化工进展 ›› 2020, Vol. 39 ›› Issue (4): 1539-1549.DOI: 10.16085/j.issn.1000-6613.2019-1124
污泥生物炭中氮硫行为及环境效应研究进展
- 昆明理工大学环境科学与工程学院,云南 昆明 650000
-
收稿日期:2019-07-15出版日期:2020-04-05发布日期:2020-04-28 -
通讯作者:陈芳媛 -
作者简介:王彦(1995—),男,硕士研究生,研究方向为土壤污染与固碳。E-mail:787959651@qq.com 。 -
基金资助:国家自然科学基金(41763016)
Behavior and environmental effects of nitrogen and sulfur in sludge biochar
Yan WANG( ),Ning ZUO,Yuanyuan JIANG,Fangyuan CHEN(
),Ning ZUO,Yuanyuan JIANG,Fangyuan CHEN( )
)
- Faculty of Environmental Science and Engineering, Kunming University of Science and Technology, Kunming 650000, Yunnan, China
-
Received:2019-07-15Online:2020-04-05Published:2020-04-28 -
Contact:Fangyuan CHEN
摘要:
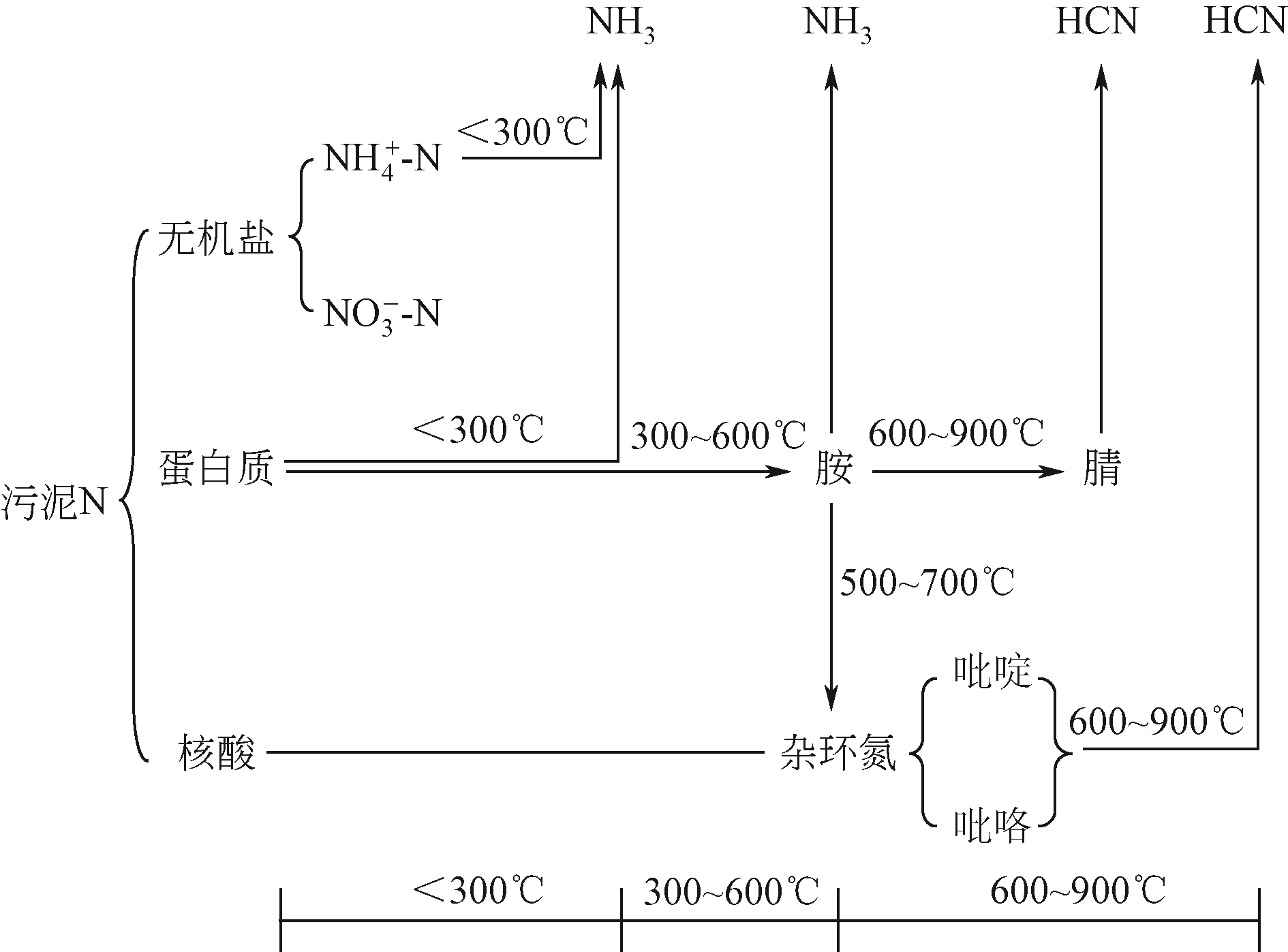
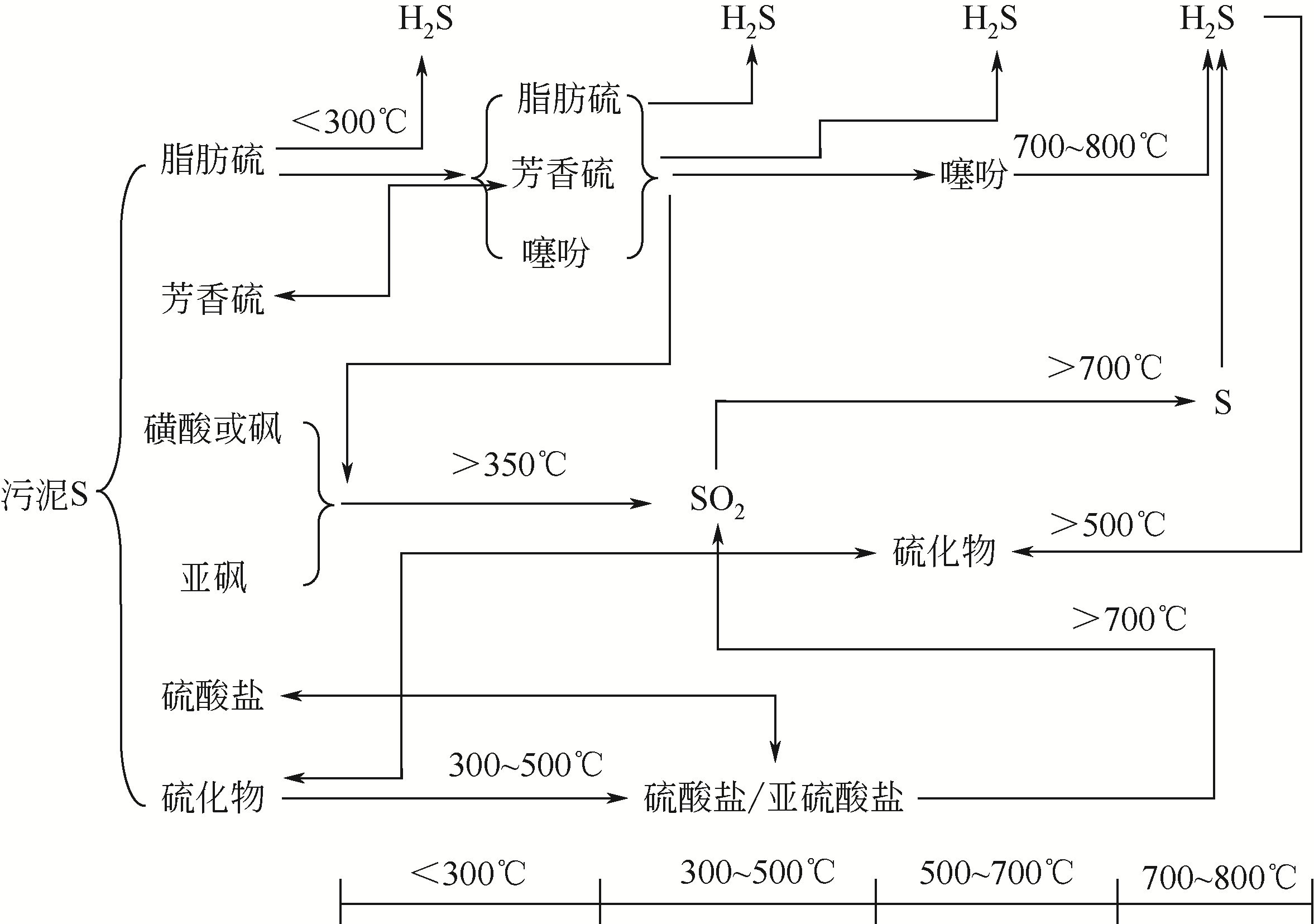
污泥生物炭中氮硫元素含量高,其氮硫行为和环境效应对全球气候变化的影响不容忽视。以往的研究中,研究者往往以富碳生物炭作为主要研究对象,关注碳对全球气候变化的行为和功效,而对氮硫元素的作用关注不够。本文从原始污泥基本性质到其热解过程,再到生物炭的老化,逐步对污泥生物炭整个生命周期内氮硫的行为及其环境效应研究进行综述,并对未来应注重开展的研究方向进行展望,为生物炭中氮硫元素固定、释放及与之关联的环境效应和温室气体排放控制研究提供理论基础。分析表明,污泥中氮元素含量普遍高于硫元素,且热解过程中氮比硫更容易转移至气相产物。氮硫元素随热解温度的增加,在三相产物中的分配都是炭中持续减少,油中先增后减,气中一直增加。高温(>800℃)条件下,气相中的氮含量高于固相,而硫元素则仍然主要存在于固相中。污泥生物炭老化及其环境效应研究表明,污泥生物炭氮硫元素与土壤的相互作用及其温室效应问题在今后的研究中应引起重视。
中图分类号:
引用本文
王彦,左宁,姜媛媛,陈芳媛. 污泥生物炭中氮硫行为及环境效应研究进展[J]. 化工进展, 2020, 39(4): 1539-1549.
Yan WANG,Ning ZUO,Yuanyuan JIANG,Fangyuan CHEN. Behavior and environmental effects of nitrogen and sulfur in sludge biochar[J]. Chemical Industry and Engineering Progress, 2020, 39(4): 1539-1549.
| 污泥来源 | 元素质量分数/% | 污泥类型 | ||||
|---|---|---|---|---|---|---|
| N | C | H | S | O | ||
| 石化工厂[ | 5.20 | 41.00 | 5.60 | 3.30 | — | 石化污泥 |
| 高雄石化废水处理厂[ | 4.75 | 39.00 | 5.70 | 1.18 | 24.40 | 石化污泥 |
| 北京清河污水处理厂[ | 9.96 | 55.90 | 8.19 | 1.16 | — | 市政污泥 |
| 武汉某市政污水处理厂[ | 4.78 | 25.93 | 4.21 | 1.03 | 22.02 | 市政污泥 |
| 昆明第四污水处理厂[ | 4.76 | 33.70 | 3.85 | 0.86 | 27.71 | 市政污泥 |
| 重庆市唐家沱污水处理厂[ | 3.52 | 20.66 | 3.61 | 0.84 | 24.14 | 市政污泥 |
| 广州市猎德污水处理厂[ | 2.95 | 15.55 | 3.18 | 0.68 | 16.48 | 市政污泥 |
| 荆州市红光污水处理厂[ | 2.37 | 12.99 | 2.54 | <0.05 | 16.30 | 市政污泥 |
| 肇庆市某污水处理厂[ | 1.57 | 12.15 | 2.74 | 0.48 | 14.49 | 市政污泥 |
| 广州市某市政污水处理厂[ | 5.3 | 19.7 | 4.3 | 0.3 | — | 市政污泥 |
| 某盐酸四环素制药厂[ | 6.00 | 34.00 | 10.00 | 0.06 | — | 制药污泥 |
| 某制药厂[ | 3.98 | 19.05 | 3.64 | 2.79 | 19.85 | 制药污泥 |
| 胜利油田孤岛采油厂联合站[ | 0.32 | 19.28 | 3.79 | 0.54 | — | 油田污泥 |
| 辽河油田采油污水处理厂[ | 0.51 | 33.43 | 4.82 | 0.97 | 7.43 | 油田污泥 |
| 山东某造纸厂[ | 0.25 | 25.31 | 2.49 | 0.13 | 28.88 | 脱墨污泥 |
| 河北某废纸制浆造纸厂[ | 1.14 | 9.74 | 2.10 | 1.06 | 43.8 | 造纸污泥 |
| 某市印染集控区[ | 0.92 | 16.49 | 3.02 | 4.07 | — | 印染污泥 |
| 某工厂污泥[ | 1.81 | 16.97 | 2.90 | 0.06 | 11.27 | 化工污泥 |
| 安徽华谊化工[ | 1.91 | 20.75 | 1.77 | 0.55 | 25.75 | 化工污泥 |
| 扬州某化工企业[ | 3.67 | 19.96 | 3.41 | 0.71 | 28.21 | 化工污泥 |
| 某石化炼油厂含油污水处理站[ | 0.74 | 42.25 | 5.45 | 1.87 | 6.46 | 化工污泥 |
| 桐君阁制药、铜梁造纸、重庆啤酒[ | 2.23 | 26.16 | 2.37 | 5.63 | 22.11 | 1∶1∶1混合工业污泥 |
| 某钢铁厂[ | 0.09 | 33.90 | 5.16 | 0.03 | — | 轧钢污泥 |
| 某铬鞣工艺牛皮革厂[ | 3.36 | 22.23 | 2.66 | 16.10 | — | 制革污泥 |
| 江苏某无铬鞣工艺制革厂[ | 2.33 | 25.19 | 3.10 | 11.90 | — | 制革污泥 |
表1 不同来源污泥元素分析结果列举
| 污泥来源 | 元素质量分数/% | 污泥类型 | ||||
|---|---|---|---|---|---|---|
| N | C | H | S | O | ||
| 石化工厂[ | 5.20 | 41.00 | 5.60 | 3.30 | — | 石化污泥 |
| 高雄石化废水处理厂[ | 4.75 | 39.00 | 5.70 | 1.18 | 24.40 | 石化污泥 |
| 北京清河污水处理厂[ | 9.96 | 55.90 | 8.19 | 1.16 | — | 市政污泥 |
| 武汉某市政污水处理厂[ | 4.78 | 25.93 | 4.21 | 1.03 | 22.02 | 市政污泥 |
| 昆明第四污水处理厂[ | 4.76 | 33.70 | 3.85 | 0.86 | 27.71 | 市政污泥 |
| 重庆市唐家沱污水处理厂[ | 3.52 | 20.66 | 3.61 | 0.84 | 24.14 | 市政污泥 |
| 广州市猎德污水处理厂[ | 2.95 | 15.55 | 3.18 | 0.68 | 16.48 | 市政污泥 |
| 荆州市红光污水处理厂[ | 2.37 | 12.99 | 2.54 | <0.05 | 16.30 | 市政污泥 |
| 肇庆市某污水处理厂[ | 1.57 | 12.15 | 2.74 | 0.48 | 14.49 | 市政污泥 |
| 广州市某市政污水处理厂[ | 5.3 | 19.7 | 4.3 | 0.3 | — | 市政污泥 |
| 某盐酸四环素制药厂[ | 6.00 | 34.00 | 10.00 | 0.06 | — | 制药污泥 |
| 某制药厂[ | 3.98 | 19.05 | 3.64 | 2.79 | 19.85 | 制药污泥 |
| 胜利油田孤岛采油厂联合站[ | 0.32 | 19.28 | 3.79 | 0.54 | — | 油田污泥 |
| 辽河油田采油污水处理厂[ | 0.51 | 33.43 | 4.82 | 0.97 | 7.43 | 油田污泥 |
| 山东某造纸厂[ | 0.25 | 25.31 | 2.49 | 0.13 | 28.88 | 脱墨污泥 |
| 河北某废纸制浆造纸厂[ | 1.14 | 9.74 | 2.10 | 1.06 | 43.8 | 造纸污泥 |
| 某市印染集控区[ | 0.92 | 16.49 | 3.02 | 4.07 | — | 印染污泥 |
| 某工厂污泥[ | 1.81 | 16.97 | 2.90 | 0.06 | 11.27 | 化工污泥 |
| 安徽华谊化工[ | 1.91 | 20.75 | 1.77 | 0.55 | 25.75 | 化工污泥 |
| 扬州某化工企业[ | 3.67 | 19.96 | 3.41 | 0.71 | 28.21 | 化工污泥 |
| 某石化炼油厂含油污水处理站[ | 0.74 | 42.25 | 5.45 | 1.87 | 6.46 | 化工污泥 |
| 桐君阁制药、铜梁造纸、重庆啤酒[ | 2.23 | 26.16 | 2.37 | 5.63 | 22.11 | 1∶1∶1混合工业污泥 |
| 某钢铁厂[ | 0.09 | 33.90 | 5.16 | 0.03 | — | 轧钢污泥 |
| 某铬鞣工艺牛皮革厂[ | 3.36 | 22.23 | 2.66 | 16.10 | — | 制革污泥 |
| 江苏某无铬鞣工艺制革厂[ | 2.33 | 25.19 | 3.10 | 11.90 | — | 制革污泥 |
| 1 | ROBERTS K G, GLOY B A, JOSEPH S, et al. Life cycle assessment of biochar systems: estimating the energetic, economic, and climate change potential[J]. Environmental Science & Technology, 2010, 44(2): 827-833. |
| 2 | MARRIS E. Putting the carbon back: black is the new green[J]. Nature, 2006, 442(7103): 624-626. |
| 3 | LEHMANN J, JOSEPH S M. Biochar for environmental management: science and technology[M]. London: Earthscan, 2009. |
| 4 | LEHMANN J. A handful of carbon[J]. Nature, 2007, 447(7141): 143-144. |
| 5 | SINGH B P, HATTON B J, BALWANT S, et al. Influence of biochars on nitrous oxide emission and nitrogen leaching from two contrasting soils[J]. Journal of Environmental Quality, 2010, 39(4): 1224-1235. |
| 6 | CHEN T, ZHANG Y, WANG H, et al. Influence of pyrolysis temperature on characteristics and heavy metal adsorptive performance of biochar derived from municipal sewage sludge[J]. Bioresour Technol, 2014, 164(7): 47-54. |
| 7 | HASSANI A H, NEJAEI A, TORABIAN A. Excess sludge minimization in conventional activated sludge pilot plant by three chemical matters[J]. International Journal of Environmental Research, 2011, 5(4): 981-988. |
| 8 | BRDJANOVIC D, SLAMET A, LOOSDRECHT M C M VAN, et al. Impact of excessive aeration on biological phosphorus removal from wastewater[J]. Water Research, 1998, 32(1): 200-208. |
| 9 | AHMAD M, RAJAPAKSHA A U, LIM J E, et al. Biochar as a sorbent for contaminant management in soil and water: a review[J]. Chemosphere, 2014, 99: 19-33. |
| 10 | YAO H, LU J, WU J, et al. Adsorption of fluoroquinolone antibiotics by wastewater sludge biochar: role of the sludge source[J]. Water, Air, & Soil Pollution, 2013, 224(1): 1370. |
| 11 | SOMMERS L E. Chemical composition of sewage sludges and analysis of their potential use as fertilizers[J]. J. Environ. Qual., 1977, 6(2): 225-232. |
| 12 | CHAO C, CHIANG H, CHEN C. Pyrolytic kinetics of sludge from a petrochemical factory wastewater treatment plant—A transition state theory approach[J]. Chemosphere, 2002, 49(4): 431-437. |
| 13 | CHIANG H, CHAO C, CHANG C Y, et al. Residue characteristics and pore development of petrochemical industry sludge pyrolysis[J]. Water Research, 2001, 35(18): 4331-4338. |
| 14 | ZHOU P, XIONG S, ZHANG Y, et al. Study on the nitrogen transformation during the primary pyrolysis of sewage sludge by Py-GC/MS and Py-FTIR[J]. International Journal of Hydrogen Energy, 2017, 29(42): 18181-18188. |
| 15 | LIU H, ZHANG Q, HU H, et al. Dual role of conditioner CaO in product distributions and sulfur transformation during sewage sludge pyrolysis[J]. Fuel, 2014, 134(9): 514-520. |
| 16 | 王霜. 城市污水污泥的热解特性与低温热解实验研究[D]. 昆明: 昆明理工大学, 2005.WANG S. Experimental study on pyrolysis characteristics and low temperature pyrolysis of municipal sewage sludge[D]. Kunming: Kunming University of Science and Technology, 2005. |
| 17 | 马蜀, 高旭, 郭劲松. 城市污水处理厂剩余污泥的元素含量分析[J]. 中国给水排水, 2007, 23(19): 60-63. |
| MA S, GAO X, GUO J S. Element analysis of excess activated sludge in WW TP[J]. China Water & Wastewater, 2007, 23(19): 60-63. | |
| 18 | 王定美, 王跃强, 余震, 等. 污泥与稻秆共热解对生物炭中碳氮固定的协同作用[J]. 环境科学学报, 2015, 35(7): 2202-2209. |
| WANG D M, WANG Y Q, YU Z, et al. Synergistic effect on carbon and nitrogen fixation of biochar during co-pyrolysis of sewage sludge and rice straw[J]. Acta Scientiae Circumstantiae, 2015, 35(7): 2202-2209. | |
| 19 | 成功, 孙蕾, 焦李, 等. 脱水污泥-松木共热解生物炭的制备及吸附性能[J]. 工业用水与废水, 2013, 44(3): 55-59. |
| CHENG G, SUN L, JIAO L, et al. Preparation of biochar from co-pyrolysis of dewatered sewage sludge-pine sawdust and its adsorption capability[J]. Industrial Water & Wastewater, 2013, 44(3): 55-59. | |
| 20 | 袁浩然, 鲁涛, 黄宏宇, 等. 市政污泥热解制备生物炭实验研究[J]. 化工学报, 2012, 63(10): 3310-3315. |
| YUAN H R, LU T, HUANG H Y, et al. Experimental study for preparing biochar by pyrolysis of municipal sludge[J]. CIESC Journal, 2012, 63(10): 3310-3315. | |
| 21 | 鲁涛, 袁浩然, 王亚琢, 等. 热解温度对污泥生物炭稳定性及养分淋溶特性影响[J]. 化工学报, 2015, 66(7): 2664-2669. |
| LU T, YUAN H R, WANG Y Z, et al. Influence of pyrolysis temperature on biochar stability and leaching properties of nutrients contained in biochar[J]. CIESC Journal, 2015, 66(7): 2664-2669. | |
| 22 | 王昕彤. 抗生素剩余污泥对重金属的吸附性能及对环境的影响[D]. 天津: 天津理工大学, 2015.WANG X T. The adsorption of heavy metals on antibiotic activated excess sludge and its impact on the environment[D]. Tianjin: Tianjin University of Technology, 2015. |
| 23 | 王山辉. 制药污泥热解特征及热解油的特性分析[D]. 保定: 河北科技大学, 2016.WANG S H. The pyrolysis characteristics of pharmacy sludge and analysis of pyrolytic oil[D]. Baoding: Hebei University of Science and Technology, 2016. |
| 24 | 祝威. 油田含油污泥热解产物分析及性能评价[J]. 环境化学, 2010, 29(1): 127-131. |
| ZHU W. Analysis and performance mensuration of pyrolysis products for oil sludge[J]. Environmental Chemistry, 2010, 29(1): 127-131. | |
| 25 | 王志强, 邢奕, 洪晨, 等. 油田含油污泥掺煤资源化试验研究[J]. 安全与环境学报, 2015, 15(5): 287-291. |
| WANG Z Q, XING Y, HONG C, et al. Probe to the anthropogenic air pollutant emission inventory in Guanzhong Region, Shaanxi[J]. Journal of Safety and Environment, 2015, 15(5): 287-291. | |
| 26 | 王圆圆. 脱墨污泥热解工艺研究[D]. 济南: 齐鲁工业大学, 2015.WANG Y Y. Study on pyrolysis technology of deinking sludge[D]. Jinan: Qilu University of Technology, 2015. |
| 27 | 戎宇舟, 葛强, 李清, 等. 制浆造纸厂富铁污泥性质及其回用为污泥调理剂研究[J]. 西安交通大学学报, 2016, 50(9): 43-48. |
| RONG Y Z, GE Q, LI Q, et al. Study on properties of iron-rich sludge in pulp and paper industry and its recycling as sludge conditioning agent[J]. Journal of Xi’an Jiaotong University, 2016, 50(9): 43-48. | |
| 28 | 陈剑峰. 印染污泥元素分析及干化焚烧方案[J]. 化学工程与装备, 2017(9): 343-346. |
| CHEN J F. Element analysis and drying incineration scheme of printing and dyeing sludge[J]. Chemical Engineering & Equipment, 2017(9): 343-346. | |
| 29 | 羊建新, 翁居轼, 秦恒飞, 等. 化工污泥的干燥焚烧工艺设计[J]. 江苏理工学院学报, 2017, 23(6): 31-34. |
| YANG J X, WENG J S, QIN H F, et al. Drying and incineration technology design of chemical plant wastewater sludge[J]. Journal of Jiangsu University of Technology, 2017, 23(6): 31-34. | |
| 30 | 徐超, 王凯, 范旭文. 煤化工装置污水处理站污泥的综合利用[J]. 化肥工业, 2015, 42(1): 56-58. |
| XU C, WANG K, FAN X W. Comprehensive utilization of sludge of wastewater treatment station of coal chemical plant[J]. Chemical Fertilizer Industry, 2015, 42(1): 56-58. | |
| 31 | 黄河涛, 岳喜龙, 朱雪锋, 等. 危废埋场污泥干化脱水的试验研究[C]//环境工程2018年全国学术年会论文集, 扬州, 2018. |
| HUANG H T, YUE X L, ZHU X F, et al. Experimental study on desiccation and dewatering of sludge in waste landfill sludge [C]// Proceedings of the 2018 National Academic Annual Conference on Environmental Engineering, Yangzhou, 2018. | |
| 32 | 高敏杰, 林青山, 娄红春, 等. 炼化厂含油污泥的理化特性分析及动力学研究[J]. 淮阴工学院学报, 2018(1): 36-40. |
| GAO M J, LIN Q S, LOU H C, et al. Physical and chemical properties and kinetics analysis of oily sludge in refinery[J]. Journal of Huaiyin Institute of Technology, 2018(1): 36-40. | |
| 33 | 冉景煜, 胡建红, 王裕明, 等. 含金属元素化合物和压力对工业污泥燃烧特性的影响[J]. 环境科学学报, 2008, 28(1): 108-113. |
| RAN J Y, HU J H, WANG Y M, et al. Effects of compounds with metal element and pressure on the combustion characteristics of mixed industrial sludge[J]. Acta Scientiae Circumstantiae, 2008, 28(1): 108-113. | |
| 34 | 鲁文涛, 何品晶, 邵立明, 等. 轧钢含油污泥的热解与动力学分析[J]. 中国环境科学, 2017, 37(3): 1024-1030. |
| LU W T, HE P J, SHAO L M, et al. Pyrolysis of rolling oil sludge and its kinetic analysis[J]. China Environmental Science, 2017, 37(3): 1024-1030. | |
| 35 | 畅浩. 制革污泥热解过程及其产物特性的研究[D]. 西安: 陕西科技大学, 2014.CHANG H. Study on the pyrolysis process and product properties of leather sludge[D]. Xi’an: Shaanxi University of Science and Technology, 2014. |
| 36 | 李智伟, 王兴栋, 林景江, 等. 污泥生物炭制备过程中氮磷钾及重金属的迁移行为[J]. 环境工程学报, 2016, 10(3): 1392-1399. |
| LI Z W, WANG X D, LIN J J, et al. Transformation of nitrogen, phosphorus, potassium and heavy metals during sewage sludge biochar preparation[J]. Chinese Journal of Environmental Engineering, 2016, 10(3): 1392-1399. | |
| 37 | 张娜. 污泥热解过程中氮的迁移特性研究[D]. 沈阳: 沈阳航空航天大学, 2013.ZHANG N. Study on the transformation characteristics of nitrogen during pyrolysis of sewage sludge[D]. Shenyang: Shenyang Aerospace University, 2013. |
| 38 | ZHANG J, TIAN Y, CUI Y, et al. Key intermediates in nitrogen transformation during microwave pyrolysis of sewage sludge: a protein model compound study[J]. Bioresource Technology, 2013, 132(2): 57-63. |
| 39 | ZHANG J, ZUO W, TIAN Y, et al. Release of hydrogen sulfide during microwave pyrolysis of sewage sludge: effect of operating parameters and mechanism[J]. Journal of Hazardous Materials, 2017, 331: 117-122. |
| 40 | ZHANG J, ZUO W, TIAN Y, et al. Sulfur transformation during microwave and conventional pyrolysis of sewage sludge[J]. Environmental Science & Technology, 2017, 51(1): 709. |
| 41 | LI P, HU Y, YU W, et al. Investigation of sulfur forms and transformation during the co-combustion of sewage sludge and coal using X-ray photoelectron spectroscopy[J]. Journal of Hazardous Materials, 2009, 167(1): 1126-1132. |
| 42 | LIU H, LUO G, HU H, et al. Emission characteristics of nitrogen- and sulfur-containing odorous compounds during different sewage sludge chemical conditioning processes[J]. Journal of Hazardous Materials, 2012, 235/236(20): 298-306. |
| 43 | CAO J, LI L, MORISHITA K, et al. Nitrogen transformations during fast pyrolysis of sewage sludge[J]. Fuel, 2013, 104: 1-6. |
| 44 | ZHANG J, TIAN Y, ZHU J, et al. Characterization of nitrogen transformation during microwave-induced pyrolysis of sewage sludge[J]. Journal of Analytical & Applied Pyrolysis, 2014, 105(5): 335-341. |
| 45 | BAGREEV A, BANDOSZ T J, LOCKE D C. Pore structure and surface chemistry of adsorbents obtained by pyrolysis of sewage sludge-derived fertilizer[J]. Carbon, 2001, 39(13): 1971-1979. |
| 46 | SÁNCHEZ M E, MENÉNDEZ J A, DOMÍNGUEZ A, et al. Effect of pyrolysis temperature on the composition of the oils obtained from sewage sludge[J]. Biomass & Bioenergy, 2009, 33(6): 933-940. |
| 47 | TSAI W, LEE M, CHANG J, et al. Characterization of bio-oil from induction-heating pyrolysis of food-processing sewage sludges using chromatographic analysis[J]. Bioresource Technology, 2009, 100(9): 2650-2654. |
| 48 | DEBONO O, VILLOT A. Nitrogen products and reaction pathway of nitrogen compounds during the pyrolysis of various organic wastes[J]. Journal of Analytical and Applied Pyrolysis, 2015, 114: 222-234. |
| 49 | HOSSAIN M K, STREZOV V, CHAN K Y, et al. Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar[J]. Journal of Environmental Management, 2011, 92(1): 223-228. |
| 50 | FULLANA A, CONESA J A, FONT R, et al. Pyrolysis of sewage sludge: nitrogenated compounds and pretreatment effects[J]. Journal of Analytical & Applied Pyrolysis, 2003, 68: 561-575. |
| 51 | LI C, TAN L. Formation of NOx and SOx precursors during the pyrolysis of coal and biomass. Part Ⅲ. Further discussion on the formation of HCN and NH3 during pyrolysis[J]. Fuel, 2000, 79: 1899-1906. |
| 52 | TIAN F, LI B, CHEN Y, et al. Formation of NOx precursors during the pyrolysis of coal and biomass. Part V. Pyrolysis of a sewage sludge[J]. Fuel, 2002, 81: 2203-2208. |
| 53 | TAN L, LI C. Formation of NOx and SOx precursors during the pyrolysis of coal and biomass. Part I. Effects of reactor configuration on the determined yields of HCN and NH3 during pyrolysis[J]. Fuel, 2000, 79(15): 1883-1889. |
| 54 | HANSSON K M, SAMUELSSON J, TULLIN C S, et al. Formation of HNCO, HCN, and NH3 from the pyrolysis of bark and nitrogen-containing model compounds[J]. Combustion and Flame, 2004, 137(3): 265-277. |
| 55 | REN Q, ZHAO C. NOx and N2O precursors from biomass pyrolysis: role of cellulose, hemicellulose and lignin[J]. Environmental Science & Technology, 2013, 47(15): 8955-8961. |
| 56 | TSUBOUCHI N, OHTSUKA Y. Nitrogen chemistry in coal pyrolysis: catalytic roles of metal cations in secondary reactions of volatile nitrogen and char nitrogen[J]. Fuel Process Technol, 2008, 89: 379-390. |
| 57 | XIE Z, FENG J, ZHAO W, et al. Formation of NOx and SOx precursors during the pyrolysis of coal and biomass. Part Ⅳ. Pyrolysis of a set of Australian and Chinese coals[J]. Fuel, 2001, 80(15): 2131-2138. |
| 58 | OHTSUKA Y. Selective conversion of fuel-bound nitrogen to N2 with iron nanoparticles[J]. J. Jpn. Petrol. Inst., 1998, 41: 182-192. |
| 59 | CHEAH S, MALONE S C, FEIK C J. Speciation of sulfur in biochar produced from pyrolysis and gasification of oak and corn stover[J]. Environmental Science & Technology, 2014, 48(15): 8474-8480. |
| 60 | LIU S, WEI M, QIAO Y, et al. Release of organic sulfur as sulfur-containing gases during low temperature pyrolysis of sewage sludge[J]. Proceedings of the Combustion Institute, 2015, 35(3): 2767-2775. |
| 61 | CHENG S, QIAO Y, HUANG J, et al. Effect of alkali addition on sulfur transformation during low temperature pyrolysis of sewage sludge[J]. Proceedings of the Combustion Institute, 2017, 36: 2253-2261. |
| 62 | GOSTELOW P, PARSONS S A, STUETZ R M. Odour measurements for sewage treatment works[J]. Water Research, 2001, 35(3): 579-597. |
| 63 | 林庆毅, 姜存仓, 张梦阳. 生物炭老化后理化性质及微观结构的表征[J]. 环境化学, 2017, 36(10): 2107-2114. |
| LIN Q Y, JIANG C C, ZHANG M Y. Characterization of the physical and chemical structures of biochar under simulated aging condition[J]. Environmental Chemistry, 2017, 36(10): 2107-2114. | |
| 64 | 林庆毅, 张梦阳, 张林, 等. 老化生物炭对红壤铝形态影响的潜在机制[J]. 生态环境学报, 2018, 27(3): 491-497. |
| LIN Q Y, ZHANG M Y, ZHANG L, et al. The potential mechanism of the effect of aging biochar on the aluminum morphology in red soil[J]. Ecology and Environmental Sciences, 2018, 27(3): 491-497. | |
| 65 | 文方园, 陈建, 田路萍, 等. 过氧化氢氧化对生物炭表面性质的改变及其对双酚A吸附的影响[J]. 生态毒理学报, 2016, 11(2): 628-635. |
| WEN F Y, CHEN J, TIAN L P, et al. Chemical oxidation of biochars and the impact on bisphenol A sorption[J]. Asian Journal of Ecotoxicology, 2016, 11(2): 628-635. | |
| 66 | 唐伟, 郭悦, 吴景贵, 等. 老化的生物质炭性质变化及对菲吸持的影响[J]. 环境科学, 2014, 35(7): 2604-2611. |
| TANG W, GUO Y, WU J G, et al. Structural changes of aged biochar and the influence on phenanthrene adsorption[J]. Environmental Science, 2014, 35(7): 2604-2611. | |
| 67 | 武丽君. 生物炭对农田土壤氮素迁移及氨氧化作用的影响[D]. 太原: 太原理工大学, 2016.WU L J. Effect of biochar on nitrogen migration and ammonia oxidation in farmland soil[D]. Taiyuan: Taiyuan University of Technology, 2016. |
| 68 | 汪艳如, 侯杰发, 郭建华, 等. 冻融循环对牦牛粪生物炭吸附氨氮的影响[J]. 农业环境科学学报, 2017, 36(3): 566-573. |
| WANG Y R, HOU J F, GUO J H, et al. Effects of freeze-thaw cycles on ammonium-nitrogen adsorption of yak dung biochar[J]. Journal of Agro-Environment Science, 2017, 36(3): 566-573. | |
| 69 | 王朝旭, 陈绍荣, 张峰, 等. 老化玉米秸秆生物炭对碱性农田土壤氨氧化作用的影响[J]. 生态环境学报, 2018, 27(1): 31-39. |
| WANG C X, CHEN S R, ZHANG F, et al. Effects of aged maize straw-derived biochars on ammonia oxidation in an alkaline farmland soil[J]. Ecology and Environmental Sciences, 2018, 38(5): 1877-1884. | |
| 70 | 高鹏, 陈昱, 梁媛. 老化作用促进生物炭已吸附Cd(Ⅱ)的进一步稳定化研究[J]. 环境科学学报, 2018, 38(5): 1877-1884. |
| GAO P, CHEN Y, LIANG Y. Study of aging effect on the stability of biochar initially adsorbed Cd(Ⅱ)[J]. Acta Scientiae Circumstantiae, 2018, 38(5): 1877-1884. | |
| 71 | 陈昱, 梁媛, 郑章琪, 等. 浮萍生物炭的老化作用对其性质及对Cd(Ⅱ)吸附的影响[J]. 环境工程, 2016, 34(10): 60-64. |
| CHEN Y, LIANG Y, ZHENG Z Q, et al. Influnce of biochar ageing on its properties and Cd(Ⅱ) adsorption[J]. Environmental Engineering, 2016, 34(10): 60-64. | |
| 72 | 陈昱, 梁媛, 郑章琪, 等. 老化作用对水稻秸秆生物炭吸附Cd( Ⅱ)能力的影响[J]. 环境化学, 2016, 35(11): 2337-2343. |
| CHEN Y, LIANG Y, ZHENG Z, et al. Effect of ageing on Cd adsorption ability by rice-straw derived biochar[J]. Environmental Chemistry, 2016, 35(11): 2337-2343. | |
| 73 | CHENG C H, LEHMANN J, THIES J E, et al. Oxidation of black carbon by biotic and abiotic processes[J]. Organic Geochemistry, 2006, 37(11): 1477-1488. |
| 74 | JOSEPH S D, ARBESTAIN M C, LIN Y, et al. An investigation into the reactions of biochar in soil[J]. Soil Research, 2010, 48(7): 501-515. |
| 75 | HILSCHER A, KNICKER H. Degradation of grass-derived pyrogenic organic material, transport of the residues within a soil column and distribution in soil organic matter fractions during a 28 month microcosm experiment[J]. Organic Geochemistry, 2012, 42(1): 42-54. |
| 76 | MIA S, DIJKSTRA F A, SINGH B. Aging induced changes in biochar's functionality and adsorption behavior for phosphate and ammonium[J]. Environmental Science & Technology, 2017, 51(15): 8359-8367. |
| 77 | HALE S E, HANLEY K, LEHMANN J, et al. Effects of chemical, biological, and physical aging as well as soil addition on the sorption of pyrene to activated carbon and biochar[J]. Environmental Science & Technology, 2011, 45(24): 10445-10453. |
| 78 | 谢祖彬, 刘琦, 许燕萍, 等. 生物炭研究进展及其研究方向[J]. 土壤, 2011, 43(6): 857-861. |
| XIE Z B, LIU Q, XU Y P, et al. Advances and perspectives of biochar research[J]. Soils, 2011, 43(6): 857-861. | |
| 79 | YAO F X, CAMPS A M, VIRGEL S, et al. Simulated geochemical weathering of a mineral ash-rich biochar in a modified Soxhlet reactor[J]. Chemosphere, 2010, 80(7): 724-732. |
| 80 | 王洪媛, 盖霞普, 翟丽梅, 等. 生物炭对土壤氮循环的影响研究进展[J]. 生态学报, 2016, 36(19): 5998-6011. |
| WANG H Y, GAI X P, ZHAI L M, et al. Effect of biochar on soil nitrogen cycling: a review[J]. Acta Ecologica Sinica, 2016, 36(19): 5998-6011. | |
| 81 | UCHIMIYA M, WARTELLE L H, KLASSON K T, et al. Influence of pyrolysis temperature on biochar property and function as a heavy metal sorbent in soil[J]. Journal of Agricultural and Food Chemistry, 2011, 59(6): 2501-2510. |
| 82 | UCHIMIYA M, CANTRELL K B, HUNT P G, et al. Retention of heavy metals in a Typic Kandiudult amended with different manure-based biochars[J]. Journal of Environmental Quality, 2012, 41(4): 1138-1149. |
| 83 | 王宁, 侯艳伟, 彭静静, 等. 生物炭吸附有机污染物的研究进展[J]. 环境化学, 2012(3): 287-295. |
| WANG N, HOU Y W, PENG J J, et al. Research progress on sorption of organic contaminants to biochar[J]. Environmental Chemistry, 2012(3): 287-295. | |
| 84 | JOULE J A. Heterocyclic chemistry[M]. MILLS K. 4th ed. Oxford: Blackwell, 2000: |
| 85 | 李力, 陆宇超, 刘娅, 等. 玉米秸秆生物炭对Cd(Ⅱ)的吸附机理研究[J]. 农业环境科学学报, 2012, 32(11): 2277-2283. |
| LI L, LU Y C, LIU Y, et al. Adsorption mechanisms of cadmium(Ⅱ) on biochars derived from corn straw[J]. Journal of Agro-Environment Science, 2012, 32(11): 2277-2283. | |
| 86 | KNUDSEN J N, JENSEN P A, LIN W, et al. Sulfur transformations during thermal conversion of herbaceous biomass[J]. Energy & Fuels, 2004, 18(3): 810-819. |
| 87 | KONG L, ZHOU Q. Influences of biochar aging processes by eco-environmental conditions[J]. Advanced Materials Research, 2013, 790: 467-470. |
| [1] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [2] | 胡喜, 王明珊, 李恩智, 黄思鸣, 陈俊臣, 郭秉淑, 于博, 马志远, 李星. 二硫化钨复合材料制备与储钠性能研究进展[J]. 化工进展, 2023, 42(S1): 344-355. |
| [3] | 张杰, 王放放, 夏忠林, 赵光金, 马双忱. “双碳”目标下SF6排放现状、减排手段分析及未来展望[J]. 化工进展, 2023, 42(S1): 447-460. |
| [4] | 李化全, 王明华, 邱贵宝. 硫酸酸解钙钛矿相精矿的行为[J]. 化工进展, 2023, 42(S1): 536-541. |
| [5] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [6] | 盛维武, 程永攀, 陈强, 李小婷, 魏嘉, 李琳鸽, 陈险峰. 微气泡和微液滴双强化脱硫反应器操作分析[J]. 化工进展, 2023, 42(S1): 142-147. |
| [7] | 孙继鹏, 韩靖, 唐杨超, 闫汉博, 张杰瑶, 肖苹, 吴峰. 硫黄湿法成型过程数值模拟与操作参数优化[J]. 化工进展, 2023, 42(S1): 189-196. |
| [8] | 赖诗妮, 江丽霞, 李军, 黄宏宇, 小林敬幸. 含碳掺氨燃料的研究进展[J]. 化工进展, 2023, 42(9): 4603-4615. |
| [9] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [10] | 许中硕, 周盼盼, 王宇晖, 黄威, 宋新山. 硫铁矿介导的自养反硝化研究进展[J]. 化工进展, 2023, 42(9): 4863-4871. |
| [11] | 杨志强, 曾纪珺, 马义丁, 尉涛, 赵波, 刘英哲, 张伟, 吕剑, 李兴文, 张博雅, 唐念, 李丽, 孙东伟. 六氟化硫替代气体的研究现状及未来发展趋势[J]. 化工进展, 2023, 42(8): 4093-4107. |
| [12] | 王云刚, 焦健, 邓世丰, 赵钦新, 邵怀爽. 冷凝换热与协同脱硫性能实验分析[J]. 化工进展, 2023, 42(8): 4230-4237. |
| [13] | 李艳玲, 卓振, 池亮, 陈曦, 孙堂磊, 刘鹏, 雷廷宙. 氮掺杂生物炭的制备与应用研究进展[J]. 化工进展, 2023, 42(7): 3720-3735. |
| [14] | 李佳, 樊星, 陈莉, 李坚. 硝酸生产尾气中NO x 和N2O联合脱除技术研究进展[J]. 化工进展, 2023, 42(7): 3770-3779. |
| [15] | 于姗, 段元刚, 张怡欣, 唐春, 付梦瑶, 黄靖元, 周莹. 分步法分解硫化氢制氢和硫黄催化剂研究进展[J]. 化工进展, 2023, 42(7): 3780-3790. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||