化工进展 ›› 2025, Vol. 44 ›› Issue (11): 6301-6315.DOI: 10.16085/j.issn.1000-6613.2024-1560
• 工业催化 • 上一篇
用于电化学氨氧化的催化剂研究进展
- 宿迁联盛科技股份有限公司,江苏 宿迁 223809
-
收稿日期:2024-09-25修回日期:2025-03-31出版日期:2025-11-25发布日期:2025-12-08 -
作者简介:韩炎(1993—),男,硕士,工程师,研究方向为有机电化学合成。E-mail:1047522239@qq.com
胡新利(1986—),男,硕士,高级工程师,研究方向为有机电化学。E-mail:huxinli2006@126.com
郑晓芹(1998—),女,硕士,研究方向为有机电化学。E-mail:zhengxiaoqinnjt@163.com。
第一联系人:所有作者对本文贡献相同。
Advances in catalysts for electrochemical ammonia oxidation
HAN Yan( ), HU Xinli(
), HU Xinli( ), ZHENG Xiaoqin(
), ZHENG Xiaoqin( )
)
- Suqian Unitech Co. , Ltd. , Suqian 223809, Jiangsu, China
-
Received:2024-09-25Revised:2025-03-31Online:2025-11-25Published:2025-12-08
摘要:
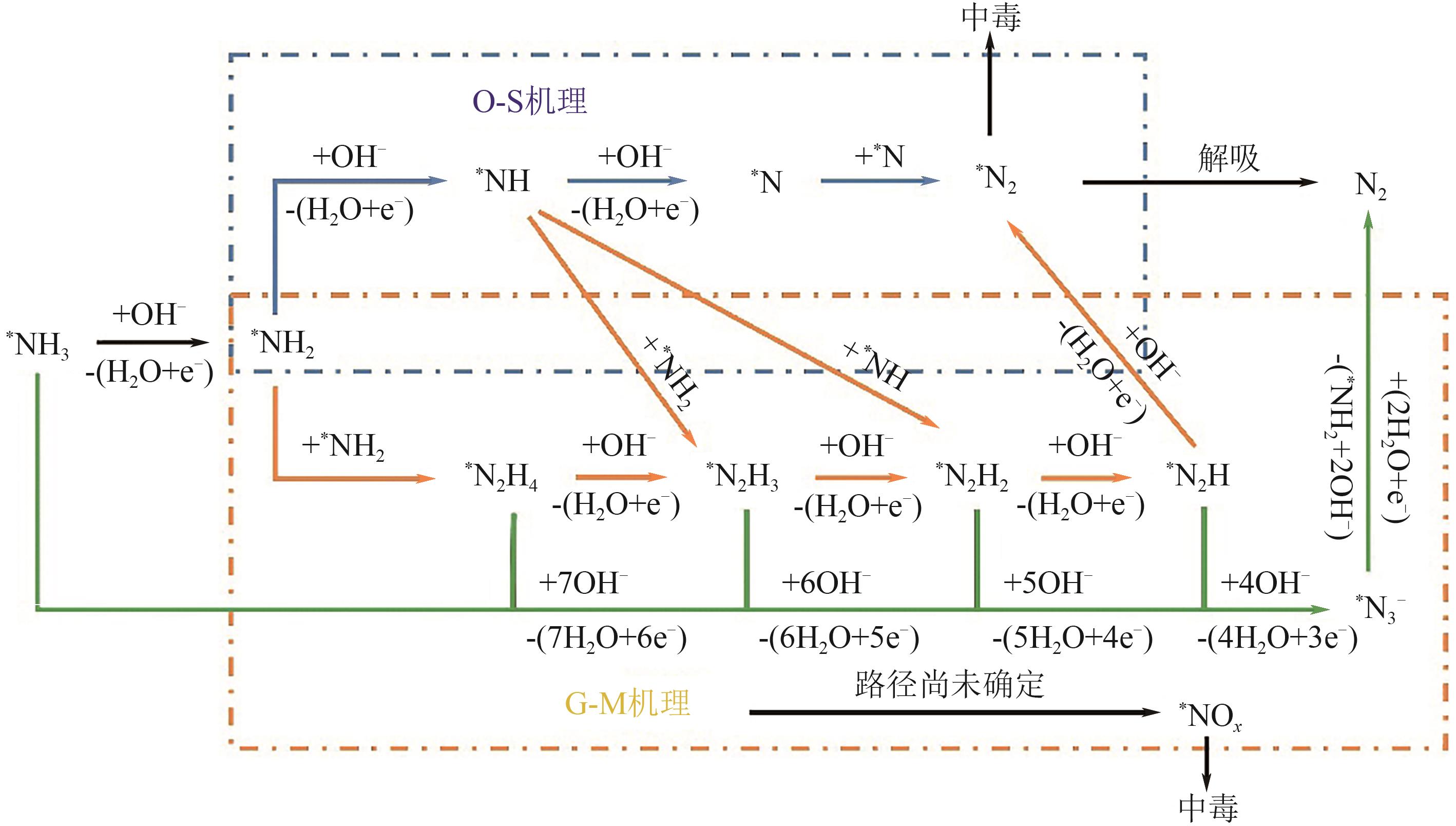
电催化氨氧化反应(eAOR)在清洁能源转化与废水脱氮领域具有重要应用,但其高过电位、动力学缓慢及催化剂中毒等问题制约了技术发展。本文系统梳理了eAOR机理研究进展,聚焦于铂基与镍基催化剂的改性策略与性能优化机制。铂基催化剂通过晶面工程、多元合金化及纳米结构调控可优化中间体吸附能,降低反应能垒并抑制表面毒化;镍基催化剂以低成本、高稳定性为优势,其活性源于表面氧化态的原位重构与双金属协同效应。尽管研究在活性与选择性上取得突破,但贵金属依赖性、N2选择性不足及反应路径争议仍是瓶颈。未来需着力开发非贵金属催化体系,结合原位光谱与理论计算揭示动态反应路径,推动eAOR在制氢、燃料电池及废水处理中的规模化应用。
中图分类号:
引用本文
韩炎, 胡新利, 郑晓芹. 用于电化学氨氧化的催化剂研究进展[J]. 化工进展, 2025, 44(11): 6301-6315.
HAN Yan, HU Xinli, ZHENG Xiaoqin. Advances in catalysts for electrochemical ammonia oxidation[J]. Chemical Industry and Engineering Progress, 2025, 44(11): 6301-6315.
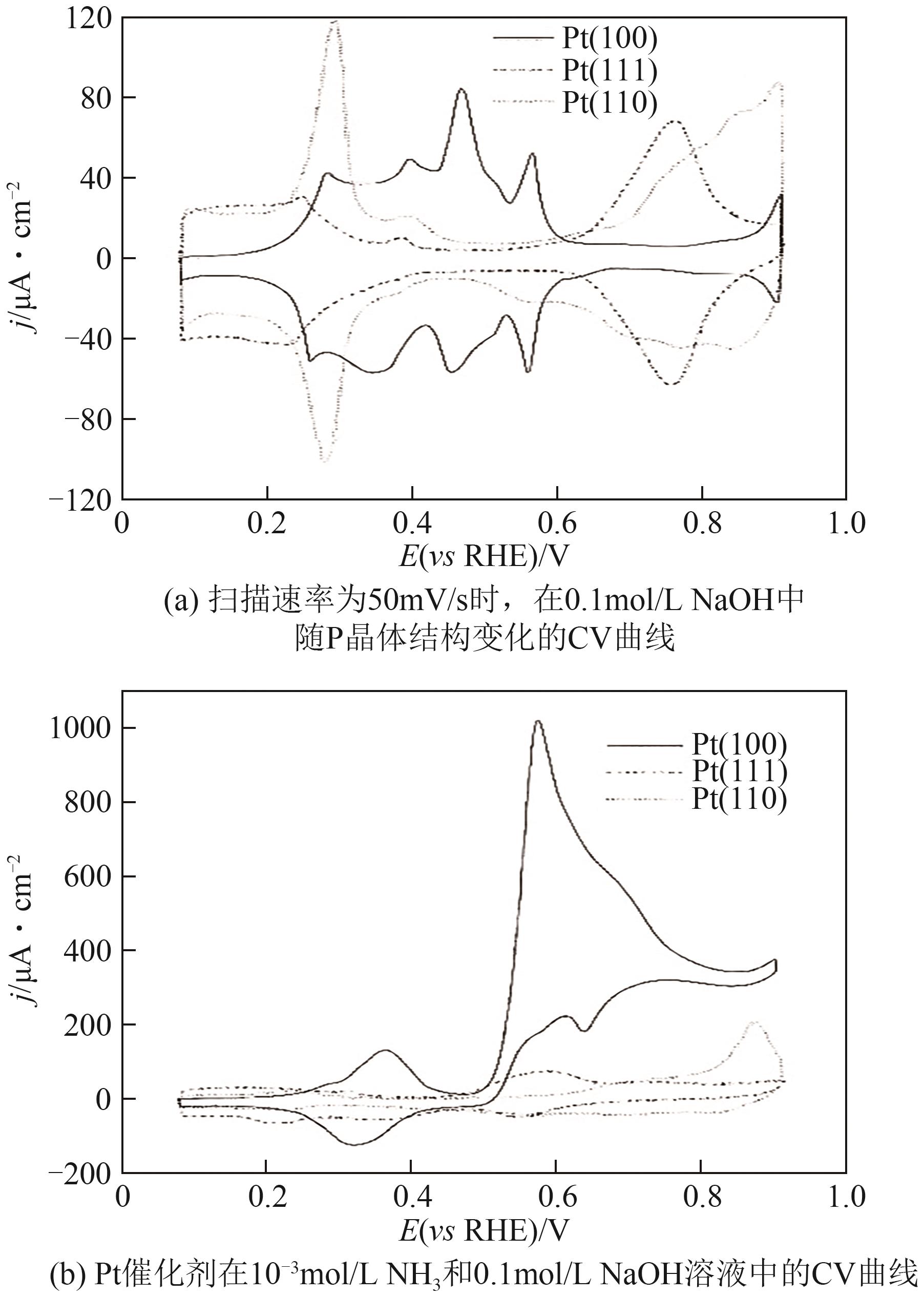
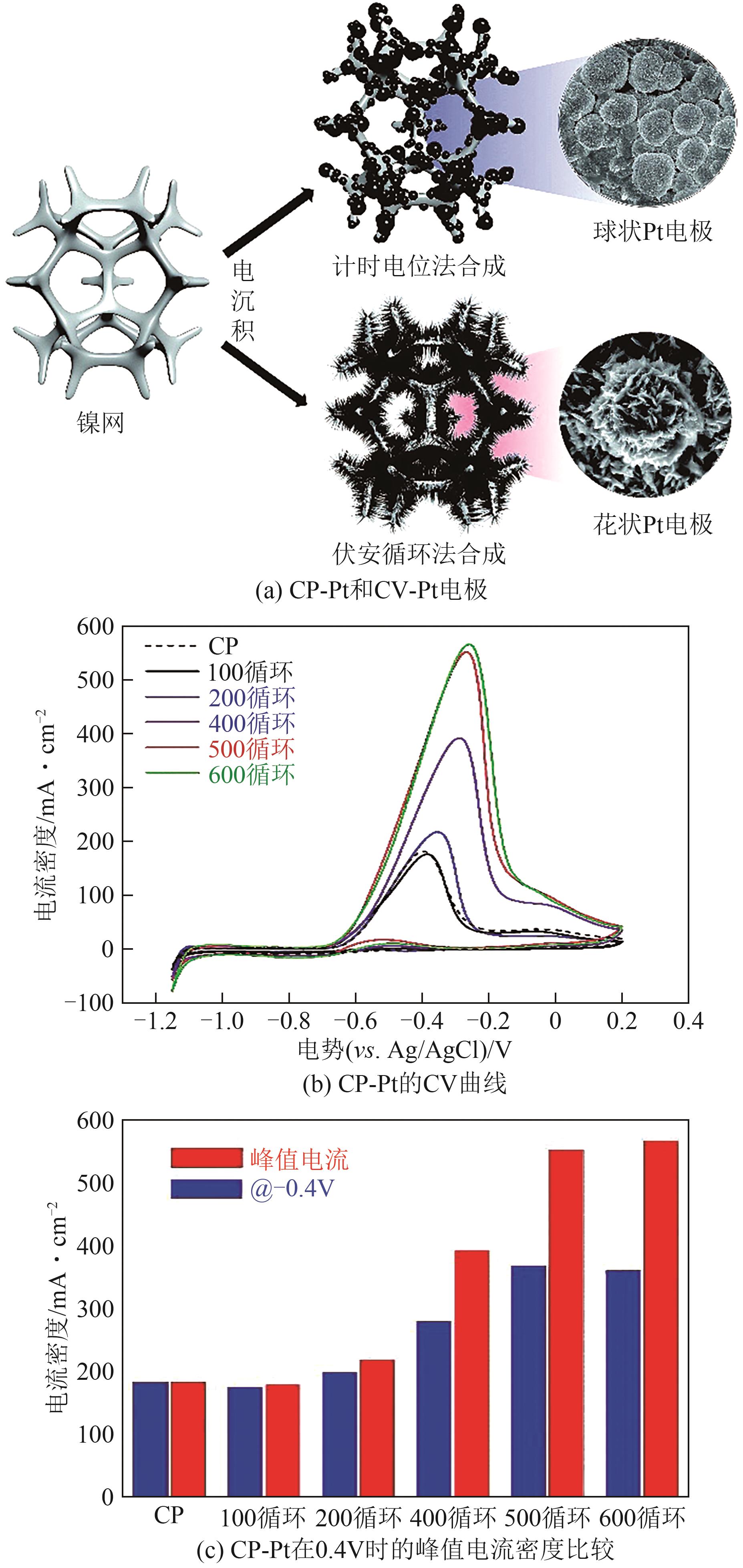
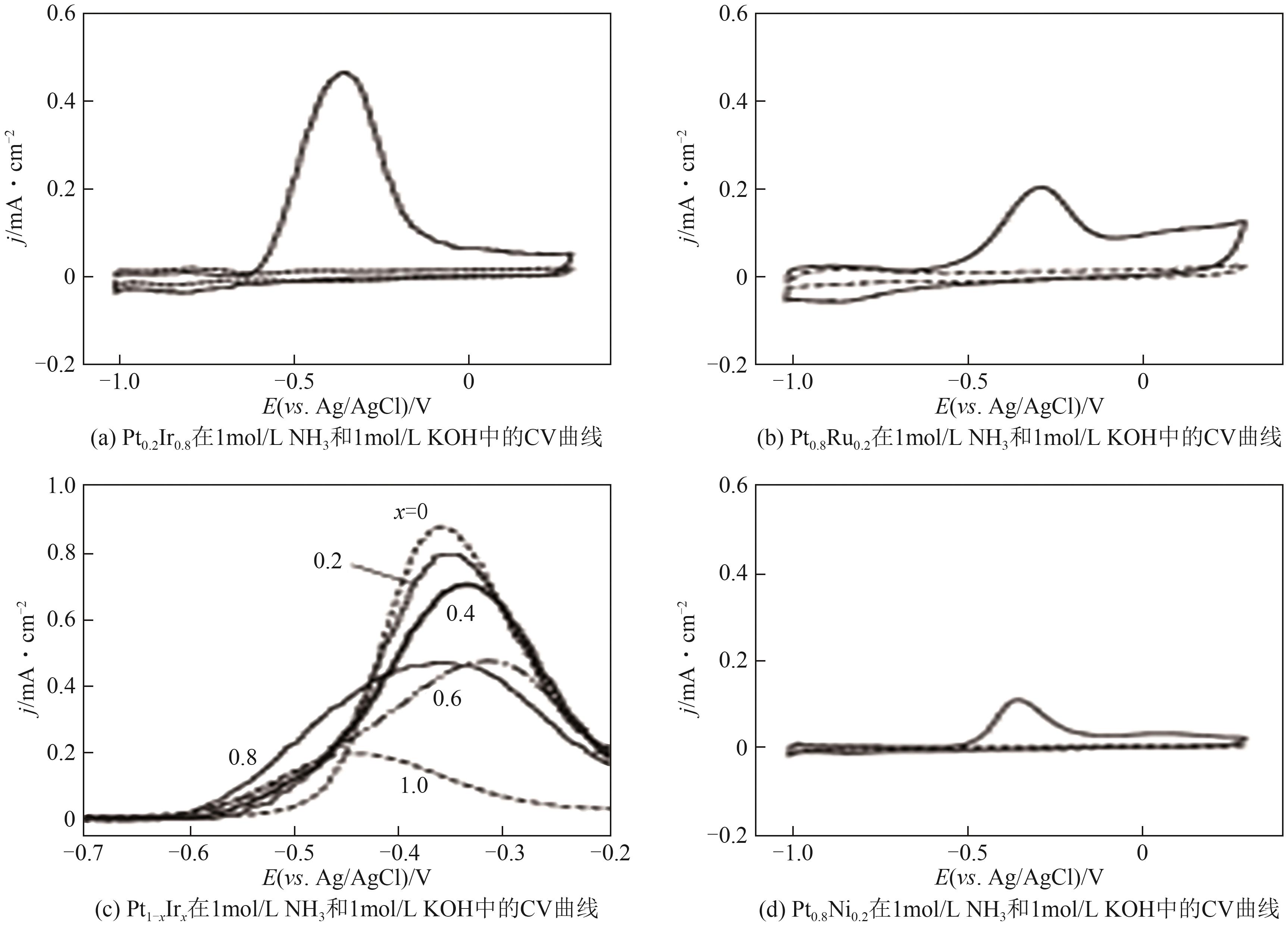
| 催化剂 | 电解液 | 起始电位(相对于RHE)/V | 峰值电流密度/mA·cm-2 | 参考文献 |
|---|---|---|---|---|
| 500CV-Pt | 1mol/L NH3+5mol/L KOH | 0.42 | 0.27 | [ |
| Pt纳米片 | 0.1mol/L NH3+1mol/L KOH | 0.57 | 0.32 | [ |
| PtZn | 0.1mol/L NH4OH+0.5mol/L KOH | 0.42 | 0.6 | [ |
| Pt薄膜 | 0.1mol/L NH3+0.2mol/L NaOH | 0.5 | 0.212 | [ |
| 花状Pt | 0.1mol/L NH3+1mol/L KOH | 0.5 | 0.48 | [ |
| Pt纳米立方体(Pt-NC) | 0.1mol/L NH4OH+1mol/L KOH | 0.5 | 5.1 | [ |
| Pt纳米颗粒(Pt NP) | 0.1mol/L NH3+0.2mol/L NaOH | 0.55 | 1.96 | [ |
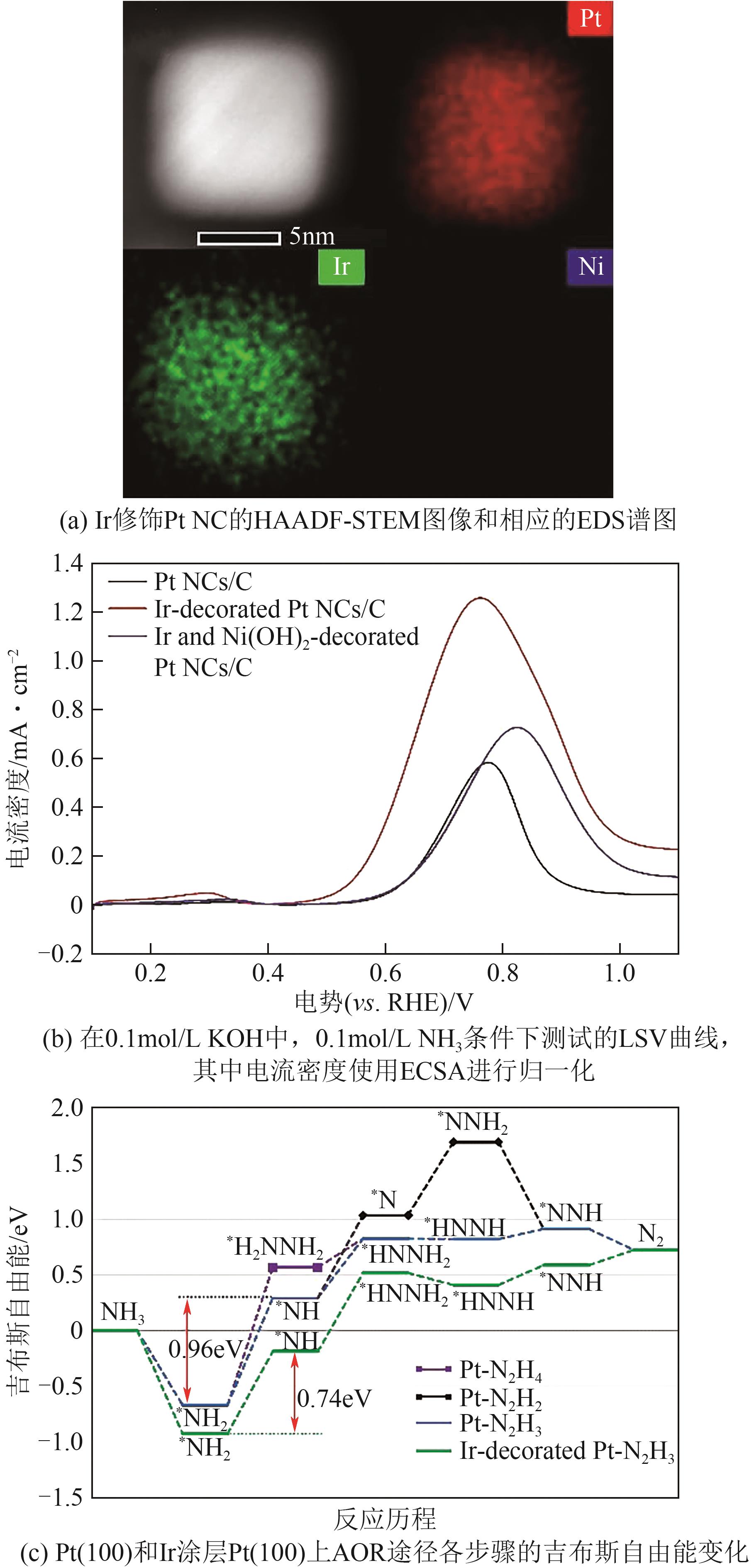
| Ir-修饰Pt | 0.1mol/L NH3 +0.1mol/L KOH | 0.43 | 1.26 | [ |
| PtIr纳米颗粒 | 0.5mol/L NH4OH+1mol/L KOH | 0.4 | 0.11 | [ |
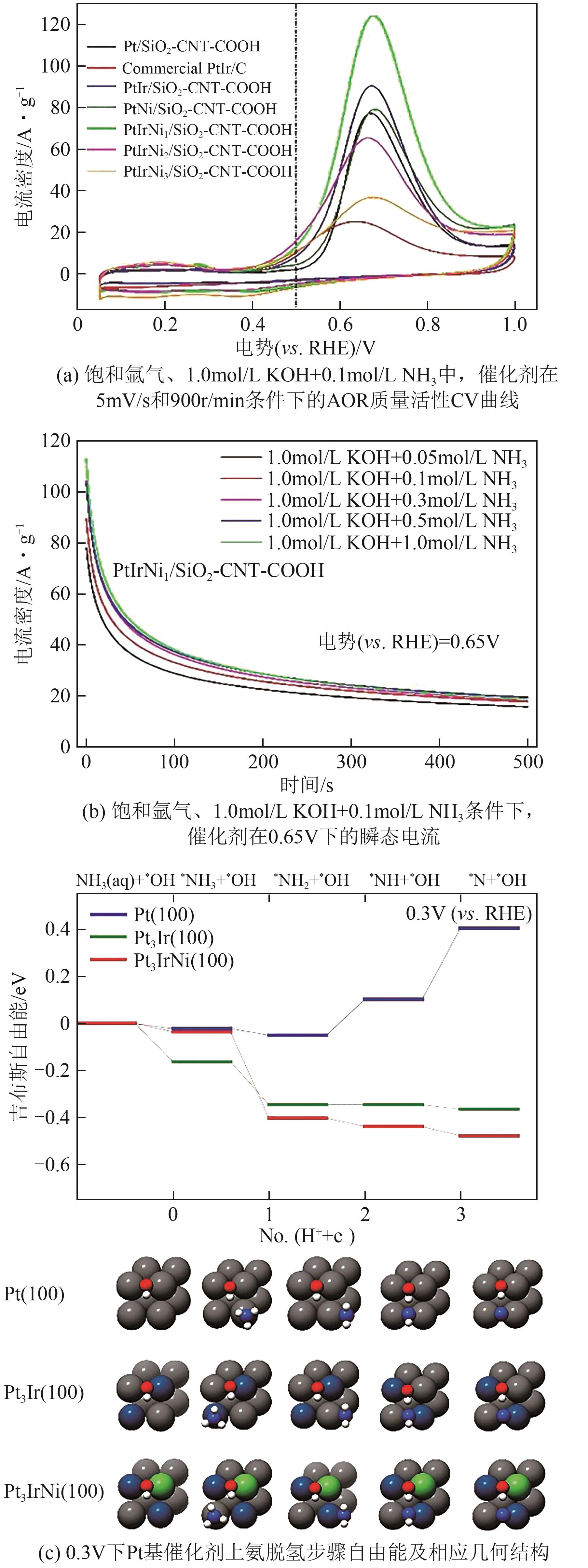
| PtIrNi/SiO2-CNT-COOH | 0.1mol/L NH3+1mol/L KOH | 0.4 | 2.48 | [ |
| PtNC/C | 0.1mol/L NH3+1mol/L KOH | 0.48 | 3.89 | [ |
| C-Pt/SnO2 | 0.1mol/L NH3+1mol/L KOH | 0.45 | 1.6 | [ |
| Pt/PBI/MWNT-CeO2 | 0.1mol/L NH3+1mol/L KOH | 0.45 | 0.26 | [ |
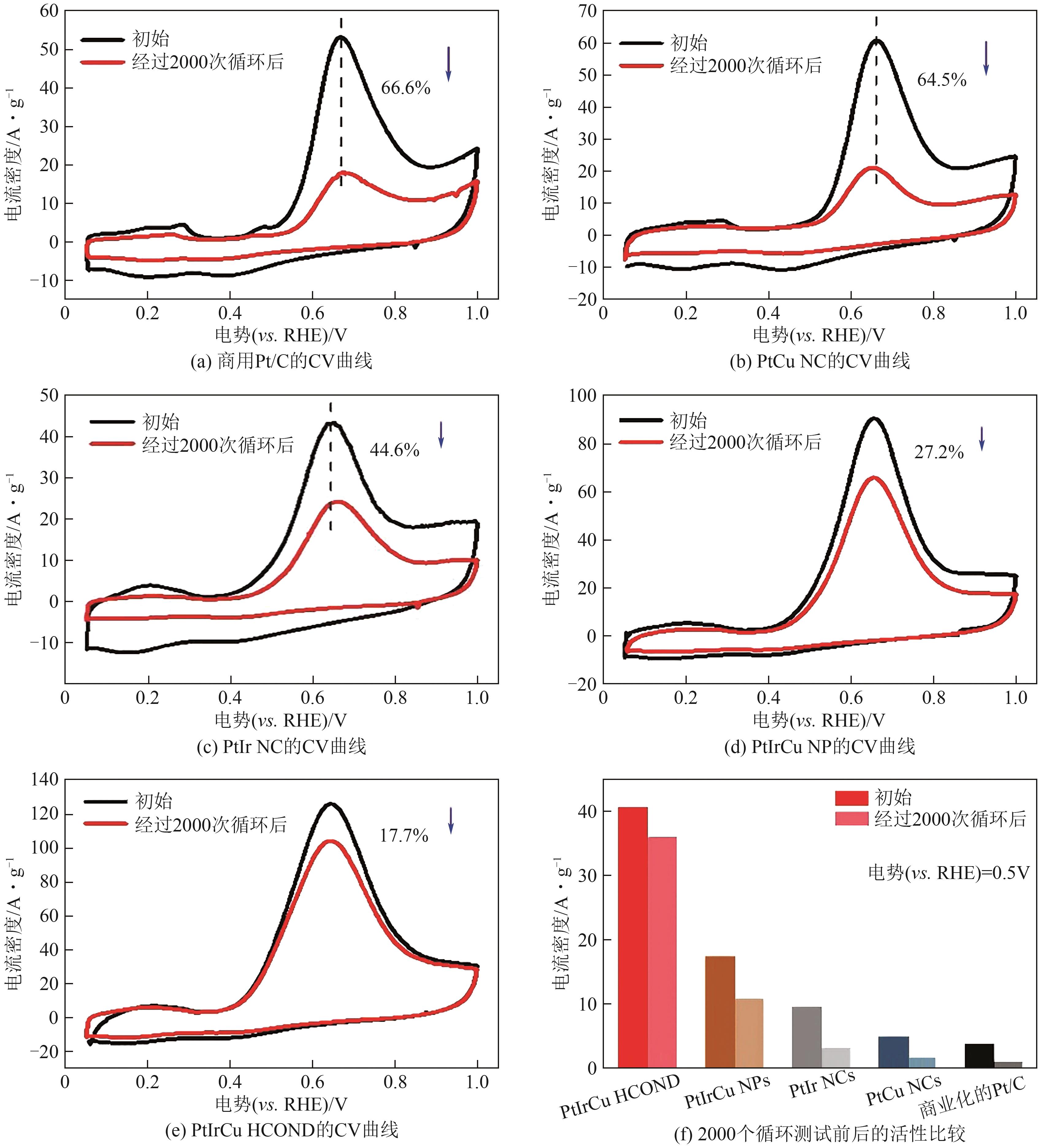
| PtIrCu HCOND | 0.1 mol/L NH3+1mol/L KOH | 0.35 | 122.9A/g | [ |
| PtIrZn2/CeO2-ZIF-8 | 0.1mol/L NH3+1mol/L KOH | 0.35 | 0.64 | [ |
| Pt85Pd15/rGO | 0.1mol/L NH3+1mol/L KOH | 0.47 | 1.46 | [ |
| Pt/Ir/MWCNT | 0.1mol/L NH3+0.1mol/L KOH | 0.38 | — | [ |
| Pt-修饰Ni NP | 0.1mol/L NH3+1mol/L KOH | 0.5 | 5.32A/g | [ |
| PtIrZn | 0.1mol/L NH4OH+0.5mol/L KOH | 0.3 | 0.56 | [ |
| PtPb/C | 1mol/L KOH+0.1mol/L NH3 | — | 191A/g | [ |
| Pt x Ru | 0.1mol/L NH3+1mol/L KOH | 0.5 | 92A/g | [ |
| Au@Pt NP | 1mol/L NaOH+0.05mol/L NH3 | 0.4 | 1.19~1.06 | [ |
表1 Pt电催化剂eAOR的性能比较
| 催化剂 | 电解液 | 起始电位(相对于RHE)/V | 峰值电流密度/mA·cm-2 | 参考文献 |
|---|---|---|---|---|
| 500CV-Pt | 1mol/L NH3+5mol/L KOH | 0.42 | 0.27 | [ |
| Pt纳米片 | 0.1mol/L NH3+1mol/L KOH | 0.57 | 0.32 | [ |
| PtZn | 0.1mol/L NH4OH+0.5mol/L KOH | 0.42 | 0.6 | [ |
| Pt薄膜 | 0.1mol/L NH3+0.2mol/L NaOH | 0.5 | 0.212 | [ |
| 花状Pt | 0.1mol/L NH3+1mol/L KOH | 0.5 | 0.48 | [ |
| Pt纳米立方体(Pt-NC) | 0.1mol/L NH4OH+1mol/L KOH | 0.5 | 5.1 | [ |
| Pt纳米颗粒(Pt NP) | 0.1mol/L NH3+0.2mol/L NaOH | 0.55 | 1.96 | [ |
| Ir-修饰Pt | 0.1mol/L NH3 +0.1mol/L KOH | 0.43 | 1.26 | [ |
| PtIr纳米颗粒 | 0.5mol/L NH4OH+1mol/L KOH | 0.4 | 0.11 | [ |
| PtIrNi/SiO2-CNT-COOH | 0.1mol/L NH3+1mol/L KOH | 0.4 | 2.48 | [ |
| PtNC/C | 0.1mol/L NH3+1mol/L KOH | 0.48 | 3.89 | [ |
| C-Pt/SnO2 | 0.1mol/L NH3+1mol/L KOH | 0.45 | 1.6 | [ |
| Pt/PBI/MWNT-CeO2 | 0.1mol/L NH3+1mol/L KOH | 0.45 | 0.26 | [ |
| PtIrCu HCOND | 0.1 mol/L NH3+1mol/L KOH | 0.35 | 122.9A/g | [ |
| PtIrZn2/CeO2-ZIF-8 | 0.1mol/L NH3+1mol/L KOH | 0.35 | 0.64 | [ |
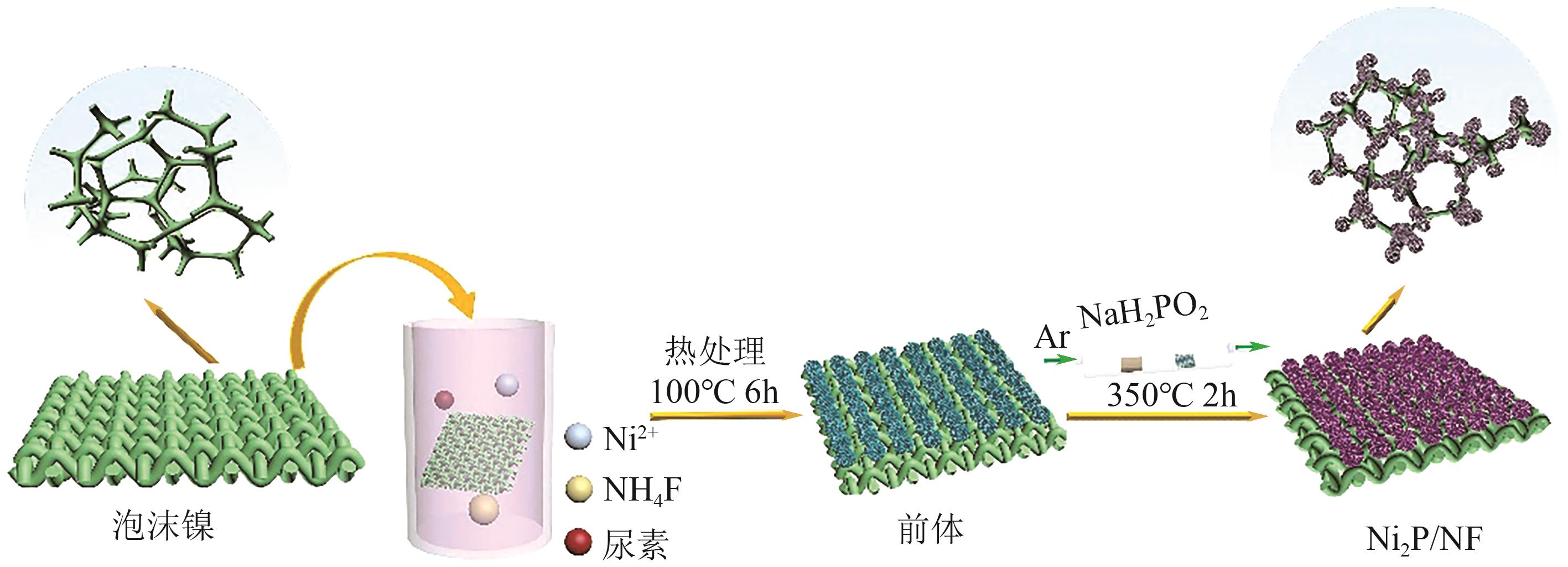
| Pt85Pd15/rGO | 0.1mol/L NH3+1mol/L KOH | 0.47 | 1.46 | [ |
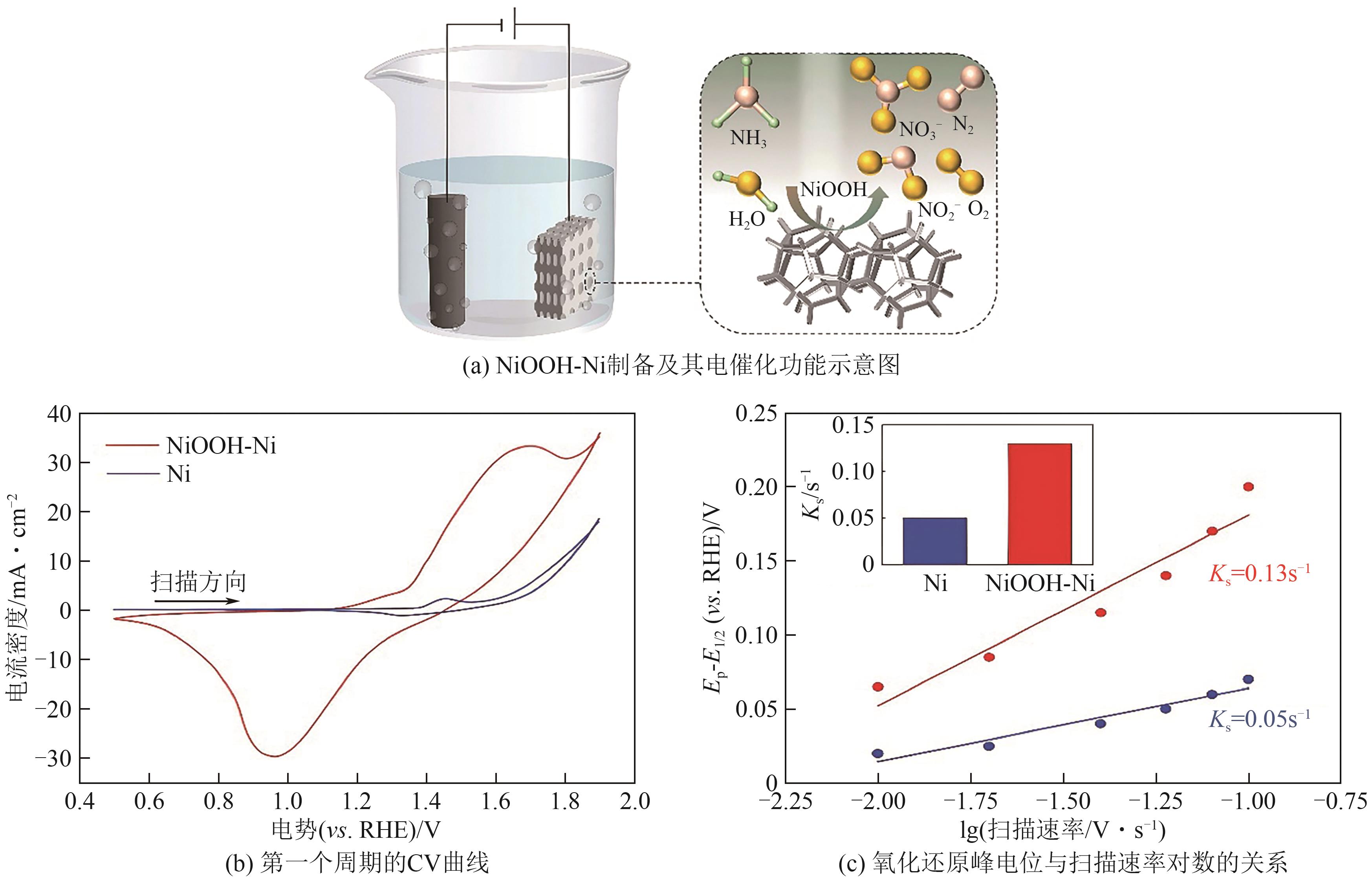
| Pt/Ir/MWCNT | 0.1mol/L NH3+0.1mol/L KOH | 0.38 | — | [ |
| Pt-修饰Ni NP | 0.1mol/L NH3+1mol/L KOH | 0.5 | 5.32A/g | [ |
| PtIrZn | 0.1mol/L NH4OH+0.5mol/L KOH | 0.3 | 0.56 | [ |
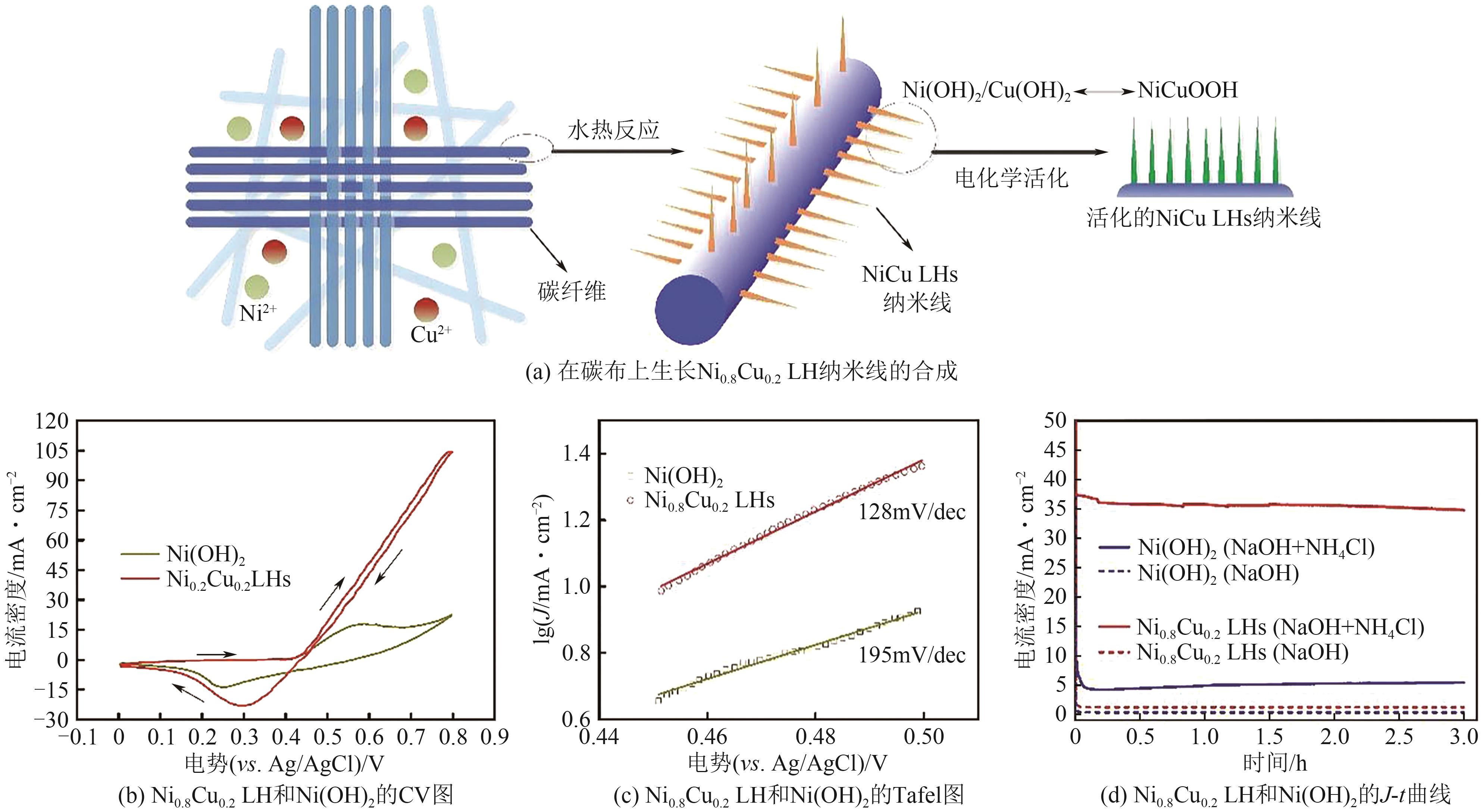
| PtPb/C | 1mol/L KOH+0.1mol/L NH3 | — | 191A/g | [ |
| Pt x Ru | 0.1mol/L NH3+1mol/L KOH | 0.5 | 92A/g | [ |
| Au@Pt NP | 1mol/L NaOH+0.05mol/L NH3 | 0.4 | 1.19~1.06 | [ |
| 催化剂 | 电解液 | 起始电位 | 参考文献 |
|---|---|---|---|
| Ag/Ni | 0.5mol/L NH3+1.5mol/L NaOH | 1.37V vs. RHE | [ |
| Ni(OH)2-Cu2O@CuO | 1mol/L NH3+1mol/L KOH | 1.37V vs. RHE | [ |
| NiCu/BDD | 0.5mol/L NH3+0.5mol/L NaOH | 1.35V vs. RHE | [ |
| NiCuCo-S-T/CP | 0.2mol/L NH4Cl+1mol/L NaOH | 1.24V vs. RHE | [ |
| Ni(OH)2/NiOOH | 3mmol/L NH3+0.1mol/L Na2SO4 | 0.65V vs. Hg/HgO | [ |
| Ni/NiOOH | 3mmol/L NH3+0.01mol/L Na2SO4 | 0.6V vs. Hg/HgO | [ |
| NiCu DHT | 0.05mol/L NH4OH+0.1mol/L NaOH | 1.31V vs. RHE | [ |
| Ni0.8Cu0.2 LH | 55mmol/L NH4Cl+0.5mol/L NaOH | 0.4V vs. Ag/AgCl | [ |
| NiCu/CP | 1mol/L NaOH+55mmol/L NH4Cl | 0.47V vs. Ag/AgCl | [ |
| Ni0.8Cu0.2氢氧化物 | 1 mmol/L NH4++0.1mol/L KOH | 1.4V vs. RHE | [ |
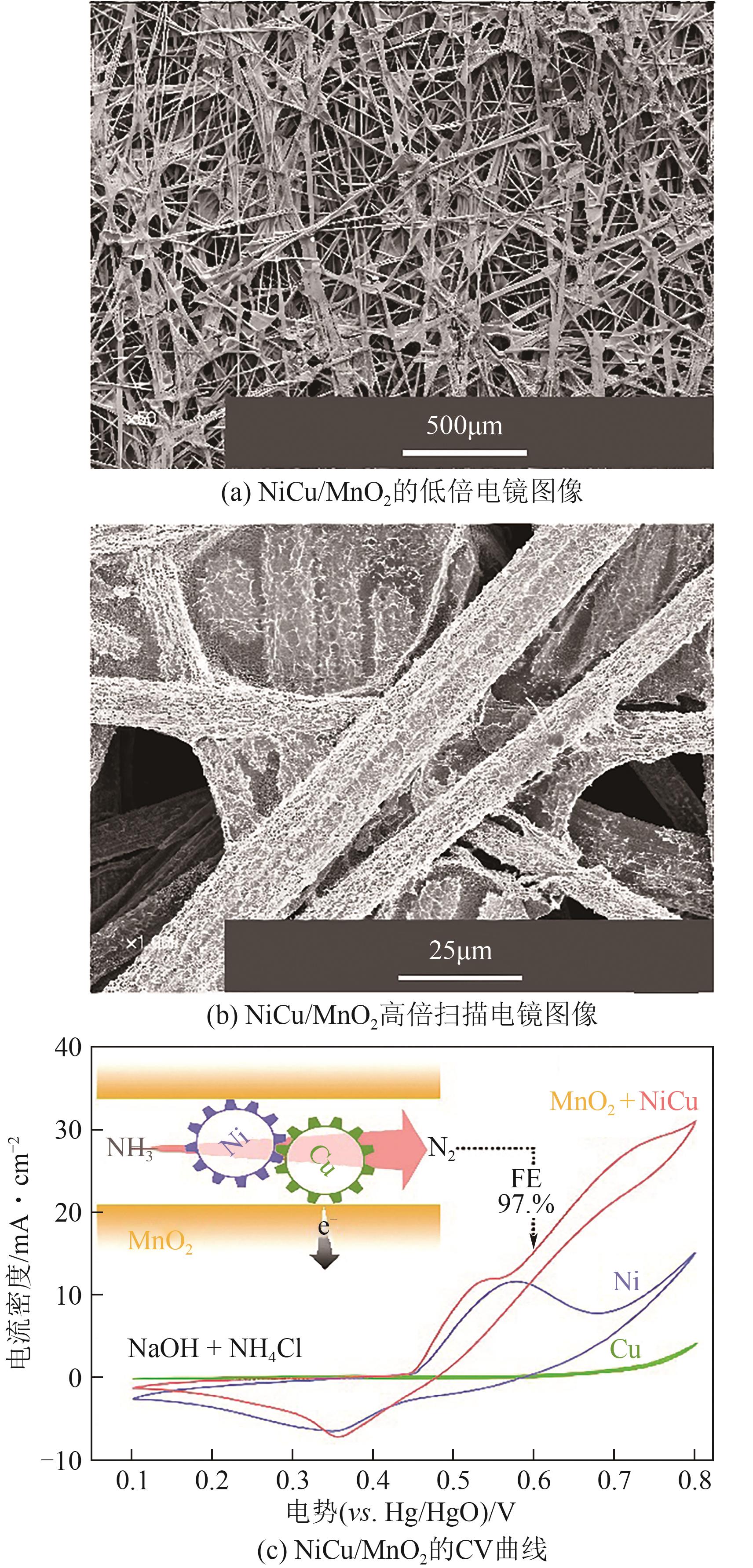
| NiCu/MnO2 | 0.5mol/L NaOH+55mmol/L NH4Cl | 0.65V vs.Hg/HgO | [ |
| NiCo2N | NH3饱和的0.1mol/L KPF6 | 0.55V vs. NHE | [ |
| NiO-TiO2 | 0.2mol/L NH4++0.1mmol/L NaNO3 | 0.5V vs. Hg/HgO | [ |
| Ni(OH)2 | 0.2mol/L NH3+0.1mol/L NaOH | 1.4V vs. RHE | [ |
| Ni1Cu3-S-T/CP | 1mol/L NaOH+0.2mol/L NH4Cl | 1.37V vs. RHE | [ |
| Ni(OH)2/SnO2 | 0.5mol/L K2SO4+10 mmol/L NH3 | 1.39V vs. RHE | [ |
| Co10/Ni-C | 0.5mol/L KOH+0.3mmol/L NH4+ | 0.35V vs. Hg/HgO | [ |
| NiCu/NF | 1mol/L KOH+0.3mol/L NH3 | 0.8V vs. Hg/HgO | [ |
表2 Ni电催化剂eAOR的性能比较
| 催化剂 | 电解液 | 起始电位 | 参考文献 |
|---|---|---|---|
| Ag/Ni | 0.5mol/L NH3+1.5mol/L NaOH | 1.37V vs. RHE | [ |
| Ni(OH)2-Cu2O@CuO | 1mol/L NH3+1mol/L KOH | 1.37V vs. RHE | [ |
| NiCu/BDD | 0.5mol/L NH3+0.5mol/L NaOH | 1.35V vs. RHE | [ |
| NiCuCo-S-T/CP | 0.2mol/L NH4Cl+1mol/L NaOH | 1.24V vs. RHE | [ |
| Ni(OH)2/NiOOH | 3mmol/L NH3+0.1mol/L Na2SO4 | 0.65V vs. Hg/HgO | [ |
| Ni/NiOOH | 3mmol/L NH3+0.01mol/L Na2SO4 | 0.6V vs. Hg/HgO | [ |
| NiCu DHT | 0.05mol/L NH4OH+0.1mol/L NaOH | 1.31V vs. RHE | [ |
| Ni0.8Cu0.2 LH | 55mmol/L NH4Cl+0.5mol/L NaOH | 0.4V vs. Ag/AgCl | [ |
| NiCu/CP | 1mol/L NaOH+55mmol/L NH4Cl | 0.47V vs. Ag/AgCl | [ |
| Ni0.8Cu0.2氢氧化物 | 1 mmol/L NH4++0.1mol/L KOH | 1.4V vs. RHE | [ |
| NiCu/MnO2 | 0.5mol/L NaOH+55mmol/L NH4Cl | 0.65V vs.Hg/HgO | [ |
| NiCo2N | NH3饱和的0.1mol/L KPF6 | 0.55V vs. NHE | [ |
| NiO-TiO2 | 0.2mol/L NH4++0.1mmol/L NaNO3 | 0.5V vs. Hg/HgO | [ |
| Ni(OH)2 | 0.2mol/L NH3+0.1mol/L NaOH | 1.4V vs. RHE | [ |
| Ni1Cu3-S-T/CP | 1mol/L NaOH+0.2mol/L NH4Cl | 1.37V vs. RHE | [ |
| Ni(OH)2/SnO2 | 0.5mol/L K2SO4+10 mmol/L NH3 | 1.39V vs. RHE | [ |
| Co10/Ni-C | 0.5mol/L KOH+0.3mmol/L NH4+ | 0.35V vs. Hg/HgO | [ |
| NiCu/NF | 1mol/L KOH+0.3mol/L NH3 | 0.8V vs. Hg/HgO | [ |
| [1] | BUSHUYEV Oleksandr S, DE LUNA Phil, DINH Cao Thang, et al. What should we make with CO2 and how can we make it?[J]. Joule, 2018, 2(5): 825-832. |
| [2] | DE LUNA Phil, HAHN Christopher, HIGGINS Drew, et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes?[J]. Science, 2019, 364(6438): eaav3506. |
| [3] | GUO Wenhan, ZHANG Kexin, LIANG Zibin, et al. Electrochemical nitrogen fixation and utilization: Theories, advanced catalyst materials and system design[J]. Chemical Society Reviews, 2019, 48(24): 5658-5716. |
| [4] | TURNER John A. Sustainable hydrogen production[J]. Science, 2004, 305(5686): 972-974. |
| [5] | SIDDIQUI O, DINCER I. Experimental investigation and assessment of direct ammonia fuel cells utilizing alkaline molten and solid electrolytes[J]. Energy, 2019, 169: 914-923. |
| [6] | TRENERRY Michael J, WALLEN Christian M, BROWN Tristan R, et al. Spontaneous N2 formation by a diruthenium complex enables electrocatalytic and aerobic oxidation of ammonia[J]. Nature Chemistry, 2021, 13(12): 1221-1227. |
| [7] | CHRISTENSEN Claus Hviid, SØRENSEN Rasmus Zink, JOHANNESSEN Tue, et al. Metal ammine complexes for hydrogen storage[J]. Journal of Materials Chemistry, 2005, 15(38): 4106-4108. |
| [8] | XI Xiaoshuang, FAN Yunying, ZHANG Kai, et al. Carbon-free sustainable energy technology: Electrocatalytic ammonia oxidation reaction[J]. Chemical Engineering Journal, 2022, 435: 134818. |
| [9] | BODIRSKY Benjamin Leon, POPP Alexander, Hermann LOTZE-CAMPEN, et al. Reactive nitrogen requirements to feed the world in 2050 and potential to mitigate nitrogen pollution[J]. Nature Communications, 2014, 5: 3858. |
| [10] | CRUZ Heidy, Ying Yu LAW, GUEST Jeremy S, et al. Mainstream ammonium recovery to advance sustainable urban wastewater management[J]. Environmental Science & Technology, 2019, 53(19): 11066-11079. |
| [11] | XUE Runmiao, DONOVAN Ariel, ZHANG Haiting, et al. Simultaneous removal of ammonia and N-nitrosamine precursors from high ammonia water by zeolite and powdered activated carbon[J]. Journal of Environmental Sciences, 2018, 64: 82-91. |
| [12] | ZHANG Zhiyong, AI Huiying, FU Minglai, et al. A new insight into catalytic ozonation of ammonia by MgO/Co3O4 composite: The effects, reaction kinetics and mechanism[J]. Chemical Engineering Journal, 2021, 418: 129461. |
| [13] | YANG Hongxin, HU Jinling, JIANG Xuesong, et al. Study on ammonia nitrogen in pesticide wastewater by breakpoint chlorination method[J]. Modern Agrochemicals, 2018, 17(5): 19-21. |
| [14] | CHANG Mingdong, LIANG Baorui, ZHANG Kuo, et al. Simultaneous shortcut nitrification and denitrification in a hybrid membrane aerated biofilms reactor (H-MBfR) for nitrogen removal from low COD/N wastewater[J]. Water Research, 2022, 211: 118027. |
| [15] | HERRON Jeffrey A, FERRIN Peter, MAVRIKAKIS Manos. Electrocatalytic oxidation of ammonia on transition-metal surfaces: A first-principles study[J]. The Journal of Physical Chemistry C, 2015, 119(26): 14692-14701. |
| [16] | KATAYAMA Yu, OKANISHI Takeou, MUROYAMA Hiroki, et al. Enhancement of ammonia oxidation activity over Y2O3-modified platinum surface: Promotion of NH2, ad dimerization process[J]. Journal of Catalysis, 2016, 344: 496-506. |
| [17] | GERISCHER H, MAUERER A. Untersuchungen zur anodischen oxidation von ammoniak an platin-elektroden[J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1970, 25(3): 421-433. |
| [18] | JIN Yongzhen, KE Changming, LIU Yang, et al. Enhancing electrocatalytic ammonia oxidation using super-concentrated electrolytes[J/OL]. ChemRxiv, 2025. . |
| [19] | JACOB S I, CHAKRABORTY A, CHAMAS A, et al. Rapid aqueous ammonia oxidation to N2 using a molecular Ru electrocatalyst[J]. ACS Energy Letters, 2023, 8(9): 3760-3766. |
| [20] | AHMED M E, STAPLES R J, UNDARI T R C, et al. Electrocatalytic ammonia oxidation by pyridyl-substituted ferrocenes[J]. Journal of the American Chemical Society, 2025, 147(8): 6514-6522. |
| [21] | OSWIN H G, SALOMON M. The anodic oxidation of ammonia at platinum black electrodes in aqueous KOH electrolyte[J]. Canadian Journal of Chemistry, 1963, 41(7): 1686-1694. |
| [22] | VIDAL-IGLESIAS F J, SOLLA-GULLÓN J, PÉREZ J M, et al. Evidence by SERS of azide anion participation in ammonia electrooxidation in alkaline medium on nanostructured Pt electrodes[J]. Electrochemistry Communications, 2006, 8(1): 102-106. |
| [23] | DE VOOYS A C A, MROZEK F, KOPER T M, et al. The nature of chemisorbates formed from ammonia on gold and palladium electrodes as discerned from surface-enhanced Raman spectroscopy[J]. Electrochemistry Communications, 2001, 3(6): 293-298. |
| [24] | WASMUS S, VASINI E J, KRAUSA M, et al. DEMS-cyclic voltammetry investigation of the electrochemistry of nitrogen compounds in 0.5mol/L potassium hydroxide[J]. Electrochimica Acta, 1994, 39(1): 23-31. |
| [25] | GOOTZEN J F E, WONDERS A H, VISSCHER W, et al. A DEMS and cyclic voltammetry study of NH3 oxidation on platinized platinum[J]. Electrochimica Acta, 1998, 43(12/13): 1851-1861. |
| [26] | ENDO Kazuki, KATAYAMA Yasushi, MIURA Takashi. A rotating disk electrode study on the ammonia oxidation[J]. Electrochimica Acta, 2005, 50(11): 2181-2185. |
| [27] | DIAZ Luis A, BOTTE Gerardine G. Mathematical modeling of ammonia electrooxidation kinetics in a polycrystalline Pt rotating disk electrode[J]. Electrochimica Acta, 2015, 179: 519-528. |
| [28] | FINKELSTEIN David A, BERTIN Erwan, GARBARINO Sébastien, et al. Mechanistic similarity in catalytic N2 production from NH3 and NO2 - at Pt(100) thin films: Toward a universal catalytic pathway for simple N-containing species, and its application to in situ removal of NH3 poisons[J]. The Journal of Physical Chemistry C, 2015, 119(18): 9860-9878. |
| [29] | PILLAI Hemanth Somarajan, XIN Hongliang. New insights into electrochemical ammonia oxidation on Pt(100) from first principles[J]. Industrial & Engineering Chemistry Research, 2019, 58(25): 10819-10828. |
| [30] | JANG Ji Hee, PARK So Young, YOUN Duck Hyun, et al. Recent advances in electrocatalysts for ammonia oxidation reaction[J]. Catalysts, 2023, 13(5): 803. |
| [31] | Erich MÜLLER, SPITZER Fritz. Über die elektrolytische oxydation des ammoniaks und ihre abhängigkeit vom anodenmaterial[J]. Zeitschrift Für Elektrochemie und Angewandte Physikalische Chemie, 1905, 11(50): 917-931. |
| [32] | Zhenhua LYU, FU Jiaju, TANG Tang, et al. Design of ammonia oxidation electrocatalysts for efficient direct ammonia fuel cells[J]. EnergyChem, 2023, 5(3): 100093. |
| [33] | KIM Hyunki, HONG Seokjin, KIM Hedam, et al. Recent progress in Pt-based electrocatalysts for ammonia oxidation reaction[J]. Applied Materials Today, 2022, 29: 101640. |
| [34] | VIDAL-IGLESIAS F J, GARCÍA-ARÁEZ N, MONTIEL V, et al. Selective electrocatalysis of ammonia oxidation on Pt(100) sites in alkaline medium[J]. Electrochemistry Communications, 2003, 5(1): 22-26. |
| [35] | VIDAL-IGLESIAS Francisco J, José SOLLA-GULLÓN, MONTIEL Vicente, et al. Ammonia selective oxidation on Pt(100) sites in an alkaline medium[J]. The Journal of Physical Chemistry B, 2005, 109(26): 12914-12919. |
| [36] | NOVELL-LERUTH G, VALCÁRCEL A, CLOTET A, et al. DFT characterization of adsorbed NH x species on Pt(100) and Pt(111) surfaces[J]. The Journal of Physical Chemistry B, 2005, 109(38): 18061-18069. |
| [37] | ROSCA Victor, KOPER Marc T M. Electrocatalytic oxidation of ammonia on Pt(111) and Pt(100) surfaces[J]. Physical Chemistry Chemical Physics, 2006, 8(21): 2513-2524. |
| [38] | SUN Huiying, XU Guangrui, LI Fumin, et al. Hydrogen generation from ammonia electrolysis on bifunctional platinum nanocubes electrocatalysts[J]. Journal of Energy Chemistry, 2020, 47: 234-240. |
| [39] | FU Gengtao, LIU Chang, WU Rui, et al. L-lysine mediated synthesis of platinum nanocuboids and their electrocatalytic activity towards ammonia oxidation[J]. Journal of Materials Chemistry A, 2014, 2(42): 17883-17888. |
| [40] | YANG Yejin, KIM Jeongwon, Hyoi JO, et al. A rigorous electrochemical ammonia electrolysis protocol with in operando quantitative analysis[J]. Journal of Materials Chemistry A, 2021, 9(19): 11571-11579. |
| [41] | LIU Jie, HU Wenbin, ZHONG Cheng, et al. Surfactant-free electrochemical synthesis of hierarchical platinum particle electrocatalysts for oxidation of ammonia[J]. Journal of Power Sources, 2013, 223: 165-174. |
| [42] | LOMOCSO Thegy L, BARANOVA Elena A. Electrochemical oxidation of ammonia on carbon-supported bi-metallic PtM (M=Ir, Pd, SnO x ) nanoparticles[J]. Electrochimica Acta, 2011, 56(24): 8551-8558. |
| [43] | BOGGS Bryan K, BOTTE Gerardine G. Optimization of Pt-Ir on carbon fiber paper for the electro-oxidation of ammonia in alkaline media[J]. Electrochimica Acta, 2010, 55(19): 5287-5293. |
| [44] | ENDO Kazuki, NAKAMURA Kyoko, KATAYAMA Yasushi, et al. Pt-Me (Me=Ir, Ru, Ni) binary alloys as an ammonia oxidation anode[J]. Electrochimica Acta, 2004, 49(15): 2503-2509. |
| [45] | XUE Qi, ZHAO Yue, ZHU Jingyi, et al. PtRu nanocubes as bifunctional electrocatalysts for ammonia electrolysis[J]. Journal of Materials Chemistry A, 2021, 9(13): 8444-8451. |
| [46] | SILVA Júlio César M, SILVA Sirlane G DA, DE SOUZA Rodrigo F B, et al. PtAu/C electrocatalysts as anodes for direct ammonia fuel cell[J]. Applied Catalysis A: General, 2015, 490: 133-138. |
| [47] | WEI Ruilin, LIU Yue, CHEN Zhen, et al. Ammonia oxidation on iridium electrode in alkaline media: An in situ ATR-SEIRAS study[J]. Journal of Electroanalytical Chemistry, 2021, 896: 115254. |
| [48] | SIDDHARTH Kumar, HONG Youngmin, QIN Xueping, et al. Surface engineering in improving activity of Pt nanocubes for ammonia electrooxidation reaction[J]. Applied Catalysis B: Environmental, 2020, 269: 118821. |
| [49] | LIN Xu, ZHANG Xiaoran, WANG Zhen, et al. Hyperbranched concave octahedron of PtIrCu nanocrystals with high-index facets for efficiently electrochemical ammonia oxidation reaction[J]. Journal of Colloid and Interface Science, 2021, 601: 1-11. |
| [50] | LI Yi, LI Xing, PILLAI Hemanth Somarajan, et al. Ternary PtIrNi catalysts for efficient electrochemical ammonia oxidation[J]. ACS Catalysis, 2020, 10(7): 3945-3957. |
| [51] | JIANG Junhua. Promotion of PtIr and Pt catalytic activity towards ammonia electrooxidation through the modification of Zn[J]. Electrochemistry Communications, 2017, 75: 52-55. |
| [52] | BERTIN Erwan, GARBARINO Sébastien, GUAY Daniel, et al. Electrodeposited platinum thin films with preferential (100) orientation: Characterization and electrocatalytic properties for ammonia and formic acid oxidation[J]. Journal of Power Sources, 2013, 225: 323-329. |
| [53] | LIU J, ZHONG C, YANG Y, et al. Electrochemical preparation and characterization of Pt particles on ITO substrate: Morphological effect on ammonia oxidation[J]. International Journal of Hydrogen Energy, 2012, 37(11): 8981-8987. |
| [54] | MARTÍNEZ-RODRÍGUEZ Roberto A, VIDAL-IGLESIAS Francisco J, José SOLLA-GULLÓN, et al. Synthesis of Pt nanoparticles in water-in-oil microemulsion: Effect of HCl on their surface structure[J]. Journal of the American Chemical Society, 2014, 136(4): 1280-1283. |
| [55] | ALLAGUI Anis, OUDAH Mohamed, TUAEV Xenia, et al. Ammonia electro-oxidation on alloyed PtIr nanoparticles of well-defined size[J]. International Journal of Hydrogen Energy, 2013, 38(5): 2455-2463. |
| [56] | ZHANG Changlin, HWANG Sang Youp, PENG Zhenmeng. Shape-enhanced ammonia electro-oxidation property of a cubic platinum nanocrystal catalyst prepared by surfactant-free synthesis[J]. Journal of Materials Chemistry A, 2013, 1(45): 14402-14408. |
| [57] | OKANISHI Takeou, KATAYAMA Yu, MUROYAMA Hiroki, et al. SnO2-modified Pt electrocatalysts for ammonia-fueled anion exchange membrane fuel cells[J]. Electrochimica Acta, 2015, 173: 364-369. |
| [58] | KATAYAMA Yu, OKANISHI Takeou, MUROYAMA Hiroki, et al. Electrochemical oxidation of ammonia over rare earth oxide modified platinum catalysts[J]. The Journal of Physical Chemistry C, 2015, 119(17): 9134-9141. |
| [59] | LI Yi, PILLAI Hemanth Somarajan, WANG Teng, et al. High-performance ammonia oxidation catalysts for anion-exchange membrane direct ammonia fuel cells[J]. Energy & Environmental Science, 2021, 14(3): 1449-1460. |
| [60] | LIU Zhenzhong, LI Yi, ZHANG Xiangsong, et al. Surface structure engineering of PtPd nanoparticles for boosting ammonia oxidation electrocatalysis[J]. ACS Applied Materials & Interfaces, 2022, 14(25): 28816-28825. |
| [61] | MORITA Seitaro, KUDO Eiji, SHIRASAKA Ryo, et al. Electrochemical oxidation of ammonia by multi-wall-carbon-nanotube-supported Pt shell-Ir core nanoparticles synthesized by an improved Cu short circuit deposition method[J]. Journal of Electroanalytical Chemistry, 2016, 762: 29-36. |
| [62] | LIU Jie, CHEN Bin, KOU Yue, et al. Pt-Decorated highly porous flower-like Ni particles with high mass activity for ammonia electro-oxidation[J]. Journal of Materials Chemistry A, 2016, 4(28): 11060-11068. |
| [63] | JIANG Zexing, YU Tianqi, CHEN Jinli, et al. Regulating competitive adsorption on Pt nanoparticles by introducing Pb to expedite hydrogen production via ammonia oxidation[J]. ACS Applied Nano Materials, 2023, 6(3): 1889-1897. |
| [64] | WANG Jun, Jaeyoung HEO, CHEN Changqiang, et al. Ammonia oxidation enhanced by photopotential generated by plasmonic excitation of a bimetallic electrocatalyst[J]. Angewandte Chemie International Edition, 2020, 59(42): 18430-18434. |
| [65] | MYERS Clifford E, FRANZEN Hugo F, ANDEREGG James W. X-ray photoelectron spectra and bonding in transition-metal phosphides[J]. Inorganic Chemistry, 1985, 24(12): 1822-1824. |
| [66] | WANG Renyu, LIU Huijuan, ZHANG Kai, et al. Ni(Ⅱ)/Ni(Ⅲ) redox couple endows Ni foam-supported Ni2P with excellent capability for direct ammonia oxidation[J]. Chemical Engineering Journal, 2021, 404: 126795. |
| [67] | LIU Hanwen, YANG Chengjie, DONG Chungli, et al. Electrocatalytic ammonia oxidation to nitrite and nitrate with NiOOH-Ni[J]. Advanced Energy Materials, 2024, 14(42): 2401675. |
| [68] | WANG Jiong, GAN Liyong, ZHANG Wenyu, et al. In situ formation of molecular Ni-Fe active sites on heteroatom-doped graphene as a heterogeneous electrocatalyst toward oxygen evolution[J]. Science Advances, 2018, 4(3): eaap7970. |
| [69] | MEDFORD Andrew J, VOJVODIC Aleksandra, HUMMELSHØJ Jens S, et al. From the Sabatier principle to a predictive theory of transition-metal heterogeneous catalysis[J]. Journal of Catalysis, 2015, 328: 36-42. |
| [70] | XU Wei, LAN Rong, DU Dongwei, et al. Directly growing hierarchical nickel-copper hydroxide nanowires on carbon fibre cloth for efficient electrooxidation of ammonia[J]. Applied Catalysis B: Environmental, 2017, 218: 470-479. |
| [71] | XU Wei, DU Dongwei, LAN Rong, et al. Electrodeposited NiCu bimetal on carbon paper as stable non-noble anode for efficient electrooxidation of ammonia[J]. Applied Catalysis B: Environmental, 2018, 237: 1101-1109. |
| [72] | ZHANG Huimin, WANG Hailong, ZHOU Luanqi, et al. Efficient and highly selective direct electrochemical oxidation of ammonia to dinitrogen facilitated by NiCu diatomic site catalysts[J]. Applied Catalysis B: Environmental, 2023, 328: 122544. |
| [73] | ZHU Mingke, YANG Yi, XI Shibo, et al. Deciphering NH3 adsorption kinetics in ternary Ni-Cu-Fe oxyhydroxide toward efficient ammonia oxidation reaction[J]. Small, 2021, 17(7): 2005616. |
| [74] | NAGITA Kenji, YUHARA Yoshiki, FUJII Kenta, et al. Ni- and Cu-co-intercalated layered manganese oxide for highly efficient electro-oxidation of ammonia selective to nitrogen[J]. ACS Applied Materials & Interfaces, 2021, 13(24): 28098-28107. |
| [75] | SHIH Yu-Jen, HSU Ching-Hsiang. Kinetics and highly selective N2 conversion of direct electrochemical ammonia oxidation in an undivided cell using NiCo oxide nanoparticle as the anode and metallic Cu/Ni foam as the cathode[J]. Chemical Engineering Journal, 2021, 409: 128024. |
| [76] | ALMOMANI Fares, SALAH SAAD Mohammed ALI H. Electrochemical oxidation of ammonia (NH4 +/NH3) on synthesized nickel-cobalt oxide catalyst[J]. International Journal of Hydrogen Energy, 2021, 46(6): 4678-4690. |
| [77] | HE Shi, CHEN Yufeng, WANG Mengdi, et al. Metal nitride nanosheets enable highly efficient electrochemical oxidation of ammonia[J]. Nano Energy, 2021, 80: 105528. |
| [78] | JIN Yongzhen, CHEN Xin, WANG Jianhui. From inert to active: A cocktail-like mediation of an Ag/Ni mixture for electrocatalytic ammonia oxidation reaction[J]. Chemical Communications, 2022, 58(76): 10631-10634. |
| [79] | HUANG Jingjing, CAI Jinmeng, WANG Jianhui. Nanostructured wire-in-plate electrocatalyst for high-durability production of hydrogen and nitrogen from alkaline ammonia solution[J]. ACS Applied Energy Materials, 2020, 3(5): 4108-4113. |
| [80] | SONG Jingjin, YANG Yinhai, JIA Yingna, et al. Improved NH3-N conversion efficiency to N2 activated by BDD substrate on NiCu electrocatalysis process[J]. Separation and Purification Technology, 2021, 276: 119350. |
| [81] | WANG Hailong, TONG Xing, ZHOU Luanqi, et al. Unique three-dimensional nanoflower-like NiCu electrodes constructed by Co, S co-doping for efficient ammonia oxidation reaction[J]. Separation and Purification Technology, 2022, 303: 122293. |
| [82] | SHIH Yu-Jen, HUANG Yaohui, HUANG C P. Electrocatalytic ammonia oxidation over a nickel foam electrode: Role of Ni(OH)2(s)-NiOOH(s) nanocatalysts[J]. Electrochimica Acta, 2018, 263: 261-271. |
| [83] | SHIH Yu-Jen, HUANG Yaohui, HUANG C P. In-situ electrochemical formation of nickel oxyhydroxide (NiOOH) on metallic nickel foam electrode for the direct oxidation of ammonia in aqueous solution[J]. Electrochimica Acta, 2018, 281: 410-419. |
| [84] | YANG Anzhou, WANG Jingchun, SU Keying, et al. Modulating hydroxyl-rich interfaces on nickel-copper double hydroxide nanotyres to pre-activate alkaline ammonia oxidation reactivity[J]. Chemistry—A European Journal, 2021, 27(15): 4869-4875. |
| [85] | JIANG Xuan, YING Diwen, LIU Xi, et al. Identification of the role of Cu site in Ni-Cu hydroxide for robust and high selective electrochemical ammonia oxidation to nitrite[J]. Electrochimica Acta, 2020, 345: 136157. |
| [86] | MEDVEDEV Jury J, TOBOLOVSKAYA Yulia, MEDVEDEVA Xenia V, et al. Pathways of ammonia electrooxidation on nickel hydroxide anodes and an alternative route towards recycled fertilizers[J]. Green Chemistry, 2022, 24(4): 1578-1589. |
| [87] | ZHANG Huimin, WANG Hailong, TONG Xing, et al. Sulfur induced surface reconfiguration of Ni1Cu3-S-T/CP anode for high-efficiency ammonia electro-oxidation[J]. Chemical Engineering Journal, 2023, 452: 139582. |
| [88] | HOU Jing, CHENG Yingying, PAN Hui, et al. Tailored bimetallic Ni-Sn catalyst for electrochemical ammonia oxidation to dinitrogen with high selectivity[J]. Inorganic Chemistry, 2023, 62(9): 3986-3992. |
| [89] | Eglė LATVYTĖ, ZHU Xuanheng, WU Liang, et al. A low-temperature ammonia electrolyser for wastewater treatment and hydrogen production[J]. International Journal of Hydrogen Energy, 2024, 52: 265-282. |
| [90] | ZHANG Shuo, ZHAO Yanchao, YAN Liting, et al. Electrochemical ammonia oxidation reaction on defect-rich TiO nanofibers: Experimental and theoretical studies[J]. International Journal of Hydrogen Energy, 2021, 46(79): 39208-39215. |
| [91] | HUANG Jingjing, CHEN Zhe, CAI Jinmeng, et al. Activating copper oxide for stable electrocatalytic ammonia oxidation reaction via in situ introducing oxygen vacancies[J]. Nano Research, 2022, 15(7): 5987-5994. |
| [92] | UDACHYAN Iranna, BHANUSHALI Jayesh T, MIZRAHI Amir, et al. Manganese carbonate as an efficient electrocatalyst for the conversion of ammonia (NH4 +/NH3) to dinitrogen[J]. Sustainable Energy & Fuels, 2023, 7(17): 4088-4093. |
| [93] | CLEETUS Annie, TELLER Hanan, SCHECHTER Alex. CuCr bimetallic catalyst for selective electrooxidation of ammonia at room temperature[J]. ChemCatChem, 2023, 15(7): e202300035. |
| [1] | 秦菲, 张志, 宋光春, 王武昌, 李玉星, 王世鑫, 何思成, 王江妍. 水合物储氢分子动力学行为研究进展[J]. 化工进展, 2025, 44(S1): 112-123. |
| [2] | 刘哲, 周顺利, 李永祥, 张成喜, 刘宜鹏. 烷基萘合成催化剂研究进展[J]. 化工进展, 2025, 44(S1): 144-158. |
| [3] | 林已杰, 乔鹏, 李心睿, 张宏斌, 王雪芹. TiO2纳米光催化剂的异质结构建策略与应用研究进展[J]. 化工进展, 2025, 44(S1): 159-177. |
| [4] | 王涛, 张雪冰, 张琪, 陈强, 张魁, 门卓武. 还原碳化温度和CO浓度对工业级费托合成沉淀铁催化剂性能的影响[J]. 化工进展, 2025, 44(S1): 178-184. |
| [5] | 包新德, 刘必烨, 黄仁伟, 洪宇豪, 关鑫, 林金国. 生物质基@CuNiOS复合催化剂的制备及其在有机染料还原中的应用[J]. 化工进展, 2025, 44(S1): 185-196. |
| [6] | 赵思阳, 李陈冉, 刘洋. 副产C4预积炭调控MTO再生催化剂双烯选择性的工艺优化[J]. 化工进展, 2025, 44(S1): 205-212. |
| [7] | 赵雨龙, 蔡凯, 于善青. 氧化铝孔结构对催化裂化烃类分子吸附扩散及反应性能的影响[J]. 化工进展, 2025, 44(S1): 213-221. |
| [8] | 李军良, 李悦, 孙道来. Cu/SiO2-Al2O3催化1,2-丁二醇加氢脱氧制备1-丁醇[J]. 化工进展, 2025, 44(S1): 222-231. |
| [9] | 刘超, 丁承奥, 吴宝顺, 雷欣宇, 王光应, 余正伟. TiO2载体粒度对RuO x -V2O5-WO3/TiO2催化剂脱硝及抗水硫中毒性能的影响[J]. 化工进展, 2025, 44(S1): 232-242. |
| [10] | 张涵林, 岳学海, 刘俊希, 殷逢俊. 钌锶铱电沉积构筑高稳定性析氧反应电催化剂[J]. 化工进展, 2025, 44(S1): 243-251. |
| [11] | 王露, 何阳东, 李雅欣, 范锐, 陈仕锦, 张杰. 高性能聚合物膜用于He/CH4和He/N2分离的结构设计与性能优化[J]. 化工进展, 2025, 44(S1): 261-276. |
| [12] | 李芮莹, 周颖, 周红军, 徐春明. 生物质衍生纳米碳基材料:电化学场景下的机遇与挑战[J]. 化工进展, 2025, 44(S1): 288-306. |
| [13] | 管思颖, 问金月, 焦守政, 郝雨薇, 孙志成. 染料敏化太阳能电池氧化还原电对[J]. 化工进展, 2025, 44(S1): 350-367. |
| [14] | 邹先志, 廖亚龙, 杨双宇. 铜电解液净化除杂研究进展[J]. 化工进展, 2025, 44(S1): 492-503. |
| [15] | 周寅聪, 熊小鹤, 郭富文, 张一楠, 谭厚章. 20G和T91钢在含硫和氯元素的熔盐环境中的热腐蚀行为[J]. 化工进展, 2025, 44(S1): 541-550. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||