin_13@163.com 。 -
基金资助:港澳及海外青年学者合作研究基金(51729801);中国博士后科学基金(2020M672532)
Advance of ultrathin 2D porous nanosheets in water treatment
HUANG Congxin1,2( ), WANG Shunteng1,2, FAN Yuying2,3, JIAN Meipeng4,5(
), WANG Shunteng1,2, FAN Yuying2,3, JIAN Meipeng4,5( ), TANG Chaochun1, LIU Ruiping2,6(
), TANG Chaochun1, LIU Ruiping2,6( )
)
- 1.School of Civil Engineering and Architecture, East China Jiaotong University, Nanchang 330013, Jiangxi, China
2.State Key Laboratory of Environmental Aquatic Chemistry, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing 100085, China
3.School of Environment, Northeast Normal University, Changchun 130117, Jilin, China
4.School of Materials and Energy, Guangdong University of Technology, Guangzhou 510006 Guangdong, China
5.Department of Chemical Engineering, Monash University, Victoria 3800, Australia
6.Center for Water and Ecology, State Key Joint Laboratory of Environment Simulation and Pollution Control, School of Environment, Tsinghua University, Beijing 100084, China
-
Received:2021-01-14Revised:2021-03-07Online:2021-12-05Published:2021-12-21 -
Contact:JIAN Meipeng,LIU Ruiping
摘要:
二维(2D)纳米片是一类非常具有前瞻性的多孔材料。作为新型的高性能吸附剂,与传统吸附材料相比,超薄2D多孔纳米片具有高比表面积、原子级厚度和几乎完全裸露的活性位点等优点,可快速有效地捕获水体中的(有机或无机)污染物质。本综述总结了两类代表性的超薄2D多孔纳米片[金属有机骨架材料(MOFs)和共价有机骨架材料(COFs)]作为优良吸附剂去除水环境中有机染料、有毒重金属和放射性元素的最新进展。介绍了该类化合物的结构特性和物理化学性质,总结了四种常用合成方法,重点分析了四种方法的优缺点以及面临的挑战,并对不同的合成方法进行了特点比较。阐述了超薄2D多孔纳米片在水中对各种污染物的吸附条件和吸附性能,对相关吸附机理做了系统总结和对比。论述了材料的再生性能,总结分析了再生过程中可能遇到的问题。最后对当前超薄2D多孔纳米片存在的不足、合成条件复杂等问题,提出了材料的合成优化、绿色无毒或低毒新技术的开发、重复使用效率的提高将会是未来的发展方向。
中图分类号:
引用本文
黄从新, 王顺藤, 范宇莹, 简美鹏, 唐朝春, 刘锐平. 超薄二维多孔纳米片在水处理中的研究进展[J]. 化工进展, 2021, 40(12): 6859-6875.
HUANG Congxin, WANG Shunteng, FAN Yuying, JIAN Meipeng, TANG Chaochun, LIU Ruiping. Advance of ultrathin 2D porous nanosheets in water treatment[J]. Chemical Industry and Engineering Progress, 2021, 40(12): 6859-6875.
| 制备方法 | 制备条件 | 特点 | 产品质量 |
|---|---|---|---|
| 微机械剥离 | 以层状块体为原料,借助透明胶带或研磨 | 缺陷少,厚度和形状难控制,适合大的层状晶体材料 | 尺寸小,产量极低 |
| 液基剥离 | 以层状粉末为原料,通过插层剂或超声的方式 | 成本较低,操作相对简单,可量化生产,不使用有毒有害的有机溶剂 | 尺寸较小,较宽的厚度,产量偏低 |
| 化学气相沉积 | 特定的CVD设备,高温高真空,挥发性前体在基体上沉积 | 衬底选择不具有任意性,不可避免地会引入残留物和缺陷,低温下合成难度大 | 产品纯度高,质量好,多为片状 |
| 水热合成 | 高温或蒸气压下,在水溶液或有机溶液中结晶 | 成本低,高产率,可大批量生产,控制参数多,获得单层纳米片难度大 | 成分可控,大型优质,纳米薄片 |
表1 几种超薄2D多孔纳米片的制备方法比较
| 制备方法 | 制备条件 | 特点 | 产品质量 |
|---|---|---|---|
| 微机械剥离 | 以层状块体为原料,借助透明胶带或研磨 | 缺陷少,厚度和形状难控制,适合大的层状晶体材料 | 尺寸小,产量极低 |
| 液基剥离 | 以层状粉末为原料,通过插层剂或超声的方式 | 成本较低,操作相对简单,可量化生产,不使用有毒有害的有机溶剂 | 尺寸较小,较宽的厚度,产量偏低 |
| 化学气相沉积 | 特定的CVD设备,高温高真空,挥发性前体在基体上沉积 | 衬底选择不具有任意性,不可避免地会引入残留物和缺陷,低温下合成难度大 | 产品纯度高,质量好,多为片状 |
| 水热合成 | 高温或蒸气压下,在水溶液或有机溶液中结晶 | 成本低,高产率,可大批量生产,控制参数多,获得单层纳米片难度大 | 成分可控,大型优质,纳米薄片 |
| 吸附剂 | 吸附剂 带电性 | 染料 | 吸附强弱 | 主要吸附机理 | 文献来源 |
|---|---|---|---|---|---|
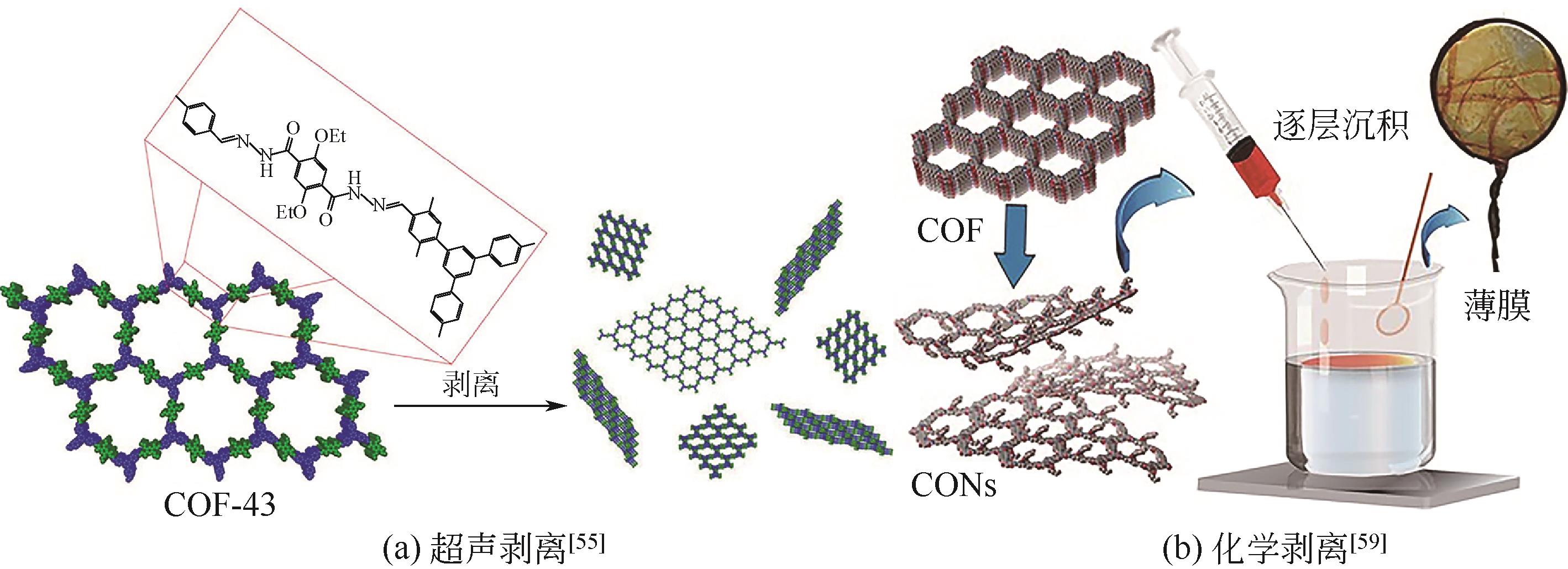
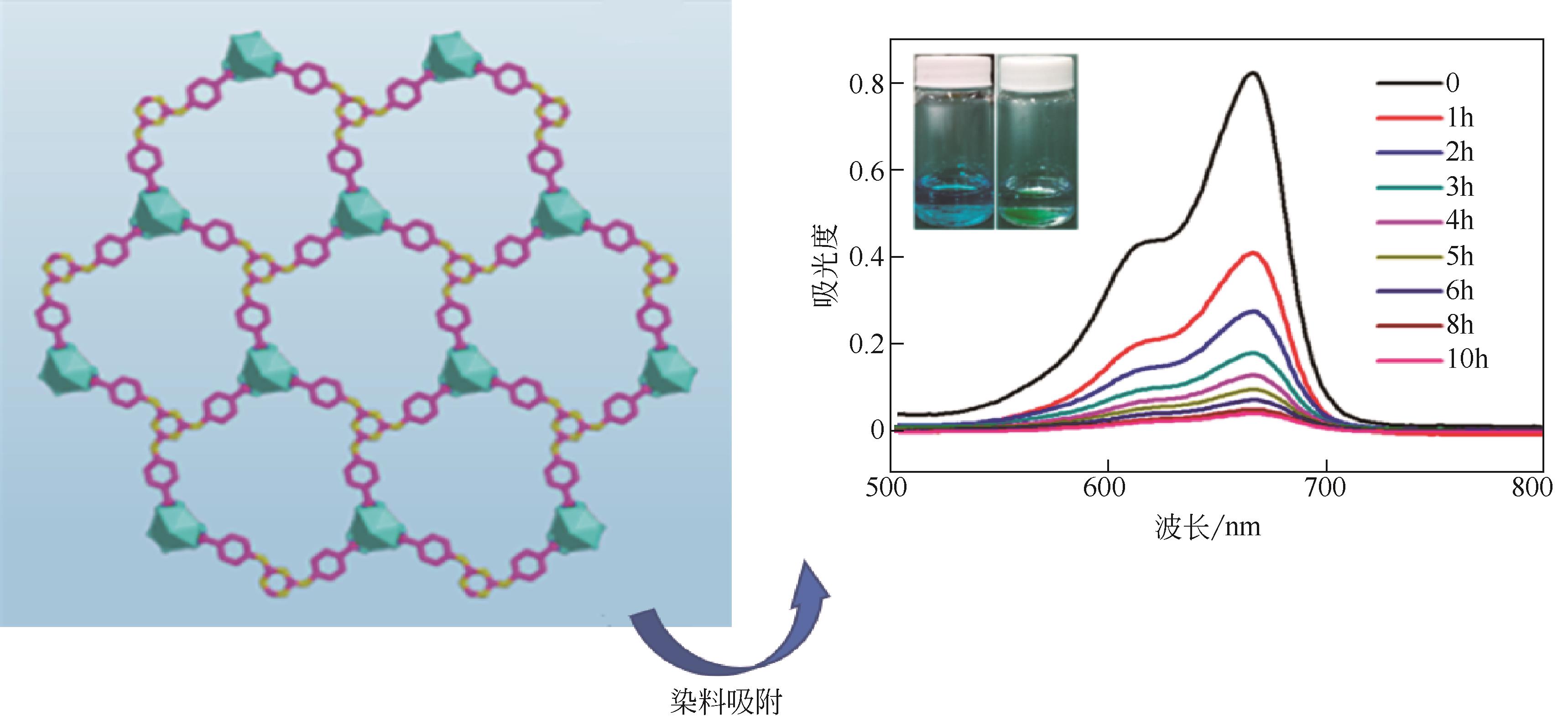
[(CH3)2NH2]2[(Ca4O)L4(H2O)4]·6DMF (SHU-1) | - | MG、CR、CV、RhB | MG>RhB>CR>CV | 尺寸排斥、酸碱相互作用、π-π相互作用 | [ |
| IPM-MOF-201 | + | MO、MB、IC、BTB | MO>IC>MB>BTB | —SO | [ |
[(CH3)2NH2][UO2(TATAB)]·2DMF·4H2O (U-TATAB) | - | MB、ST、茜素、MO、EY | MB>ST>茜素>MO>EY | 层间不配位阳离子与阳离子染料离子交换作用、仲胺基团与染料相互作用 | [ |
| Cu-TCPP-MOF | - | RhB、MB、CR | MB>CR>RhB | —COO-与染料分子相互作用、静电作用 | [ |
| Ni-MOF | - | MB、CR | MB>CR | 静电引力、π-π相互作用、氢键相互作用 | [ |
[Zn2(tpdc)2(H2O)2](H2O)2(DMF)5 (Zn-MOF) | 中性 | NR、BR-2、MO | NR>BR-2>MO | 尺寸选择 | [ |
| Ttba-TPDA-COF | - | RhB | — | 三嗪基和亚氨基与RhB分子的静电作用 | [ |
| PC-COF | + | MO、AG-25、DFBM、AR-27 | AR-27>MO>DFBM>AG-25 | 染料与层间Cl-交换作用 | [ |
表2 各种超薄2D多孔纳米片吸附水中染料的差异对比
| 吸附剂 | 吸附剂 带电性 | 染料 | 吸附强弱 | 主要吸附机理 | 文献来源 |
|---|---|---|---|---|---|
[(CH3)2NH2]2[(Ca4O)L4(H2O)4]·6DMF (SHU-1) | - | MG、CR、CV、RhB | MG>RhB>CR>CV | 尺寸排斥、酸碱相互作用、π-π相互作用 | [ |
| IPM-MOF-201 | + | MO、MB、IC、BTB | MO>IC>MB>BTB | —SO | [ |
[(CH3)2NH2][UO2(TATAB)]·2DMF·4H2O (U-TATAB) | - | MB、ST、茜素、MO、EY | MB>ST>茜素>MO>EY | 层间不配位阳离子与阳离子染料离子交换作用、仲胺基团与染料相互作用 | [ |
| Cu-TCPP-MOF | - | RhB、MB、CR | MB>CR>RhB | —COO-与染料分子相互作用、静电作用 | [ |
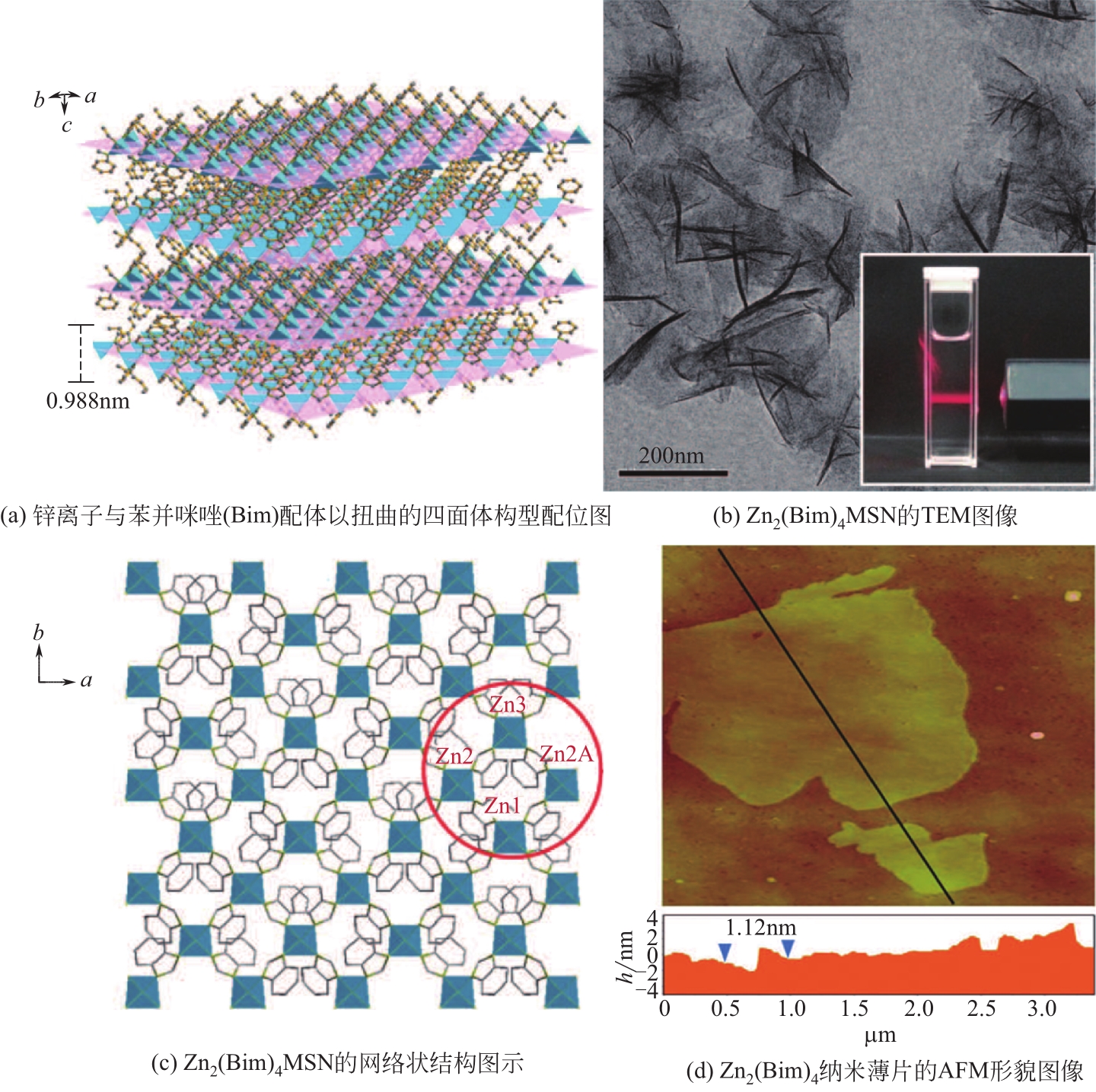
| Ni-MOF | - | MB、CR | MB>CR | 静电引力、π-π相互作用、氢键相互作用 | [ |
[Zn2(tpdc)2(H2O)2](H2O)2(DMF)5 (Zn-MOF) | 中性 | NR、BR-2、MO | NR>BR-2>MO | 尺寸选择 | [ |
| Ttba-TPDA-COF | - | RhB | — | 三嗪基和亚氨基与RhB分子的静电作用 | [ |
| PC-COF | + | MO、AG-25、DFBM、AR-27 | AR-27>MO>DFBM>AG-25 | 染料与层间Cl-交换作用 | [ |
| 金属离子 | 吸附剂 | 吸附强弱 | 主要吸附机理 | 文献 来源 |
|---|---|---|---|---|
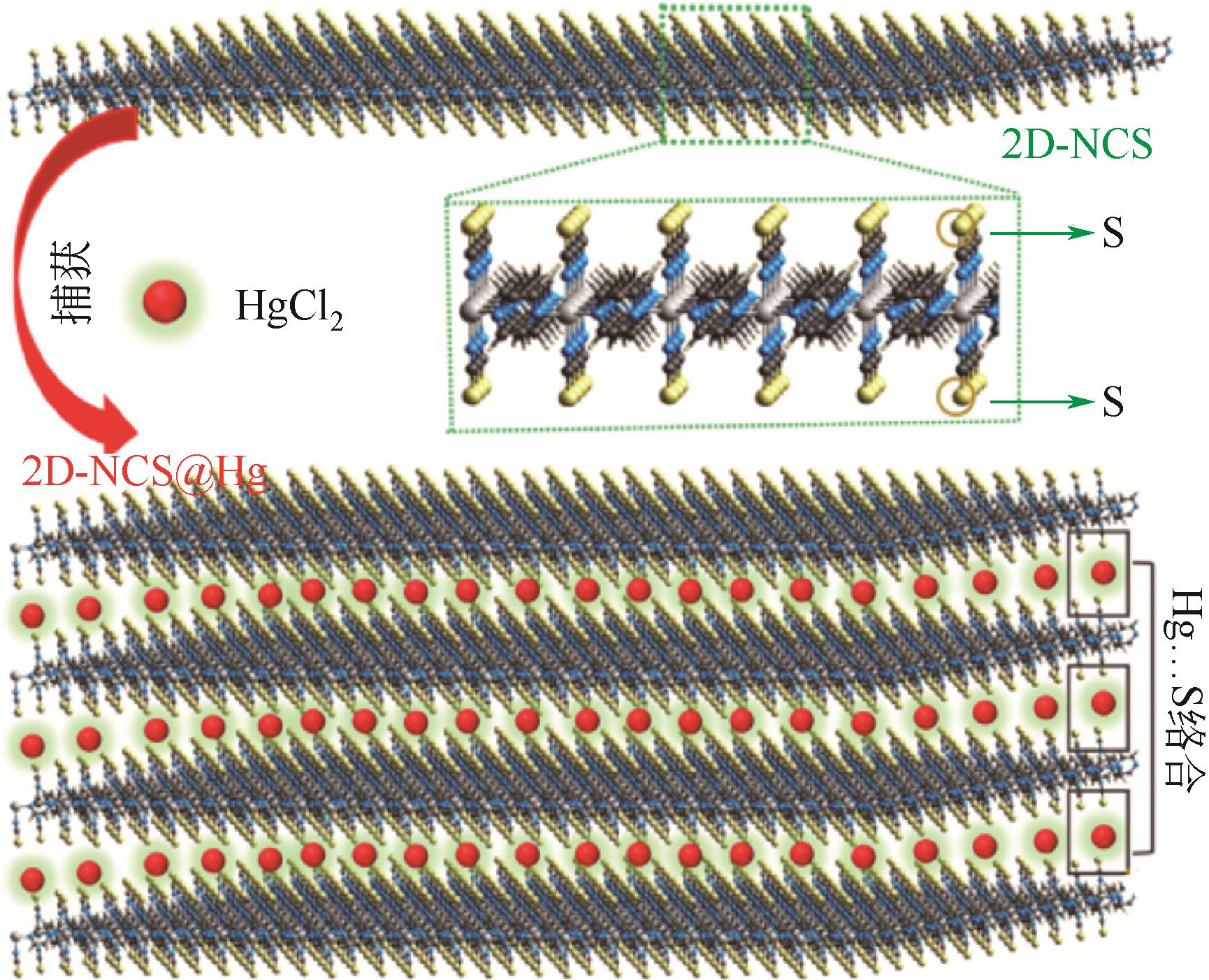
| Hg2+ | {[Co(NCS)2(pyz)2]}n (2D-NCS) | 高亲和力(Kd=2.26×106mL/g) | 2D-NCS的吡嗪环之间的阳离子-π相互作用,硫氰酸根与Hg2+的螯合作用 | [ |
| Hg2+、Pb2+、Cu2+、Zn2+ | COF-S-SH | Hg2+>Pb2+>Cu2+>Zn2+ | 含硫基团与Hg2+的螯合作用 | [ |
| Pb2+、Cu2+、Fe3+、Cd2+、Mn2+、Cr6+ | COF-SH | Pb2+>Cu2+>Fe3+>Cd2+>Mn2+>Cr6+ | Pb2+与巯基之间的静电吸引或螯合作用 | [ |
| Pb2+、Cu2+、Ni2+、Co2+、Cd2+ | Zn(Bim)(OAc)-NS | Pb2+>Cu2+>Ni2+>Co2+>Cd2+ | —NH2、—OH与重金属离子的络合作用 | [ |
| Pb2+、Ni2+、Zn2+、Cu2+、Cd2+ | [Ca(H4L)(DMA)2]·2DMA (Ca-MOF) | Pb2+>Cd2+>Ni2+>Cu2+>Zn2+ | Ca2+与重金属离子之间的离子交换作用 | [ |
| Cu2+、Hg2+、Pb2+、Cd2+、Cr2+ | TpODH-COF | Cu2+>Hg2+>Pb2+>Cr2+>Cd2+ | Cu2+与酰胺、氨基和羰基之间的配位作用 | [ |
| UO | MPCOF | UO | 颗粒内扩散和阳离子筛分效应 | [ |
表3 各种超薄2D多孔纳米片用于水中重金属离子吸附的差异对比
| 金属离子 | 吸附剂 | 吸附强弱 | 主要吸附机理 | 文献 来源 |
|---|---|---|---|---|
| Hg2+ | {[Co(NCS)2(pyz)2]}n (2D-NCS) | 高亲和力(Kd=2.26×106mL/g) | 2D-NCS的吡嗪环之间的阳离子-π相互作用,硫氰酸根与Hg2+的螯合作用 | [ |
| Hg2+、Pb2+、Cu2+、Zn2+ | COF-S-SH | Hg2+>Pb2+>Cu2+>Zn2+ | 含硫基团与Hg2+的螯合作用 | [ |
| Pb2+、Cu2+、Fe3+、Cd2+、Mn2+、Cr6+ | COF-SH | Pb2+>Cu2+>Fe3+>Cd2+>Mn2+>Cr6+ | Pb2+与巯基之间的静电吸引或螯合作用 | [ |
| Pb2+、Cu2+、Ni2+、Co2+、Cd2+ | Zn(Bim)(OAc)-NS | Pb2+>Cu2+>Ni2+>Co2+>Cd2+ | —NH2、—OH与重金属离子的络合作用 | [ |
| Pb2+、Ni2+、Zn2+、Cu2+、Cd2+ | [Ca(H4L)(DMA)2]·2DMA (Ca-MOF) | Pb2+>Cd2+>Ni2+>Cu2+>Zn2+ | Ca2+与重金属离子之间的离子交换作用 | [ |
| Cu2+、Hg2+、Pb2+、Cd2+、Cr2+ | TpODH-COF | Cu2+>Hg2+>Pb2+>Cr2+>Cd2+ | Cu2+与酰胺、氨基和羰基之间的配位作用 | [ |
| UO | MPCOF | UO | 颗粒内扩散和阳离子筛分效应 | [ |
| 污染物 | 吸附剂 | 解吸剂 | 再生性能 | 文献来源 |
|---|---|---|---|---|
| CR、RhB、MB、MO | {[Mn3(L1)2(L2)2(H2O)8]·4H2O}n(Mn-MOF) | DMF | 95%(5次) | [ |
| CR、MO | {[Zn2(5-OH-BDC)2L2]·1.5H2O}n(Zn-MOF) | — | 86.79%(7次) | [ |
| MB | [(1,2-DPE)Co2Cl2]n(HT-1) | 乙醇 | >80%(5次) | [ |
| Hg2+、Pb2+、Zn2+、Fe3+ | TAPB-BMTTPA-COF | 6mol/L的盐酸 | 92%(6次) | [ |
| Cu2+、Hg2+、Pb2+、Cd2+、Cr2+ | TpODH-COF | 6mmol/L的盐酸 | 51%(2次) | [ |
| Hg2+ | {Zn(BDC)(L?)}·DMF(TMU-40) | 0.5mol/L的硝酸 | 90%(3次) | [ |
表4 各种超薄2D多孔纳米片用于水中污染物吸附再生性能对比
| 污染物 | 吸附剂 | 解吸剂 | 再生性能 | 文献来源 |
|---|---|---|---|---|
| CR、RhB、MB、MO | {[Mn3(L1)2(L2)2(H2O)8]·4H2O}n(Mn-MOF) | DMF | 95%(5次) | [ |
| CR、MO | {[Zn2(5-OH-BDC)2L2]·1.5H2O}n(Zn-MOF) | — | 86.79%(7次) | [ |
| MB | [(1,2-DPE)Co2Cl2]n(HT-1) | 乙醇 | >80%(5次) | [ |
| Hg2+、Pb2+、Zn2+、Fe3+ | TAPB-BMTTPA-COF | 6mol/L的盐酸 | 92%(6次) | [ |
| Cu2+、Hg2+、Pb2+、Cd2+、Cr2+ | TpODH-COF | 6mmol/L的盐酸 | 51%(2次) | [ |
| Hg2+ | {Zn(BDC)(L?)}·DMF(TMU-40) | 0.5mol/L的硝酸 | 90%(3次) | [ |
| 1 | XU J, CAO Z, ZHANG Y, et al. A review of functionalized carbon nanotubes and graphene for heavy metal adsorption from water: preparation, application, and mechanism[J]. Chemosphere, 2018, 195(3): 351-364. |
| 2 | SHERLALA A I A, RAMAN A A A, BELLO M M, et al. A review of the applications of organo-functionalized magnetic graphene oxide nanocomposites for heavy metal adsorption[J]. Chemosphere, 2018, 193(11): 1004-1017. |
| 3 | UPADHYAY R, SOIN N, ROY S. Role of graphene/metal oxide composites as photocatalysts, adsorbents and disinfectants in water treatment: a review[J]. RSC Advances, 2014, 4(29): 3823-3851. |
| 4 | LI T, LIN G, PODOLA B, et al. Continuous removal of zinc from wastewater and mine dump leachate by a microalgal biofilm PSBR[J]. Journal of Hazardous Materials, 2015, 297(10): 112-118. |
| 5 | 李萱萱. 高级氧化技术在处理染料废水中的应用[J]. 化工进展, 2012, 31(S2): 219-222. |
| LI Xuanxuan. Application of advanced oxidation in dye wastewater treatment[J]. Chemical Industry and Engineering Progress, 2012, 31(S2): 219-222. | |
| 6 | 黄智辉, 纪志永, 陈希, 等. 过硫酸盐高级氧化降解水体中有机污染物研究进展[J]. 化工进展, 2019, 38(5): 2461-2470. |
| HUANG Zhihui, JI Zhiyong, CHEN Xi, et al. Degradation of organic pollutants in water by persulfate advanced oxidation[J]. Chemical Industry and Engineering Progress, 2019, 38(5): 2461-2470. | |
| 7 | 唐朝春, 许荣明. 电化学法除镍研究进展[J]. 工业水处理, 2018, 38(12): 10-14, 19. |
| TANG Chaochun, Xu Rongming. Research progress in nickel removal by electrochemical method[J]. Industrial Water Treatment, 2018, 38(12): 10-14, 19. | |
| 8 | 王重庆, 王晖, 江小燕, 等. 生物炭吸附重金属离子的研究进展[J]. 化工进展, 2019, 38(1): 692-706. |
| WANG Chongqing, WANG Hui, JIANG Xiaoyan, et al. Research advances on adsorption of heavy metals by biochar[J]. Chemical Industry and Engineering Progress, 2019, 38(1): 692-706. | |
| 9 | 杨光绪, 龚正刚, 罗小林, 等. 氯代UiO-66吸附染色纸废水中罗丹明B和刚果红[J]. 化工进展, 2019, 38(7): 3434-3442. |
| YANG Guangxu, GONG Zhenggang, LUO Xiaolin, et al. Adsorption of Rhodamine B and Congo red in dyeing paper wastewater by chlorinesubstituted UiO-66[J]. Chemical Industry and Engineering Progress, 2019, 38(7): 3434-3442. | |
| 10 | MATLOCK M M, HOWERTON B S, ATWOOD D A. Chemical precipitation of heavy metals from acid mine drainage[J]. Water Research, 2002, 36(19): 4757-4764. |
| 11 | 覃发梅, 邱学青, 孙川, 等. 纳米纤维素去除水体系重金属离子的研究进展[J]. 化工进展, 2019, 38(7): 3390-3401. |
| QIN Famei, QIU Xueqing, SUN Chuan, et al. Research progress in nanocellulose for the removal of heavy metal ions in water[J]. Chemical Industry and Engineering Progress, 2019, 38(7): 3390-3401. | |
| 12 | 张帆, 李菁, 谭建华, 等. 吸附法处理重金属废水的研究进展[J]. 化工进展, 2013, 32(11): 2749-2756. |
| ZHANG Fan,LI Jing,TAN Jianhua,et al. Advance of the treatment of heavy metal wastewater by adsorption[J]. Chemical Industry and Engineering Progress, 2013, 32(11): 2749-2756. | |
| 13 | HO Y S. Review of second-order models for adsorption systems[J]. Journal of Hazardous Materials, 2006, 136(3): 681-689. |
| 14 | ALI I. New generation adsorbents for water treatment[J]. Chemical Reviews, 2012, 112(10): 5073-5091. |
| 15 | 文永林, 刘攀, 汤琪. 农林废弃物吸附脱除废水中重金属研究进展[J]. 化工进展, 2016, 35(4): 1208-1215. |
| WEN Yonglin, LIU Pan, TANG Qi. Review of removal of heavy metal ions from wastewater with agricultural and forestry waste as adsorbent[J]. Chemical Industry and Engineering Progress, 2016, 35(4): 1208-1215. | |
| 16 | 申朋飞, 朱颖颖, 李信宝, 等. 植物基活性炭的制备及吸附应用研究进展[J]. 化工进展, 2019, 38(8): 3763-3773. |
| SHEN Pengfei, ZHU Yingying, LI Xinbao, et al. Review on preparation of plant-based activated carbon and its adsorption application[J]. Chemical Industry and Engineering Progress, 2019, 38(8): 3763-3773. | |
| 17 | ERSAN G, APUL O G, PERREAULT F, et al. Adsorption of organic contaminants by graphene nanosheets: a review[J]. Water Research, 2017, 126(10): 385-398. |
| 18 | LI A, SUN H X, TAN D Z, et al. Superhydrophobic conjugated microporous polymers for separation and adsorption[J]. Energy & Environment Science, 2011, 4(6): 2062-2065. |
| 19 | WANG H S, LIU H L, WANG K, et al. Insight into the unique fluorescence quenching property of metal-organic frameworks upon DNA binding[J]. Analytical Chemistry, 2017, 89(21): 11366-11371. |
| 20 | SONG W J. Intracellular DNA and microRNA sensing based on metal-organic framework nanosheets with enzyme-free signal amplification[J]. Talanta, 2017, 170(8): 74-80. |
| 21 | WANG H S, LI J, LI J Y, et al. Lanthanide-based metal-organic framework nanosheets with unique fluorescence quenching properties for two-color intracellular adenosine imaging in living cells[J]. NPG Asia Materials, 2017, 9(3): 354-362. |
| 22 | ZHAO M, HUANG Y, PENG Y, et al. Two-dimensional metal-organic framework nanosheets: synthesis and applications[J]. Chemical Society Reviews, 2018, 47(16): 6267-6295. |
| 23 | YOO E, KIM J, HOSONO E, et al. Large reversible Li storage of graphene nanosheet families for use in rechargeable lithium ion batteries[J]. Nano Letters, 2008, 8(8): 2277-2282. |
| 24 | DERIA P, MONDLOCH J E, KARAGIARIDI O, et al. Cheminform abstract: beyond post-synthesis modification: evolution of metal-organic frameworks via building block replacement[J]. ChemInform, 2014, 45(43): 5896-5912. |
| 25 | PARK I H, JU H, HERNG T S, et al. Supramolecular isomerism and polyrotaxane-based two-dimensional coordination polymers[J]. Crystal Growth & Design, 2016, 16(12): 7278-7287. |
| 26 | AHMED F, ROY S, NASKAR K, et al. Halogen…halogen interactions in the supramolecular assembly of 2D coordination polymers and the CO2 sorption behavior[J]. Crystal Growth & Design, 2016, 16(9): 5514-5535. |
| 27 | ZHAO M, LU Q, MA Q, et al. Two-dimensional metal-organic framework nanosheets[J]. Small Methods, 2016, 1(1/2): 1600030-1600037. |
| 28 | BAI W, LI S, MA J, et al. Ultrathin 2D metal-organic framework (nanosheets and nanofilms)-based xD-2D hybrid nanostructures as biomimetic enzymes and supercapacitors[J]. Journal of Materials Chemistry A, 2019, 7(15): 9086-9098. |
| 29 | PENG Y, LI Y, BAN Y, et al. Metal-organic framework nanosheets as building blocks for molecular sieving membranes[J]. Science, 2014, 346(6215): 1356-1359. |
| 30 | DOONAN C J, TRANCHEMONTAGNE D J, GLOVER T G, et al. Exceptional ammonia uptake by a covalent organic framework[J]. Nature Chemistry, 2010, 2(3): 235-238. |
| 31 | ZOU X Q, REN H, ZHU G S, et al. Topology-directed design of porous organic frameworks and their advanced applications[J]. Chemical Communications Royal Society of Chemistry, 2013, 49(38): 3925-3936. |
| 32 | COTE A P, BENIN A I, OCKWIG N W, et al. Porous, crystalline, covalent organic frameworks[J]. Science, 2005, 310(5751): 1166-1170. |
| 33 | KUHN P, ANTONIETTI M, Porous THOMAS A., covalent triazine-based frameworks prepared by ionothermal synthesis[J]. Angewandte Chemie, 2008, 47(18): 3450-3453. |
| 34 | FANG Q, ZHUANG Z, GU S, et al. Designed synthesis of large-pore crystalline polyimide covalent organic frameworks[J]. Nature Communications, 2014, 5(1): 4503-4510. |
| 35 | DING S, WANG W. Covalent organic frameworks (COFs): from design to applications[J]. Chemical Society Reviews, 2013, 42(2): 548-568. |
| 36 | HUANG N, ZHAI L, COUPRY D E, et al. Multiple-component covalent organic frameworks[J]. Nature Communications, 2016, 7(1): 12325. |
| 37 | FENG X, DING X, JIANG D. Covalent organic frameworks[J]. Chemical Society Reviews, 2012, 41(18): 6010-6022. |
| 38 | WALLER P J, GANDARA F, YAGHI O M. Chemistry of covalent organic frameworks[J]. Accounts of Chemical Research, 2015, 48(12): 3053-3063. |
| 39 | HOU Y, ZHANG X, WANG C, et al. Novel imine-linked porphyrin covalent organic frameworks with good adsorption removing properties of RhB[J]. New Journal of Chemistry, 2017, 41(14): 6145-6151. |
| 40 | GAO J, JIANG D. Covalent organic frameworks with spatially confined guest molecules in nanochannels and their impacts on crystalline structures[J]. Chemical Communications, 2016, 52(7): 1498-1500. |
| 41 | WU M X, YANG Y W. Applications of covalent organic frameworks (COFs): from gas storage and separation to drug delivery[J]. Chinese Chemical Letters, 2017, 28(6): 1135-1143. |
| 42 | CROWE J W, BALDWIN L A, MCGRIER P L. Luminescent covalent organic frameworks containing a homogeneous and heterogeneous distribution of dehydrobenzoannulene vertex units[J]. Journal of the American Chemical Society, 2016, 138(32): 10120-10123. |
| 43 | YUE J Y, MO Y P, LI S Y, et al. Simultaneous construction of two linkages for the on-surface synthesis of imine-boroxine hybrid covalent organic frameworks[J]. Chemical Science, 2017, 8(3): 2169-2174. |
| 44 | LIU Y, MA Y, ZHAO Y, et al. Weaving of organic threads into a crystalline covalent organic framework[J]. Science, 2016, 351(6271): 365-369. |
| 45 | VYAS V S, HAASE F, STEGBAUER L, et al. A tunable azine covalent organic framework platform for visible light-induced hydrogen generation[J]. Nature Communications, 2015, 6(1): 8508-8516. |
| 46 | LANNI L M, TILFORD R W, BHARATHY M, et al. Enhanced hydrolytic stability of self-assembling alkylated two-dimensional covalent organic frameworks[J]. Journal of the American Chemical Society, 2011, 133(35): 13975-13983. |
| 47 | NAGAI A, GUO Z, FENG X, et al. Pore surface engineering in covalent organic frameworks[J]. Nature Communications, 2011, 2(1): 536-543. |
| 48 | BANERJEE R, BISWAL B P, KANDAMBETH S, et al. Pore surface engineering in porous, chemically stable covalent organic frameworks for water adsorption[J]. Journal of Materials Chemistry A, 2015, 3(47): 1-7. |
| 49 | PENG Y, HUANG Y, ZHU Y, et al. Ultrathin two-dimensional covalent organic framework nanosheets: preparation and application in highly sensitive and selective DNA detection[J]. Journal of the American Chemical Society, 2017, 139(25): 8698-8704. |
| 50 | ZHU Y, MURALI S, CAI W, et al. Graphene and graphene oxide: synthesis, properties, and applications[J]. Advanced Materials, 2010, 22(35): 3906-3924. |
| 51 | NOVOSELOV K S, JIANG D, SCHEDIN F, et al. Two-dimensional atomic crystals[J]. Proceeding of the National Academy Sciences of the United States of America, 2005, 102(30): 10451-10453. |
| 52 | WANG S, WANG Q, SHAO P, et al. Exfoliation of covalent organic frameworks into few-layer redox-active nanosheets as cathode materials for lithium-ion batteries[J]. Journal of the American Chemical Society, 2017, 139(12): 4258-4261. |
| 53 | NIU L, COLEMAN J N, ZHANG H, et al. Production of two-dimensional nanomaterials via liquid-based direct exfoliation[J]. Small, 2016, 12(3): 272-293. |
| 54 | LI P Z, MAEDA Y, XU Q. Top-down fabrication of crystalline metal-organic framework nanosheets[J]. Chemical Communications, 2011, 47(29): 8436-8438. |
| 55 | BUNCK D N, DICHTEL W R. Bulk synthesis of exfoliated two-dimensional polymers using hydrazone-linked covalent organic frameworks[J]. Journal of the American Chemical Society, 2013, 135(40): 14952-14955. |
| 56 | HUMMERS W S, OFFEMAN R E. Preparation of graphitic oxide[J]. Journal of the American Chemical Society, 1958, 80(6): 1339. |
| 57 | SHIN Y R, JUNG S M, JEON I Y, et al. The oxidation mechanism of highly ordered pyrolytic graphite in a nitric acid/sulfuric acid mixture[J]. Carbon, 2013, 52(2): 493-498. |
| 58 | DREYER D R, PARK S, BIELAWSKI C W, et al. The chemistry of graphene oxide[J]. Chemical Society Reviews, 2010, 39(1): 228-240. |
| 59 | KHAYUM M A, KANDAMBETH S, MITRA S, et al. Chemically delaminated free-standing ultrathin covalent organic nanosheets[J]. Angewandte Chemie, 2016, 128(50): 15833-15837. |
| 60 | HANLON D, BACKES C, HIGGINS T M, et al. Production of molybdenum trioxide nanosheets by liquid exfoliation and their application in high-performance supercapacitors[J]. Chemistry of Materials, 2014, 26(4): 1751-1763. |
| 61 | BACKES C, SMITH R J, MCEVOY N, et al. Edge and confinement effects allow in situ measurement of size and thickness of liquid-exfoliated nanosheets[J]. Nature Communications, 2014, 5(1): 4576-4585. |
| 62 | CHHOWALLA M, LIU Z, ZHANG H. Two-dimensional transition metal dichalcogenide (TMD) nanosheets[J]. Chemical Society Reviews, 2015, 44(9): 2584-2586. |
| 63 | STEPANOW S, LINGENFELDER M, DMITRIEV A, et al. Steering molecular organization and host-guest interactions using two-dimensional nanoporous coordination systems[J]. Nature Materials, 2004, 3(4): 229-233. |
| 64 | CHHOWALLA M, SHIN H S, EDA G, et al. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets[J]. Nature Chemistry, 2013, 5(4): 263-275. |
| 65 | TAM K H, CHEUNG C K, LEUNG Y H, et al. Defects in ZnO nanorods prepared by a hydrothermal method[J]. The Journal of Physical Chemistry B, 2006, 110(42): 20865-20871. |
| 66 | LEE Y H, ZHANG X Q, ZHANG W, et al. Synthesis of large-area MoS2 atomic layers with chemical vapor deposition[J]. Advanced Materials, 2012, 24(17): 2320-2325. |
| 67 | JIAN M, LIU H, WILLIAMS T, et al. Temperature-induced oriented growth of large area, few-layer 2D metal-organic framework nanosheets[J]. Chemical Communications, 2017, 53(98): 13161-13164. |
| 68 | LIU W, LI X, WANG C, et al. A scalable general synthetic approach toward ultrathin imine-linked two-dimensional covalent organic framework nanosheets for photocatalytic CO2 reduction [J]. Journal of the American Chemical Society, 2019, 141(43): 17431-17440. |
| 69 | EVANS A E V, MATEO-SAGASTA J, QADIR M, et al. Agricultural water pollution: key knowledge gaps and research needs[J]. Current Opinion in Environmental Sustainability, 2019, 36(2): 20-27. |
| 70 | GAO Q, XU J, BU X H. Recent advances about metal-organic frameworks in the removal of pollutants from wastewater[J]. Coordination Chemistry Reviews, 2019, 378(1): 17-31. |
| 71 | SUN Y, LEI C, KHAN E, et al. Aging effects on chemical transformation and metal(loid) removal by entrapped nanoscale zero-valent iron for hydraulic fracturing wastewater treatment[J]. Science of the Total Environment, 2018, 615(2): 498-507. |
| 72 | POUREBRAHIM F, GHAEDI M, DASHTIAN K, et al. Optimization of solid phase dispersive field-assisted ultrasonication for the extraction of auramine O and crystal violet dyes using central composite design[J]. Applied Organometallic Chemistry, 2018, 32(3): 4181-4191. |
| 73 | KAYKHAII M, SASANI M, MARGHZARI S. Removal of dyes from the environment by adsorption process[J]. Chemical and Materials Engineering, 2018, 6(2): 31-35. |
| 74 | CUI K, YAN B, XIE Y, et al. Regenerable urchin-like Fe3O4@PDA-Ag hollow microspheres as catalyst and adsorbent for enhanced removal of organic dyes[J]. Journal of Hazardous Materials, 2018, 350(5): 66-75. |
| 75 | DONG B, TANG M, LIU W, et al. Solvent- and temperature-induced multiple crystal phases: crystal structure, selective adsorption, and separation of organic dye in three S-containing {[Cd(MIPA)]n}n- homologues[J]. Crystal Growth & Design, 2016, 16(11): 6363-6370. |
| 76 | WANG X S, LIANG J, LI L, et al. An anion metal-organic framework with Lewis basic sites-rich toward charge-exclusive cationic dyes separation and size-selective catalytic reaction[J]. Inorg. Chem., 2016, 55(5): 2641-2649. |
| 77 | WU M K, YI F, FANG Y, et al. An ultrastable metal-organic framework with open coordinated sites realizing selective separation toward cationic dyes in aqueous solution[J]. Crystal Growth & Design, 2017, 17(10): 5458-5464. |
| 78 | SONG Y, FAN R Q, XING K, et al. Insight into the controllable synthesis of Cu(Ⅰ)/Cu(Ⅱ) metal organic complexes: size-exclusive selective dye adsorption and semiconductor properties[J]. Crystal Growth & Design, 2017, 17(5): 2549-2559. |
| 79 | LI H, FANG X, MA S, et al. A two-dimensional porous framework: solvent-induced structural transformation and selective adsorption towards malachite green[J]. Dalton Transactions, 2017, 46(26): 8350-8353. |
| 80 | DESAI A, ROY A, SAMANTA P, et al. Base resistant ionic metal-organic framework as a porous ion-exchange sorbent[J]. iScience, 2018, 3(4): 21-30. |
| 81 | ZHANG N, XING Y, BAI F. A uranyl-organic framework featuring two-dimensional graphene-like layered topology for efficient iodine and dyes capture[J]. Inorganic Chemistry, 2019, 58(10): 6866-6876. |
| 82 | BAI Z, WANG Y, LI Y, et al. First cationic uranyl-organic framework with anion-exchange capabilities[J]. Inorganic Chemistry, 2016, 55(13): 6358-6360. |
| 83 | TAN J, ZHOU B, LIANG C, et al. Secondary-amine-functionalized isoreticular metal-organic frameworks for controllable and selective dye capture[J]. Materials Chemistry Frontiers, 2018, 2(1): 129-135. |
| 84 | CHEN Q, HE Q, LYU M, et al. Selective adsorption of cationic dyes by UiO-66-NH2[J]. Applied Surface Science, 2015, 327(103): 77-85. |
| 85 | ZHAO S, LI S, ZHAO Z, et al. Microwave-assisted hydrothermal assembly of 2D copper-porphyrin metal-organic frameworks for the removal of dyes and antibiotics from water[J]. Environmental Science and Pollution Research, 2020, 27(31): 39186-39197. |
| 86 | SALAM H M ABD EL, ZAKI T. Removal of hazardous cationic organic dyes from water using nickel-based metal-organic frameworks[J]. Inorganica Chimica Acta, 2018, 471(40): 203-210. |
| 87 | YANG J, XIONG P, ZHENG C, et al. Metal-organic frameworks: a new promising class of materials for a high performance supercapacitor electrode[J]. Journal of Materials Chemistry A, 2014, 2(39): 16640-16644. |
| 88 | LI Y, YUAN H H, LI C P, et al. A 2D Zn(Ⅱ) metal-organic framework to show selective removal of Neutral red (NR) from water[J]. Inorganic Chemistry Communications, 2017, 80(6): 36-40. |
| 89 | XU T, AN S, PENG C, et al. Construction of large-pore crystalline covalent organic framework as high-performance adsorbent for Rhodamine B dye removal[J]. Industrial & Engineering Chemistry Research, 2020, 59(4): 8315-8322. |
| 90 | WANG T, KAILASAM K, XIAO P, et al. Adsorption removal of organic dyes on covalent triazine framework (CTF)[J]. Microporous and Mesoporous Materials, 2014, 187(16): 63-70. |
| 91 | YU S B, LYU H, TIAN J, et al. A polycationic covalent organic framework: a robust adsorbent for anionic dye pollutants[J]. Polymer Chemistry, 2016, 7(20): 3392-3397. |
| 92 | BURAKOV A E, GALUNIN E, BURAKOVA I V, et al. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: a review[J]. Ecotoxicology and Environmental Safety, 2018, 148(1): 702-712. |
| 93 | ABNEY C W, MAYES R T, SAITO T, et al. Materials for the recovery of uranium from seawater[J]. Chemical Reviews, 2017, 117(23): 13935-14013. |
| 94 | MORI N, YASUTAKE A, MARUMOTO M, et al. Methylmercury inhibits electron transport chain activity and induces cytochrome c release in cerebellum mitochondria[J]. Journal of Toxicological Ences, 2011, 36(3): 253-259. |
| 95 | LI Y K, YANG T, CHEN M L, et al. Recent advances in nanomaterials for analysis of trace heavy metals[J]. Critical Reviews in Analytical Chemistry, 2020, 5(17): 1-20. |
| 96 | FLEGAL A R, GALLON C, GANGULI P M, et al. All the lead in China[J]. Critical Reviews in Environmental Science and Technology, 2013, 43(17): 1869-1944. |
| 97 | WANG C, HE C, LUO Y H, et al. Efficient mercury chloride capture by ultrathin 2D metal-organic framework nanosheets[J]. Chemical Engineering Journal, 2020, 379(1): 122337-122344. |
| 98 | SUN Q, AGUILA B, PERMAN J A, et al. Postsynthetically modified covalent organic frameworks for efficient and effective mercury removal[J]. Journal of the American Chemical Society, 2017, 139(7): 2786-2793. |
| 99 | CAO Y, HU X, ZHU C, et al. Sulfhydryl functionalized covalent organic framework as an efficient adsorbent for selective Pb(Ⅱ) removal[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2020, 600(9): 125004-125032. |
| 100 | XU R, JIAN M, JI Q, et al. 2D water-stable zinc-benzimidazole framework nanosheets for ultrafast and selective removal of heavy metals[J]. Chemical Engineering Journal, 2020, 382(8): 122658-122668. |
| 101 | MARGARITI A, RAPTI S, KATSENIS A D, et al. Cu2+ sorption from aqueous media by a recyclable Ca2+ framework[J]. Inorganic Chemistry Frontiers, 2017, 4(5): 773-781. |
| 102 | LI Y, WANG C, MA S, et al. Fabrication of hydrazone-linked covalent organic frameworks using alkyl amine as building block for high adsorption capacity of metal ions[J]. ACS Applied Materials & Interfaces, 2019, 11(12): 11706-11714. |
| 103 | ZHANG S, ZHAO X, LI B, et al. “Stereoscopic” 2D super-microporous phosphazene-based covalent organic framework: design, synthesis and selective sorption towards uranium at high acidic condition[J]. Journal of Hazardous Materials, 2016, 314(8): 95-104. |
| 104 | LIU J, ZHANG X Y, HOU J X, et al. Functionalized Mn(Ⅱ)-MOF based on host-guest interaction for selective and rapid capture of Congo red from water[J]. Journal of Solid State Chemistry, 2019, 270(39): 697-704. |
| 105 | LIU L L, CHEN J, ZHANG Y, et al. Fabrication of ultrathin single-layer 2D metal-organic framework nanosheets with excellent adsorption performance via a facile exfoliation approach[J]. Journal of Materials Chemistry A, 2021, 9(1): 546-555. |
| 106 | SALEH H A M, MANTASHA I, QASEM K M A, et al. A two dimensional Co(Ⅱ) metal-organic framework with bey topology for excellent dye adsorption and separation: exploring kinetics and mechanism of adsorption[J]. Inorganica Chimica Acta, 2020, 512(27): 119900-119908. |
| 107 | HUANG N, ZHAI L, XU H, et al. Stable covalent organic frameworks for exceptional mercury removal from aqueous solutions[J]. Journal of the American Chemical Society, 2017, 139(6): 2428-2434. |
| 108 | ROUHANI F, MORSALI A. Fast and selective heavy metal removal by a novel metal-organic framework designed with in-situ ligand building block fabrication bearing free nitrogen[J]. Chemistry: A European Journal, 2018, 24(21): 5529-5537. |
| [1] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [2] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [3] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [4] | 李世霖, 胡景泽, 王毅霖, 王庆吉, 邵磊. 电渗析分离提取高值组分的研究进展[J]. 化工进展, 2023, 42(S1): 420-429. |
| [5] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [6] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [7] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [8] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [9] | 李卫华, 于倩雯, 尹俊权, 吴寅凯, 孙英杰, 王琰, 王华伟, 杨玉飞, 龙於洋, 黄启飞, 葛燕辰, 何依洋, 赵灵燕. 酸雨环境下填埋飞灰吨袋破损后重金属的溶出行为[J]. 化工进展, 2023, 42(9): 4917-4928. |
| [10] | 李志远, 黄亚继, 赵佳琪, 于梦竹, 朱志成, 程好强, 时浩, 王圣. 污泥与聚氯乙烯共热解重金属特性[J]. 化工进展, 2023, 42(9): 4947-4956. |
| [11] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [12] | 姜晶, 陈霄宇, 张瑞妍, 盛光遥. 载锰生物炭制备及其在环境修复中应用研究进展[J]. 化工进展, 2023, 42(8): 4385-4397. |
| [13] | 张振, 李丹, 陈辰, 吴菁岚, 应汉杰, 乔浩. 吸附树脂对唾液酸的分离纯化[J]. 化工进展, 2023, 42(8): 4153-4158. |
| [14] | 王知彩, 刘伟伟, 周璁, 潘春秀, 闫洪雷, 李占库, 颜井冲, 任世彪, 雷智平, 水恒福. 基于煤基腐殖酸的高效减水剂合成与性能表征[J]. 化工进展, 2023, 42(7): 3634-3642. |
| [15] | 于静文, 宋璐娜, 刘砚超, 吕瑞东, 武蒙蒙, 冯宇, 李忠, 米杰. 一种吲哚基超交联聚合物In-HCP对水中碘的吸附作用[J]. 化工进展, 2023, 42(7): 3674-3683. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||