化工进展 ›› 2021, Vol. 40 ›› Issue (10): 5730-5746.DOI: 10.16085/j.issn.1000-6613.2020-2039
餐饮油烟中典型VOCs催化氧化研究进展
隗晶慧1( ), 冯勇超1, 于庆君1,2(
), 冯勇超1, 于庆君1,2( ), 易红宏1,2, 唐晓龙1,2, 张媛媛1, 孟宪政1, 袁雨婷1
), 易红宏1,2, 唐晓龙1,2, 张媛媛1, 孟宪政1, 袁雨婷1
- 1.北京科技大学能源与环境工程学院,北京 100083
2.工业典型污染物资源化处理北京市重点实验室,北京 100083
-
收稿日期:2020-10-10修回日期:2021-03-17出版日期:2021-10-10发布日期:2021-10-25 -
通讯作者:于庆君 -
作者简介:隗晶慧(1996—),女,硕士研究生,研究方向为大气污染控制。E-mail:weijh0523@163.com 。 -
基金资助:北京市科技计划(首都蓝天行动培育专项)(Z181100005418008);中央高校基本科研业务费专项资金(FRF-TP-18-011A3);中央引导地方科技发展专项资金(19943816G);重质油国家重点实验室开放基金(SKLOP202002001)
Research progress of catalytic oxidation of typical VOCs in cooking oil fumes
WEI Jinghui1( ), FENG Yongchao1, YU Qingjun1,2(
), FENG Yongchao1, YU Qingjun1,2( ), YI Honghong1,2, TANG Xiaolong1,2, ZHANG Yuanyuan1, MENG Xianzheng1, YUAN Yuting1
), YI Honghong1,2, TANG Xiaolong1,2, ZHANG Yuanyuan1, MENG Xianzheng1, YUAN Yuting1
- 1.School of Energy and Environmental Engineering, University of Science and Technology Beijing, Beijing 100083, China
2.Beijing Key Laboratory of Resource-oriented Treatment of Industrial Pollutants, Beijing 100083, China
-
Received:2020-10-10Revised:2021-03-17Online:2021-10-10Published:2021-10-25 -
Contact:YU Qingjun
摘要:
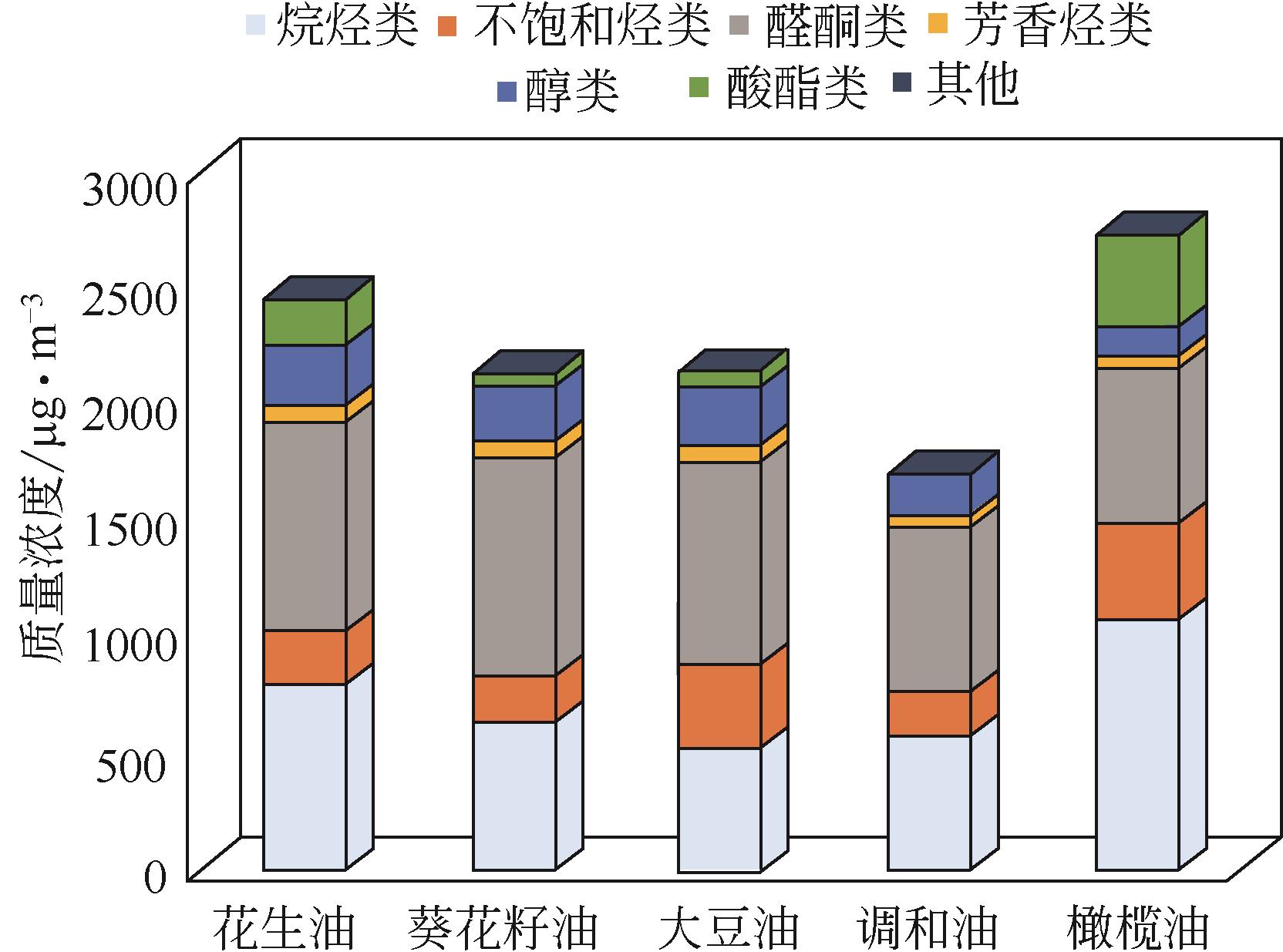
近年来,餐饮油烟逐渐成为城市大气污染的主要来源之一,油烟中挥发性有机化合物(VOCs)对人体及环境均产生非常大的危害,对其治理刻不容缓。催化燃烧技术因具备去除效率高、无二次污染等优点在有机废气治理方面有着广阔的应用前景。鉴于此,本文首先总结并分析了油烟中典型挥发性有机物的成分,发现油烟中烃类、醛酮类污染物含量较高,其次包括酸酯类、醇类和少量的多环芳烃类污染物。在此基础上,重点综述了催化燃烧技术对净化上述典型挥发性有机污染物的研究进展,并对近年来常用的催化剂包括贵金属催化剂、非贵金属催化剂及特定结构的催化剂进行了归纳和整理。最后结合油烟VOCs成分及其特点,总结了不同种类型的催化剂在油烟治理中的适用性,并对催化燃烧技术在油烟治理方面的研究前景作出了展望。
中图分类号:
引用本文
隗晶慧, 冯勇超, 于庆君, 易红宏, 唐晓龙, 张媛媛, 孟宪政, 袁雨婷. 餐饮油烟中典型VOCs催化氧化研究进展[J]. 化工进展, 2021, 40(10): 5730-5746.
WEI Jinghui, FENG Yongchao, YU Qingjun, YI Honghong, TANG Xiaolong, ZHANG Yuanyuan, MENG Xianzheng, YUAN Yuting. Research progress of catalytic oxidation of typical VOCs in cooking oil fumes[J]. Chemical Industry and Engineering Progress, 2021, 40(10): 5730-5746.
| VOCs | 组分 | 油烟来源 | 参考文献(年份) |
|---|---|---|---|
烃类 (49%~100%) | 正庚烷、正辛烷、正戊烷、辛烯、柠檬烯、庚烯、甲苯、二甲苯、乙苯等 | 花生油、葵花子油、大豆油、 调和油、橄榄油 | |
十二烷、十三烷、十五烷、十六烷、十二烷烯、十六烷烯、十七烷烯、 十八烷烯、十基环氧己烷、壬基环丙烷等 | 大豆油 | ||
| 十一烷、十二烷、十八烷、二十烷、甲苯、二甲苯、丙乙烯等 | 大豆油、菜籽油、玉米油、花生油 | ||
| 正己烷、甲基环戊烷、环己烷、正庚烷、苯、甲苯、乙苯等 | 大豆油、调和油、花生油 | ||
醛酮类 (50%~80%) | 戊醛、己醛、庚烯醛、壬醛、庚醛、丙烯醛、癸烯醛等 | 花生油、葵花子油、大豆油、 调和油、橄榄油等 | |
| 甲醛、丙醛、戊醛、丁烯醛等 | 大豆油 | ||
| 己醛、己烯醛、庚醛、庚烯醛、辛醛、庚二烯醛等 | 色拉油、猪油 | ||
| 正己醛、2-乙基己醛、正辛醛、正壬醛、正癸醛等 | 大豆油、调和油、花生油 | ||
| 己醛、3-甲基-2-丁烯醛、庚醛、辛醛等 | 菜籽油、色拉油、豆油、猪油 | ||
| 酸酯类 | 棕榈酸、油酸乙酯等 | 花生油、葵花子油、大豆油、 调和油、橄榄油等 | |
苯二甲酸二丁酯、壬酸甲酯、9-十八碳烯酸甲酯、15-十八碳烯酸甲酯、 油酸、亚麻酸等 | 大豆油 | ||
| 醇类 | 戊醇、乙基乙醇、十二醇等 | 花生油、葵花子油、大豆油、 调和油、橄榄油 |
表1 餐饮油烟中典型污染物汇总
| VOCs | 组分 | 油烟来源 | 参考文献(年份) |
|---|---|---|---|
烃类 (49%~100%) | 正庚烷、正辛烷、正戊烷、辛烯、柠檬烯、庚烯、甲苯、二甲苯、乙苯等 | 花生油、葵花子油、大豆油、 调和油、橄榄油 | |
十二烷、十三烷、十五烷、十六烷、十二烷烯、十六烷烯、十七烷烯、 十八烷烯、十基环氧己烷、壬基环丙烷等 | 大豆油 | ||
| 十一烷、十二烷、十八烷、二十烷、甲苯、二甲苯、丙乙烯等 | 大豆油、菜籽油、玉米油、花生油 | ||
| 正己烷、甲基环戊烷、环己烷、正庚烷、苯、甲苯、乙苯等 | 大豆油、调和油、花生油 | ||
醛酮类 (50%~80%) | 戊醛、己醛、庚烯醛、壬醛、庚醛、丙烯醛、癸烯醛等 | 花生油、葵花子油、大豆油、 调和油、橄榄油等 | |
| 甲醛、丙醛、戊醛、丁烯醛等 | 大豆油 | ||
| 己醛、己烯醛、庚醛、庚烯醛、辛醛、庚二烯醛等 | 色拉油、猪油 | ||
| 正己醛、2-乙基己醛、正辛醛、正壬醛、正癸醛等 | 大豆油、调和油、花生油 | ||
| 己醛、3-甲基-2-丁烯醛、庚醛、辛醛等 | 菜籽油、色拉油、豆油、猪油 | ||
| 酸酯类 | 棕榈酸、油酸乙酯等 | 花生油、葵花子油、大豆油、 调和油、橄榄油等 | |
苯二甲酸二丁酯、壬酸甲酯、9-十八碳烯酸甲酯、15-十八碳烯酸甲酯、 油酸、亚麻酸等 | 大豆油 | ||
| 醇类 | 戊醇、乙基乙醇、十二醇等 | 花生油、葵花子油、大豆油、 调和油、橄榄油 |
| 催化剂 | 污染物 | 体积分数 /×10-6 | 空速 /h-1 | 转化温度 /℃ | 转化率 /% | 参考 文献 |
|---|---|---|---|---|---|---|
| Au/CeO2 | 丙烯 | 1000 | 35000 | 100 | 90 | [ |
| CeO2/Au/Al2O3 | 正己烷 | 120 | 9700 | 370 | 100 | [ |
| Mn-Pt/Al2O3 | 正己烷 | 1500 | 17500 | 247 | 80 | [ |
| Cu1Co4 | 正庚烷 | 1800 | 15000 | 185.61 | 90 | [ |
| La1Co4 | 正庚烷 | 1800 | 15000 | 152 | 90 | [ |
| Mn0.9Ce0.1 | 正庚烷 | 1000 | 11000 | 330 | 90 | [ |
表2 烃类污染物催化氧化条件和性能
| 催化剂 | 污染物 | 体积分数 /×10-6 | 空速 /h-1 | 转化温度 /℃ | 转化率 /% | 参考 文献 |
|---|---|---|---|---|---|---|
| Au/CeO2 | 丙烯 | 1000 | 35000 | 100 | 90 | [ |
| CeO2/Au/Al2O3 | 正己烷 | 120 | 9700 | 370 | 100 | [ |
| Mn-Pt/Al2O3 | 正己烷 | 1500 | 17500 | 247 | 80 | [ |
| Cu1Co4 | 正庚烷 | 1800 | 15000 | 185.61 | 90 | [ |
| La1Co4 | 正庚烷 | 1800 | 15000 | 152 | 90 | [ |
| Mn0.9Ce0.1 | 正庚烷 | 1000 | 11000 | 330 | 90 | [ |
| 催化剂 | 污染物 | 体积分数/×10-6 | 空速/h-1 | 转化温度/℃ | 转化率/% | 参考文献 |
|---|---|---|---|---|---|---|
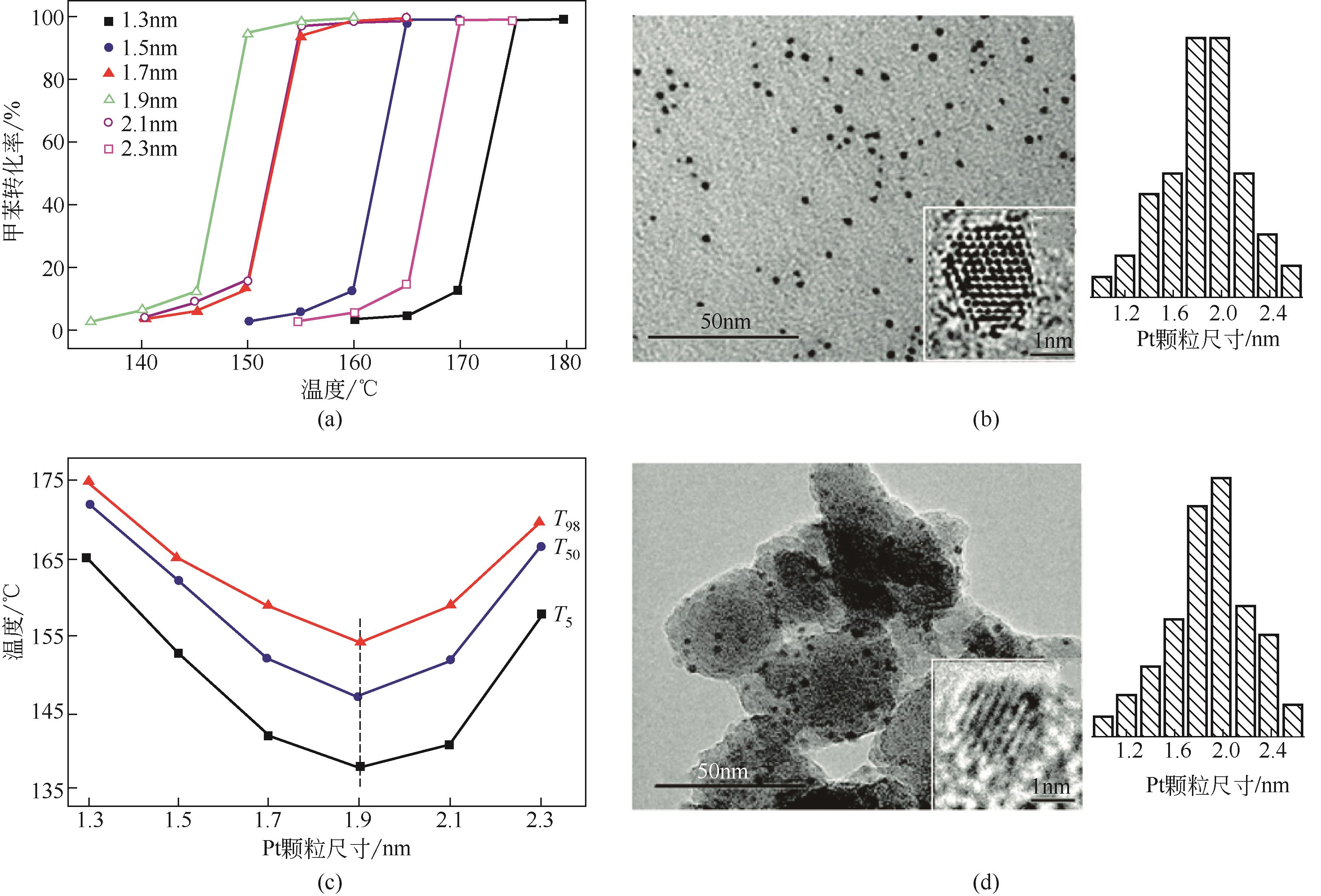
| Pt/Al2O3 | 甲苯 | 1000 | 24000 | 180 | 100 | [ |
| Pt-R/meso-KZSM-5 | 甲苯 | 1000 | 60000 | 175 | 98 | [ |
| Pt-1.2/Al2O3 | 苯 | 2800 | 32000 | 145 | 99 | [ |
| Pt-1.9/ZSM-5 | 甲苯 | 1000 | 3000 | 155 | 98 | [ |
| Pt/MnO2(单原子) | 甲苯 | 10 | 300 | 80 | 100 | [ |
| Pt/meso-Fe2O3(单原子) | 苯 | 1000 | 20000 | 198 | 90 | [ |
| Pt-SA/MgO | 甲苯 | 1000 | 36000 | 220 | 90 | [ |
| CeO2(棒状) | 邻二甲苯 | 250 | 60000 | 239 | 90 | [ |
| Pt/CeO2(棒状) | 甲苯 | 1000 | 48000 | 150 | 90 | [ |
| MnO2(棒状) | 甲苯 | 800 | 99200 | 176 | 90 | [ |
表3 芳香烃类污染物催化氧化条件和性能
| 催化剂 | 污染物 | 体积分数/×10-6 | 空速/h-1 | 转化温度/℃ | 转化率/% | 参考文献 |
|---|---|---|---|---|---|---|
| Pt/Al2O3 | 甲苯 | 1000 | 24000 | 180 | 100 | [ |
| Pt-R/meso-KZSM-5 | 甲苯 | 1000 | 60000 | 175 | 98 | [ |
| Pt-1.2/Al2O3 | 苯 | 2800 | 32000 | 145 | 99 | [ |
| Pt-1.9/ZSM-5 | 甲苯 | 1000 | 3000 | 155 | 98 | [ |
| Pt/MnO2(单原子) | 甲苯 | 10 | 300 | 80 | 100 | [ |
| Pt/meso-Fe2O3(单原子) | 苯 | 1000 | 20000 | 198 | 90 | [ |
| Pt-SA/MgO | 甲苯 | 1000 | 36000 | 220 | 90 | [ |
| CeO2(棒状) | 邻二甲苯 | 250 | 60000 | 239 | 90 | [ |
| Pt/CeO2(棒状) | 甲苯 | 1000 | 48000 | 150 | 90 | [ |
| MnO2(棒状) | 甲苯 | 800 | 99200 | 176 | 90 | [ |
| 催化剂 | 污染物 | 体积分数 /×10-6 | 空速 /h-1 | 转化温度 /℃ | 转化率 /% | 参考 文献 |
|---|---|---|---|---|---|---|
| Pt-Ce/TiO2 | 丙酮 | 1000 | 30000 | 243 | 100 | |
| MnCe/Al-MSP | 乙醛 | — | 18000 | 150 | 100 | |
| MnOx/TiO2 | 丙酮 | 500 | 36000 | 290 | 90 | |
| Cu15-Mn15/TiO2 | 己醛 | 500 | 25000 | 225 | 90 | |
| MnCe/Al-MSP | 丙酮 | 1000 | 15000 | 195 | 99 | |
| SrMn0.8Ce0.2O3 | 丙酮 | 1000 | 5100 | 200 | 90 | |
| 5∶1CoAlO-300 | 丙酮 | 1000 | 33000 | 222 | 90 | |
| 5∶1CoAlO-200 | 丙酮 | 1000 | 33000 | 225 | 90 |
表4 醛酮类污染物催化氧化条件和性能
| 催化剂 | 污染物 | 体积分数 /×10-6 | 空速 /h-1 | 转化温度 /℃ | 转化率 /% | 参考 文献 |
|---|---|---|---|---|---|---|
| Pt-Ce/TiO2 | 丙酮 | 1000 | 30000 | 243 | 100 | |
| MnCe/Al-MSP | 乙醛 | — | 18000 | 150 | 100 | |
| MnOx/TiO2 | 丙酮 | 500 | 36000 | 290 | 90 | |
| Cu15-Mn15/TiO2 | 己醛 | 500 | 25000 | 225 | 90 | |
| MnCe/Al-MSP | 丙酮 | 1000 | 15000 | 195 | 99 | |
| SrMn0.8Ce0.2O3 | 丙酮 | 1000 | 5100 | 200 | 90 | |
| 5∶1CoAlO-300 | 丙酮 | 1000 | 33000 | 222 | 90 | |
| 5∶1CoAlO-200 | 丙酮 | 1000 | 33000 | 225 | 90 |
| 项目 | 转化温度/℃ | ||
|---|---|---|---|
| 甲苯 | 丙酮 | 乙酸乙酯 | |
| 单组分 | |||
| T50 | 155 | 150 | 125 |
| T90 | 270 | 252 | 223 |
| 双组分 | |||
| T50 | 164 | 163 | 133 |
| T90 | 290 | 299 | 237 |
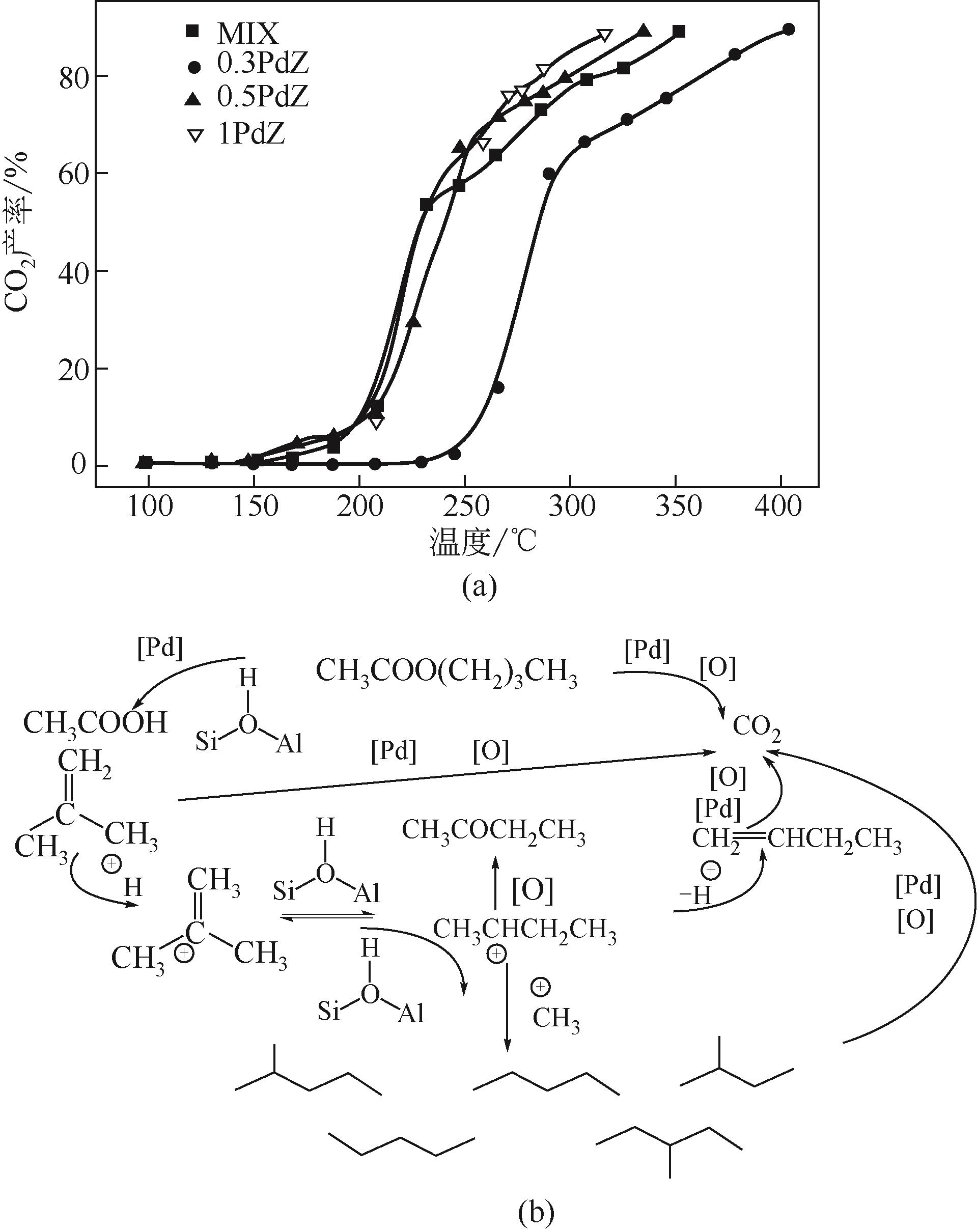
表5 VOCs在单组分和二元催化系统中MnCeOx/沸石催化氧化的T50和T90[91]
| 项目 | 转化温度/℃ | ||
|---|---|---|---|
| 甲苯 | 丙酮 | 乙酸乙酯 | |
| 单组分 | |||
| T50 | 155 | 150 | 125 |
| T90 | 270 | 252 | 223 |
| 双组分 | |||
| T50 | 164 | 163 | 133 |
| T90 | 290 | 299 | 237 |
| 催化剂 | 污染物 | 体积分数/×10-6 | 空速/h-1 | 转化温度/℃ | 转化率/% | 参考文献 |
|---|---|---|---|---|---|---|
| 0.1Pd/γ-Al2O3 | 油烟 | — | 28000 | 360 | 93.1 | [ |
| 2%CeO2- 0.1Pd/γ-Al2O3 | 油烟 | — | 28000 | 300 | 93.7 | [ |
| Fe-MEL | 油烟 | 2000 | 20000 | 400 | 96 | [ |
| La0.8Ce0.2CoO3/Al2O3 | 油烟 | — | — | 300 | 88 | [ |
| Mn-MEL | 油烟 | 2000 | 20000 | 400 | 99 | [ |
| Mn/堇青石 | 油烟 | 5000 | 12000 | 400 | 87.8 | [ |
| Mn-CeOx/MeOx | 油烟 | 1500 | 12000 | 400 | 93.6 | [ |
表6 油烟的催化氧化条件和性能
| 催化剂 | 污染物 | 体积分数/×10-6 | 空速/h-1 | 转化温度/℃ | 转化率/% | 参考文献 |
|---|---|---|---|---|---|---|
| 0.1Pd/γ-Al2O3 | 油烟 | — | 28000 | 360 | 93.1 | [ |
| 2%CeO2- 0.1Pd/γ-Al2O3 | 油烟 | — | 28000 | 300 | 93.7 | [ |
| Fe-MEL | 油烟 | 2000 | 20000 | 400 | 96 | [ |
| La0.8Ce0.2CoO3/Al2O3 | 油烟 | — | — | 300 | 88 | [ |
| Mn-MEL | 油烟 | 2000 | 20000 | 400 | 99 | [ |
| Mn/堇青石 | 油烟 | 5000 | 12000 | 400 | 87.8 | [ |
| Mn-CeOx/MeOx | 油烟 | 1500 | 12000 | 400 | 93.6 | [ |
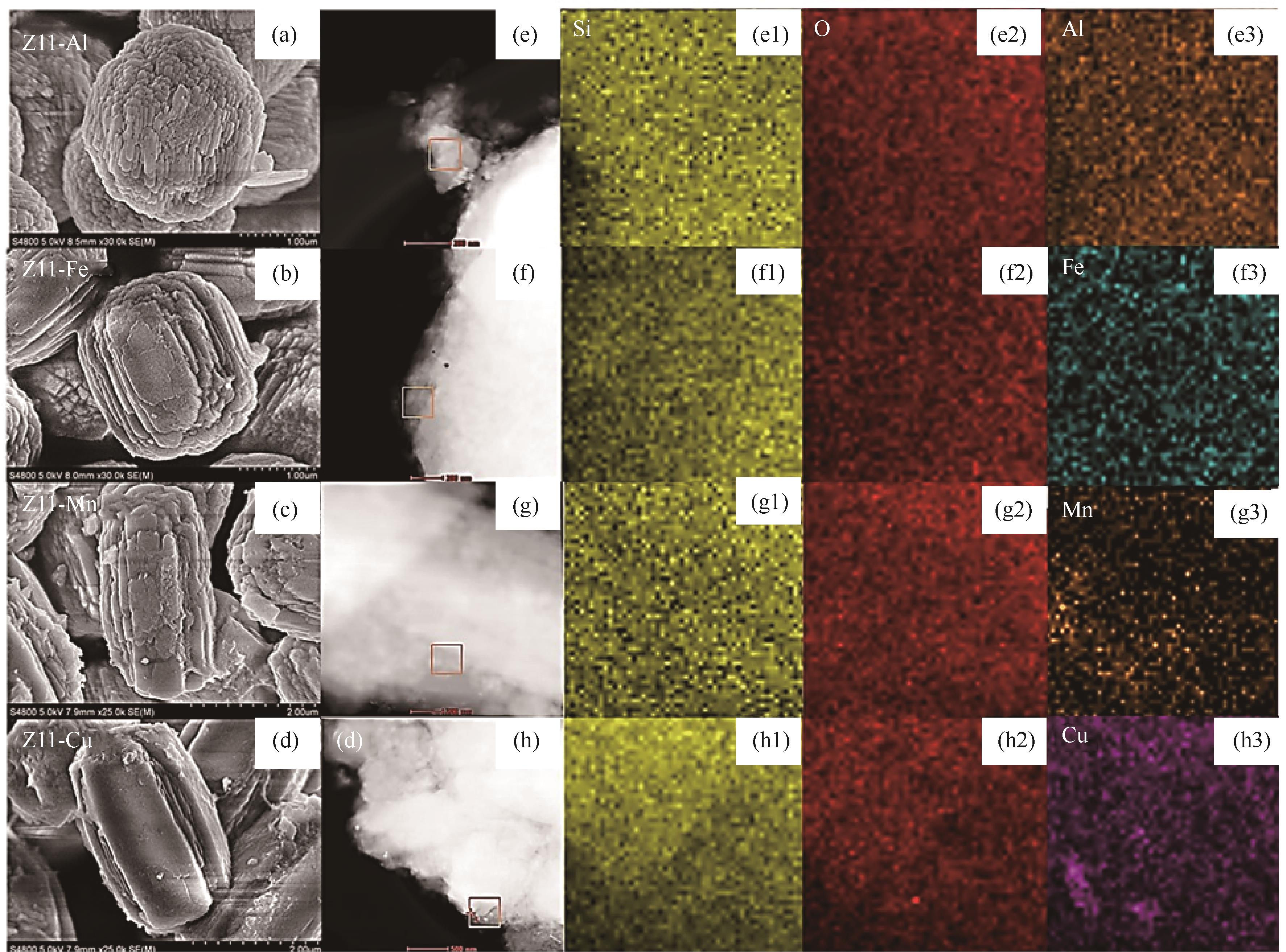
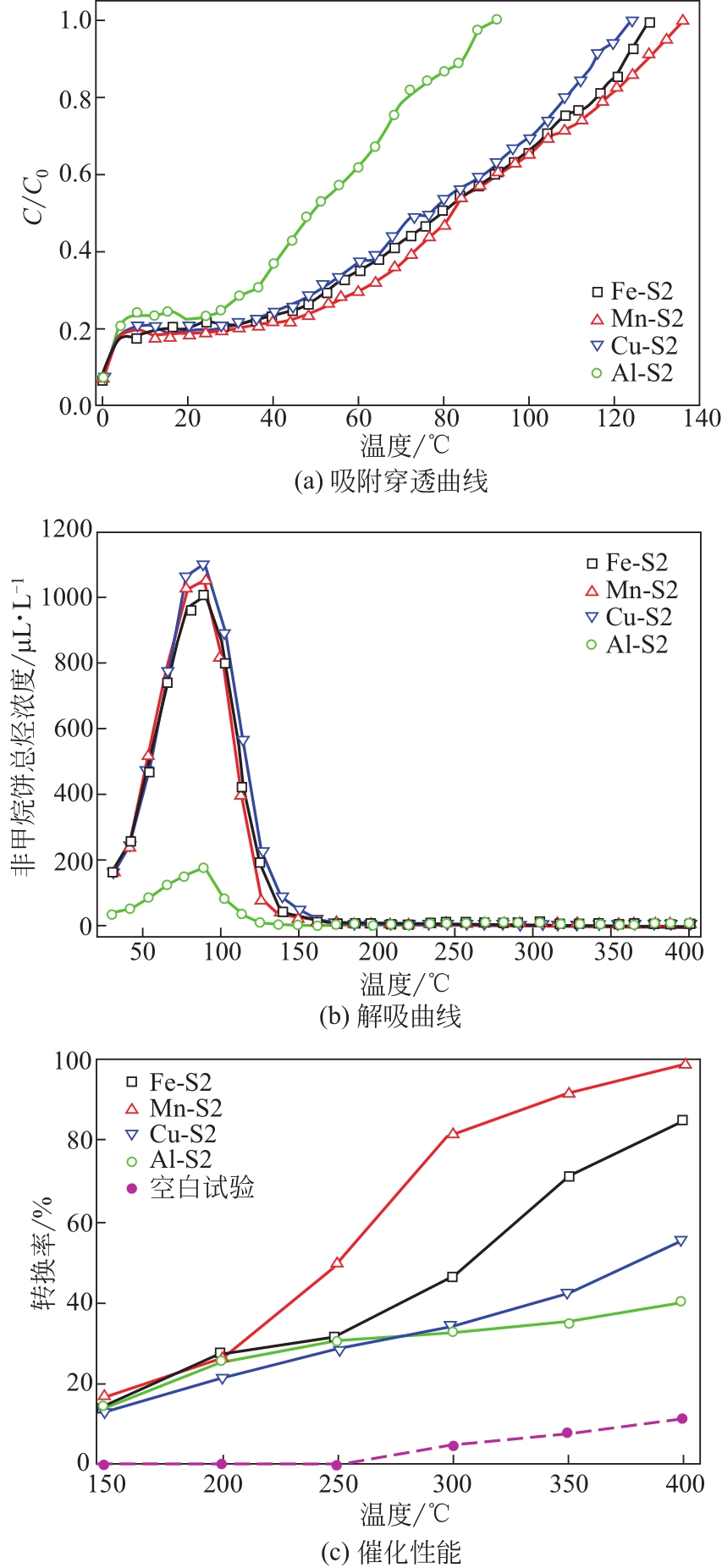
图8 (a)、(b)、(c)、(d)分别为Z11-Al、Z11-Fe、Z11-Mn、Z11-Cu透射电子显微镜图像;(e)、(f)、(g)、(h)分别为Z11-Al、Z11-Fe、Z11-Mn、Z11-Cu扫描透射电子显微镜图像[(e1)~(h1) Si,(e2)~(h2) O,(e3) Al,(f3) Fe,(g3) Mn,(h3) Cu][96]
| 1 | HOU J, SUN H, ZHOU Y, et al. Environmental exposure to polycyclic aromatic hydrocarbons, kitchen ventilation, fractional exhaled nitric oxide, and risk of diabetes among Chinese females[J]. Indoor Air, 2018, 28(3): 383-393. |
| 2 | ARI A, ARI E P, YENİSOY-KARAKAS S, et al. Source characterization and risk assessment of occupational exposure to volatile organic compounds (VOCs) in a barbecue restaurant[J]. Building and Environment, 2020, 174: 106791. |
| 3 | YANG Y, WANG Y H, YAO D, et al. Significant decreases in the volatile organic compound concentration, atmospheric oxidation capacity and photochemical reactivity during the National Day holiday over a suburban site in the North China Plain[J]. Environmental Pollution, 2020, 263: 114657. |
| 4 | GARMASH O, RISSANEN M P, PULLINET I, et al. Multi-generation OH oxidation as a source for highly oxygenated organic molecules from aromatics[J]. Atmospheric Chemistry and Physics, 2020, 20(1): 515-537. |
| 5 | HE Z R, WANG X M, LING Z H, et al. Contributions of different anthropogenic volatile organic compound sources to ozone formation at a receptor site in the Pearl River Delta region and its policy implications[J]. Atmospheric Chemistry and Physics, 2019, 19(13): 8801-8816. |
| 6 | HUANG X F, ZHANG B, XIA S Y, et al. Sources of oxygenated volatile organic compounds (OVOCs) in urban atmospheres in North and South China[J]. Environmental Pollution, 2020, 261: 114152. |
| 7 | SVEDAHL S R, HILT B, SVENDSEN K. Work environment factors and respiratory complaints in Norwegian cooks[J]. International Archives of Occupational and Environmental Health, 2020, 93(2): 205-212. |
| 8 | ZHANG D C, LIN J J, JIA L Z, et al. Speciation of VOCs in the cooking fumes from five edible oils and their corresponding health risk assessments[J]. Atmospheric Environment, 2019, 211: 6-17. |
| 9 | 张星,钱振清,张德峰,等. 餐饮油烟排放特征与净化技术研究进展[J]. 环境工程, 2020, 38(1): 37-41. |
| ZHANG X, QIAN Z Q, ZHANG D F, et al. Research progress of cooking fume emission characteristics and purification technologies[J]. Environmental Engineering, 2020, 38(1): 37-41. | |
| 10 | 冯铁成,易红宏,唐晓龙,等. 餐饮油烟污染及其净化技术研究进展[J]. 现代化工, 2017, 37(3): 20-23. |
| FENG T C, YI H H, TANG X L, et al. Research progress of the pollution from cooking oil fume and its purification technology[J]. Modern Chemical Industry, 2017, 37(3): 20-23. | |
| 11 | 柴美彤,张润铎. 餐饮油烟催化净化技术的研究进展[J]. 工业催化, 2018, 26(5): 12-19. |
| CHAI M T, ZHANG R D. Progress on catalytic control techniques of cooking oil fumes[J]. Industrial Catalysis, 2018, 26(5): 12-19. | |
| 12 | 黄永海,易红宏,唐晓龙,等. 催化燃烧技术用于油烟废气净化的研究进展[J]. 化工进展, 2017, 36(4): 1270-1277. |
| HUANG Y H, YI H H, TANG X L, et al. Research progress in the removal of cooking oil fumes by catalytic combustion[J]. Chemical Industry and Engineering Progress, 2017, 36(4): 1270-1277. | |
| 13 | 何万清,聂磊,田刚,等. 基于GC-MS的烹调油烟VOCs的组分研究[J]. 环境科学, 2013, 34(12): 4605-4611. |
| HE W Q, NIE L, TIAN G, et al. Study on the chemical compositions of VOCs emitted by cooking oils based on GC-MS[J]. Environmental Science, 2013, 34(12): 4605-4611. | |
| 14 | 刘中文,孙咏梅,袭著革. 烹调油烟雾中有机成分的分析[J]. 中国公共卫生, 2002(9): 26-28. |
| LIU Z W, SUN Y M, XI Z G. Analysis on organic compounds of cooking oil fum[J]. China Public Health, 2002(9): 26-28. | |
| 15 | 汪笃权,左晓斌,李小玲,等. 饮食油烟检测及成分分析[J]. 现代农业科技, 2010(9): 284-285. |
| WANG D Q, ZUO X B, LI X L, et al. Detection and composition analysis of cooking fume[J]. Modern Agricultural Science and Technology, 2010(9): 284-285. | |
| 16 | 史宝成, 刘景泰. 植物油烟组分的色质联机分析[J]. 中国环境监测, 2001(11): 34-36. |
| SHI B C, LIU J T. Analysing the composition of vegetable oil smoke by using gas chromatograph-mass spectrometer[J]. Environmental Monitoring in China, 2001(11): 34-36. | |
| 17 | 王凯雄,朱杏冬. 烹调油烟气的成分及其分析方法[J]. 上海环境科学, 1999(11): 3-5. |
| WANG K X, ZHU X D. The constituents in cooking-oil smoke and their analytical of methods[J]. Shanghai Environmental Science, 1999(11): 3-5. | |
| 18 | 梁衍魁. 餐饮业烹调油烟气的组成与危害及净化方法探讨[J]. 能源与环境, 2004(1): 43-44. |
| LIANG Y K. Composition, harm and purification methods of cooking oil smoke in catering industry[J]. Energy and Environment, 2004(1): 43-44. | |
| 19 | 谭晓风,孙晓钰,刘德全,等. 厨房烹调油烟的有机物定性定量GC/MS分析[J]. 质谱学报, 2003(1): 270-274. |
| TAN X F, SUN X Y, LIU D Q, et al. Qualitative and quantitative GC/MS analysis of organic matter in kitchen cooking fume[J]. Journal of Chinese Mass Spectrometry Society, 2003 (1): 270-274. | |
| 20 | PENG C Y, LAN C H, LIN P C, et al. Effects of cooking method, cooking oil, and food type on aldehyde emissions in cooking oil fumes[J]. Journal of Hazardous Materials, 2017, 324: 160-167. |
| 21 | 朱杏冬, 王凯雄, 朱军林, 等. 烹调油烟冷凝物的紫外吸收光谱和色谱/质谱分析研究[J]. 中国粮油学报, 2001(5): 59-62. |
| ZHU X D, WANG K X, ZHU J L, et al. Study on UV absorption spectrum and GC/MS analysis of cooking oil fume condensate[J]. Journal of the Chinese Cereals and Oils Association, 2001(5): 59-62. | |
| 22 | 史纯珍,姜锡,姚志良,等. 烹饪油烟羰基化合物排放特征[J]. 环境工程学报, 2015, 9(3): 1376-1380. |
| SHI C Z, JIANG X, YAO Z L, et al. Carbonyl compounds emission characteristics in cooking fumes[J]. Chinese Journal of Environmental Engineering, 2015, 9(3): 1376-1380. | |
| 23 | 徐幽琼,IGNATIUS T S Y,林捷,等. 不同食用油和烹调方式的油烟成分分析[J]. 中国卫生检验杂志, 2012, 22(10): 2271-2274. |
| XU Y Q, IGNATITUS T S Y, LIN J, et al. The composition of cooking fumes with different oils,cooking methods and foods[J]. Chinese Journal of Health Laboratory Technology, 2012, 22(10): 2271-2274. | |
| 24 | 黄永海. 餐饮油烟中VOCs代表物的排放特征及催化氧化研究[D]. 北京: 北京科技大学, 2020. |
| HUANG Y H. Investing of the emission characteristics and catalytic oxidation of the representatives of VOCs in the cooking oil fumes[D]. BeiJing: University of Science and Technology BeiJing, 2020. | |
| 25 | ZHANG D C, LIU J L, JIA L Z, et al. Speciation of VOCs in the cooking fumes from five edible oils and their corresponding health risk assessments[J]. Atmospheric Environment, 2019, 211: 6-17. |
| 26 | 李勤勤,龚道程,吴爱华,等. 餐饮油烟VOCs排放特征研究进展[J]. 环境科学与技术, 2018, 41(12): 113-121. |
| LI Q Q, GONG D C, WU A H, et al. Emission characteristics of VOCs from commercial cooking fumes: a review[J]. Environmental Science & Technology, 2018, 41(12): 113-121. | |
| 27 | HE C, CHENG J, ZHANG X, et al. Recent advances in the catalytic oxidation of volatile organic compounds: a review based on pollutant sorts and sources[J]. Chemical Reviews, 2019, 119(7): 4471-4568. |
| 28 | YANG C T, MIAO G, PI Y H, et al. Abatement of various types of VOCs by adsorption/catalytic oxidation: a review[J]. Chemical Engineering Journal, 2019, 370: 1128-1153. |
| 29 | YAN Y, YE B, CHEN M, et al. Site-specific deposition creates electron-rich Pd atoms for unprecedented C—H activation in aerobic alcohol oxidation[J]. Chinese Journal of Catalysis, 2020, 41(8): 1240-1247. |
| 30 | XIA Y, WANG Z W, FENG Y, et al. In situ molten salt derived iron oxide supported platinum catalyst with high catalytic performance for o-xylene elimination[J]. Catalysis Today, 2020, 351: 30-36. |
| 31 | YAN D W, LI Q R, ZHANG H, et al. A highly dispersed mesoporous zeolite@TiO2-supported Pt for enhanced sulfur-resistance catalytic CO oxidation[J]. Catalysis Communications, 2020, 142: 106042. |
| 32 | OUSMANE M, LIOTTA L F, CARLO G D, et al. Supported Au catalysts for low-temperature abatement of propene and toluene, as model VOCs: support effect[J]. Applied Catalysis B: Environmental, 2011, 101(3-4): 629-637. |
| 33 | HUA W, GAO Z. Catalytic combustion of n-pentane on Pt supported on solid superacids[J]. Applied Catalysis B: Environmental, 1998, 17(1): 37-42. |
| 34 | HOSSEINI M, BARAKAT T, COUSIN R, et al. Catalytic performance of core-shell and alloy Pd-Au nanoparticles for total oxidation of VOC: the effect of metal deposition[J]. Applied Catalysis B: Environmental, 2012, 111/112: 218-224. |
| 35 | CENTENO M A, PAULIS M, MONTES M, et al. Catalytic combustion of volatile organic compounds on Au/CeO2/Al2O3 and Au/Al2O3 catalysts[J]. Applied Catalysis A: General, 2002, 234(1-2): 65-78. |
| 36 | ANIĆ M, RADIĆ N, GRBIĆ B, et al. Catalytic activity of Pt catalysts promoted by MnOx for n-hexane oxidation[J]. Applied Catalysis B: Environmental, 2011, 107(3-4): 327-332. |
| 37 | AGUILERA D A, PEREZ A, MOLINA R, et al. Cu-Mn and Co-Mn catalysts synthesized from hydrotalcites and their use in the oxidation of VOCs[J]. Applied Catalysis B: Environmental, 2011, 104(1/2): 144-150. |
| 38 | LI W B, WANG J X, GONG H. Catalytic combustion of VOCs on non-noble metal catalysts[J]. Catalysis Today, 2009, 148(1/2): 81-87. |
| 39 | ZHAO H J, HAN W L, TANG Z C. Tailored design of high-stability CoMn1.5Ox@TiO2 double-wall nanocages derived from Prussian blue analogue for catalytic combustion of o-dichlorobenzene[J]. Applied Catalysis B: Environmental, 2020, 276: 119133. |
| 40 | MORALES M, BARBERO B, CADUS L. Total oxidation of ethanol and propane over Mn-Cu mixed oxide catalysts[J]. Applied Catalysis B: Environmental, 2006, 67(3/4): 229-236. |
| 41 | MORALES M R, YESTE M P, VIDAl H, et al. Insights on the combustion mechanism of ethanol and n-hexane in honeycomb monolithic type catalysts: influence of the amount and nature of Mn-Cu mixed oxide[J]. Fuel, 2017, 208: 637-646. |
| 42 | 徐思遥,李森,彭东辉,等. Cu/Co复合氧化物催化降解正庚烷[J]. 精细化工, 2018, 35(3): 402-409. |
| XU S Y, LI S, PENG D H, et al. Catalytic degradation of n-heptane over Cu /Co composite oxides[J]. Fine Chemicals, 2018, 35(3): 402-409. | |
| 43 | 李森, 徐思遥, 唐俊杰, 等. La-Co复合氧化物催化降解VOCs[J]. 应用技术学报, 2019, 19(2): 110-118. |
| LI S, XU S Y, TANG J J, et al. Catalytic degradation of VOCs over La-Co composite Oxides[J]. Journal of Technology, 2019, 19(2): 110-118. | |
| 44 | DÍAZ C C, PILAR Y M, VIDAL H, et al. In situ generation of Mn1-xCex system on cordierite monolithic supports for combustion of n-hexane: effects on activity and stability[J]. Fuel, 2020, 262: 116564. |
| 45 | FU X R, LIU Y, YAO W Y, et al. One-step synthesis of bimetallic Pt-Pd/MCM-41 mesoporous materials with superior catalytic performance for toluene oxidation[J]. Catalysis Communications, 2016, 83: 22-26. |
| 46 | WANG H, YANG W, TIAN P H, et al. A highly active and anti-coking Pd-Pt/SiO2 catalyst for catalytic combustion of toluene at low temperature[J]. Applied Catalysis A: General, 2017, 529: 60-67. |
| 47 | GAN T, CHU X F, QI H, et al. Pt/Al2O3 with ultralow Pt-loading catalyze toluene oxidation: promotional synergistic effect of Pt nanoparticles and Al2O3 support[J]. Applied Catalysis B: Environmental, 2019, 257: 117943. |
| 48 | CHEN C Y, WANG X, ZHANG J, et al. Superior performance in catalytic combustion of toluene over KZSM-5 zeolite supported platinum catalyst[J]. Catalysis Letters, 2014, 144(11): 1851-1859. |
| 49 | CHEN Z Y, MAO J X, ZHOU R X. Preparation of size-controlled Pt supported on Al2O3 nanocatalysts for deep catalytic oxidation of benzene at lower temperature[J]. Applied Surface Science, 2019, 465: 15-22. |
| 50 | CHEN C Y, CHEN F, ZHANG L, et al. Importance of platinum particle size for complete oxidation of toluene over Pt/ZSM-5 catalysts[J]. Chemical Communications, 2015, 51(27): 5936-5938. |
| 51 | DERITA L, DAI S, LOPEZ-ZEPEDA K, et al. Catalyst architecture for stable single atom dispersion enables site-specific spectroscopic and reactivity measurements of CO adsorbed to Pt atoms, oxidized Pt clusters, and metallic Pt clusters on TiO2[J]. Journal of the American Chemical Society, 2017, 139(40): 14150-14165. |
| 52 | MOSES-DEBUSK M, YOON M, ALLARD L F, et al. CO oxidation on supported single Pt atoms: experimental and ab initio density functional studies of CO interaction with Pt atom on θ-Al2O3(010) surface[J]. Journal of the American Chemical Society, 2013, 135(34): 12634-12645. |
| 53 | ZHANG H Y, SUI S H, ZHENG X M, et al. One-pot synthesis of atomically dispersed Pt on MnO2 for efficient catalytic decomposition of toluene at low temperatures[J]. Applied Catalysis B: Environmental, 2019, 257: 117878. |
| 54 | YANG K, LIU Y X, DENG J G, et al. Three-dimensionally ordered mesoporous iron oxide-supported single-atom platinum: highly active catalysts for benzene combustion[J]. Applied Catalysis B: Environmental, 2019, 244: 650-659. |
| 55 | ZHAO S Z, WEN Y F, LIU X J, et al. Formation of active oxygen species on single-atom Pt catalyst and promoted catalytic oxidation of toluene[J]. Nano Research, 2020, 13(6): 1544-1551. |
| 56 | GOODMAN E D, SCHWALBE J A, CARGNELLO M. Mechanistic understanding and the rational design of sinter-resistant heterogeneous catalysts[J]. ACS Catalysis, 2017, 7(10): 7156-7173. |
| 57 | ZOU X L, RUI Z B, JI H B. Core-shell NiO@PdO nanoparticles supported on alumina as an advanced catalyst for methane oxidation[J]. ACS Catalysis, 2017, 7(3): 1615-1625. |
| 58 | JI W K, WANG X Y, TANG M N, et al. Strategy for stabilizing noble metal nanoparticles without sacrificing active sites[J]. Chemical Communications, 2019, 55: 6846-6850. |
| 59 | WANG L, WANG Y F, ZHANG Y, et al. Shape dependence of nanoceria on complete catalytic oxidation of o-xylene[J]. Catalysis Science & Technology, 2016, 6(13): 4840-4848. |
| 60 | PENG R S, SUN X B, LI S J, et al. Shape effect of Pt/CeO2 catalysts on the catalytic oxidation of toluene[J]. Chemical Engineering Journal, 2016, 306: 1234-1246. |
| 61 | 廖银念, 张璇, 牛文浩, 等. 不同形貌氧化锰催化降解甲苯的性能研究[J]. 环境工程, 2018, 36(1): 62-66. |
| LIAO Y N, ZHANG X, NIU W H, et al. Catalytic decomposition of toluene by manganese[J]. Environmental Engineering, 2018, 36(1): 62-66. | |
| 62 | 李俊洁,刘建英,胡晓东,等. Pd/MCM-41对乙醇汽油车尾气排放乙醛的吸附和催化氧化性能[J]. 环境工程学报, 2018, 12(9): 2558-2565. |
| LI J J, LIU J Y, HU X D, et al. Adsorption and catalytic oxidation properties of Pd/MCM-41 for acetaldehyde in gasohol exhausts[J]. Chinese Journal of Environmental Engineering, 2018, 12(9): 2558-2565. | |
| 63 | GE Y L, FU K X, ZHAO Q, et al. Performance study of modified Pt catalysts for the complete oxidation of acetone[J]. Chemical Engineering Science, 2019, 206: 499-506. |
| 64 | TAN X, LAN H, XIE H M, et al. Role of surface oxygen species of mesoporous CeCu oxide catalyst in OVOCs catalytic combustion[J]. Journal of Environmental Chemical Engineering, 2017, 5(2): 2068-2076. |
| 65 | WANG J G, ZHANG C, YANG S F, et al. Highly improved acetone oxidation activity over mesoporous hollow nanospherical MnxCo3-xO4 solid solutions[J]. Catalysis Science & Technology, 2019, 9(22): 6379-6390. |
| 66 | SUN Y J, LI N, XING X, et al. Catalytic oxidation performances of typical oxygenated volatile organic compounds (acetone and acetaldehyde) over MAlO (M = Mn, Co, Ni, Fe) hydrotalcite-derived oxides[J]. Catalysis Today, 2019, 327: 389-397. |
| 67 | LIN L Y, BAI H L. Promotional effects of manganese on the structure and activity of Ce-Al-Si based catalysts for low-temperature oxidation of acetone[J]. Chemical Engineering Journal, 2016, 291: 94-105. |
| 68 | 李悦,张婷婷,王娟,等. TiO2负载Cu-Mn复合氧化物催化燃烧正己醛[J]. 物理化学学报, 2016, 32(8): 2084-2092. |
| LI Y, ZHANG T T, WANG J, et al. Catalytic combustion of n-hexanal using Cu-Mn composite oxide supported on TiO2[J]. Acta Physico-Chimica Sinica, 2016, 32(8): 2084-2092. | |
| 69 | REZLESCU N, REZLESCU E, POPA P D, et al. Partial substitution of manganese with cerium in SrMnO3 nano-perovskite catalyst: effect of the modification on the catalytic combustion of dilute acetone[J]. Materials Chemistry and Physics, 2016, 182: 332-337. |
| 70 | LIN L Y, WANG C Y, BAI H L. A comparative investigation on the low-temperature catalytic oxidation of acetone over porous aluminosilicate-supported cerium oxides[J]. Chemical Engineering Journal, 2015, 264: 835-844. |
| 71 | ZHU X C, ZHANG S, YU X N, et al. Controllable synthesis of hierarchical MnOx/TiO2 composite nanofibers for complete oxidation of low-concentration acetone[J]. Journal of Hazardous Materials, 2017, 337: 105-114. |
| 72 | ZHAO Q, GE Y L, FU K X, et al. Oxidation of acetone over Co-based catalysts derived from hierarchical layer hydrotalcite: influence of Co/Al molar ratios and calcination temperatures[J]. Chemosphere, 2018, 204: 257-266. |
| 73 | YUE L, HE C, HAO Z P, et al. Effects of metal and acidic sites on the reaction by-products of butyl acetate oxidation over palladium-based catalysts[J]. Journal of Environmental Sciences, 2014, 26(3): 702-707. |
| 74 | CAMPESI M A, MARIANI N J, BRESSA S P, et al. Kinetic study of the combustion of ethanol and ethyl acetate mixtures over a MnCu catalyst[J]. Fuel Processing Technology, 2012, 103: 84-90. |
| 75 | LIU X L, HAN Q Z, SHI W B, et al. Catalytic oxidation of ethyl acetate over Ru-Cu bimetallic catalysts: further insights into reaction mechanism via in situ FTIR and DFT studies[J]. Journal of Catalysis, 2019, 369: 482-492. |
| 76 | HOSSEINI S A, NIAEI A, SALARI D, et al. Nanostructure copper-exchanged ZSM-5 catalytic activity for conversion of volatile organic compounds (toluene and ethyl acetate)[J]. Chinese Journal of Chemistry, 2010, 28(2): 143-148. |
| 77 | ZHOU Y, ZHANG H P, YAN Y. Catalytic oxidation of ethyl acetate over CuO/ZSM-5 catalysts: effect of preparation method[J]. Journal of the Taiwan Institute of Chemical Engineers, 2018, 84: 162-172. |
| 78 | KONSOLAKIS M, CARABINEIRO S A C, MARNELLOS G E, et al. Volatile organic compounds abatement over copper-based catalysts: effect of support[J]. Inorganica Chimica Acta, 2017, 455: 473-482. |
| 79 | ZHOU Y, ZHANG H P, YAN Y. Catalytic oxidation of ethyl acetate over CuO/ZSM-5 zeolite membrane coated on stainless steel fibers by chemical vapor deposition[J]. Chemical Engineering Research and Design, 2020, 157: 13-24. |
| 80 | NIU J R, DENG J G, LIU W, et al. Nanosized perovskite-type oxides La1-xSrxMO3-δ (M=Co, Mn; x=0, 0.4) for the catalytic removal of ethylacetate[J]. Catalysis Today, 2007, 126(3/4): 420-429. |
| 81 | QIN Y, SHEN F X, ZHU T L, et al. Catalytic oxidation of ethyl acetate over LaBO3 (B = Co, Mn, Ni, Fe) perovskites supported silver catalysts[J]. RSC Advances, 2018, 8(58): 33425-33431. |
| 82 | SEMUSHINA Y P, PECHENYUK S I, KUZMICH L F, et al. Relationship between the catalytic properties of the products of the oxidative thermolysis of certain complexes and the porous structures of samples in the oxidation reactions of volatile organic compounds[J]. Russian Journal of Physical Chemistry A, 2017, 91(1): 26-29. |
| 83 | ANKE S, BENDT G, SINEV I, et al. Selective 2-propanol oxidation over unsupported Co3O4 spinel nanoparticles: mechanistic insights into aerobic oxidation of alcohols[J]. ACS Catalysis, 2019, 9(7): 5974-5985. |
| 84 | SANTOS V P, CARABINEIRO S A C, Tavares P B, et al. Oxidation of CO, ethanol and toluene over TiO2 supported noble metal catalysts[J]. Applied Catalysis B: Environmental, 2010, 99(1/2): 198-205. |
| 85 | SABOUR S, ESPECEL C, FONTAINE C, et al. Catalytic oxidation of n-butanol over platinum supported mesoporous silica CMI-1[J]. Journal of Molecular Catalysis A: Chemical, 2016, 420: 50-55. |
| 86 | RAMÍREZ-LÓPEZ R, ELIZALDE-MARTINEZ I, BALDERAS-TAPIA L. Complete catalytic oxidation of methane over Pd/CeO2-Al2O3: the influence of different ceria loading[J]. Catalysis Today, 2010, 150(3/4): 358-362. |
| 87 | SEDJAME H J, FONTAINE C, LAFAYE G, et al. On the promoting effect of the addition of ceria to platinum based alumina catalysts for VOCs oxidation[J]. Applied Catalysis B: Environmental, 2014, 144: 233-242. |
| 88 | BAI B Y, LI J H, HAO J M. 1D-MnO2, 2D-MnO2 and 3D-MnO2 for low-temperature oxidation of ethanol[J]. Applied Catalysis B: Environmental, 2015, 164: 241-250. |
| 89 | MORALS M R, BARBERO B P, CADUS L E. Evaluation and characterization of Mn-Cu mixed oxide catalysts for ethanol total oxidation: influence of copper content[J]. Fuel, 2008, 87(7): 1177-1186. |
| 90 | KIM S C, SHIM W G. Catalytic combustion of VOCs over a series of manganese oxide catalysts[J]. Applied Catalysis B: Environmental, 2010, 98(3/4): 180-185. |
| 91 | 曹利, 连子, 黄学敏. MnCeOx/沸石催化剂对工业典型VOC的催化性能[J]. 环境工程, 2020, 38(1): 48-53. |
| CAO L, LIAN Z, HUANG X M. Catalytic performance of typical VOCs over MnCeOx /zeolite catalyst[J]. Environmental Engineering, 2020, 38 (1): 48-53. | |
| 92 | BEAUCHET R, MAGNOUX P, MIJOIN J. Catalytic oxidation of volatile organic compounds (VOCs) mixture (isopropanol/o-xylene) on zeolite catalysts[J]. Catalysis Today, 2007, 124(3/4): 118-123. |
| 93 | 叶长明. 油烟的催化净化研究[D]. 郑州: 郑州大学, 2002. |
| YE C M. Study on catalytic purification of lampblack[D]. Zhengzhou: Zhengzhou University, 2002. | |
| 94 | HUANG Y H, YI H H, TANG X L, et al. Cordierite-supported metal oxide for non-methane hydrocarbon oxidation in cooking oil fumes[J]. Environmental Technology, 2019, 40(25): 3358-3363. |
| 95 | YI H H, HUANG Y H, TANG X L, et al. Mn-CeOx/MeOx(Ti, Al)/cordierite preparation with ultrasound-assisted for non-methane hydrocarbon removal from cooking oil fumes[J]. Ultrasonics Sonochemistry, 2019, 53: 126-133. |
| 96 | YI H H, FENG Y C, YU Q J, et al. Synthesis of divalent metal-silicalite MEL zeolites as efficient bi-functional adsorbents/catalysts for non-methane hydrocarbon in cooking oil fumes elimination[J]. Separation and Purification Technology, 2020, 251: 117363. |
| 97 | YU Q J, FENG Y C, TANG X L, et al. A novel ferrisilicate MEL zeolite with bi-functional adsorption/catalytic oxidation properties for non-methane hydrocarbon removal from cooking oil fumes[J]. Microporous and Mesoporous Materials, 2020, 11(20):11-14. |
| 98 | 左乐, 李彩亭, 曾光明, 等. La0.8Ce0.2CoO3/Al2O3对油烟的催化净化[J]. 环境科学与技术, 2008, 31(12): 136-139. |
| ZUO L, LI C T, ZENG G M, et al. Catalysis purifying of cooking oil fumes over supported La0.8Ce0.2CoO3/Al2O3 catalyst[J]. Environmental Science and Technology, 2008, 31 (12): 136-139. | |
| 99 | 吕丽. 厨房油烟净化催化剂的研究[D]. 长春: 吉林大学, 2010. |
| LYU L. Investigation of a new catalyst for removing the kitchen cooking oil fume[D]. Changchun: Jilin University, 2010. |
| [1] | 许友好, 王维, 鲁波娜, 徐惠, 何鸣元. 中国炼油创新技术MIP的开发策略及启示[J]. 化工进展, 2023, 42(9): 4465-4470. |
| [2] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [3] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [4] | 潘宜昌, 周荣飞, 邢卫红. 高效分离同碳数烃的先进微孔膜:现状与挑战[J]. 化工进展, 2023, 42(8): 3926-3942. |
| [5] | 常印龙, 周启民, 王青月, 王文俊, 李伯耿, 刘平伟. 废弃聚烯烃的高值化学回收研究进展[J]. 化工进展, 2023, 42(8): 3965-3978. |
| [6] | 杨静, 李博, 李文军, 刘晓娜, 汤刘元, 刘月, 钱天伟. 焦化污染场地中萘降解菌的分离及降解特性[J]. 化工进展, 2023, 42(8): 4351-4361. |
| [7] | 刘柏成, 李法云, 赵琦慧, 吝美霞. 禾本科植物修复多环芳烃污染土壤研究进展[J]. 化工进展, 2023, 42(7): 3736-3748. |
| [8] | 谭利鹏, 申峻, 王玉高, 刘刚, 徐青柏. 煤沥青和石油沥青共混改性的研究进展[J]. 化工进展, 2023, 42(7): 3749-3759. |
| [9] | 韩恒文, 韩伟, 李明丰. 烯烃水合反应工艺与催化剂研究进展[J]. 化工进展, 2023, 42(7): 3489-3500. |
| [10] | 李若琳, 何少林, 苑宏英, 刘伯约, 纪冬丽, 宋阳, 刘博, 余绩庆, 徐英俊. 原位热解对油页岩物性及地下水水质影响探索[J]. 化工进展, 2023, 42(6): 3309-3318. |
| [11] | 吴和平, 曹宁, 徐圆圆, 曹云波, 李裕东, 杨强, 卢浩. 氢氟酸与烷基化油快速分离[J]. 化工进展, 2023, 42(6): 2845-2853. |
| [12] | 李栋先, 王佳, 蒋剑春. 皂脚热解-催化气态加氢制备生物燃料[J]. 化工进展, 2023, 42(6): 2874-2883. |
| [13] | 殷鹏镇, 吴芹, 黎汉生. 甲基芳烃液相选择性催化氧化催化剂研究进展[J]. 化工进展, 2023, 42(6): 2916-2943. |
| [14] | 王子健, 柯明, 宋昭峥, 李佳涵, 童燕兵, 孙巾茹. 分子筛催化汽油烷基化降苯技术研究进展[J]. 化工进展, 2023, 42(5): 2371-2389. |
| [15] | 王科菊, 赵成, 胡晓玫, 云军阁, 魏凝涵, 姜雪迎, 邹昀, 陈志航. 金属氧化物低温催化氧化VOCs的研究进展[J]. 化工进展, 2023, 42(5): 2402-2412. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||