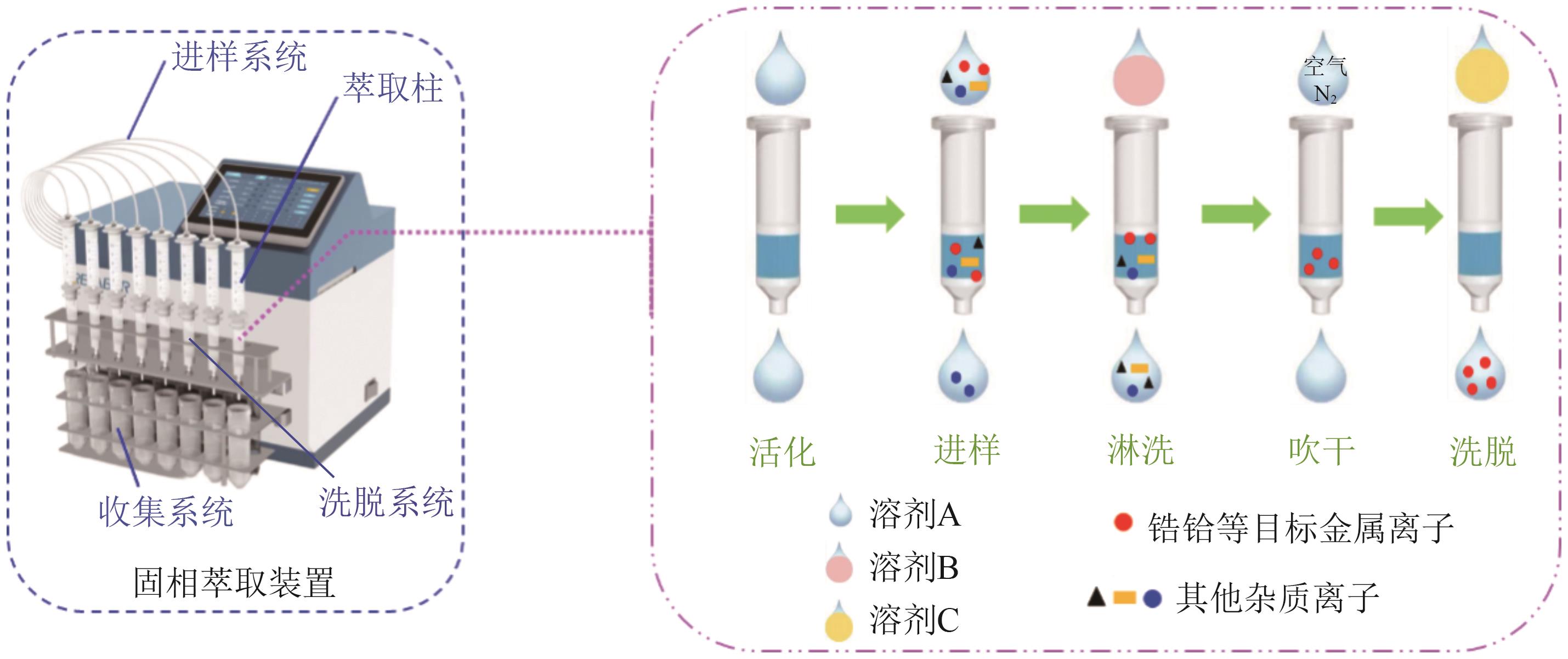
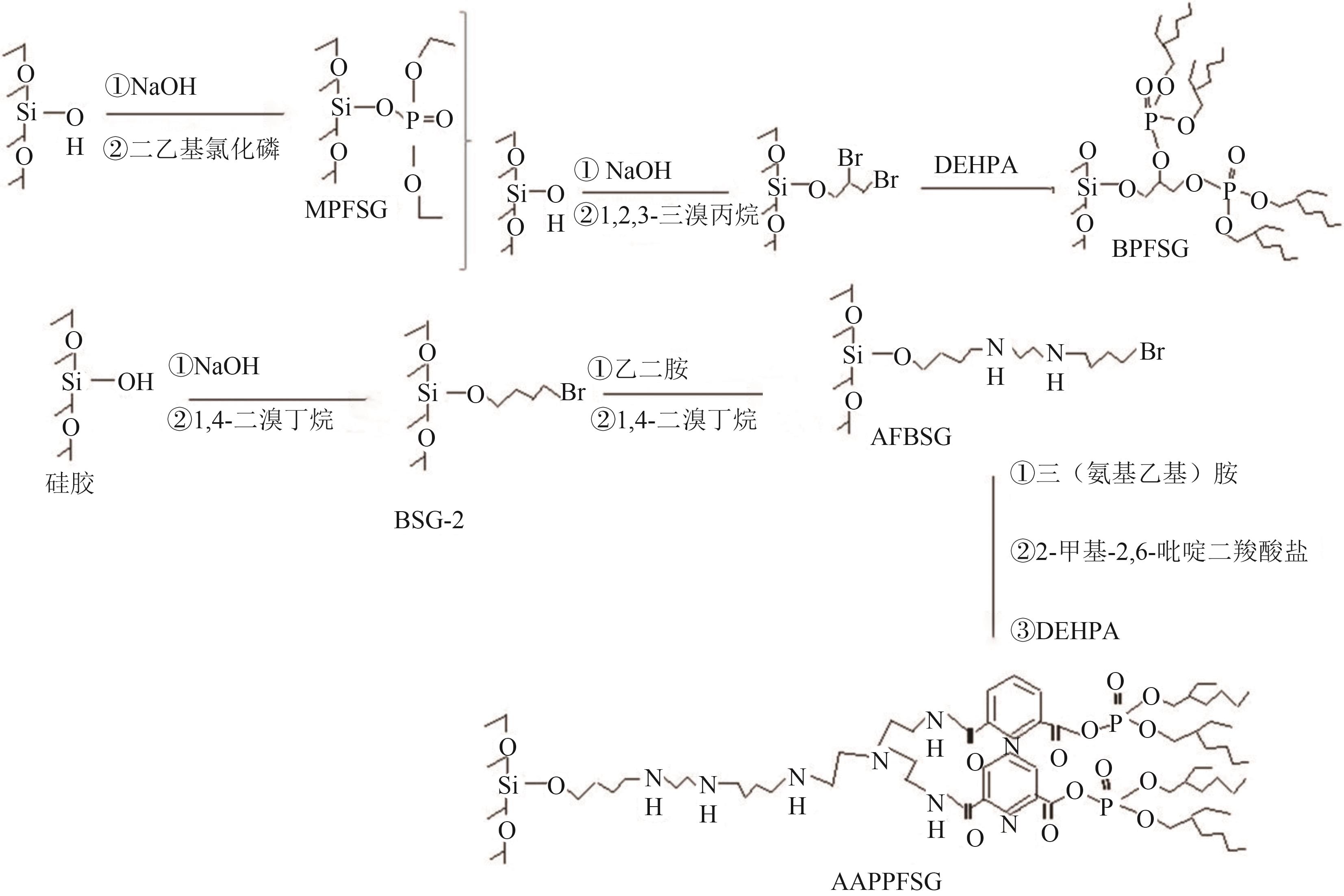
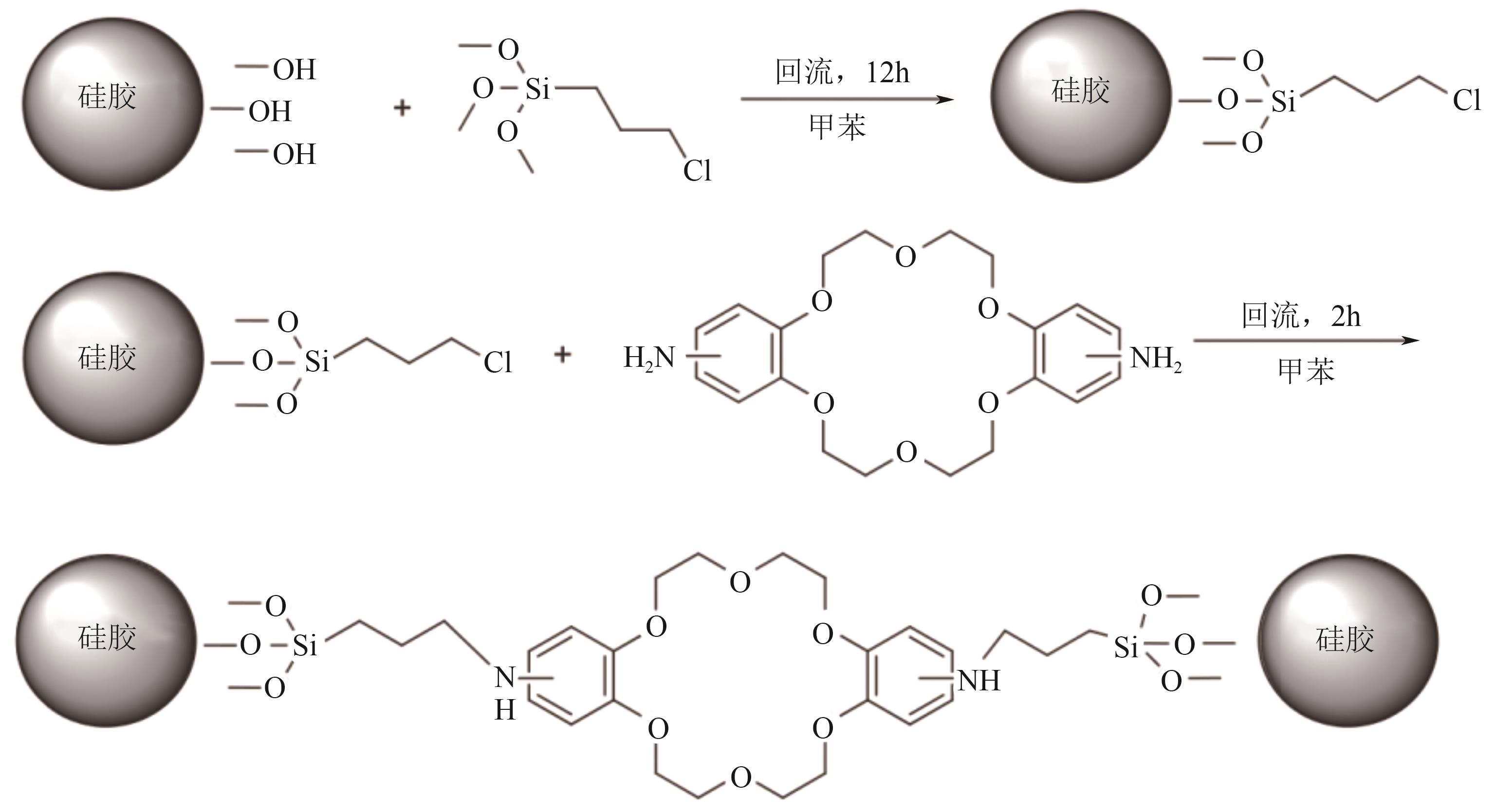
化工进展 ›› 2025, Vol. 44 ›› Issue (10): 5991-6003.DOI: 10.16085/j.issn.1000-6613.2024-1443
• 资源与环境化工 • 上一篇
基于萃取色层法分离锆铪的萃淋树脂制备、结构与性能
杨秀敏1( ), 胡成强1, 王俊莲1(
), 胡成强1, 王俊莲1( ), 李勇2, 刘新宇3
), 李勇2, 刘新宇3
- 1.北京科技大学土木与资源工程学院,北京 100083
2.自然资源部咨询研究中心,北京 100035
3.北方矿业有限责任公司,北京 100053
-
收稿日期:2024-09-03修回日期:2024-11-18出版日期:2025-10-25发布日期:2025-11-10 -
通讯作者:王俊莲 -
作者简介:杨秀敏(2000—),女,硕士研究生,研究方向为锆铪萃取分离。E-mail:xiuminyang2022@163.com。 -
基金资助:国家自然科学基金(51974026)
Preparation, structures and performance of extraction resins based on zirconium and hafnium separation with extraction chromatography
YANG Xiumin1( ), HU Chengqiang1, WANG Junlian1(
), HU Chengqiang1, WANG Junlian1( ), LI Yong2, LIU Xinyu3
), LI Yong2, LIU Xinyu3
- 1.School of Civil and Resource Engineering, University of Science and Technology Beijing, Beijing 100083, China
2.Consulting and Research Center of Ministry of Natural Resources, Beijing 100035, China
3.Norin Mining Co. , Ltd. , Beijing 100053, China
-
Received:2024-09-03Revised:2024-11-18Online:2025-10-25Published:2025-11-10 -
Contact:WANG Junlian
摘要:
锆、铪是核工业中无法替代的材料,其分离技术的发展在高端技术领域至关重要。萃取色层法结合了离子交换法和溶剂萃取法的优点,生产能力大、分离效率高、选择性好,在锆铪分离中展现出巨大潜力,其中起关键作用的是萃淋树脂。本文按照萃取官能团的酸碱性,将已报道的锆铪分离萃淋树脂按照中性基团(酮类、中性磷类、冠醚类、酰胺类)、酸性基团(有机磷酸类、磺酸类)和碱性基团(含N类、季铵盐类)修饰的萃淋树脂进行分类,对其制备方法、结构与性能进行了讨论与总结。酮类基团修饰的萃淋树脂对锆铪吸附能力很弱,锆铪分离效率低;中性磷类基团、含N类和季铵盐类基团修饰的萃淋树脂需在高酸度下分离锆铪,分离体系腐蚀性大,对设备要求高;冠醚类基团可识别并匹配锆、铪金属离子,但价格十分昂贵,其应用受限;酰胺类基团修饰的萃淋树脂对锆、铪的饱和吸附量较大,但研究有限;酸性基团修饰的萃淋树脂对锆、铪具有强吸附能力和高饱和吸附量,但锆铪分离系数较低,且反萃困难。鉴于此,开发支链较多的二烷基次膦酸官能团修饰的萃淋树脂可有利于实现锆铪的高效吸附分离。
中图分类号:
引用本文
杨秀敏, 胡成强, 王俊莲, 李勇, 刘新宇. 基于萃取色层法分离锆铪的萃淋树脂制备、结构与性能[J]. 化工进展, 2025, 44(10): 5991-6003.
YANG Xiumin, HU Chengqiang, WANG Junlian, LI Yong, LIU Xinyu. Preparation, structures and performance of extraction resins based on zirconium and hafnium separation with extraction chromatography[J]. Chemical Industry and Engineering Progress, 2025, 44(10): 5991-6003.
| 树脂 | 官能团 | 基底 | 制备方法 | 体系最佳条件 | 选择性 | Qmax(Hf)/mg·g-1 | Qmax(Zr)/mg·g-1 | 分离系数β | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| SIR-MIBK/HPD100 | 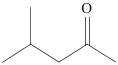 | HPD100 | 浸渍法 | [NH4SCN]=0.5mol/L,[HCl]=1.1mol/L | Hf | 14.33 | 473.80 | — | [ |
| SIR-TBP/PEG-coated-SPIONs | 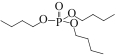 | PEG-coated-γ-Fe2O3 | 浸渍法 | [HNO3]=4.0mol/L | Zr | — | — | βZr/Hf=5 | [ |
| [HCl]=4.0mol/L | Hf | — | — | βHf/Zr=1.4 | |||||
| UTEVA | 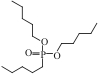 | — | 浸渍法 | [HCl]=5.6mol/L | Zr | — | — | βZr/Hf>9.4 | [ |
| [HNO3]=4.9mol/L | Zr | — | — | βZr/Hf=10±1 | |||||
| [H2SO4]=12.1mol/L | Hf | — | — | βHf/Zr=2.0±0.2 | |||||
| MPFSG | 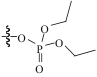 | SiO2 | 接枝法 | HNO3, pH=1.8 | Zr | 11.8 | 12.3 | βZr/Hf=1.1 | [ |
| BPFSG | 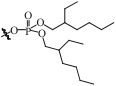 | SiO2 | 接枝法 | HNO3, pH=1.8 | Zr | 15 | 16.7 | βZr/Hf=1.2 | [ |
| AAPPFSG | 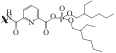 | SiO2 | 接枝法 | HNO3, pH=1.8 | Zr | 24 | 29 | βZr/Hf=11.0 | [ |
| SGN18 | 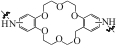 | SiO2 | 接枝法 | HCl, pH=1.5 | Zr | 3.3 | 38.3 | βZr/Hf=5.72 | [ |
| SIR-Calix[ | 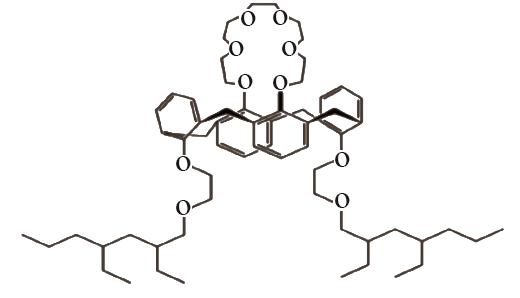 | SiO2-P | 浸渍法 | [HCl]=1mol/L | Zr | — | — | βZr/Hf=1.4 | [ |
| SIR-TODGA/SiO2-P | 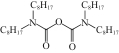 | SiO2-P | 浸渍法 | [HCl]=1.5mol/L | Zr | 75.85 | 44.24 | βZr/Hf=5.2 | [ |
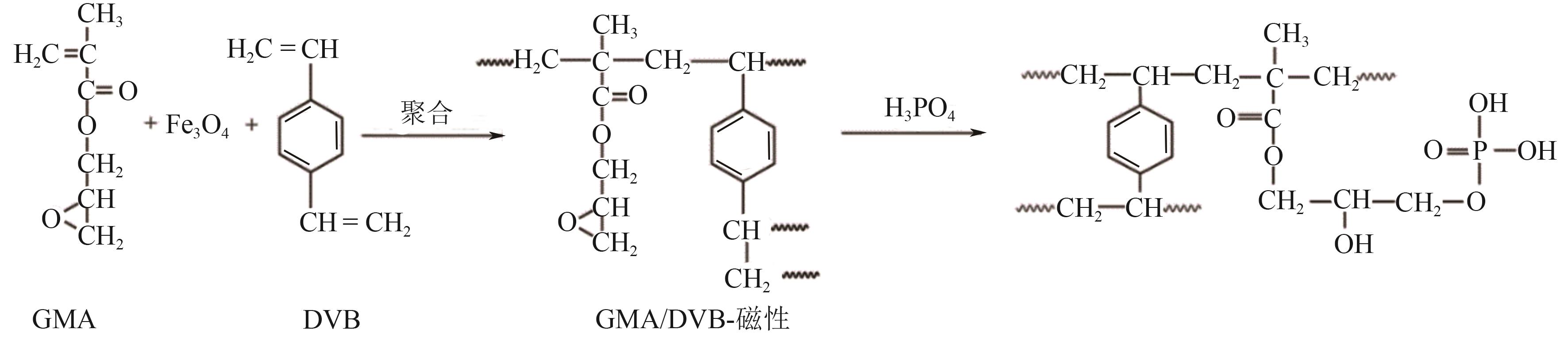
| R-PO4H2 | 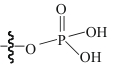 | GMA/DVB 修饰的 Fe3O4 | 接枝法 | HCl, pH=2.5 | Zr | 42.88 | 85.75 | βZr/Hf=6 | [ |
| TVEX-P507 | 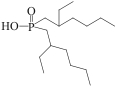 | 苯乙烯-二乙烯基共聚物 | 原位聚合法 | [H2SO4]=0.08mol/L | Hf | 0.21 | — | βHf/Zr=6.81 | [ |
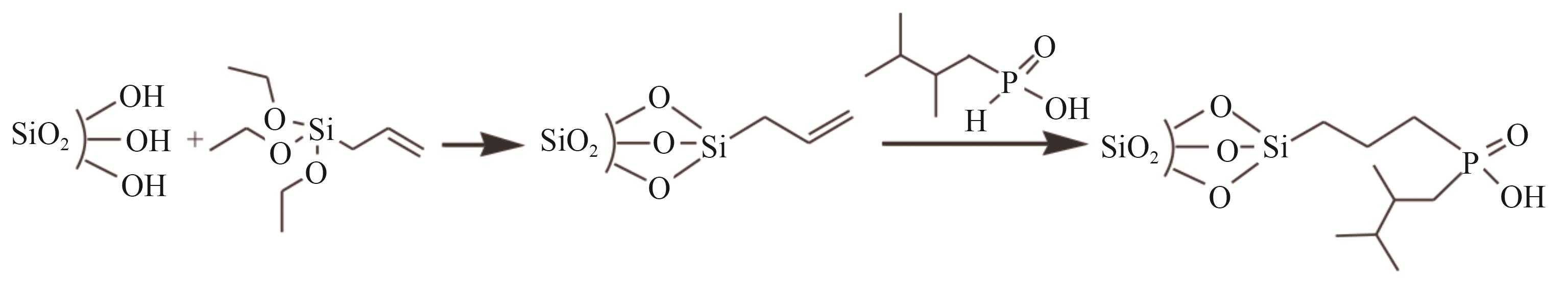
| SiO2-POOH | 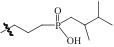 | SiO2 | 接枝法 | [H2SO4]=0.5mol/L | Hf | 31.62 | — | βHf/Zr =4.69 | [ |
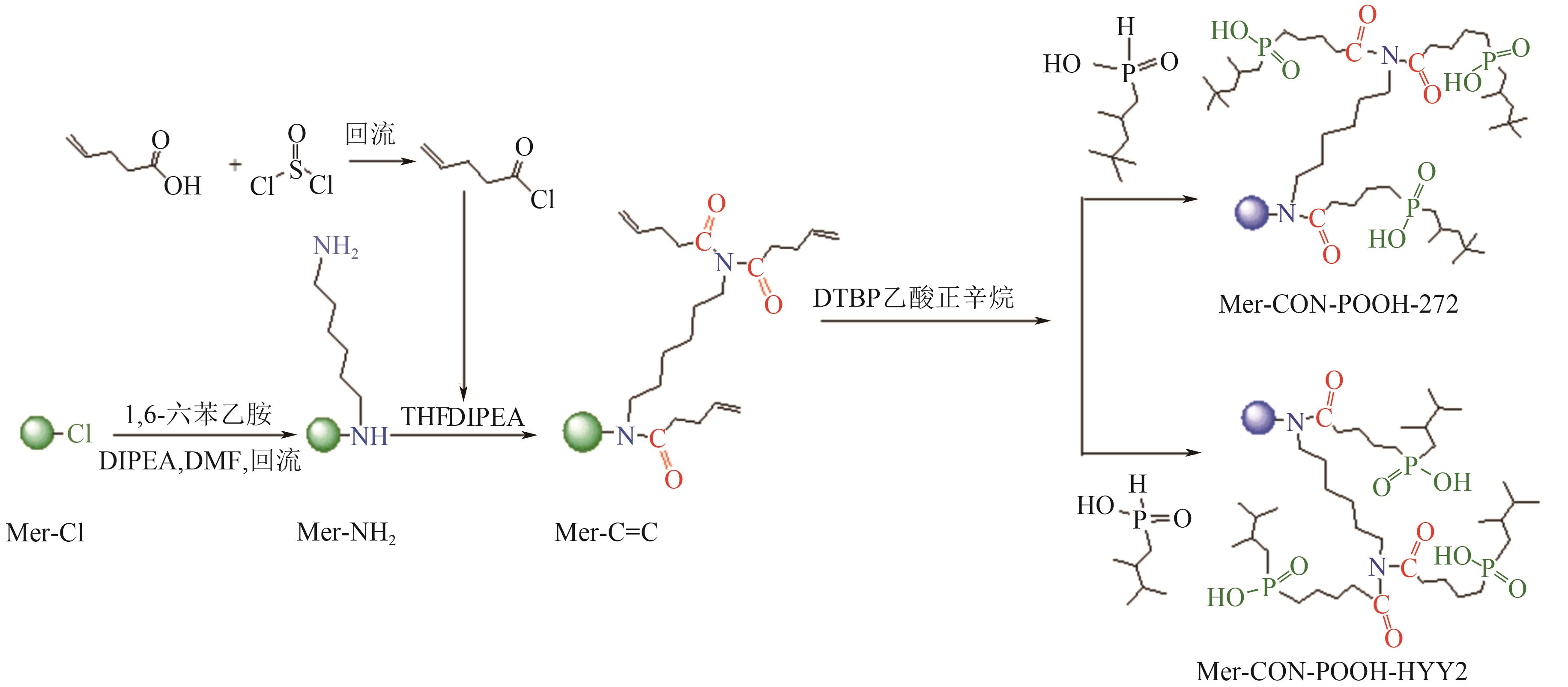
| Mer-CON-POOH-HYY2 | 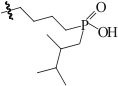 | 氯球 | 接枝法 | [H2SO4]=0.3mol/L | Hf | 13.16 | — | βHf/Zr=4.33 | [ |
| Mer-CON-POOH-272 | 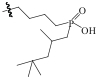 | 氯球 | 接枝法 | [H2SO4]=0.3mol/L | Hf | 11.11 | — | βHf/Zr=2.96 | |
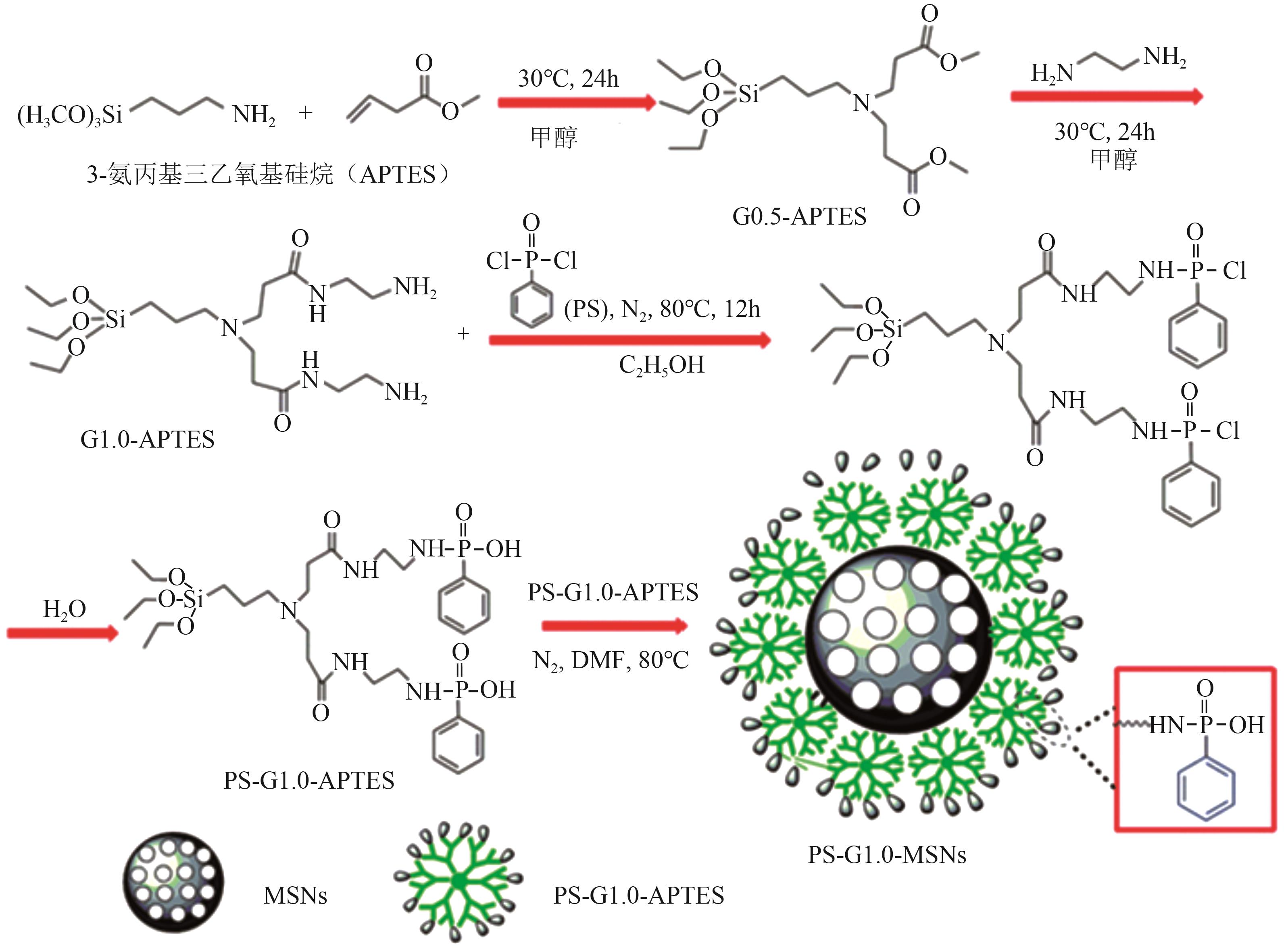
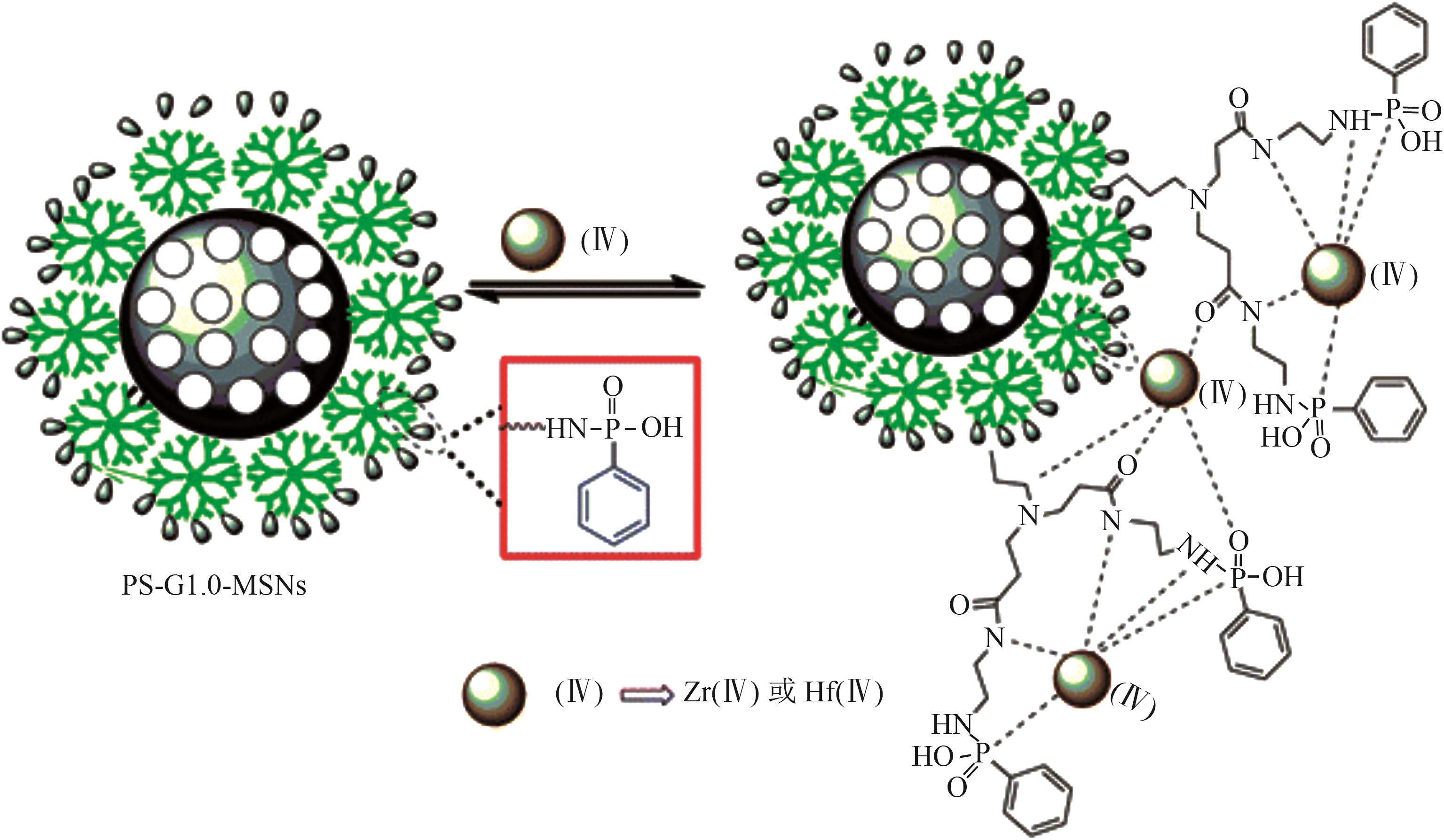
| PS-G1.0-MSNs | 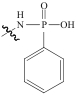 | SiO2 | 接枝法 | HNO3, pH=0.5 | Hf | 5.36 | 25.7 | βHf/Zr=2.0 | [ |
| Marathon C | —SO3H | 苯乙烯-二乙烯基共聚物 | 接枝法 | H2SO4, pH=2.5 | Hf | 24.92 | 103.74 | βHf/Zr=2.63 | [ |
| Diphonix | —PO4H2、—SO3H、—COOH | 苯乙烯-二乙烯基共聚物 | 接枝法 | [H2SO4]=1mol/L | Hf | — | — | βHf/Zr=1.47 | [ |
| Purolite S-957 | —PO4H2、—SO3H | 苯乙烯-二乙烯基共聚物 | 接枝法 | [H2SO4]=0.5mol/L | Hf | — | — | βHf/Zr=2.8 | [ |
| SIR-BTP/ SiO2-P | 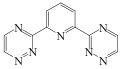 | SiO2-P | 浸渍法 | [HCl]=1mol/L | Hf | — | — | βHf/Zr=1.1 | [ |
| Reillex PVP | 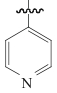 | 苯乙烯-二乙烯基共聚物 | 接枝法 | [HCl]=9.5mol/L | Zr | — | — | βZr/Hf=10.4 | [ |
| TEVA | 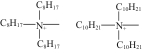 | — | 浸渍法 | [HCl]=8.4mol/L | Zr | — | — | βZr/Hf=18±8 | [ |
| Amberjet 4200 Cl | 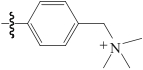 | 苯乙烯-二乙烯基共聚物 | 接枝法 | [HCl]=9.5mol/L | Zr | — | 86 | βZr/Hf=11.4 | [ |
| Reillex HPQ | 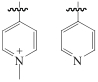 | 聚乙烯基吡咯烷酮-二乙烯基苯 | — | [HCl]=9.5mol/L | Zr | — | — | βZr/Hf=9.7 | [ |
表1 基于锆铪分离的萃淋树脂结构与性能
| 树脂 | 官能团 | 基底 | 制备方法 | 体系最佳条件 | 选择性 | Qmax(Hf)/mg·g-1 | Qmax(Zr)/mg·g-1 | 分离系数β | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| SIR-MIBK/HPD100 |  | HPD100 | 浸渍法 | [NH4SCN]=0.5mol/L,[HCl]=1.1mol/L | Hf | 14.33 | 473.80 | — | [ |
| SIR-TBP/PEG-coated-SPIONs |  | PEG-coated-γ-Fe2O3 | 浸渍法 | [HNO3]=4.0mol/L | Zr | — | — | βZr/Hf=5 | [ |
| [HCl]=4.0mol/L | Hf | — | — | βHf/Zr=1.4 | |||||
| UTEVA |  | — | 浸渍法 | [HCl]=5.6mol/L | Zr | — | — | βZr/Hf>9.4 | [ |
| [HNO3]=4.9mol/L | Zr | — | — | βZr/Hf=10±1 | |||||
| [H2SO4]=12.1mol/L | Hf | — | — | βHf/Zr=2.0±0.2 | |||||
| MPFSG |  | SiO2 | 接枝法 | HNO3, pH=1.8 | Zr | 11.8 | 12.3 | βZr/Hf=1.1 | [ |
| BPFSG |  | SiO2 | 接枝法 | HNO3, pH=1.8 | Zr | 15 | 16.7 | βZr/Hf=1.2 | [ |
| AAPPFSG |  | SiO2 | 接枝法 | HNO3, pH=1.8 | Zr | 24 | 29 | βZr/Hf=11.0 | [ |
| SGN18 |  | SiO2 | 接枝法 | HCl, pH=1.5 | Zr | 3.3 | 38.3 | βZr/Hf=5.72 | [ |
| SIR-Calix[ |  | SiO2-P | 浸渍法 | [HCl]=1mol/L | Zr | — | — | βZr/Hf=1.4 | [ |
| SIR-TODGA/SiO2-P |  | SiO2-P | 浸渍法 | [HCl]=1.5mol/L | Zr | 75.85 | 44.24 | βZr/Hf=5.2 | [ |
| R-PO4H2 |  | GMA/DVB 修饰的 Fe3O4 | 接枝法 | HCl, pH=2.5 | Zr | 42.88 | 85.75 | βZr/Hf=6 | [ |
| TVEX-P507 |  | 苯乙烯-二乙烯基共聚物 | 原位聚合法 | [H2SO4]=0.08mol/L | Hf | 0.21 | — | βHf/Zr=6.81 | [ |
| SiO2-POOH |  | SiO2 | 接枝法 | [H2SO4]=0.5mol/L | Hf | 31.62 | — | βHf/Zr =4.69 | [ |
| Mer-CON-POOH-HYY2 |  | 氯球 | 接枝法 | [H2SO4]=0.3mol/L | Hf | 13.16 | — | βHf/Zr=4.33 | [ |
| Mer-CON-POOH-272 |  | 氯球 | 接枝法 | [H2SO4]=0.3mol/L | Hf | 11.11 | — | βHf/Zr=2.96 | |
| PS-G1.0-MSNs |  | SiO2 | 接枝法 | HNO3, pH=0.5 | Hf | 5.36 | 25.7 | βHf/Zr=2.0 | [ |
| Marathon C | —SO3H | 苯乙烯-二乙烯基共聚物 | 接枝法 | H2SO4, pH=2.5 | Hf | 24.92 | 103.74 | βHf/Zr=2.63 | [ |
| Diphonix | —PO4H2、—SO3H、—COOH | 苯乙烯-二乙烯基共聚物 | 接枝法 | [H2SO4]=1mol/L | Hf | — | — | βHf/Zr=1.47 | [ |
| Purolite S-957 | —PO4H2、—SO3H | 苯乙烯-二乙烯基共聚物 | 接枝法 | [H2SO4]=0.5mol/L | Hf | — | — | βHf/Zr=2.8 | [ |
| SIR-BTP/ SiO2-P |  | SiO2-P | 浸渍法 | [HCl]=1mol/L | Hf | — | — | βHf/Zr=1.1 | [ |
| Reillex PVP |  | 苯乙烯-二乙烯基共聚物 | 接枝法 | [HCl]=9.5mol/L | Zr | — | — | βZr/Hf=10.4 | [ |
| TEVA | 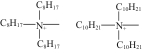 | — | 浸渍法 | [HCl]=8.4mol/L | Zr | — | — | βZr/Hf=18±8 | [ |
| Amberjet 4200 Cl |  | 苯乙烯-二乙烯基共聚物 | 接枝法 | [HCl]=9.5mol/L | Zr | — | 86 | βZr/Hf=11.4 | [ |
| Reillex HPQ |  | 聚乙烯基吡咯烷酮-二乙烯基苯 | — | [HCl]=9.5mol/L | Zr | — | — | βZr/Hf=9.7 | [ |
| [1] | SHATALOV V V, NIKONOV V I, KOTSAR M L. Prospects for zirconium and hafnium supplies for nuclear power in Russia up to 2030[J]. Atomic Energy, 2008, 105(4): 242-247. |
| [2] | XU L, XIAO Y, VAN SANDWIJK A, et al. Production of nuclear grade zirconium: A review[J]. Journal of Nuclear Materials, 2015, 466: 21-28. |
| [3] | 王俊莲, 付家帅, 许文, 等. 基于锆铪溶剂萃取分离的萃取剂性能[J]. 稀有金属, 2020, 44(6): 658-667. |
| WANG Junlian, FU Jiashuai, XU Wen, et al. Performance of various extractants for zirconium and hafnium separation by solvent extraction[J]. Chinese Journal of Rare Metals, 2020, 44(6): 658-667. | |
| [4] | XU L, XIAO Y, VAN SANDWIJK A, et al. Separation of zirconium and hafnium: A review[C]//Energy Materials 2014. Cham: Springer International Publishing, 2014: 451-457. |
| [5] | 郭宁, 俞中华. 钽铌锆铪行业“十三五”前瞻[J]. 中国有色金属, 2016(11): 48-49. |
| GUO Ning, YU Zhonghua. Prospects of the tantalum, niobium, zirconium and hafnium industries during the period of the 13th Five-Years Plan[J]. China Nonferrous Metals, 2016(11): 48-49. | |
| [6] | NISELSON Lev A, EGOROV Egor A, CHUVILINA Elena L, et al. Solid-liquid and liquid-vapor equilibria in the Zr(Hf)Cl4-KalCl4 systems: A basis for the extractive distillation separation of zirconium and hafnium tetrachlorides[J]. Journal of Chemical & Engineering Data, 2009, 54(3): 726-729. |
| [7] | 徐亮. 熔盐萃取锆铪分离和锆在熔盐中的电化学行为[D]. 沈阳: 东北大学, 2017. |
| XU Liang. Separation of zirconium and hafnium with molten salt extraction and electrochemical behavior of zirconium in molten salt[D]. Shenyang: Northeastern University, 2017. | |
| [8] | 柴延全. 熔盐萃取法分离锆铪的研究[D]. 马鞍山: 安徽工业大学, 2017. |
| CHAI Yanquan. Research on the separation of zirconium and hafnium by molten salt extraction[D]. Ma’anshan: Anhui University of Technology, 2017. | |
| [9] | BRANKEN D J, LACHMANN G, KRIEG H M, et al. A density-functional theory approach to the separation of K2ZrF6 and K2HfF6 via fractional crystallization[J]. International Journal of Quantum Chemistry, 2011, 111(3): 682-693. |
| [10] | WANG Junlian, LIU Hui, ZHAO Hongru, et al. Selective extraction of Hf over Zr by a novel extractant (n-octyl)(2,4,4′-trimethylpentyl)phosphinic acid (INET-1) from sulfuric acid media[J]. Journal of Radioanalytical and Nuclear Chemistry, 2023, 332(7): 2473-2485. |
| [11] | ZHAO Hongru, HU Chengqiang, SUI Na, et al. Evaluation of novel solvent extraction systems for Zr/Hf separation with MIBK homologues[J]. Arabian Journal of Chemistry, 2024, 17(1): 105425. |
| [12] | TANG Tingting, YANG Fan, XIE Meiying, et al. Highly efficient separation and enrichment of hafnium from zirconium oxychloride solutions by advanced ion-imprinted membrane separation technology[J]. Journal of Membrane Science, 2023, 668: 121237. |
| [13] | ALFONSO M C, BENNETT M E, FOLDEN C M. Extraction chromatography of the Rf homologs, Zr and Hf, using TEVA and UTEVA resins in HCl, HNO3, and H2SO4 media[J]. Journal of Radioanalytical and Nuclear Chemistry, 2016, 307(2): 1529-1536. |
| [14] | XU Zhigao, WU Yanke, ZHANG Jiandong, et al. Equilibrium and kinetic data of adsorption and separation for zirconium and hafnium onto MIBK extraction resin[J]. Transactions of Nonferrous Metals Society of China, 2010, 20(8): 1527-1533. |
| [15] | 王俊莲, 孙春宝, 徐盛明. 基于稀土分离的萃淋树脂制备与应用研究[J]. 中国稀土学报, 2015, 33(2): 129-145. |
| WANG Junlian, SUN Chunbao, XU Shengming. Advances in preparation and application of solid-liquid extraction resins based on rare earth separation[J]. Journal of the Chinese Society of Rare Earths, 2015, 33(2): 129-145. | |
| [16] | FAN Jinlong, DUAN Li, WANG Yufeng, et al. Assembly of a polymer-based extraction resin and separation of minor actinides[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 610: 125473. |
| [17] | HUANG Weini, LIN Zian. Recent advances in sample pretreatment techniques for chromatographic analysis[J]. Se Pu=Chinese Journal of Chromatography, 2021, 39(1): 1-3. |
| [18] | Semih ÖTLES, KARTAL Canan. Solid-phase extraction (SPE): Principles and applications in food samples[J]. Acta Scientiarum Polonorum Technologia Alimentaria, 2016, 15(1): 5-15. |
| [19] | 李华昌, 周春山, 符斌. 萃淋树脂技术及其在湿法冶金中的应用[J]. 有色金属, 2001(1): 70-73. |
| LI Huachang, ZHOU Chunshan, FU Bin. Extraction resin technology and its application in wet metallurgy[J]. Nonferrous Metals, 2001(1): 70-73. | |
| [20] | 熊洁, 许云书, 黄玮. 偕胺肟基螯合吸附分离材料研究进展[J]. 材料导报, 2006, 20(7): 102-104, 108. |
| XIONG Jie, XU Yunshu, HUANG Wei. Recent development of absorption and separation materials with amidoxime group[J]. Materials Review, 2006, 20(7): 102-104, 108. | |
| [21] | Nadia A ALI, ABD-ELNAIEM Alaa M, HUSSEIN Seenaa I, et al. Thermal and mechanical properties of epoxy resin functionalized copper and graphene hybrids using in situ polymerization method[J]. Current Nanoscience, 2021, 17(3): 494-502. |
| [22] | LANASA Jacob A, TORRES Vincent M, HICKEY Robert J. In situ polymerization and polymer grafting to stabilize polymer-functionalized nanoparticles in polymer matrices[J]. Journal of Applied Physics,2020, 127(13): 134701. |
| [23] | BORAI Emad, KARESOJA Mikko, HARJULA Risto. Separation of cobalt from europium with mesoporous hybrid silica-poly(styrene) impregnated chelating resin[J]. Mineral Processing and Extractive Metallurgy Review, 2013, 34(1): 57-72. |
| [24] | ZHANG Xiaofeng, Zihao OU, XIANG Jinxin. Fabrication of magnetic activated carbon from waste macroporous resin via Fenton’s reagent impregnation[J]. Journal of Porous Materials, 2021, 28(1): 165-170. |
| [25] | THEBAULT Marion, KUTUZOVA Larysa, JURY Sandra, et al. Effect of phenolation, lignin-type and degree of substitution on the properties of lignin-modified phenol-formaldehyde impregnation resins: Molecular weight distribution, wetting behavior, rheological properties and thermal curing profiles[J]. Journal of Renewable Materials, 2020, 8(6): 603-630. |
| [26] | ZHANG Zhen, ZHOU Yuedi, ZHOU Jingbo, et al. Synthesis of TOPO/XAD-16 impregnated resins and effective adsorption of uranium (Ⅵ) in acidic solution[J]. Journal of Radioanalytical and Nuclear Chemistry, 2023, 332(4): 1149-1162. |
| [27] | MATSUNAGA Hideyuki, ISMAIL Adel ALI, WAKUI Yoshito, et al. Extraction of rare earth elements with 2-ethylhexyl hydrogen 2-ethylhexyl phosphonate impregnated resins having different morphology and reagent content[J]. Reactive and Functional Polymers, 2001, 49(3): 189-195. |
| [28] | GENG Shiyu, WEI Jiayuan, Yvonne AITOMÄKI, et al. Well-dispersed cellulose nanocrystals in hydrophobic polymers by in situ polymerization for synthesizing highly reinforced bio-nanocomposites[J]. Nanoscale, 2018, 10(25): 11797-11807. |
| [29] | 柏雨婷, 严岑琪, 李祯, 等. 采用原位成孔法制备热闭孔特性的高强度聚酰亚胺多孔薄膜[J]. 物理化学学报, 2024, 40(9): 83-89. |
| BAI Yuting, YAN Cenqi, LI Zhen, et al. Preparation of high strength polyimide porous films with thermal closed pore characteristics by in situ porosity method [J]. Journal of Physical Chemistry, 2024, 40(9): 83-89. | |
| [30] | WANG Jinhui, YANG Shaowei, MA Fubin, et al. RuCo alloy nanoparticles embedded within N-doped porous two-dimensional carbon nanosheets: A high-performance hydrogen evolution reaction catalyst[J]. Tungsten, 2024, 6(1): 114-123. |
| [31] | 郎倩, 竹筱歆, 张丽新, 等. 原位聚合法制备可降解纤维基地膜及机理分析[J]. 农业工程学报, 2023, 39(15): 249-258. |
| LANG Qian, ZHU Xiaoxin, ZHANG Lixin, et al. Fabrication and mechanism analysis of degradable fiber membrane by in situ polymerization[J]. Transactions of the Chinese Society of Agricultural Engineering, 2023, 39(15): 249-258. | |
| [32] | LIU Yisi, CHEN Zhicheng, LI Zongxu, et al. CoNi nanoalloy-Co-N4 composite active sites embedded in hierarchical porous carbon as bi-functional catalysts for flexible Zn-air battery[J]. Nano Energy, 2022, 99: 107325. |
| [33] | FU Yihan, PENG Yuanyou, ZHAO Lei, et al. Vanadium nitride quantum dots@carbon skeleton anode material synthesized via in situ oxidation initiation strategy[J]. Tungsten, 2024, 6(3): 561-573. |
| [34] | KAUCZOR H W, MEYER A. Structure and properties of levextrel resins[J]. Hydrometallurgy, 1978, 3(1): 65-73. |
| [35] | TAMANG Aditya Moktan, SINGH Nitesh, CHANDRAKER Sandip Kumar, et al. Solvent impregnated resin a potential alternative material for separation dyes, metal and phenolic compounds: A review[J]. Current Research in Green and Sustainable Chemistry, 2022, 5: 100232. |
| [36] | 吴明. DIBK-TBP体系萃取分离锆和铪的动力学研究[D]. 武汉: 武汉工程大学, 2012. |
| WU Ming. Kinetic study on extraction and separation of zirconium and hafnium by DIBK-TBP system[D]. Wuhan: Wuhan Institute of Technology, 2012. | |
| [37] | 林振汉. 用甲基异丁基酮萃取分离锆铪的工艺评价[J]. 稀有金属快报, 2007, 26(1): 93-96. |
| LIN Zhenhan. Extraction separation of zirconium and hafnium with methyl isobutyl ketone (MIBK)[J]. 2007, 26(1): 93-96. | |
| [38] | 徐志高, 王力军, 池汝安, 等. DIBK溶剂萃取法分离锆铪[J]. 有色金属(冶炼部分), 2012(3): 35-38, 42. |
| XU Zhigao, WANG Lijun, CHI Ruan, et al. Solvent extraction and separation of hafnium from zirconium with DIBK[J]. Nonferrous Metals (Extractive Metallurgy), 2012(3): 35-38, 42. | |
| [39] | 林振汉. 用TBP萃取分离和的工艺研究[J]. 稀有金属快报, 2004, 23(11): 21-25. |
| LIN Zhenhan. Study of the technological process for extractive separation of zirconium-hafnium with tributyl phosphate[J]. Rare Metals Letters, 2004, 23(11): 21-25. | |
| [40] | ALIAKBARI Mohsen, SABERYAN Kamal, NOAPARAST Mohammad, et al. Separation of hafnium and zirconium using TBP modified ferromagnetic nanoparticles: Effects of acid and metals concentrations[J]. Hydrometallurgy, 2014, 146: 72-75. |
| [41] | Amrita DAS, CHANDRAKUMAR K R S, PAUL Bhaskar, et al. Enhanced adsorption and separation of zirconium and hafnium under mild conditions by phosphoric acid based ligand functionalized silica gels: Insights from experimental and theoretical investigations[J]. Separation and Purification Technology, 2020, 239: 116518. |
| [42] | JAL P K, PATEL S, MISHRA B K. Chemical modification of silica surface by immobilization of functional groups for extractive concentration of metal ions[J]. Talanta, 2004, 62(5): 1005-1028. |
| [43] | SMITH Jacob B, KERR Stewart H, WHITE Peter S, et al. Thermodynamic studies of cation-macrocycle interactions in nickel pincer-crown ether complexes enable switchable ligation[J]. Organometallics, 2017, 36(16): 3094-3103. |
| [44] | YIN Xiangbiao, WEI Yuezhou, ZU Jianhua. Adsorption behavior of Zr(Ⅳ) and Hf(Ⅳ) on a silica-based macroporous TODGA adsorbent[J]. Nuclear Science and Techniques, 2013, 24(4): 11-18. |
| [45] | QIN Wei, XU Shengming, XU Gang, et al. Preparation of silica gel bound crown ether and its extraction performance towards zirconium and hafnium[J]. Chemical Engineering Journal, 2013, 225: 528-534. |
| [46] | KUMARI Shikha, CARMONA Angelica V, TIWARI Amit K, et al. Amide bond bioisosteres: Strategies, synthesis, and successes[J]. Journal of Medicinal Chemistry, 2020, 63(21): 12290-12358. |
| [47] | Sandra AGUDO-ÁLVAREZ, DÍAZ-MÍNGUEZ Sandra S, Raúl BENITO-ARENAS. The amide group and its preparation methods by acid-amine coupling reactions: An overview[J]. Pure and Applied Chemistry, 2024, 96(5): 691-707. |
| [48] | WANG Ling yun, LEE Man Seung. A review on the aqueous chemistry of Zr(Ⅳ) and Hf(Ⅳ) and their separation by solvent extraction[J]. Journal of Industrial and Engineering Chemistry, 2016, 39: 1-9. |
| [49] | BANDA Raju, LEE Man Seung. Solvent extraction for the separation of Zr and Hf from aqueous solutions[J]. Separation & Purification Reviews, 2015, 44(3): 199-215. |
| [50] | DONIA A M, ATIA A A, DAHER A M, et al. Extraction and separation of zirconium(Ⅳ) and hafnium(Ⅳ) from chloride media using magnetic resin with phosphoric acid functionality[J]. Journal of Dispersion Science and Technology, 2011, 32(2): 193-202. |
| [51] | 周新木, 董雪平, 陈慧勤, 等. 改进的P507在H2SO4体系中对锆铪的静态吸附实验[J]. 有色金属(冶炼部分), 2009(4): 26-29. |
| ZHOU Xinmu, DONG Xueping, CHEN Huiqin, et al. Captive adsorption of zirconium and hafnium in H2SO4 with improved P507[J]. Nonferrous Metals (Extractive Metallurgy), 2009(4): 26-29. | |
| [52] | WANG Junlian, LIU Hui, XU Wen, et al. Selective extraction of hafnium over zirconium with dialkylphosphinic acids from H2SO4 media[J]. Journal of the Brazilian Chemical Society, 2023: 34(7): 1003-1012. |
| [53] | BANDA Raju, MIN Soo Hwan, LEE Man Seung. Selective extraction of Hf(Ⅳ) over Zr(Ⅳ) from aqueous H2SO4 solutions by solvent extraction with acidic organophosphorous based extractants[J]. Journal of Chemical Technology & Biotechnology, 2014, 89(11): 1712-1719. |
| [54] | 刘辉, 王俊莲, 徐国栋, 等. 二烷基次膦酸改性的SiO2基萃淋树脂的制备及吸附分离锆铪的性能[J]. 离子交换与吸附, 2023, 39(1): 1-16. |
| LIU Hui, WANG Junlian, XU Guodong, et al. Dialkylphosphinic acid modified SiO2-based extraction resin: Preparation and its adsorption and separation performance for zirconium and hafnium[J]. Ion Exchange and Adsorption, 2023, 39(1): 1-16. | |
| [55] | HU Chengqiang, ZHAO Hongru, SUN Ruiyi, et al. Novel dialkylphosphinic acid modified Merrifield resins: Synthesis, characterization, and their adsorption and separation behaviors for zirconium and hafnium[J]. Separation and Purification Technology, 2025, 353: 128539. |
| [56] | WANG Junlian, CHEN Guang, XU Shengming, et al. Synthesis of novel nonsymmetric dialkylphosphinic acid extractants and studies on their extraction-separation performance for heavy rare earths[J]. Hydrometallurgy, 2015, 154: 129-136. |
| [57] | WANG Junlian, XU Shengming, LI Linyan, et al. Synthesis of organic phosphinic acids and studies on the relationship between their structure and extraction-separation performance of heavy rare earths from HNO3 solutions[J]. Hydrometallurgy, 2013, 137: 108-114. |
| [58] | QIN Wei, XU Kaixuan, WANG Junwei, et al. Phosphorous-functionalized PAMAM dendrimers supported on mesoporous silica for Zr(Ⅳ) and Hf(Ⅳ) separation[J]. RSC Advances, 2021, 11(55): 34754-34765. |
| [59] | FELIPE Elaine C B, LADEIRA Ana Claudia Q. Separation of zirconium from hafnium by ion exchange[J]. Separation Science and Technology, 2018, 53(2): 330-336. |
| [60] | SMOLIK M, JAKÓBIK-KOLON A, PORAŃSKI M. Separation of zirconium and hafnium using Diphonix® chelating ion-exchange resin[J]. Hydrometallurgy, 2009, 95(3/4): 350-353. |
| [61] | SMOLIK Marek, Łukasz SIEPIETOWSKI, Agata JAKÓBIK-KOLON. The effects of concentrations of zirconium(Ⅳ) sulphate and sulphuric acid on sorption of zirconium(Ⅳ) and hafnium(Ⅳ) on purolite S-957 resin. adsorption isotherms of zirconium(Ⅳ) and hafnium(Ⅳ) ions[J]. Solvent Extraction and Ion Exchange, 2014, 32(4): 437-446. |
| [62] | KANG Jingu, KIM Yung-Uk, Sung-Ho JOO, et al. Behavior of extraction, stripping, and separation possibilities of rhenium and molybdenum from molybdenite roasting dust leaching solution using amine based extractant tri-otyl-amine (TOA)[J]. Materials Transactions, 2013, 54(7): 1209-1212. |
| [63] | DU PREEZ Jan G H. Recent advances in amines as separating agents for metal ions[J]. Solvent Extraction and Ion Exchange, 2000, 18(4): 679-701. |
| [64] | TEZCAN Fatma, DONAT Ramazan. Single and selective transport of Zr(Ⅳ) ions with trioctyl amine dissolved in kerosene using a multidropped liquid membrane technique[J]. Turkish Journal of Chemistry, 2022, 46(5): 1594-1606. |
| [65] | KRAMER Jurjen, DRIESSEN Willem L, KOCH Klaus R, et al. Highly selective extraction of platinum group metals with silica-based (poly)amine ion exchangers applied to industrial metal refinery effluents[J]. Hydrometallurgy, 2002, 64(1): 59-68. |
| [66] | MAITY Tarun, AGGARWAL Abhishek, DASGUPTA Subhadeep, et al. Efficient removal of uranyl ions using PAMAM dendrimer: Simulation and experiment[J]. Langmuir, 2023, 39(19): 6794-6802. |
| [67] | PORIEL L, PELLET-ROSTAING S, LAMOTTE V, et al. Zirconium and hafnium separation, part 2. solid/liquid extraction in hydrochloric acid aqueous solution with anion exchange resins[J]. Separation Science and Technology, 2006, 41(12): 2711-2722. |
| [68] | FAVRE-RÉGUILLON A, FIATY K, LAURENT P, et al. Solid/liquid extraction of zirconium and hafnium in hydrochloric acid aqueous solution with anion exchange ResinKinetic study and equilibrium analyses[J]. Industrial & Engineering Chemistry Research, 2007, 46(4): 1286-1291. |
| [69] | PEARSON Ralph G. Absolute electronegativity and hardness: Application to inorganic chemistry[J]. Inorganic Chemistry, 1988, 27(4): 734-740. |
| [1] | 符红梅, 刘定华, 刘晓勤. MOF材料在芳烃同分异构体分离中的研究进展[J]. 化工进展, 2025, 44(9): 5006-5017. |
| [2] | 张博, 马骏, 张维隆, 贾世川, 张智飞, 丁宇, 潘有华, 王俊宇, 张兰河. α-ZrP/PDMS超疏水防腐涂层的制备及其耐腐蚀性能[J]. 化工进展, 2025, 44(9): 5130-5139. |
| [3] | 洪凯, 樊欢, 田佳, 张荥斐. 硫化沉淀法处理铜砷多金属酸性废水研究进展[J]. 化工进展, 2025, 44(9): 5301-5314. |
| [4] | 杜璇, 王战宏, 郑彬, 许卫, 王硕, 石鹏, 高国. 钴铁酸浸液分离及电池级磷酸铁回收技术进展[J]. 化工进展, 2025, 44(9): 5327-5338. |
| [5] | 王晓光, 董青, 郎文丽, 洪翔鑫, 黄振祥, 谭凤玉, 雷以柱, 余子夷. 超低浓度甲烷减排与资源化利用研究进展[J]. 化工进展, 2025, 44(9): 5363-5376. |
| [6] | 杨证禄, 杨立峰, 路晓飞, 锁显, 张安运, 崔希利, 邢华斌. 机器学习加速多孔吸附剂筛选发现的研究进展[J]. 化工进展, 2025, 44(8): 4288-4301. |
| [7] | 杨傲, 邓苇, 黎勇, 罗婧, 王梓霖, 张俊, 申威峰. 基于热力学拓扑理论的三塔变压精馏分离四氢呋喃/甲醇/乙醇多目标优化设计[J]. 化工进展, 2025, 44(8): 4582-4593. |
| [8] | 高岩, 李永帅, 李高洋, 潘慧, 凌昊. Agrawal分壁精馏塔的动态控制[J]. 化工进展, 2025, 44(8): 4594-4605. |
| [9] | 汪国超, 丁晖殿, 师丽, 李强, 夏涛, 苑杨. 复合精馏序列的温度推断控制[J]. 化工进展, 2025, 44(8): 4720-4731. |
| [10] | 唐轩, 白晓炜, 张飞飞, 李晋平, 杨江峰. 沸石分子筛用于CO2-N2-CH4筛分分离的研究进展[J]. 化工进展, 2025, 44(7): 3938-3949. |
| [11] | 陈倩, 仝坤, 谢加才, 邵志国, 聂凡, 李成涛. 含聚油泥处理技术研究进展[J]. 化工进展, 2025, 44(7): 4158-4168. |
| [12] | 付江, 孙姣霞, 付俊杰, 朱敏, 宋品学, 周怡宁, 樊建新. 疏水改性聚酯纤维织物的自清洁作用及油水分离性能[J]. 化工进展, 2025, 44(6): 3121-3131. |
| [13] | 王恒, 卢春喜. 3.6Mt/a催化裂化旋风分离装置结构优选及运行效果分析[J]. 化工进展, 2025, 44(6): 3238-3246. |
| [14] | 张磊, 张新儒, 王永洪, 李晋平, 刘春波. 二维纳米材料混合基质膜在渗透汽化有机物分离的研究进展[J]. 化工进展, 2025, 44(6): 3324-3335. |
| [15] | 柳永兵, 王亚军, 谷平, 张永民, 郭怀勇, 刘凯. 浆态床反应器中多相分离研究进展[J]. 化工进展, 2025, 44(6): 3345-3363. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||