化工进展 ›› 2019, Vol. 38 ›› Issue (02): 1075-1084.DOI: 10.16085/j.issn.1000-6613.2018-0885
牛血清白蛋白纳米球接枝杨梅单宁的制备以及吸附去除水体中Pb2+的性能
冯健1( ),余杰1,周建1,2(
),余杰1,周建1,2( ),张永德2,林晓艳2,罗学刚1,2
),张永德2,林晓艳2,罗学刚1,2
- 1. 西南科技大学生命科学与工程学院,四川 绵阳 621010
2. 西南科技大学生物质教育部工程研究中心,四川;绵阳 621010
-
收稿日期:2018-04-28修回日期:2018-06-22出版日期:2019-02-05发布日期:2019-02-05 -
通讯作者:周建 -
作者简介:<named-content content-type="corresp-name">冯健</named-content>(1996—),男,本科生。E-mail:<email>1764503194@qq.com</email>。|周建,副研究员,硕士生导师,研究方向为生物质化学与工程。E-mail:<email>zhoujian@swust.edu.cn</email>。 -
基金资助:国家自然科学基金(21406182);四川省教育厅科研项目(17ZB0443);核废物与环境安全国防重点学科开放基金(15kffk02)
Removal of Pb2+ from aqueous solution using bovine serum albumin nanospheres grafted with bayberry tannin
Jian FENG1( ),Jie YU1,Jian ZHOU1,2(
),Jie YU1,Jian ZHOU1,2( ),Yongde ZHANG2,Xiaoyan LIN2,Xuegang LUO1,2
),Yongde ZHANG2,Xiaoyan LIN2,Xuegang LUO1,2
- 1. School of Life Sciences and Engineering, Southwest University of Science and Technology, Mianyang 621010, Sichuan, China
2. Biomass Engineering Research Center of the Ministry of Education, Southwest University of Science and Technology, Mianyang 621010, Sichuan, China
-
Received:2018-04-28Revised:2018-06-22Online:2019-02-05Published:2019-02-05 -
Contact:Jian ZHOU
摘要:
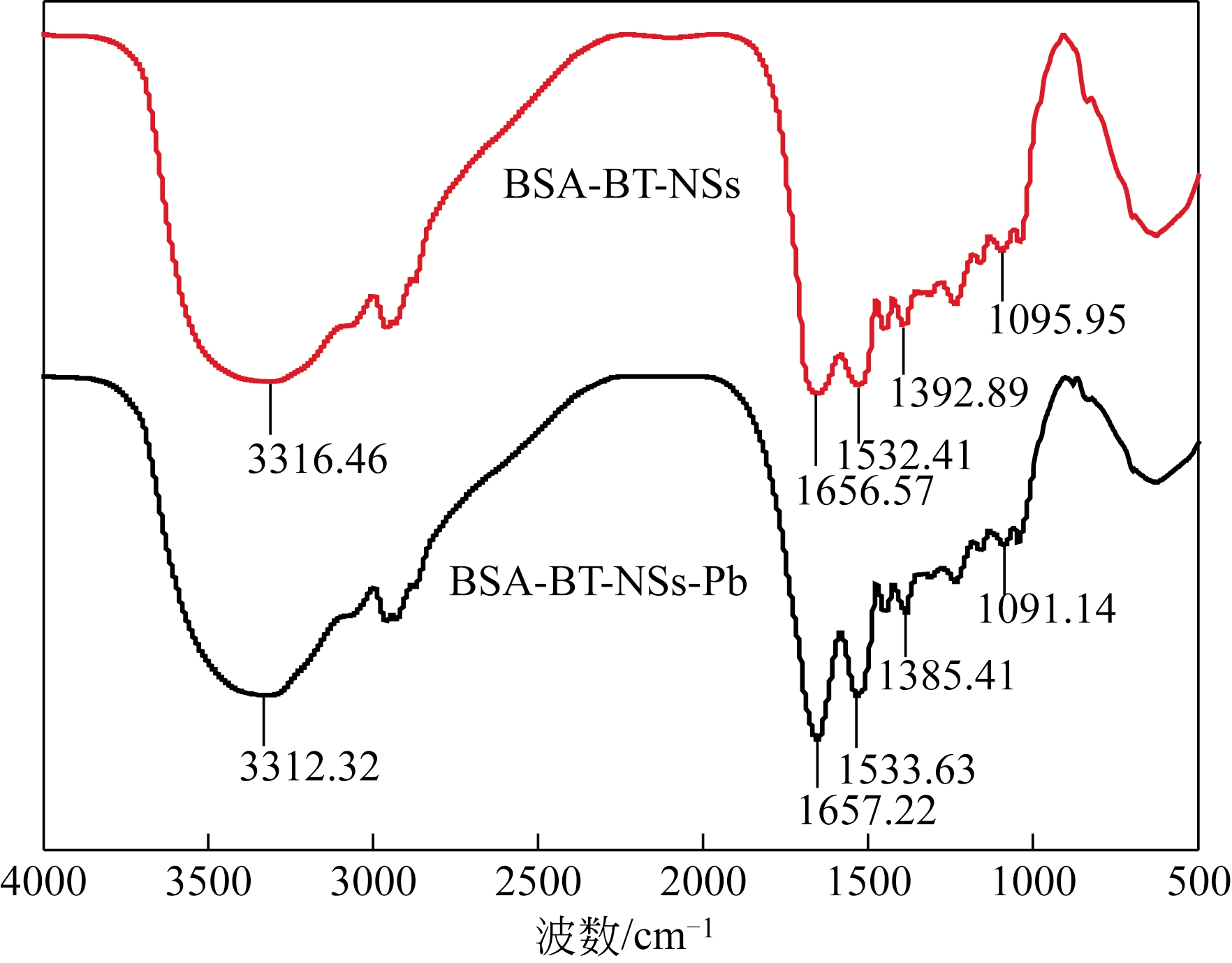
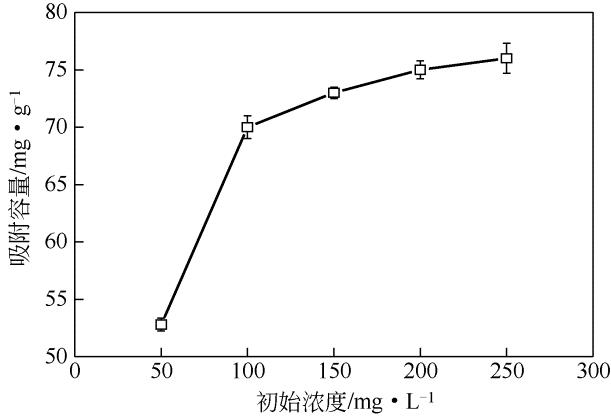
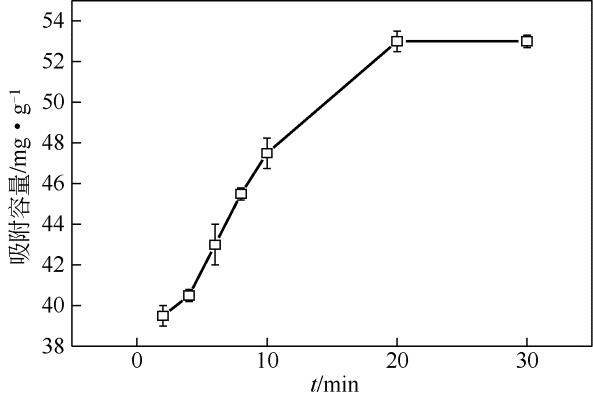
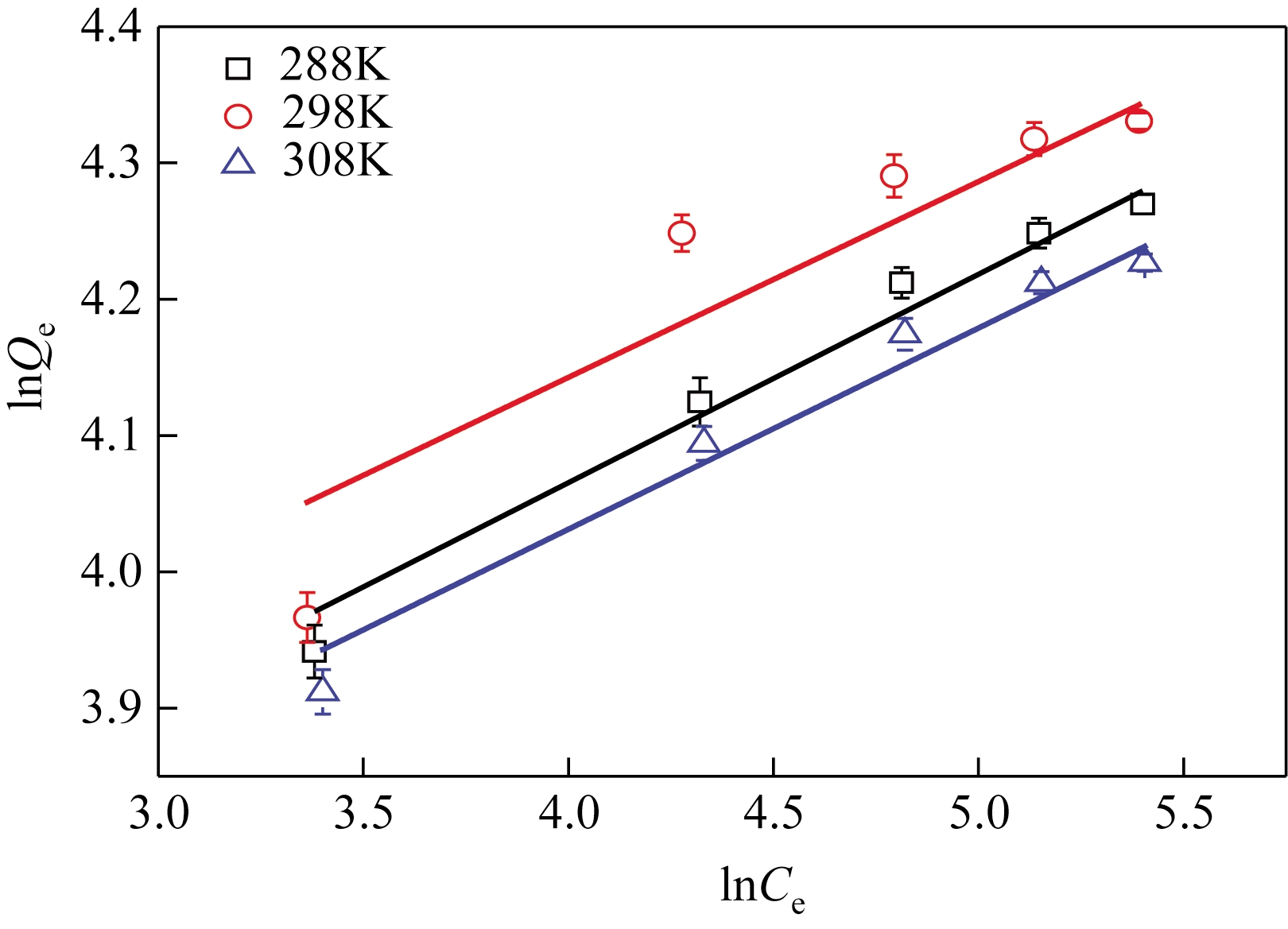
采用去溶剂法和杨梅单宁-戊二醛固化接枝制备得到杨梅单宁(BT)接枝牛血清白蛋白(BSA)纳米球(BSA-BT-NSs)吸附材料,并系统探讨了其在不同吸附条件下对水体中Pb2+的吸附去除性能。研究结果表明:50%用量杨梅单宁(基于BSA-NSs量)接枝固化得到的BSA-BT-NSs具有较好的球形结构和良好的分散性。在吸附实验中,Pb2+初始浓度为250mg/L、pH 5.0、温度为298K 条件下吸附20min,BSA-BT-NSs(0.4g/L)对Pb2+的吸附效果最佳,最大吸附容量为76mg/g,优于多数同类型吸附材料。BSA-BT-NSs对Pb2+吸附过程符合Langmuir方程和准二级吸附动力学模型,且吸附后的BSA-BT-NSs经0.1mol/L 硝酸进行解吸取得了92.04%的良好解吸效果,并可再次重复使用。进一步分析其Pb2+吸附机理,表明BSA-BT-NSs中的氨基氮原子、羟基和羧基氧原子作为电子供体参与了与Pb2+的空轨道发生配位作用。
中图分类号:
引用本文
冯健, 余杰, 周建, 张永德, 林晓艳, 罗学刚. 牛血清白蛋白纳米球接枝杨梅单宁的制备以及吸附去除水体中Pb2+的性能[J]. 化工进展, 2019, 38(02): 1075-1084.
Jian FENG, Jie YU, Jian ZHOU, Yongde ZHANG, Xiaoyan LIN, Xuegang LUO. Removal of Pb2+ from aqueous solution using bovine serum albumin nanospheres grafted with bayberry tannin[J]. Chemical Industry and Engineering Progress, 2019, 38(02): 1075-1084.
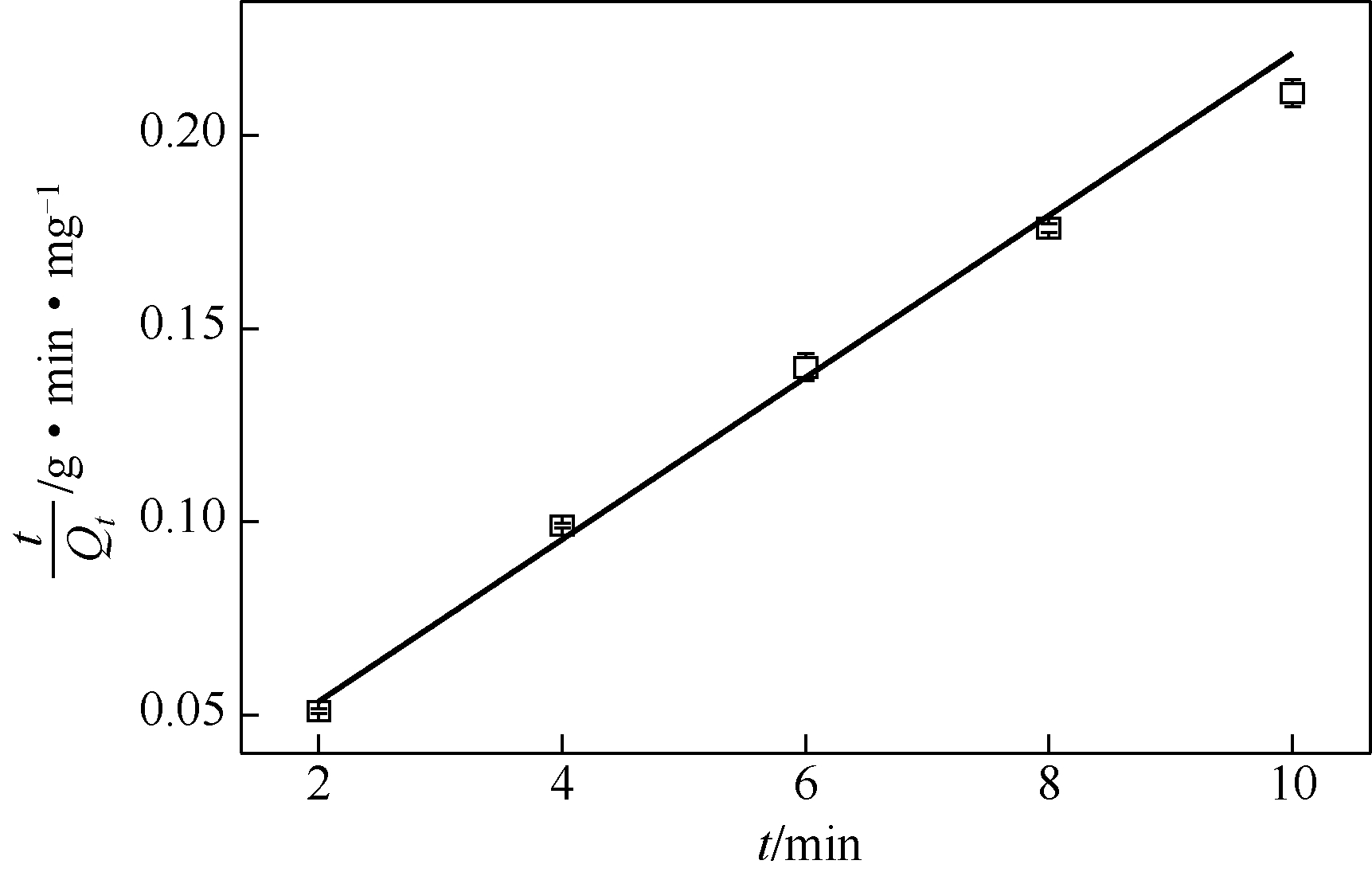
| 吸附动力学模型 | 动力学常数 k | Q e | 相关系数 R 2 |
|---|---|---|---|
| 准一级动力学 | 0.2308 | 18.5780 | 0.9604 |
| 准二级动力学 | 0.0067 | 61.3497 | 0.9954 |
表1 BSA-BT-NSs吸附Pb2+的动力学方程拟合参数
| 吸附动力学模型 | 动力学常数 k | Q e | 相关系数 R 2 |
|---|---|---|---|
| 准一级动力学 | 0.2308 | 18.5780 | 0.9604 |
| 准二级动力学 | 0.0067 | 61.3497 | 0.9954 |
| T/K | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Q m/mg·g-1 | K L/L·mg-1 | R2 | K f | 1/n | R2 | |
| 288 | 76.3359 | 0.0634 | 0.9997 | 29.7521 | 0.1662 | 0.9837 |
| 298 | 80.6452 | 0.0752 | 0.9996 | 30.4717 | 0.1772 | 0.8905 |
| 308 | 72.9927 | 0.0665 | 0.9998 | 29.3297 | 0.1612 | 0.9786 |
表2 Langmuir和Freundlich方程拟合参数
| T/K | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Q m/mg·g-1 | K L/L·mg-1 | R2 | K f | 1/n | R2 | |
| 288 | 76.3359 | 0.0634 | 0.9997 | 29.7521 | 0.1662 | 0.9837 |
| 298 | 80.6452 | 0.0752 | 0.9996 | 30.4717 | 0.1772 | 0.8905 |
| 308 | 72.9927 | 0.0665 | 0.9998 | 29.3297 | 0.1612 | 0.9786 |
| 生物质吸附剂 | pH | 温度/K | Q max/mg·g-1 |
|---|---|---|---|
| 棕榈壳[ | 3.0 | 300 | 95.2 |
| BSA-BT-NSs | 5.0 | 298 | 80.65 |
| 椰子壳[ | 5.6 | 298 | 76.66 |
| 干面包酵母[ | 5.0 | 303 | 60.24 |
| 狐尾藻[ | 5.0 | 298 | 53.87 |
| 菜豆废弃物[ | 5.0 | 293 | 42.77 |
| 大豆皮[ | 5.0 | 296 | 39.37 |
| 松果[ | — | 298 | 27.53 |
| 凤眼莲[ | 4.84 | 303 | 26.32 |
| 杏核[ | 6.5 | 298 | 22.85 |
| 樟子松锯末[ | 5.0 | 298 | 22.22 |
| HNO3处理的橄榄树[ | 5.0 | 298 | 14.15 |
| 茶叶废料[ | 5.8 | 303 | 1.35 |
表3 BSA-BT-NSs与其他生物质材料对Pb2+最大吸附
| 生物质吸附剂 | pH | 温度/K | Q max/mg·g-1 |
|---|---|---|---|
| 棕榈壳[ | 3.0 | 300 | 95.2 |
| BSA-BT-NSs | 5.0 | 298 | 80.65 |
| 椰子壳[ | 5.6 | 298 | 76.66 |
| 干面包酵母[ | 5.0 | 303 | 60.24 |
| 狐尾藻[ | 5.0 | 298 | 53.87 |
| 菜豆废弃物[ | 5.0 | 293 | 42.77 |
| 大豆皮[ | 5.0 | 296 | 39.37 |
| 松果[ | — | 298 | 27.53 |
| 凤眼莲[ | 4.84 | 303 | 26.32 |
| 杏核[ | 6.5 | 298 | 22.85 |
| 樟子松锯末[ | 5.0 | 298 | 22.22 |
| HNO3处理的橄榄树[ | 5.0 | 298 | 14.15 |
| 茶叶废料[ | 5.8 | 303 | 1.35 |
| 合成吸附剂 | pH | 温度 /K | Q max /mg·g-1 |
|---|---|---|---|
| 合成矿物吸附剂[ | 3.0 | 328 | 157.9 |
| 三亚乙基四胺改性聚苯乙烯树脂[ | 6.0 | 353 | 154.71 |
| 改性的磁赤铁矿纳米粒子[ | 6.0 | 298 | 118.51 |
| 磁性Fe3O4 /粉末活性炭复合材料(Fe3O4/PAC)[ | 6.0 | 323 | 94.3 |
| 壳聚糖修饰的MCM-41-A[ | 6.0 | — | 90.91 |
| BSA-BT-NSs | 5.0 | 298 | 80.65 |
| Pb2+离子印迹聚合物(Pb2+-IIP)[ | 6.0 | 288 | 42.55 |
| Pb2+-印迹聚甲基丙烯酸(IIP/PAN)[ | 5.7 | 298 | 41.4 |
| 纳米复合水凝胶(丙烯酸 - 丙烯酰胺)[ | 6.0 | — | 35.94 |
表4 BSA-BT-NSs与其他合成类材料对Pb2+最大吸附
| 合成吸附剂 | pH | 温度 /K | Q max /mg·g-1 |
|---|---|---|---|
| 合成矿物吸附剂[ | 3.0 | 328 | 157.9 |
| 三亚乙基四胺改性聚苯乙烯树脂[ | 6.0 | 353 | 154.71 |
| 改性的磁赤铁矿纳米粒子[ | 6.0 | 298 | 118.51 |
| 磁性Fe3O4 /粉末活性炭复合材料(Fe3O4/PAC)[ | 6.0 | 323 | 94.3 |
| 壳聚糖修饰的MCM-41-A[ | 6.0 | — | 90.91 |
| BSA-BT-NSs | 5.0 | 298 | 80.65 |
| Pb2+离子印迹聚合物(Pb2+-IIP)[ | 6.0 | 288 | 42.55 |
| Pb2+-印迹聚甲基丙烯酸(IIP/PAN)[ | 5.7 | 298 | 41.4 |
| 纳米复合水凝胶(丙烯酸 - 丙烯酰胺)[ | 6.0 | — | 35.94 |
| 1 | LI Z Y , MA Z W, KUIJP T J VAN DER , et al . A review of soil heavy metal pollution from mines in China: pollution and health risk assessment[J]. Science of the Total Environment, 2014, 468: 843-853. |
| 2 | ZHANG X W , YANG L S , LI Y H , et al . Impacts of lead/zinc mining and smelting on the environment and human health in China[J]. Environmental Monitoring and Assessment, 2012, 184(4): 2261-2273. |
| 3 | XU C B , YANG W J , SUN H L , et al . Performance and mechanism of Pb2+ removal by expanded graphite loaded with zero-valent iron[J]. Journal of Inorganic Materials, 2018, 33(1): 41-47. |
| 4 | DIMITROVA SV . Use of granular slag columns for lead removal[J]. Water Research, 2002, 36(16): 4001-4008. |
| 5 | ABOU-SHADY A , PENG C S , BI J J , et al . Recovery of Pb2+ and removal of NO3 - from aqueous solutions using integrated electrodialysis, electrolysis, and adsorption process[J]. Desalination, 2012, 286(4): 304-315. |
| 6 | DEMIRBAS A . Heavy metal adsorption onto agro-based waste materials: a review[J]. Journal of Hazardous Materials, 2008, 157(2/3): 220-229. |
| 7 | RONDA A , DELLA Z M , MARTIN-LARA M A , et al . Combustion of a Pb2+-loaded olive tree pruning used as biosorbent[J]. Journal of Hazardous Materials, 2016, 308: 285-293. |
| 8 | ZHAO D D , YU Y , CHEN J P . Treatment of lead contaminated water by a PVDF membrane that is modified by zirconium, phosphate and PVA[J]. Water Research, 2016, 101: 564-573. |
| 9 | GHERASIM C V , MIKULASEK P . Influence of operating variables on the removal of heavy metal ions from aqueous solutions by nanofiltration[J]. Desalination, 2014, 343(s1): 67-74. |
| 10 | CAO X D , MA L N, GAO B , et al . Dairy-manure derived biochar effectively sorbs lead and atrazine[J]. Environmental Science & Technology, 2009, 43(9): 3285-3291. |
| 11 | REN X M , SHAO D D , YANG S T , et al . Comparative study of Pb2+ sorption on XC-72 carbon and multi-walled carbon nanotubes from aqueous solutions[J]. Chemical Engineering Journal, 2011, 170(1): 170-177. |
| 12 | SHARIFPOUR E , KHAFRI H Z , GHAEDI M , et al . Isotherms and kinetic study of ultrasound-assisted adsorption of malachite green and Pb2+ from aqueous samples by copper sulfide nanorods loaded on activated carbon: experimental design optimization[J]. Ultrasonics Sonochemistry, 2018, 40: 373-382. |
| 13 | BRADL H B . Adsorption of heavy metal ions on soils and soils constituents[J]. Journal of Colloid and Interface Science, 2004, 277(1): 1-18. |
| 14 | MOHAMMAD A M , ELDIN T A S , HASSAN M A , et al . Efficient treatment of lead-containing wastewater by hydroxyapatite/chitosan nanostructures[J]. Arabian Journal of Chemistry, 2017, 10(5): 683-690. |
| 15 | 马贺伟,廖学品,石碧 .皮胶原固化单宁膜材料的制备及其对U(Ⅵ)的吸附研究[J]. 膜科学与技术, 2005, 25(2): 20-24. |
| MA H W, LIAO X P , SHI B .Preparation of collagen immobilized tannin membrane and its adsorption to U6+ [J].Membrane Science and Technology, 2005, 25(2):20-24. | |
| 16 | 霍子微 . 纳米纤维素接枝超支化聚酰胺交联增强单宁胶黏剂研究[D].南京:南京林业大学, 2017. |
| HUO Z W .Crosslinking and reinfocement of tannic adhesive by cellulose nanofibrils grafted with hyperbranched polyamides abstract [D]. Nanjing:Nanjing Forestry University,2017. | |
| 17 | 廖学品, 邓慧, 陆忠兵, 等 . 胶原纤维固化单宁及其对Cu2+的吸附[J]. 林产化学与工业, 2003(4): 11-16. |
| LIAO X P , DENG H , LU Z B ,et al .Collagen immobilized tannins and their adsorption for Cu2+ [J]. Chemistry and Industry of Forest Products, 2003(4): 11-16. | |
| 18 | 何春光, 廖洋, 廖学品, 等 . 胶原纤维固化杨梅单宁对Pr3+, Nd3+的吸附特性[J]. 稀有金属材料与工程, 2006(12):1928-1931. |
| HE C G , LIAO Y , LIAO X P , et al . Study on adsorption property of rare earth elements by myrtan tannins immodilized on collagen fiber[J].Rare Metal Materials and Engineering, 2006(12): 1928-1931. | |
| 19 | LI X H , XU H X , GAO B , et al . Efficient biosorption of Pb2+ from aqueous solutions by a PAH-degrading strain Herbaspirillum chlorophenolicum FA1[J]. Journal of Industrial and Engineering Chemistry, 2018, 57: 64-71. |
| 20 | SHAHBAZI A , YOUNESI H , BADIEI A . Functionalized SBA-15 mesoporous silica by melamine-based dendrimer amines for adsorptive characteristics of Pb2+, Cu2+ and Cd2+ heavy metal ions in batch and fixed bed column[J]. Chemical Engineering Journal, 2011, 168(2): 505-518. |
| 21 | 张景 . 复合MnO2-Fe3O4吸附水中重金属及其循环利用的研究[D].上海:上海大学, 2016. |
| ZHANG J .Compound MnO2-Fe3O4 adsorption of heavy metals from aqueous solutions and its research of recycling[D]. Shanghai: Shanghai University, 2016. | |
| 22 | DEMEY H , VINCENT T , GUIBAL E . A novel algal-based sorbent for heavy metal removal[J]. Chemical Engineering Journal , 2018, 332: 582-595. |
| 23 | LI D D , XU X J , YU H W , et al . Characterization of Pb2+ biosorption by psychrotrophic strain Pseudomonas sp. I3 isolated from permafrost soil of Mohe wetland in Northeast China[J]. Journal of Environmental Management, 2017, 196: 8-15. |
| 24 | HUANG J , YE M , QU Y Q , et al . Pb2+ removal from aqueous media by EDTA-modified mesoporous silica SBA-15[J]. Journal of Colloid and Interface Science, 2012, 385: 137-146. |
| 25 | DENG S B , TING Y P . Characterization of PEI-modified biomass and biosorption of Cu2+ , Pb2+ and Ni2+ [J]. Water Research, 2005, 39(10): 2167-2177. |
| 26 | RAMRAKHIANI L , HALDER A , MAJUMDER A , et al . Industrial waste derived biosorbent for toxic metal remediation: mechanism studies and spent biosorbent management[J]. Chemical Engineering Journal , 2017, 308: 1048-1064. |
| 27 | PAP S, RADONIC J , TRIFUNOVIC S , et al . Evaluation of the adsorption potential of eco-friendly activated carbon prepared from cherry kernels for the removal of Pb2+, Cd2+ and Ni2+ from aqueous wastes[J]. Journal of Environmental Management, 2016, 184: 297-306. |
| 28 | WANG B , WEN J L , SUN S L , et al . Chemosynthesis and structural characterization of a novel lignin-based bio-sorbent and its strong adsorption for Pb2+ [J]. Industrial Crops and Products, 2017, 108: |
| 72-80. | |
| 29 | RAO M M , RAO G P C , SESHAIAH K , et al . Activated carbon from Ceiba pentandra hulls, an agricultural waste, as an adsorbent in the removal of lead and zinc from aqueous solutions[J]. Waste Management, 2008, 28(5): 849-858. |
| 30 | LALHRUAITLUANGA H , JAYARAM K , PRASAD M N V , et al . Pb2+ adsorption from aqueous solutions by raw and activated charcoals of Melocanna baccifera Roxburgh (bamboo): a comparative study[J]. Journal of Hazardous Materials, 2010, 175(1/2/3): 311-318. |
| 31 | OLU-OWOLABI B I , DIAGBOYA P N , UNUABONAH E I , et al . Fractal-like concepts for evaluation of toxic metals adsorption efficiency of feldspar-biomass composites[J]. Journal of Cleaner Production , 2018, 171: 884-891. |
| 32 | WANG G Y , ZHANG S R , YAO P , et al . Removal of Pb2+ from aqueous solutions by Phytolacca americana L. biomass as a low cost biosorbent[J]. Arabian Journal of Chemistry, 2018, 11(1): 99-110. |
| 33 | KALINKE C , MANGRICH A S , MARCOLINO-JUNIOR L H , et al . Biochar prepared from castor oil cake at different temperatures: a voltammetric study applied for Pb2+, Cd2+ and Cu2+ ions preconcentration[J]. Journal of Hazardous Materials, 2016, 318: 526-532. |
| 34 | JI Z H , FENG C L , WU X F , et al . Composite of biomass and lead resistent Aspergillus oryzae for highly efficient aqueous phase Pb2+ adsorption[J]. Environmental Progress & Sustainable Energy , 2017, 36(6): 1658-1666. |
| 35 | PARLAYICI S , PEHLIVAN E . Removal of metals by Fe3O4 loaded activated carbon prepared from plum stone (Prunus nigra): kinetics and modelling study[J]. Powder Technology, 2017, 317: 23-30. |
| 36 | ISSABAYEVA G , AROUA M K , SULAIMAN N M N . Removal of lead from aqueous solutions on palm shell activated carbon[J]. Bioresource Technology, 2006, 97(18): 2350-2355. |
| 37 | KIKUCHI Y , QING Q , MACHIDA M, et al . Effect of ZnO loading to activated carbon on Pb2+ adsorption from aqueous solution[J]. Carbon, 2006, 44(2): 195-202. |
| 38 | GOKSUNGUR Y , UREN S , GUVENC U . Biosorption of cadmium and lead ions by ethanol treated waste baker’s yeast biomass[J]. Bioresource Technology , 2005, 96(1): 103-109. |
| 39 | YAN C Z , LI G X , XUE P Y , et al . Competitive effect of Cu2+ and Zn2+ on the biosorption of Pb2+ by Myriophyllum spicatum [J]. Journal of Hazardous Materials, 2010, 179(1/2/3): 721-728 |
| 40 | OZCAN A S , TUNALI S , AKAR T , et al . Biosorption of Pb2+ onto waste biomass of Phaseolus vulgaris L.: estimation of the equilibrium, kinetic and thermodynamic parameters[J]. Desalination, 2009, 244(1/2/3): |
| 188-198. | |
| 41 | JOHNS M M , MARSHALL W E , TOLES C A . Agricultural by-products as granular activated carbons for adsorbing dissolved metals and organics[J]. J. Chem. Technol. Biotechnol., 1998, 71(2): 131-140. |
| 42 | MOMCILOVIC M , PURENOVIC M , BOJIC A , et al . Removal of Pb2+ from aqueous solutions by adsorption onto pine cone activated carbon[J]. Desalination , 2011, 276(1/2/3): 53-59. |
| 43 | MAHAMADI C , NHARINGO T . Competitive adsorption of Pb2+, Cd2+ and Zn2+ ions onto Eichhornia crassipes in binary and ternary systems[J]. Bioresource Technology , 2010, 101(3): 859-864. |
| 44 | KOBYA M , DEMIRBAS E , SENTURK E , et al . Adsorption of heavy metal ions from aqueous solutions by activated carbon prepared from apricot stone[J]. Bioresource Technology, 2005, 96(13): 1518-1521. |
| 45 | TATY C V , FAUDUET H , PORTE C , et al . Removal of Cd2+ and Pb2+, from aqueous solutions, by adsorption onto sawdust of Pinus sylvestris [J]. Journal of Hazardous Materials , 2003, 105(1/2/3): 121-142. |
| 46 | RONDA A , MARTIN-LARA M A , CALERO M , et al . Analysis of the kinetics of lead biosorption using native and chemically treated olive tree pruning[J]. Ecological Engineering , 2013, 5: 278-285. |
| 47 | MONDAL M K . Removal of Pb2+ from aqueous solution by adsorption using activated tea waste[J]. Korean Journal of Chemical Engineering, 2010, 27(1): 144-151. |
| 48 | CHEN G N , SHAH K J , SHI L , et al . Removal of Cd2+ and Pb2+ from aqueous solutions by synthetic mineral adsorbent: performance and mechanisms[J]. Applied Surface Science , 2017, 409: 296-305. |
| 49 | XIONG C H , YAO C P . Synthesis,characterization and application of triethylenetetramine modified polystyrene resin in removal of mercury, cadmium and lead from aqueous solutions[J]. Chemical Engineering Journal , 2009, 155(3): 844-850. |
| 50 | MADRAKIAN T , AFKHAMI A , ZADPOUR B , et al . New synthetic mercaptoethylamino homopolymer-modified maghemite nanoparticles for effective removal of some heavy metal ions from aqueous solution[J]. Journal of Industrial and Engineering Chemistry, 2015, 21: 1160-1166. |
| 51 | KAKAVANDI B , JAFARI A J , KALANTARY R R , et al . Simultaneous adsorption of lead and aniline onto magnetically recoverable carbon: optimization, modeling and mechanism[J]. Journal of Chemical Technology and Biotechnology, 2016, 91(12): 3000-3010. |
| 52 | GUO Y G , LIU D F , ZHAO Y , et al . Synthesis of chitosan-functionalized MCM-41-A and its performance in Pb2+ removal from synthetic water[J]. Journal of The Taiwan Institute of Chemical Engineers, 2017, 71: 537-545. |
| 53 | LIU Y , LIU Z C , GAO J , et al . Selective adsorption behavior of Pb2+ by mesoporous silica SBA-15-supported Pb2+-imprinted polymer based on surface molecularly imprinting technique[J]. Journal of Hazardous Materials, 2011, 186(1): 197-205. |
| 54 | BASAGLIA A M , CORAZZA M Z , SEGATELLI M G , et al . Synthesis of Pb2+-imprinted poly(methacrylic acid) polymeric particles loaded with |
| 1-(2-pyridylazo)-2naphthol (PAN) for micro-solid phase preconcentration of Pb2+ on-line coupled to flame atomic absorption spectrometry[J]. RSC Advances, 2017, 7(52): 33001-33011. | |
| 55 | LIU P , JIANG L P , ZHU L X , et al . Synthesis of covalently crosslinked attapulgite/poly (acrylic acid-co-acrylamide) nanocomposite hydrogels and their evaluation as adsorbent for heavy metal ions[J]. Journal of Industrial and Engineering Chemistry, 2015, 23: 188-193. |
| 56 | MOROSANU I , TEODOSIU C , PADURARU C , et al . Biosorption of lead ions from aqueous effluents by rapeseed biomass[J]. New Biotechnology, 2017, 39: 110-124. |
| 57 | VARSHNEY S , JAIN P , SRIVASTAVA S . Application of ameliorated wood pulp to recover Cd2+, Pb2+, and Ni2+ from e-waste[J]. Journal of Material Cycles and Waste Management, 2017, 19(4): 1446-1456. |
| 58 | ZHOU N , CHEN H G , XI J T , et al . Biochars with excellent Pb2+ adsorption property produced from fresh and dehydrated banana peels via hydrothermal carbonization[J]. Bioresource Technology, 2017, 232: 204-210. |
| [1] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [2] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [3] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [4] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [5] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [6] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [7] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [8] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [9] | 姜晶, 陈霄宇, 张瑞妍, 盛光遥. 载锰生物炭制备及其在环境修复中应用研究进展[J]. 化工进展, 2023, 42(8): 4385-4397. |
| [10] | 张振, 李丹, 陈辰, 吴菁岚, 应汉杰, 乔浩. 吸附树脂对唾液酸的分离纯化[J]. 化工进展, 2023, 42(8): 4153-4158. |
| [11] | 于静文, 宋璐娜, 刘砚超, 吕瑞东, 武蒙蒙, 冯宇, 李忠, 米杰. 一种吲哚基超交联聚合物In-HCP对水中碘的吸附作用[J]. 化工进展, 2023, 42(7): 3674-3683. |
| [12] | 李艳玲, 卓振, 池亮, 陈曦, 孙堂磊, 刘鹏, 雷廷宙. 氮掺杂生物炭的制备与应用研究进展[J]. 化工进展, 2023, 42(7): 3720-3735. |
| [13] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| [14] | 张雪伟, 黄亚继, 许月阳, 程好强, 朱志成, 李金壘, 丁雪宇, 王圣, 张荣初. 碱性吸附剂对燃煤烟气中SO3的吸附特性[J]. 化工进展, 2023, 42(7): 3855-3864. |
| [15] | 陆洋, 周劲松, 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛. CeO2/TiO2吸附剂煤气脱汞产物的浸出规律[J]. 化工进展, 2023, 42(7): 3875-3883. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||