化工进展 ›› 2021, Vol. 40 ›› Issue (11): 6102-6112.DOI: 10.16085/j.issn.1000-6613.2020-2226
FeM双金属用于甲烷催化裂解制纯氢气和碳纳米材料
- 南京理工大学化学与化工学院,江苏 南京 210094
-
收稿日期:2020-11-08修回日期:2020-12-20出版日期:2021-11-05发布日期:2021-11-19 -
通讯作者:周吕 -
作者简介:钱敬侠(1994—),女,博士研究生,研究方向为能源催化。E-mail:2680531609@qq.com 。 -
基金资助:南京理工大学自主科研项目(AE89891);中央高校基本科研业务费专项项目(30920010006)
Methane decomposition to produce pure hydrogen and carbon nano materials over FeM catalysts
QIAN Jingxia( ), CHEN Tianwen, LIU Dabin, ZHOU Lyu(
), CHEN Tianwen, LIU Dabin, ZHOU Lyu( )
)
- School of Chemistry and Chemical Engineering, Nanjing University of Science and Technology, Nanjing 210094, Jiangsu, China
-
Received:2020-11-08Revised:2020-12-20Online:2021-11-05Published:2021-11-19 -
Contact:ZHOU Lyu
摘要:
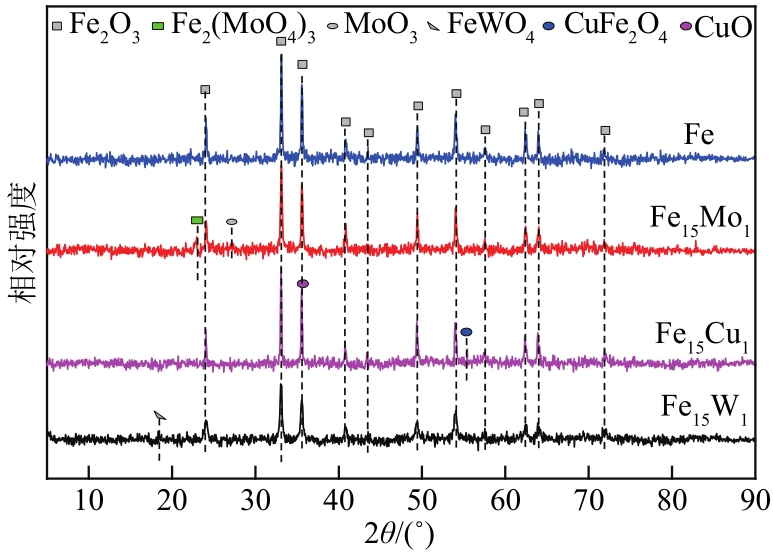
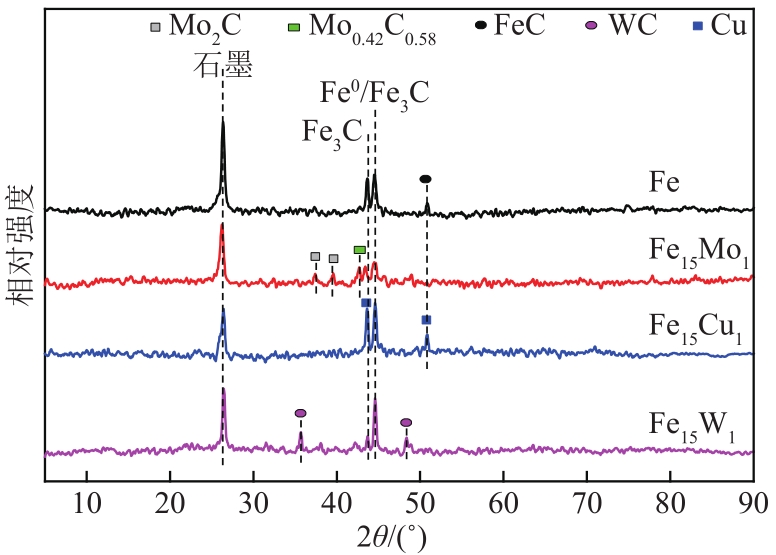
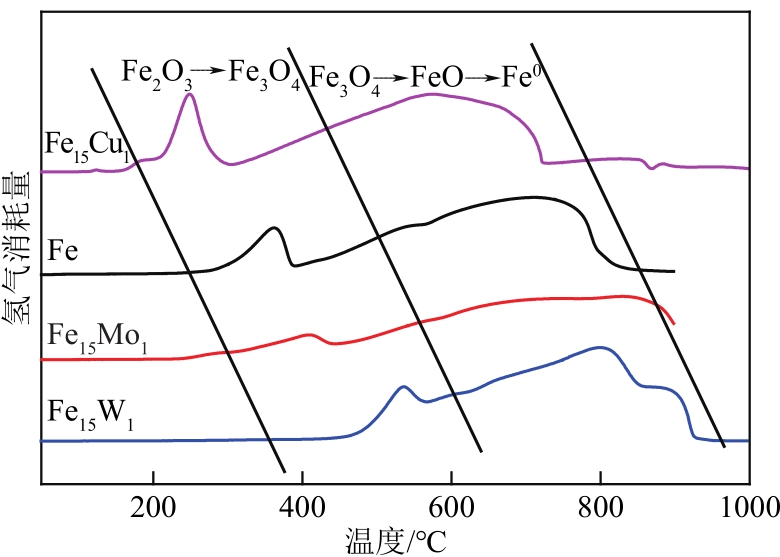
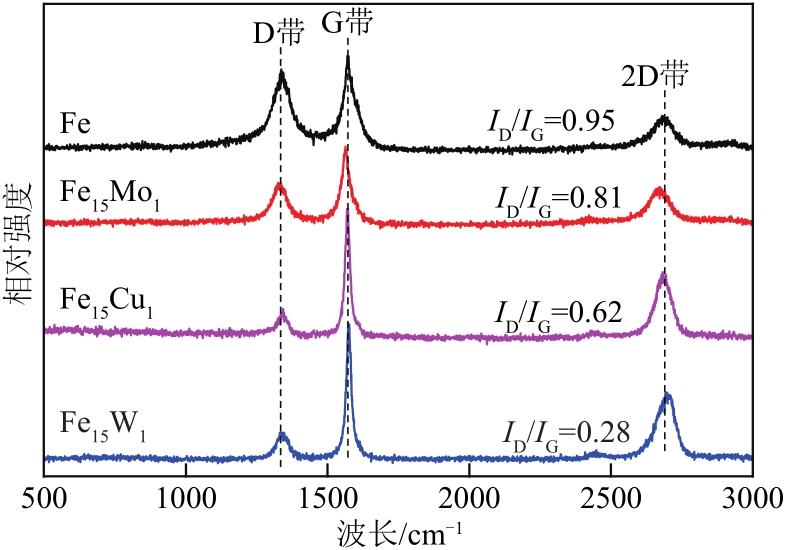
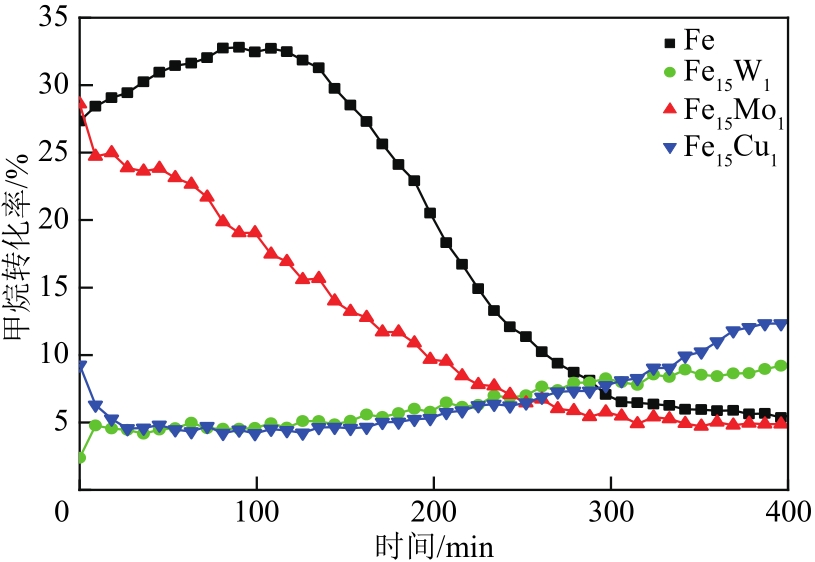
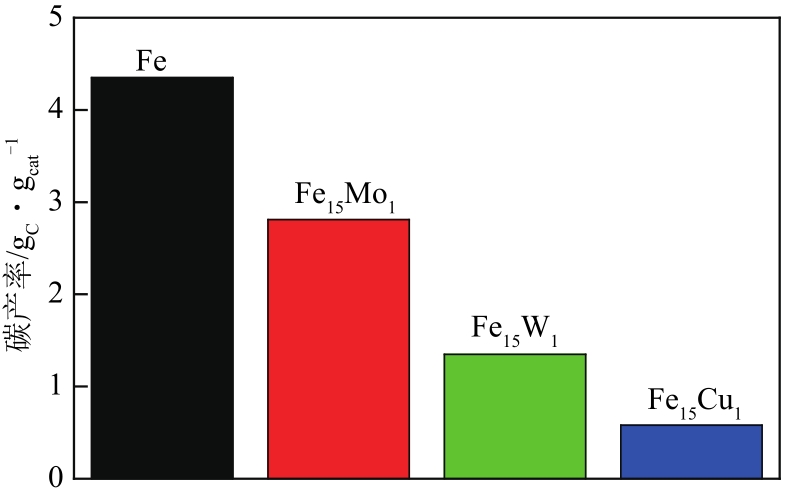
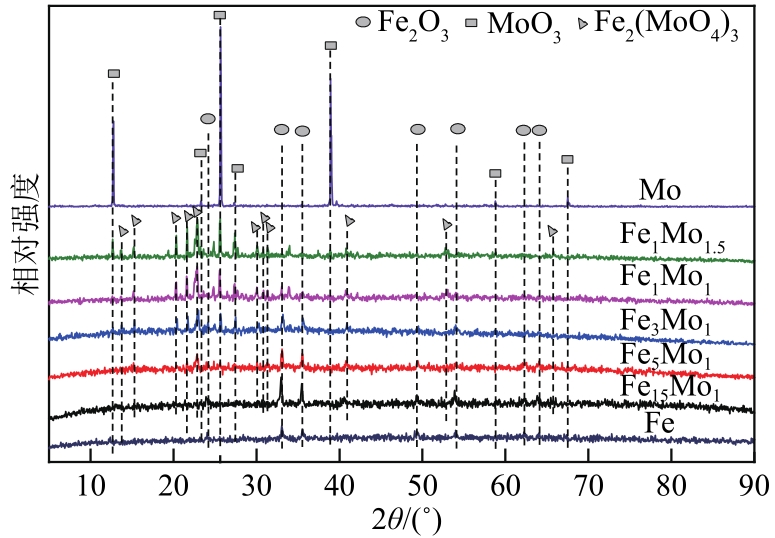
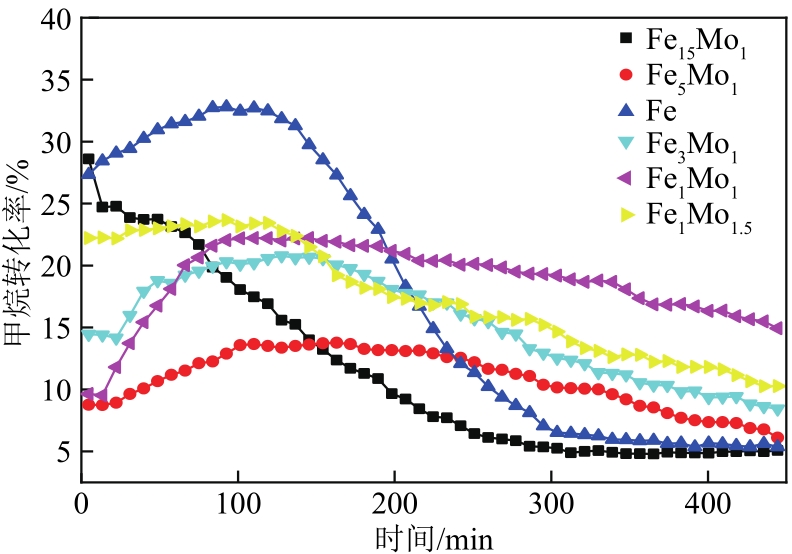
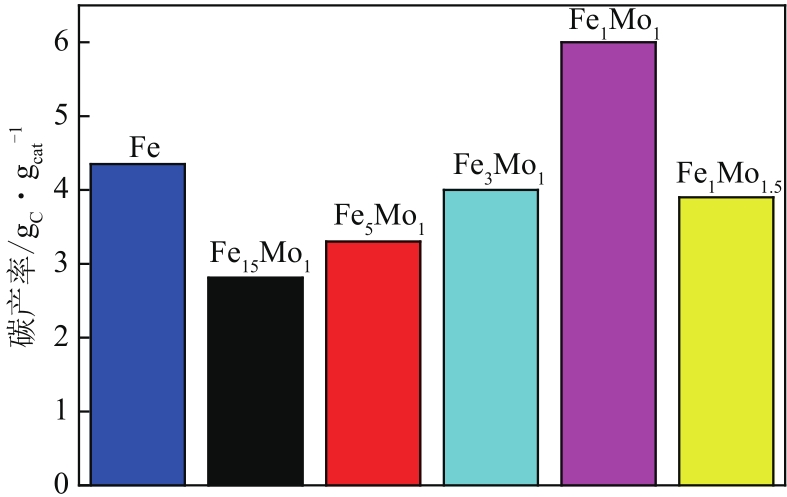
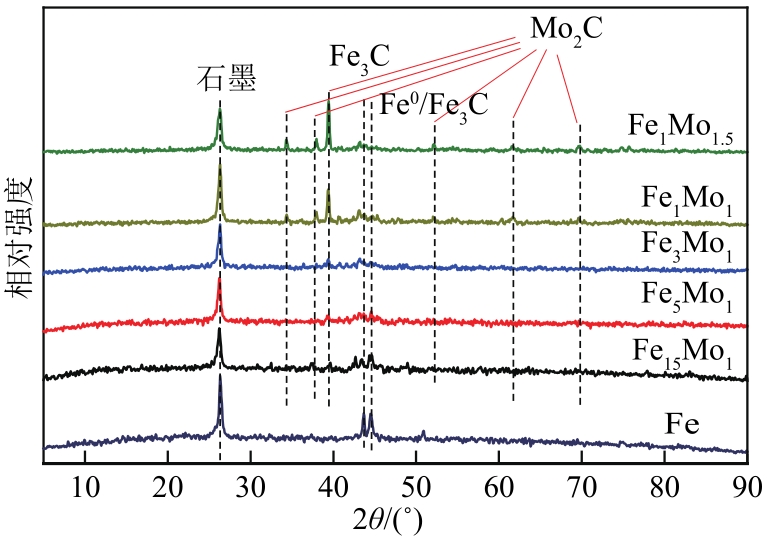
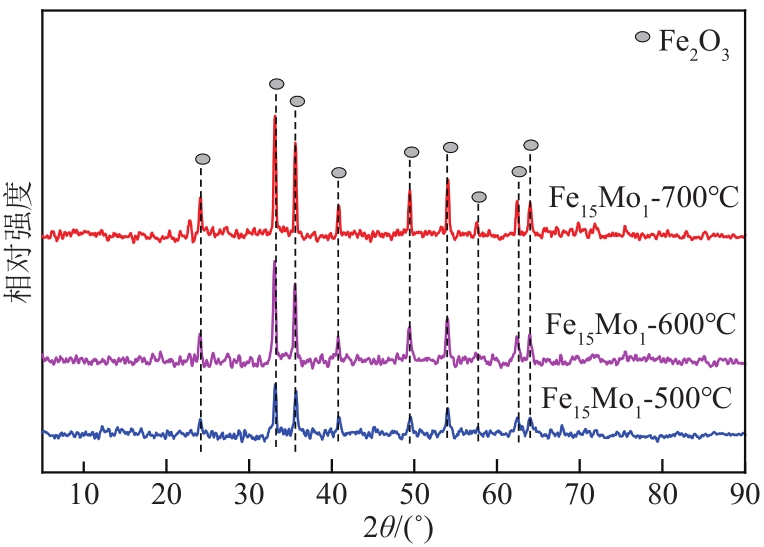
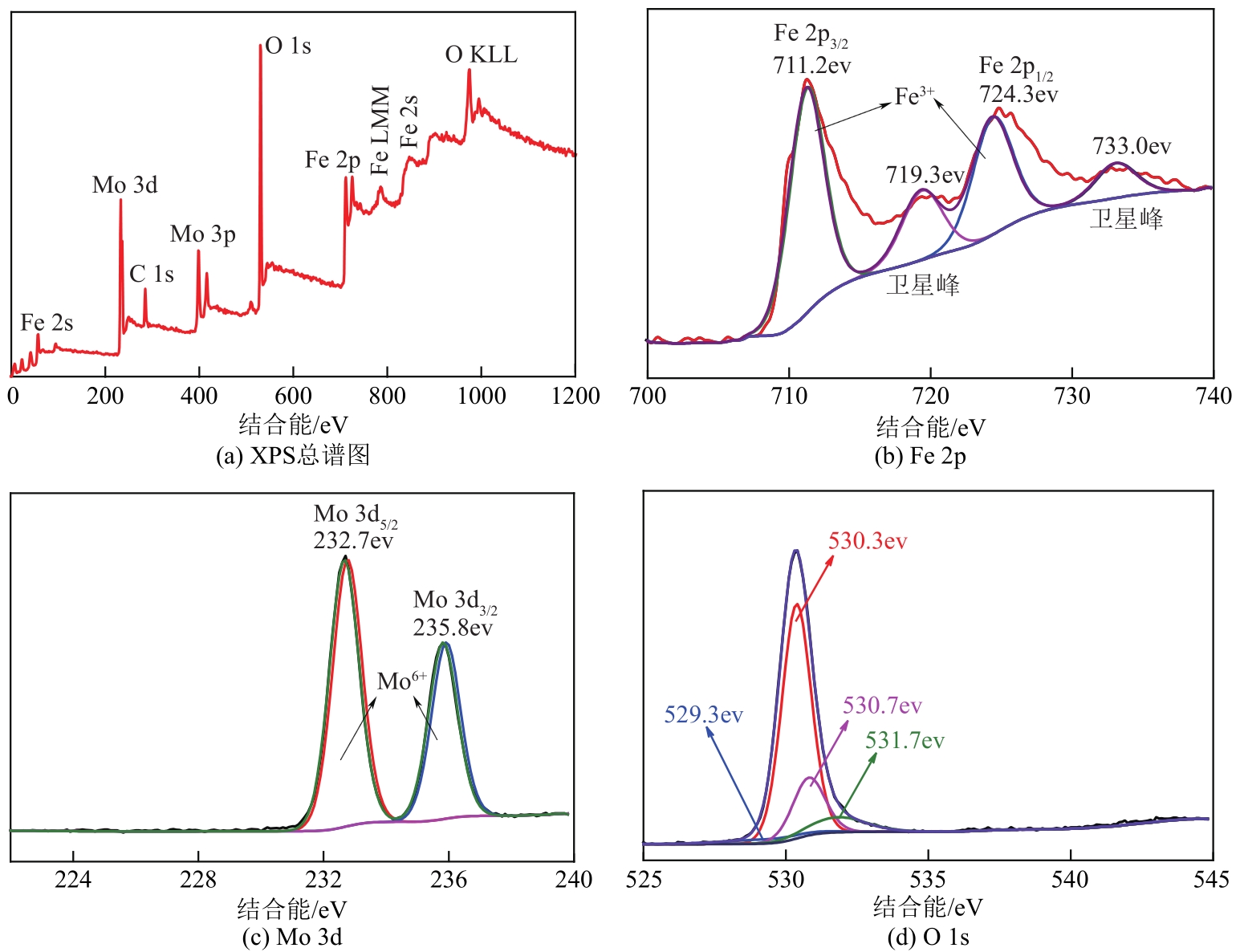
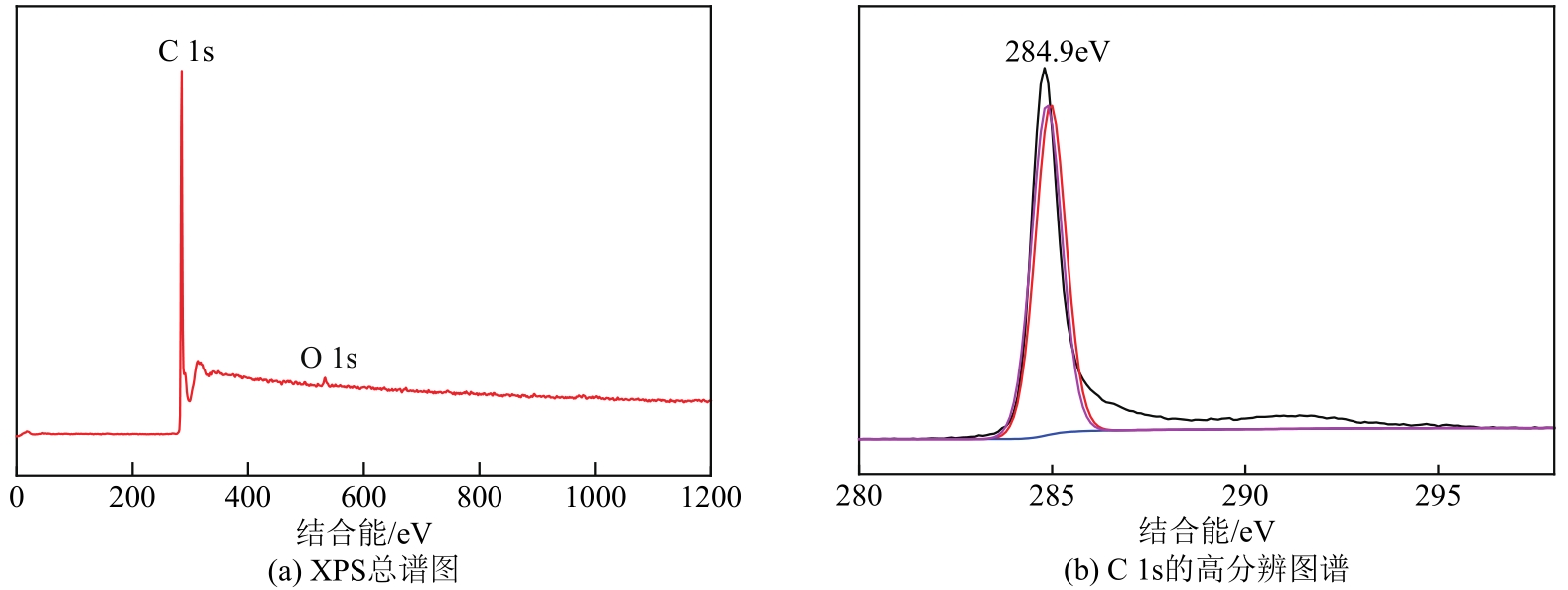
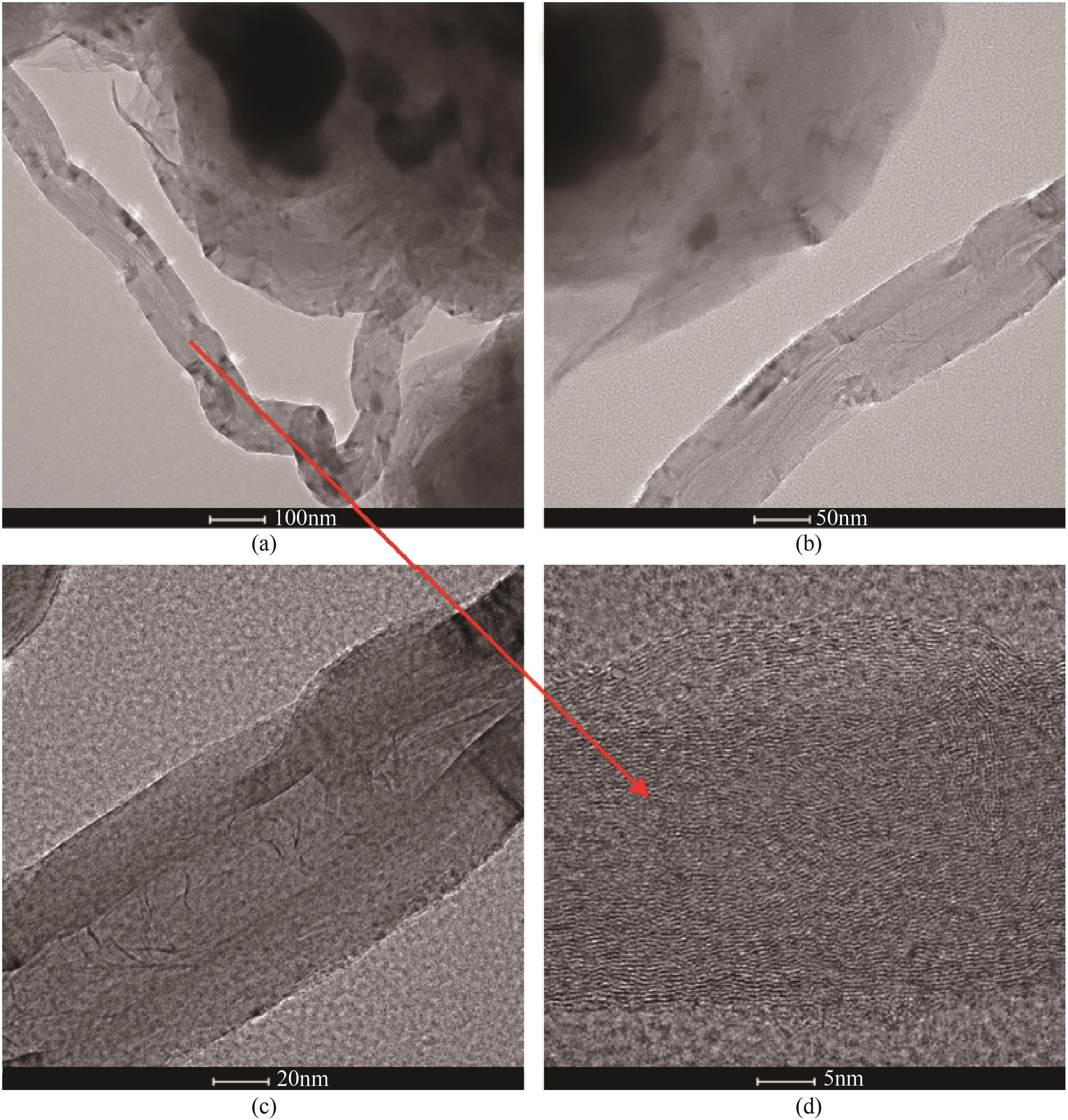
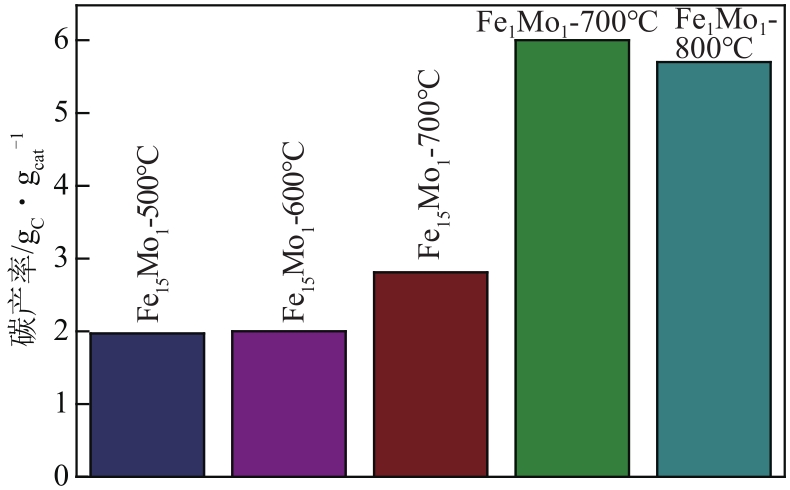
采用熔融法以铁、钼、铜和钨硝酸盐为原料,制备出一系列的FexMy(M=Mo、Cu、W)双金属催化剂。首先考察了一系列的Fe15M1(M=Mo、Cu、W)双金属催化剂的甲烷催化裂解(CDM)活性,Fe15Mo1的催化活性远高于Fe15Cu1和Fe15W1。通过比表面积测试(BET)、X射线衍射(XRD)、H2程序升温还原(H2-TPR)和拉曼光谱(Raman)等分析方法对Fe15M1的物理特性、结构组成、还原特性和副产物碳纳米材料(CNMs)的石墨化度等进行表征。进一步考察了金属Mo的掺杂量和焙烧温度对FexMoy双金属催化剂的甲烷转化率和碳产率的影响。Fe1Mo1表现出优异的催化性能,其碳产率(6gC/gcat)高于纯Fe的碳产率(4.35gC/gcat)。XRD和X射线光电子能谱(XPS)分析表明,Fe1Mo1双金属形成Fe2(MoO4)3相,提高了其催化活性和稳定性;透射电镜(TEM)结果表明,Fe1Mo1催化剂CDM反应后的CNMs为竹节状的碳纳米管。
中图分类号:
引用本文
钱敬侠, 陈天文, 刘大斌, 周吕. FeM双金属用于甲烷催化裂解制纯氢气和碳纳米材料[J]. 化工进展, 2021, 40(11): 6102-6112.
QIAN Jingxia, CHEN Tianwen, LIU Dabin, ZHOU Lyu. Methane decomposition to produce pure hydrogen and carbon nano materials over FeM catalysts[J]. Chemical Industry and Engineering Progress, 2021, 40(11): 6102-6112.
| 催化剂 | 比表面积/m2·g-1 | 孔容/cm3·g-1 | 孔径/nm |
|---|---|---|---|
| Fe | 2.7 | 0.02 | 40.3 |
| Fe15Mo1 | 11.1 | 0.07 | 40.5 |
| Fe15Cu1 | 0.4 | — | — |
| Fe15W1 | 16.4 | 0.09 | 29.1 |
表1 新鲜催化剂的物理特性
| 催化剂 | 比表面积/m2·g-1 | 孔容/cm3·g-1 | 孔径/nm |
|---|---|---|---|
| Fe | 2.7 | 0.02 | 40.3 |
| Fe15Mo1 | 11.1 | 0.07 | 40.5 |
| Fe15Cu1 | 0.4 | — | — |
| Fe15W1 | 16.4 | 0.09 | 29.1 |
| 催化剂 | 比表面积/m2·g-1 | 孔容/cm3·g-1 | 孔径/nm |
|---|---|---|---|
| Fe | 2.7 | 0.02 | 40.3 |
| Fe15Mo1 | 11.1 | 0.07 | 40.5 |
| Fe5Mo1 | 8.8 | 0.06 | 33.5 |
| Fe3Mo1 | 7.8 | 0.06 | 38.7 |
| Fe1Mo1 | 0.7 | 0.01 | 38.8 |
| Fe1Mo1.5 | 0.07 | — | — |
表2 新鲜催化剂FexMoy的物理特性
| 催化剂 | 比表面积/m2·g-1 | 孔容/cm3·g-1 | 孔径/nm |
|---|---|---|---|
| Fe | 2.7 | 0.02 | 40.3 |
| Fe15Mo1 | 11.1 | 0.07 | 40.5 |
| Fe5Mo1 | 8.8 | 0.06 | 33.5 |
| Fe3Mo1 | 7.8 | 0.06 | 38.7 |
| Fe1Mo1 | 0.7 | 0.01 | 38.8 |
| Fe1Mo1.5 | 0.07 | — | — |
| 项目 | Fe 2p | O 1s | Mo 3d | ||||
|---|---|---|---|---|---|---|---|
| Fe 2p3/2 | Fe 2p3/2卫星峰 | Fe 2p1/2 | Fe 2p1/2卫星峰 | Mo 3d5/2 | Mo 3d3/2 | ||
| Fe2O3 | — | — | — | — | 3.6 | — | — |
| MoO3 | — | — | — | — | 18.6 | — | — |
| Fe2(MoO4)3 | — | — | — | — | 69.0 | — | — |
| 表面吸附氧 | 8.8 | ||||||
| Fe2O3/Fe2(MoO4)3 | 48.9 | 14.6 | 28.1 | 8.4 | — | — | — |
| MoO3/Fe2(MoO4)3 | — | — | — | — | — | 60.4 | 39.6 |
表3 Fe1Mo1的XPS谱图中每种元素的质量分数分析 (%)
| 项目 | Fe 2p | O 1s | Mo 3d | ||||
|---|---|---|---|---|---|---|---|
| Fe 2p3/2 | Fe 2p3/2卫星峰 | Fe 2p1/2 | Fe 2p1/2卫星峰 | Mo 3d5/2 | Mo 3d3/2 | ||
| Fe2O3 | — | — | — | — | 3.6 | — | — |
| MoO3 | — | — | — | — | 18.6 | — | — |
| Fe2(MoO4)3 | — | — | — | — | 69.0 | — | — |
| 表面吸附氧 | 8.8 | ||||||
| Fe2O3/Fe2(MoO4)3 | 48.9 | 14.6 | 28.1 | 8.4 | — | — | — |
| MoO3/Fe2(MoO4)3 | — | — | — | — | — | 60.4 | 39.6 |
| 1 | IBRAHIM A A, AL‐FATESH A S, KHAN W U, et al. Influence of support type and metal loading in methane decomposition over iron catalyst for hydrogen production[J]. Journal of the Chinese Chemical Society, 2015, 62(7): 592-599. |
| 2 | 王迪, 胡燕, 高卫民, 等. 甲烷催化裂解制氢和碳纳米材料研究进展[J]. 化工进展, 2018, 37(S1): 80-93. |
| WANG Di, HU Yan, GAO Weimin, et al. Progress of methane catalytic decomposition for hydrogen and carbon nanomaterials production[J]. Chemical Industry and Engineering Progress, 2018, 37(S1): 80-93. | |
| 3 | 彭乔. 甲烷裂解制氢催化剂的制备及其性能与模拟研究[D]. 武汉: 华中师范大学, 2016. |
| PENG Qiao. The preparation of methane cracking catalyst for hydrogen production and its performance and simulation studies[D]. Wuhan: Central China Normal University, 2016. | |
| 4 | AL-FATESH A S, IBRAHIM A A, ALSHAREKH A M, et al. Methane decomposition over strontium promoted iron catalyst: effect of different ratio of Al/Si support on hydrogen yield[J]. Chemical Engineering Communications, 2020, 207(8): 1148-1156. |
| 5 | PUDUKUDY M, YAAKOB Z, JIA Q M, et al. Catalytic decomposition of methane over rare earth metal (Ce and La) oxides supported iron catalysts[J]. Applied Surface Science, 2019, 467/468: 236-248. |
| 6 | GENG S, HAN Z, HU Y, et al. Methane decomposition kinetics over Fe2O3 catalyst in micro fluidized bed reaction analyzer[J]. Industrial & Engineering Chemistry Research, 2018, 57(25): 8413-8423. |
| 7 | KARAISMAILOGLU M, FIGEN H E, BAYKARA S Z. Hydrogen production by catalytic methane decomposition over yttria doped nickel based catalysts[J]. International Journal of Hydrogen Energy, 2019, 44(20): 9922-9929. |
| 8 | UPHAM D C, AGARWAL V, KHECHFE A, et al. Catalytic molten metals for the direct conversion of methane to hydrogen and separable carbon[J]. Science, 2017, 358(6365): 917-921. |
| 9 | ASHIK U P M, WAN DAUD W M A, ABBAS H F. Production of greenhouse gas free hydrogen by thermocatalytic decomposition of methane—A review[J]. Renewable and Sustainable Energy Reviews, 2015, 44: 221-256. |
| 10 | QIAN J X, CHEN T W, ENAKONDA L R, et al. Methane decomposition to pure hydrogen and carbon nano materials: state-of-the-art and future perspectives[J]. International Journal of Hydrogen Energy, 2020, 45(32): 15721-15743. |
| 11 | ZHOU L, ENAKONDA L R, HARB M, et al. Fe catalysts for methane decomposition to produce hydrogen and carbon nano materials[J]. Applied Catalysis B: Environmental, 2017, 208: 44-59. |
| 12 | ALLAEDINI G, TASIRIN S M, AMINAYI P, et al. Bulk production of bamboo-shaped multi-walled carbon nanotubes via catalytic decomposition of methane over tri-metallic Ni-Co-Fe catalyst[J]. Reaction Kinetics, Mechanisms and Catalysis, 2015, 116(2): 385-396. |
| 13 | CALAFAT Á, SÁNCHEZ N. Production of carbon nanotubes through combination of catalyst reduction and methane decomposition over Fe-Ni/ZrO2 catalysts prepared by the citrate method[J]. Applied Catalysis A: General, 2016, 528: 14-23. |
| 14 | OLIVEIRA P F, RIBEIRO L P, ROSMANINHO M G, et al. Effect of Sn on methane decomposition over Fe supported catalysts to produce carbon[J]. Hyperfine Interactions, 2011, 203(1/2/3): 67-74. |
| 15 | FAKEEHA A H, IBRAHIM A A, NAEEM M A, et al. Methane decomposition over Fe supported catalysts for hydrogen and nano carbon yield[J]. Catalysis for Sustainable Energy, 2015, 2(1): 71-82. |
| 16 | TORRES D, PINILLA J L, LÁZARO M J, et al. Hydrogen and multiwall carbon nanotubes production by catalytic decomposition of methane: thermogravimetric analysis and scaling-up of Fe-Mo catalysts[J]. International Journal of Hydrogen Energy, 2014, 39(8): 3698-3709. |
| 17 | ZHOU L, ENAKONDA L R, LI S, et al. Iron ore catalysts for methane decomposition to make COx free hydrogen and carbon nano material[J]. Journal of the Taiwan Institute of Chemical Engineers, 2018, 87: 54-63. |
| 18 | FAKEEHA A H, IBRAHIM A A, KHAN W U, et al. Hydrogen production via catalytic methane decomposition over alumina supported iron catalyst[J]. Arabian Journal of Chemistry, 2018, 11(3): 405-414. |
| 19 | IBRAHIM A A, FAKEEHA A H, AL-FATESH A S, et al. Methane decomposition over iron catalyst for hydrogen production[J]. International Journal of Hydrogen Energy, 2015, 40(24): 7593-7600. |
| 20 | SIMON A, SEYRING M, KAMNITZ S, et al. Carbon nanotubes and carbon nanofibers fabricated on tubular porous Al2O3 substrates[J]. Carbon, 2015, 90: 25-33. |
| 21 | TORRES D, PINILLA J, SUELVES I. Co-, Cu- and Fe-doped Ni/Al2O3 catalysts for the catalytic decomposition of methane into hydrogen and carbon nanofibers[J]. Catalysts, 2018, 8(8): 300. |
| 22 | ZHANG C, ZHANG W, DREWETT N E, et al. Integrating catalysis of methane decomposition and electrocatalytic hydrogen evolution with Ni/CeO2 for improved hydrogen production efficiency[J]. ChemSusChem, 2019, 12(5): 1000-1010. |
| 23 | CALGARO C O, PEREZ-LOPEZ O W. Graphene and carbon nanotubes by CH4 decomposition over CoAl catalysts[J]. Materials Chemistry and Physics, 2019, 226: 6-19. |
| 24 | PUDUKUDY M, YAAKOB Z, AKMAL Z S. Direct decomposition of methane over Pd promoted Ni/SBA-15 catalysts[J]. Applied Surface Science, 2015, 353: 127-136. |
| 25 | WANG J, JIN L, LI Y, et al. Preparation of Fe-doped carbon catalyst for methane decomposition to hydrogen[J]. Industrial & Engineering Chemistry Research, 2017, 56(39): 11021-11027. |
| 26 | NISHII H, MIYAMOTO D, UMEDA Y, et al. Catalytic activity of several carbons with different structures for methane decomposition and by-produced carbons[J]. Applied Surface Science, 2019, 473: 291-297. |
| 27 | QIAN J X, CHEN T W, ENAKONDA L R, et al. Methane decomposition to produce COx-free hydrogen and nano-carbon over metal catalysts: a review[J]. International Journal of Hydrogen Energy, 2020, 45(15): 7981-8001. |
| 28 | TANG L, YAMAGUCHI D, BURKE N, et al. Methane decomposition over ceria modified iron catalysts[J]. Catalysis Communications, 2010, 11(15): 1215-1219. |
| 29 | PUDUKUDY M, KADIER A, YAAKOB Z, et al. Non-oxidative thermocatalytic decomposition of methane into COx free hydrogen and nanocarbon over unsupported porous NiO and Fe2O3 catalysts[J]. International Journal of Hydrogen Energy, 2016, 41(41): 18509-18521. |
| 30 | AWADALLAH A E, ABOUL-ENEIN A A, KANDIL U F, et al. Facile and large-scale synthesis of high quality few-layered graphene nano-platelets via methane decomposition over unsupported iron family catalysts[J]. Materials Chemistry and Physics, 2017, 191: 75-85. |
| 31 | PUDUKUDY M, YAAKOB Z, DAHANI N, et al. Production of COx free hydrogen and nanocarbon via methane decomposition over unsupported porous nickel and iron catalysts[J]. Journal of Cluster Science, 2017, 28(3): 1579-1594. |
| 32 | ASHIK U P M, DAUD W M A W. Stabilization of Ni, Fe, and Co nanoparticles through modified Stöber method to obtain excellent catalytic performance: preparation, characterization, and catalytic activity for methane decomposition[J]. Journal of the Taiwan Institute of Chemical Engineers, 2016, 61: 247-260. |
| 33 | WANG H Y, LUA A C. Methane decomposition using Ni-Cu alloy nano-particle catalysts and catalyst deactivation studies[J]. Chemical Engineering Journal, 2015, 262: 1077-1089. |
| 34 | PUDUKUDY M, YAAKOB Z, TAKRIFF M S. Methane decomposition over unsupported mesoporous nickel ferrites: effect of reaction temperature on the catalytic activity and properties of the produced nanocarbon[J]. RSC Advances, 2016, 6(72): 68081-68091. |
| 35 | QIAN J X, ENAKONDA L R, WANG W J, et al. Optimization of a fluidized bed reactor for methane decomposition over Fe/Al2O3 catalysts: activity and regeneration studies[J]. International Journal of Hydrogen Energy, 2019, 44(60): 31700-31711. |
| 36 | ZHOU L, ENAKONDA L R, SAIH Y, et al. Catalytic methane decomposition over Fe-Al2O3[J]. ChemSusChem, 2016, 9(11): 1243-1248. |
| 37 | REDDY ENAKONDA L, ZHOU L, SAIH Y, et al. Methane-induced activation mechanism of fused ferric oxide-alumina catalysts during methane decomposition[J]. ChemSusChem, 2016, 9(15): 1911-1915. |
| 38 | ZHANG X, NIU Y, LI Y, et al. Synthesis, optical and magnetic properties of α-Fe2O3 nanoparticles with various shapes[J]. Materials Letters, 2013, 99: 111-114. |
| 39 | WIRTH C T, BAYER B C, GAMALSKI A D, et al. The phase of iron catalyst nanoparticles during carbon nanotube growth[J]. Chemistry of Materials, 2012, 24(24): 4633-4640. |
| 40 | EBERT D Y, DOROFEEVA N V, SAVEL’EVA A S, et al. Silica-supported Fe-Mo-O catalysts for selective oxidation of propylene glycol[J]. Catalysis Today, 2019, 333: 133-139. |
| 41 | YUE Y, LIU B, LY N, et al. Direct synthesis of hierarchical FeCu‐ZSM-5 zeolite with wide temperature window in selective catalytic reduction of NO by NH3[J]. ChemCatChem, 2019, 11(19): 4744-4754. |
| 42 | AL-FATESH A S, DE KASIM S O, IBRAHIM A A, et al. Catalytic methane decomposition over ZrO2 supported iron catalysts: effect of WO3 and La2O3 addition on catalytic activity and stability[J]. Renewable Energy, 2020, 155: 969-978. |
| 43 | DECK C P, VECCHIO K. Prediction of carbon nanotube growth success by the analysis of carbon-catalyst binary phase diagrams[J]. Carbon, 2006, 44(2): 267-275. |
| 44 | FAKEEHA A H, AL-FATESH A S, CHOWDHURY B, et al. Bi-metallic catalysts of mesoporous Al2O3 supported on Fe, Ni and Mn for methane decomposition: effect of activation temperature[J]. Chinese Journal of Chemical Engineering, 2018, 26(9): 1904-1911. |
| 45 | PINILLA J L, UTRILLA R, KARN R K, et al. High temperature iron-based catalysts for hydrogen and nanostructured carbon production by methane decomposition[J]. International Journal of Hydrogen Energy, 2011, 36(13): 7832-7843. |
| 46 | WROBEL R, HELMINIAK A, ARABCZYK W, et al. Studies on the kinetics of carbon deposit formation on nanocrystalline iron stabilized with structural promoters[J]. The Journal of Physical Chemistry C, 2014, 118(28): 15434-15439. |
| 47 | PUDUKUDY M, YAAKOB Z, KADIER A, et al. One-pot sol-gel synthesis of Ni/TiO2 catalysts for methane decomposition into COx free hydrogen and multiwalled carbon nanotubes[J]. International Journal of Hydrogen Energy, 2017, 42(26): 16495-16513. |
| 48 | CHAI S P, LEE K Y, ICHIKAWA S, et al. Synthesis of carbon nanotubes by methane decomposition over Co-Mo/Al2O3: process study and optimization using response surface methodology[J]. Applied Catalysis A: General, 2011, 396(1/2): 52-58. |
| 49 | AWADALLAH A E, ABOUL-ENEIN A A, ABOUL-GHEIT A K. Effect of progressive Co loading on commercial Co-Mo/Al2O3 catalyst for natural gas decomposition to COx-free hydrogen production and carbon nanotubes[J]. Energy Conversion and Management, 2014, 77: 143-151. |
| 50 | AWADALLAH A E, ABOUL-ENEIN A A, AZAB M A, et al. Influence of Mo or Cu doping in Fe/MgO catalyst for synthesis of single-walled carbon nanotubes by catalytic chemical vapor deposition of methane[J]. Fullerenes, Nanotubes and Carbon Nanostructures, 2017, 25(4): 256-264. |
| 51 | DENG J L, LIU J X, SONG W Y, et al. Selective catalytic reduction of NO with NH3 over Mo-Fe/beta catalysts: the effect of Mo loading amounts[J]. RSC Advances, 2017, 7(12): 7130-7139. |
| 52 | NOVOTNÝ P, YUSUF S, LI F, et al. Oxidative dehydrogenation of ethane using MoO3/Fe2O3 catalysts in a cyclic redox mode[J]. Catalysis Today, 2018, 317: 50-55. |
| 53 | BAEK M, GUAN-WOO K, PARK T, et al. NiMoFe and NiMoFeP as complementary electrocatalysts for efficient overall water splitting and their application in PV-electrolysis with STH 12.3[J]. Small, 2019, 15(49): e1905501. |
| 54 | BOWKER M, BROOKES C, CARLEY A F, et al. Evolution of active catalysts for the selective oxidative dehydrogenation of methanol on Fe2O3 surface doped with Mo oxide[J]. Physical Chemistry Chemical Physics, 2013, 15(29): 12056. |
| 55 | LI Y K, ZHANG G, LU W T, et al. Amorphous Ni-Fe-Mo suboxides coupled with Ni network as porous nanoplate array on nickel foam: a highly efficient and durable bifunctional electrode for overall water splitting[J]. Advanced Science, 2020, 7(7): 1902034. |
| 56 | LU Y, ZHU Z P, SU D S, et al. Formation of bamboo-shape carbon nanotubes by controlled rapid decomposition of picric acid[J]. Carbon, 2004, 42(15): 3199-3207. |
| 57 | KUDUS M H A, AKIL H M, MOHAMAD H, et al. Effect of catalyst calcination temperature on the synthesis of MWCNT-alumina hybrid compound using methane decomposition method[J]. Journal of Alloys and Compounds, 2011, 509(6): 2784-2788. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [6] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [7] | 李宁, 李金科, 董金善. 乙烯裂解炉多孔介质燃烧器的研究与开发[J]. 化工进展, 2023, 42(S1): 73-83. |
| [8] | 赖诗妮, 江丽霞, 李军, 黄宏宇, 小林敬幸. 含碳掺氨燃料的研究进展[J]. 化工进展, 2023, 42(9): 4603-4615. |
| [9] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [10] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [11] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [12] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [13] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [14] | 吴海波, 王希仑, 方岩雄, 纪红兵. 3D打印催化材料开发与应用进展[J]. 化工进展, 2023, 42(8): 3956-3964. |
| [15] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||