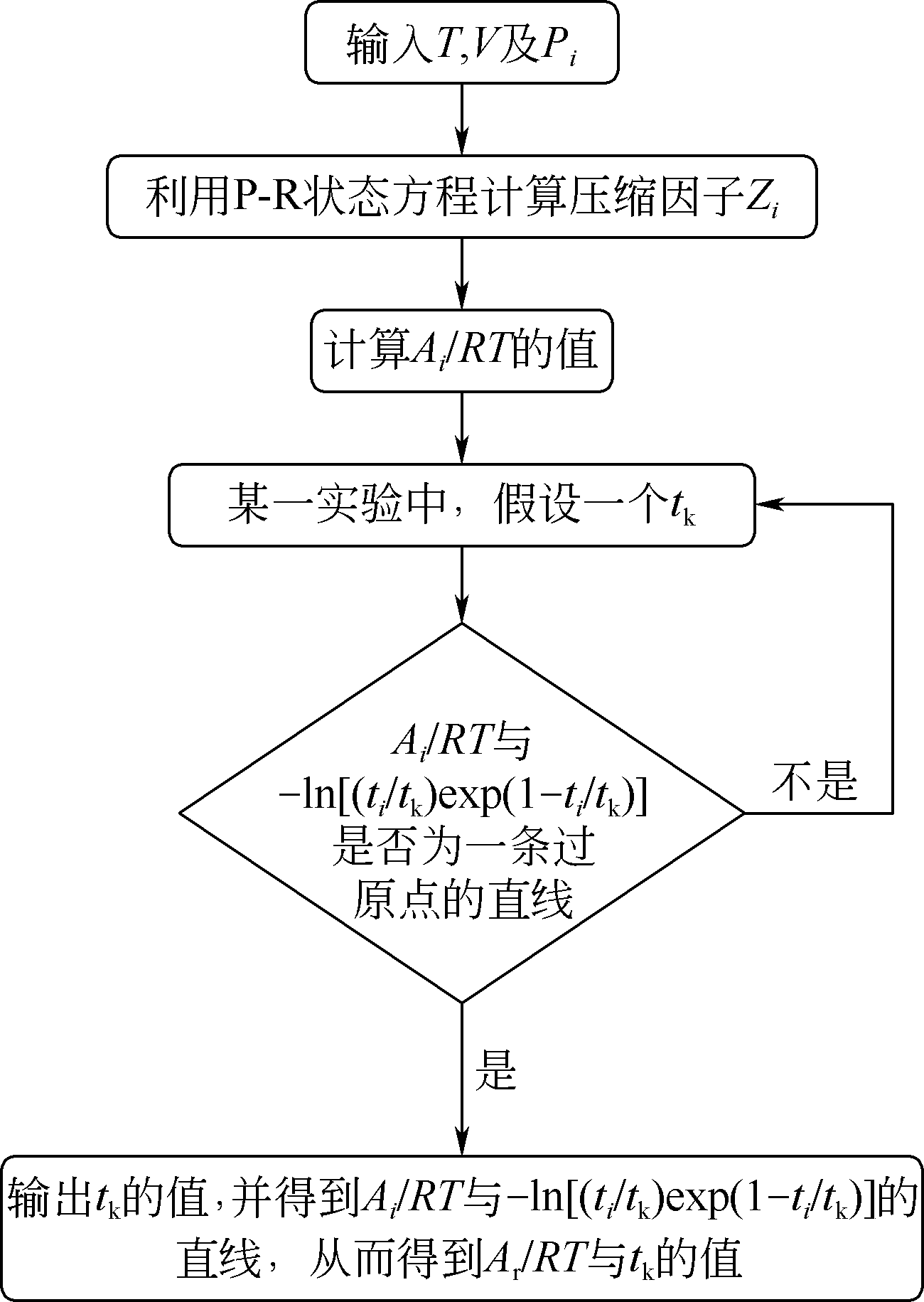
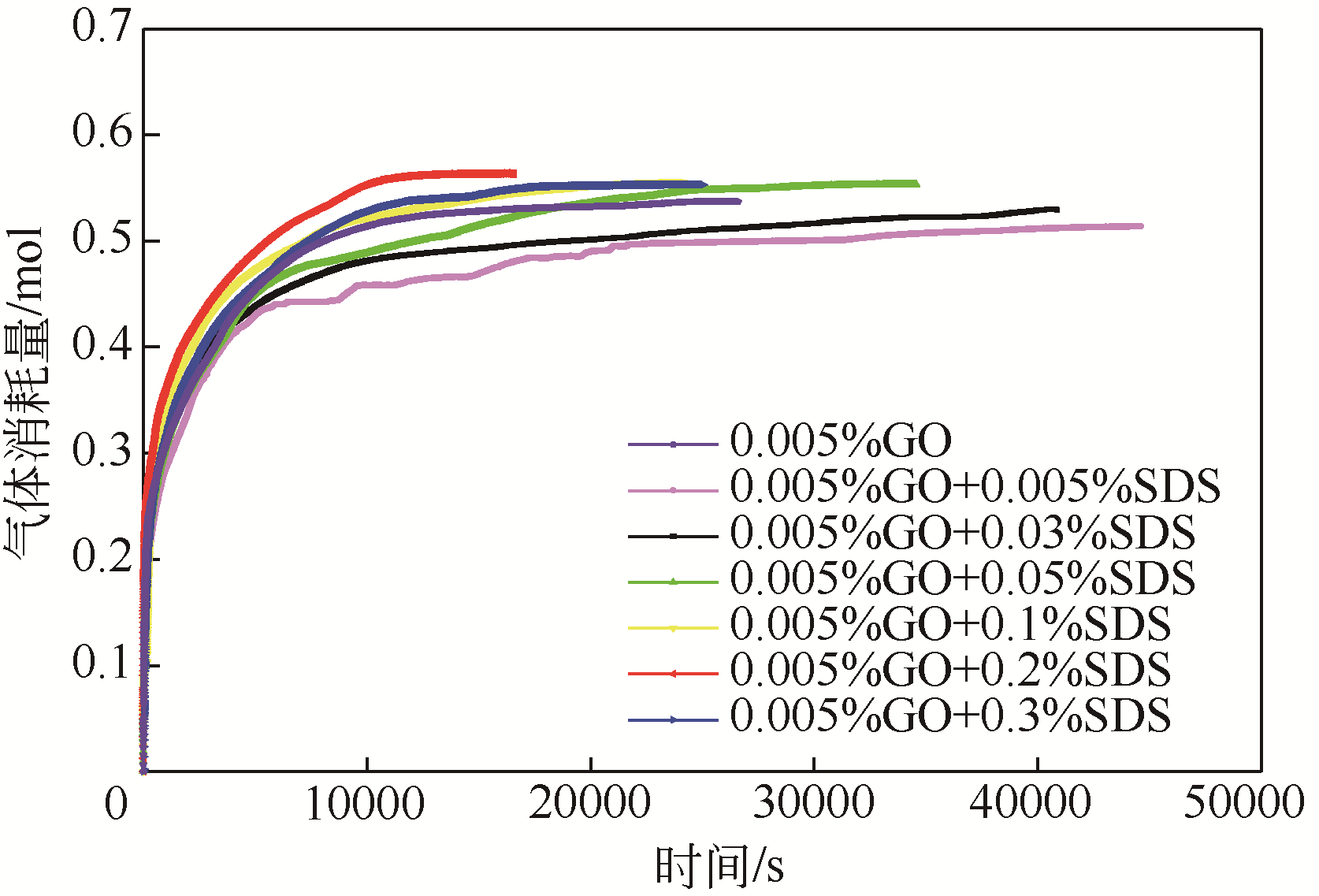
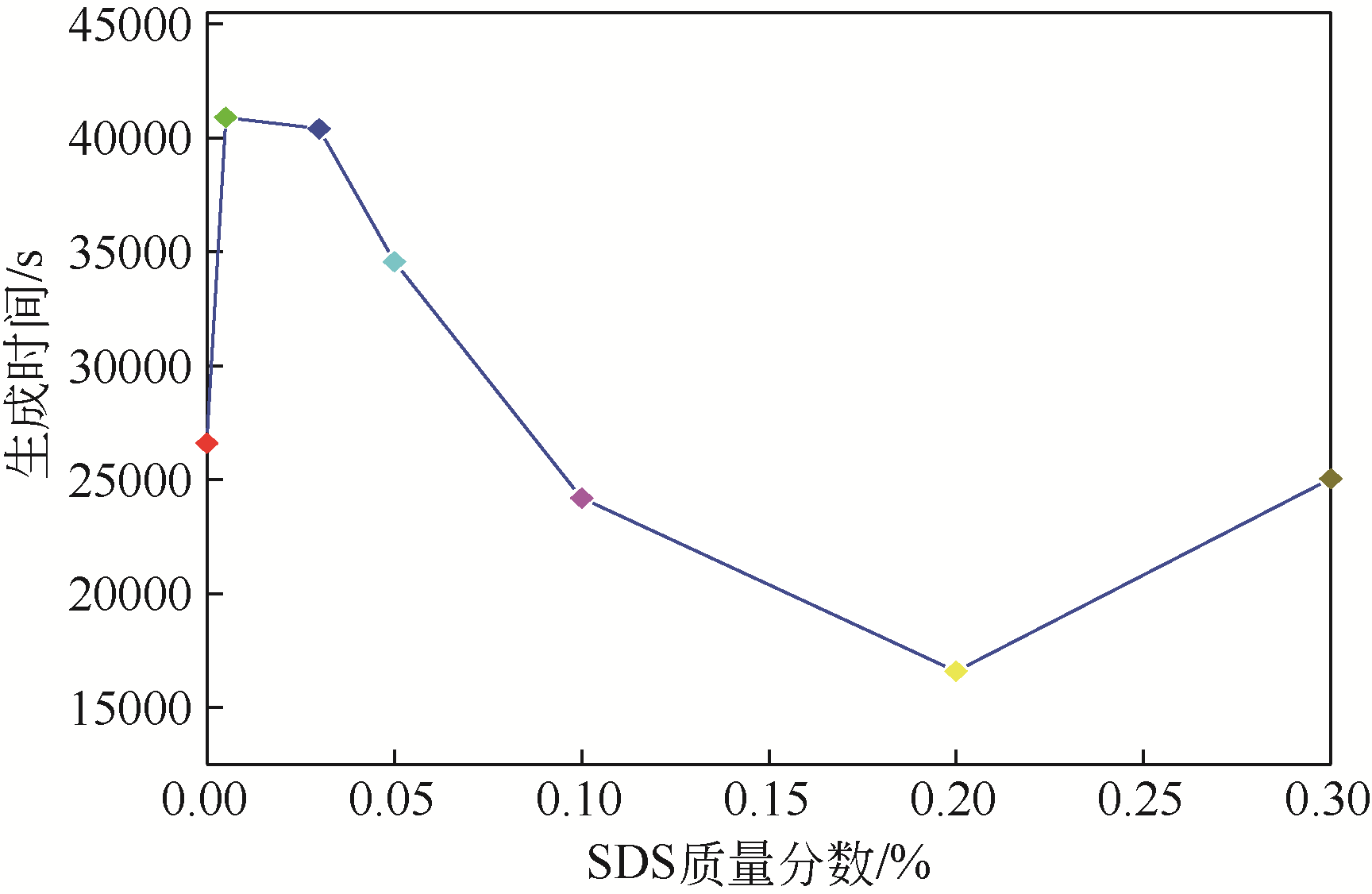
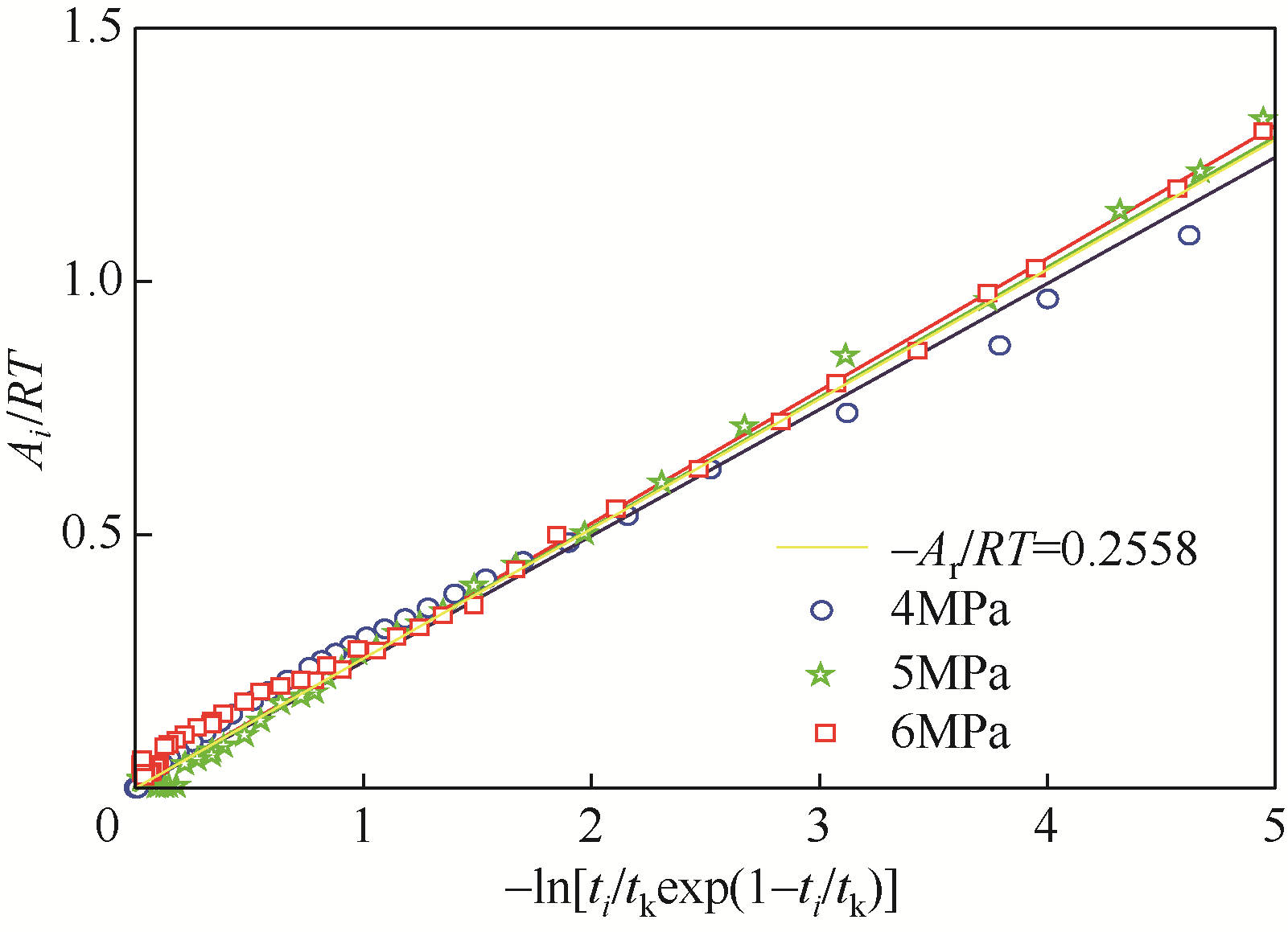
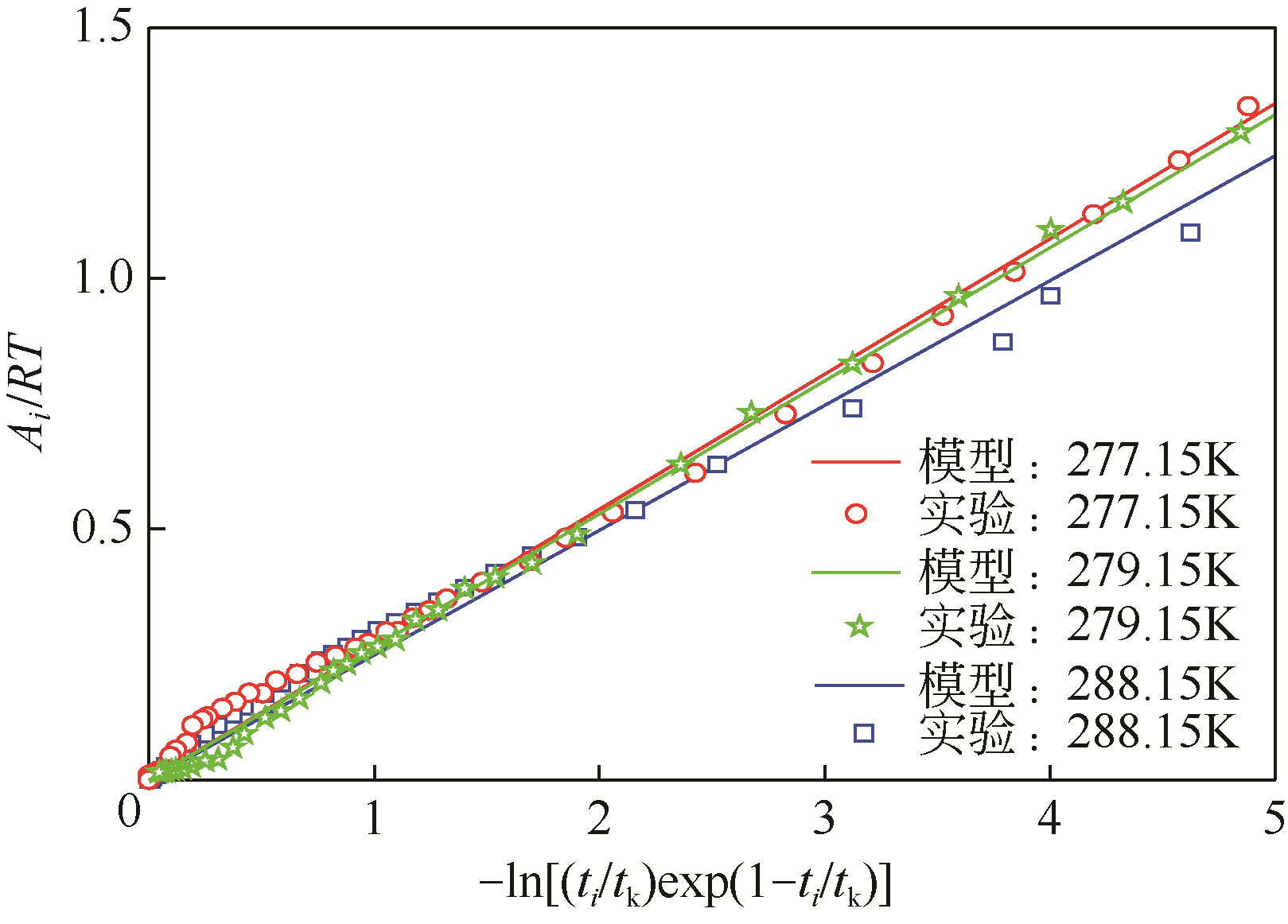
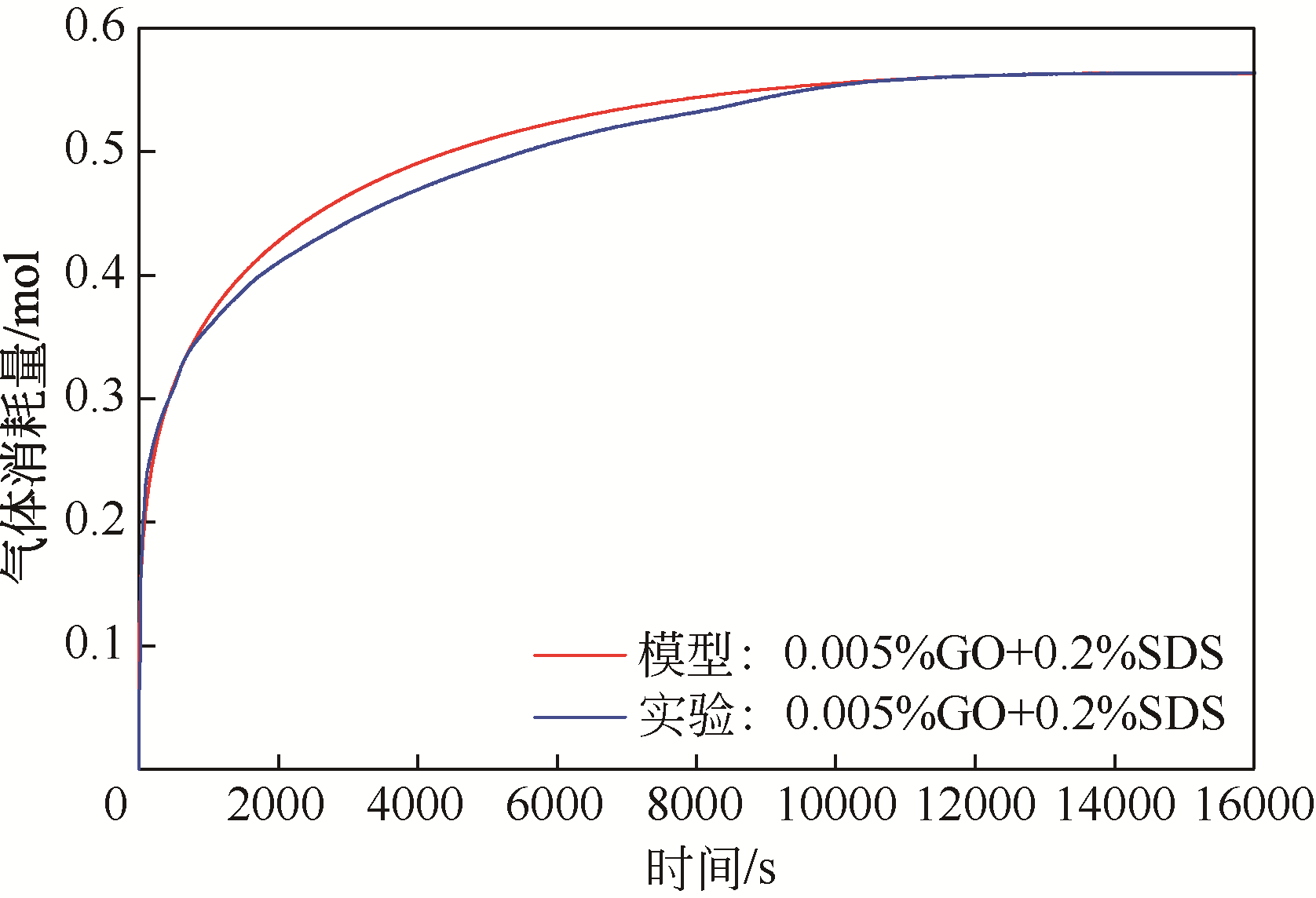
| 1 |
SLOAN E D,CAROLYN C K.Clathrate hydrates of natural gases[M].Boca Raton:Taylor & Francis Group,2008:4-8.
|
| 2 |
HAO W F,WANG J Q,FAN S S,et al.Evaluation and analysis method for natural gas hydrate storage and transportation processes[J].Energy Convers. Manage.,2008,49:2546-2553.
|
| 3 |
ZHANG C S,FAN S S,LIANG D Q,et al.Effect of additives on formation of natural gas hydrate[J].Fuel,2004,83:2115-2121.
|
| 4 |
CHATTI I,DELAHAVE A,FOUMAISON L,et al.Benefits and drawbacks of clathrate hydrates: a review of their areas of interest[J].Energy Convers. Manage.,2005,26:1333-1343.
|
| 5 |
ZHANG W X,WANG Y H,LANG X M,et al.Performance analysis of hydrate-based refrigeration system[J].Energy Convers. Manage.,2017,146:43-51.
|
| 6 |
李士凤,谭哲,申延明,等.水合物溶液分离技术研究进展[J].化工进展,2014,33(6):1387-1391, 1396.
|
|
LI Shifeng,TAN Zhe,SHEN Yanming,et al.Progress in aqueous solution concentration by forming clathrate hydrate[J].Chemical Industry and Engineering Progress,2014,33(6):1387-1391, 1396.
|
| 7 |
PARK S,LEE S,LEE Y,et al.Hydrate-based pre-combustion capture of carbon dioxide in the presence of a thermodynamic promoter and porous silica gels[J].Int.J.Greenh.Gas Con.,2013,14:193-199.
|
| 8 |
施政灼,李玉星,王武昌,等.天然气水合物储运技术中水合物反应器的研究进展[J].化工进展,2018,37(9):3326-3336.
|
|
SHI Zhengzhuo,LI Yuxing,WANG Wuchang,et al.Review of hydrate reactor in natural gas hydrate storage and transportation[J].Chemical Industry and Engineering Progress,2018,37(9):3326-3336.
|
| 9 |
JIANG L L,LI A R,TANG S Y.An experimental study on carbon dioxide hydrate formation using a gas-inducing agitated reactor[J].Energy,2017,134:629-637.
|
| 10 |
LV Q N,LI X S,XU C G,et al.Experimental investigation of the formation of cyclopentane-methane hydrate in a novel and large-size bubble column reactor[J].Ind. Eng. Chem. Res.,2012,51:5967-5975.
|
| 11 |
赵建忠,赵阳升,石定贤.喷射雾化方式下气体水合物生成的实验研究[J].化工进展,2008,27(s1):609-612.
|
|
ZHAO Jianzhong,ZHAO Yangsheng,SHI Dingxian.Experimental study on gas hydrate formation under spray atomization[J].Chemical Industry and Engineering Progress,2008,27(s1):609-612.
|
| 12 |
ROSSI F,FILIPPONI M,CASTELLANI B.Investigation on a novel reactor for gas hydrate production[J].Appl. Energy,2012,99:167-172.
|
| 13 |
JAVANMARDI J,NASRIFAR K,NAJIBI S H,et al.Economic evaluation of natural gas hydrate as an alternative for natural gas transportation[J].Appl. Therm. Eng.,2005,25(11/12):1708-1723.
|
| 14 |
LINGA P,KUMAR R,ENGLEZOS P.The clathrate hydrate process for post and pre-combustion capture of carbon dioxide[J].J. Hazard. Master.,2007,149(3):625-629.
|
| 15 |
梁海峰,朱耀剑,赵阳升,等.水逸度模型预测THF添加剂体系下气体水合物相平衡[J].化工进展,2016,35(3):700-705.
|
|
LIANG Haifeng,ZHU Yaojian,ZHAO Yangsheng,et al.Phase equilibrium study of gas mixtures hydrate formation with additives THF based on water fugacity model[J].Chemical Industry and Engineering Progress,2016,35(3):700-705.
|
| 16 |
POSTERARO D,PASIEKA J,MARIC M,et al.The effect of hydrate promoter SDS on methane dissolution rates at the three phase (H-Lw-V) equilibrium condition[J]. J. Nat. Gas Sci. Eng.,2016,35:1579-1586.
|
| 17 |
WANG F,JIA Z Z,LUO S J,et al.Effects of different anionic surfactants on methane hydrate formation[J].Chem. Eng. Sci.,2015,137:896-903.
|
| 18 |
BAGHBAN A,AHMADI M A,POULADI B,et al.Phase equilibrium modeling of semi-clathrate hydrates of seven commonly gases in the presence of TBAB ionic liquid promoter based on a low parameter connectionist technique[J].J. Supercrit. Fluid,2015,101:184-192.
|
| 19 |
丁家祥,史伶俐,申小冬,等.SDS对甲烷水合物生成动力学和微观结构的影响[J].化工学报,2017,68(12):4802-4808.
|
|
DING Jiaxiang,SHI Linli,SHEN Xiaodong,et al.SDS effect on formation kinetics and microstructure of methane hydrate [J].CIESC Jorunal,2017,68(12):4802-4808.
|
| 20 |
LEE J W,KANG Y T.CO2 absorption enhancement by Al2O3 nanoparticles in NaCl aqueous solution[J].Energy,2013,53:206-211.
|
| 21 |
FOTUKIAN S M,ESFAHANY M N.Experimental study of turbulent convective heat transfer and pressure drop of dilute CuO/water nanofluid inside a circular tube[J].Int. Commun. Heat Mass Tran.,2010,37:214-219.
|
| 22 |
ISRAEL T P,JAE W L,INHWA J,et al.CO2 absorption enhancement by methanol-based Al2O3 and SiO2 nanofluids in a tray column absorber[J].Int. J. Refri.,2012,35:1402-1409.
|
| 23 |
LI D L,PENG H,LIANG D Q.Thermal conductivity enhancement of clathrate hydrate with nanoparticles[J].Int. J. Heat. Mass. Tran.,2017,104:566-573.
|
| 24 |
YAN S,DAI W J,WANG S L,et al.Graphene oxide: an effective promoter for CO2 hydrate formation[J].Energies,2018,11:1756-1769.
|
| 25 |
周诗岽,于雪薇,李青岭,等.纳米石墨颗粒与SDS复配对水合物生成特性的影响[J].天然气化工,2017,42(2):50-53, 118.
|
|
ZHOU Shidong,YU Xuewei,LI Qingling,et al.Effect of graphite nanoparticles and SDS on hydrate formation characteristics[J].Nat. Gas Chem. Ind.,2017,42(2):50-53, 118.
|
| 26 |
CLARKE M A,BISHNOI P R.Determination of the intrinsic kinetics of CO2 gas hydrate formation using in situ particle size analysis[J].Chem. Eng. Sci.,2005,60:695-709.
|
| 27 |
VISNIAUSKAAS A,BISHNOI P R.Kinetics of ethane hydrate formation[J].Chem. Eng. Sci.,1985,40:299-303.
|
| 28 |
ROOSTA H,KHOSHARAY S,VARAMINIAN F.Experimental study of methane hydrate formation kinetics with or without additives and modeling based on chemical affinity[J].Energy Convers. Manage.,2013,76:499-505.
|
| 29 |
KARAMODDIN M,VARAMINIAN F,DARAEE M.Kinetic study on the process of CHClF2 (R22) hydrate formation in the presence of SDS surfactant based on chemical affinity[J]. J. Nat. Gas Sci. Eng.,2014,19:46-51.
|
| 30 |
FAN Shuanshi,LIANG Deqing,GUO Kaihua.Hydrate conditions for cyclopentane and a quaternary cyclopentane—rich mixture[J].Journal of Chemical and Engineering Data,2001,46:930-932.
|
 ),黄俊尧1,闫朔2,饶永超1(
),黄俊尧1,闫朔2,饶永超1( ),贾茹1,刘滨2
),贾茹1,刘滨2
 ),Junyao HUANG1,Shuo YAN2,Yongchao RAO1(
),Junyao HUANG1,Shuo YAN2,Yongchao RAO1( ),Ru JIA1,Bin LIU2
),Ru JIA1,Bin LIU2