化工进展 ›› 2024, Vol. 43 ›› Issue (11): 6553-6562.DOI: 10.16085/j.issn.1000-6613.2023-1864
• 资源与环境化工 • 上一篇
矩阵分析法优化臭氧膜接触传质技术
姚福春1,2( ), 毕莹莹1(
), 毕莹莹1( ), 刘超1,2, 唐晨2, 李泽莹1, 张耀宗2(
), 刘超1,2, 唐晨2, 李泽莹1, 张耀宗2( ), 孙晓明1(
), 孙晓明1( )
)
- 1.中国环境科学研究院国家环境保护生态工业重点实验室,北京 100012
2.华北理工大学建筑工程学院,河北 唐山 063000
-
收稿日期:2023-10-23修回日期:2023-11-10出版日期:2024-11-15发布日期:2024-12-07 -
通讯作者:张耀宗,孙晓明 -
作者简介:姚福春(1996—),男,硕士研究生,研究方向为水污染控制与废水资源化。E-mail:386439071@qq.com
毕莹莹(1990—),女,硕士,工程师,研究方向为环境科学与资源利用。E-mail:biyyjob@163.com。 -
基金资助:国家重点研发计划(2022YFC3901301);黄河流域生态保护和高质量发展联合研究一期项目(2022-YRUC-01-0404)
Matrix analysis method to optimize the ozone membrane contact mass transfer technology
YAO Fuchun1,2( ), BI Yingying1(
), BI Yingying1( ), LIU Chao1,2, TANG Chen2, LI Zeying1, ZHANG Yaozong2(
), LIU Chao1,2, TANG Chen2, LI Zeying1, ZHANG Yaozong2( ), SUN Xiaoming1(
), SUN Xiaoming1( )
)
- 1.Protection Key Laboratory of Ecological Industry Chinese Research Academy of Environmental Sciences State Environmental, Beijing 100012, China
2.School of Architecture and Civil Engineering, North China University of Science and Technology, Tangshan 063000, Hebei, China
-
Received:2023-10-23Revised:2023-11-10Online:2024-11-15Published:2024-12-07 -
Contact:ZHANG Yaozong, SUN Xiaoming
摘要:
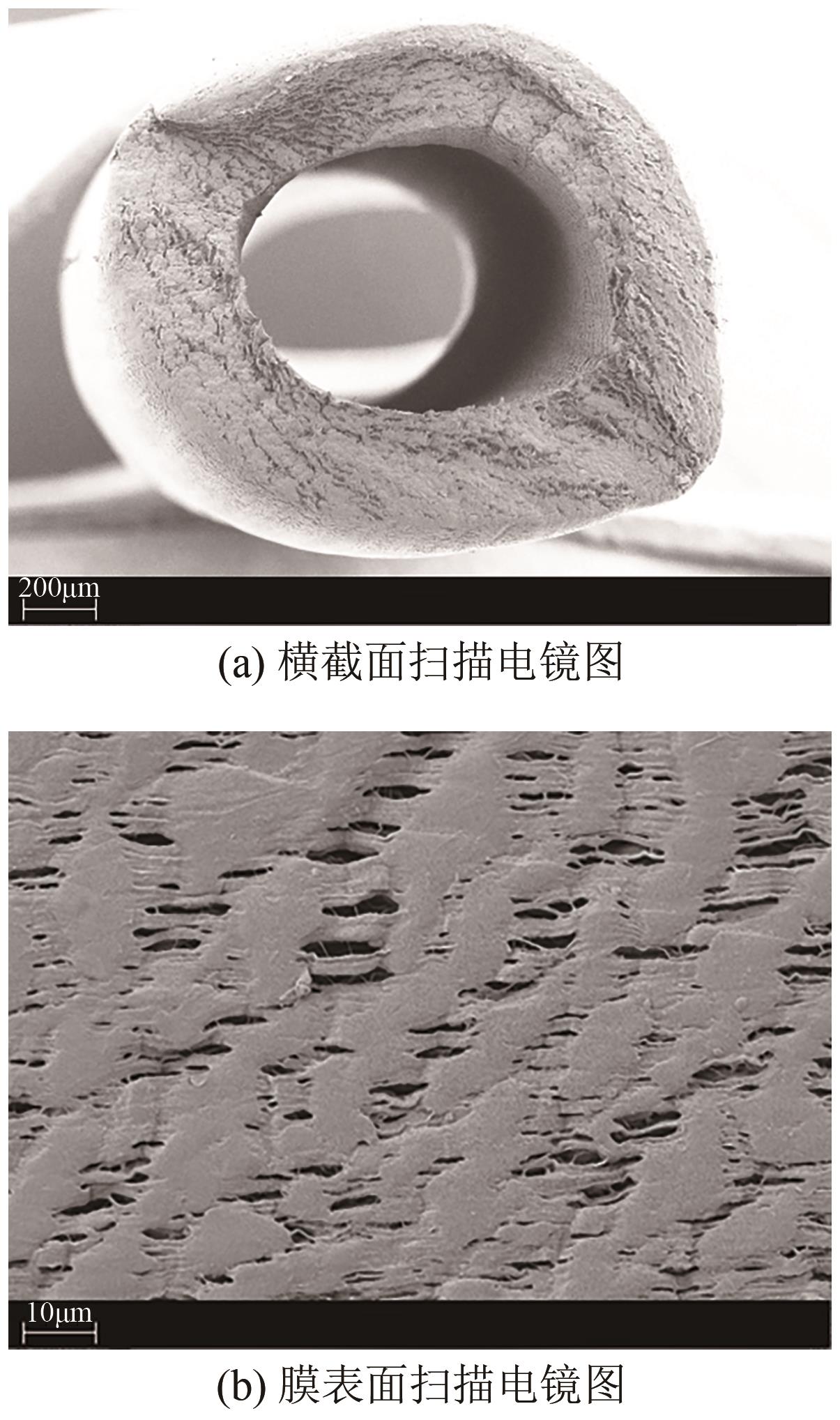
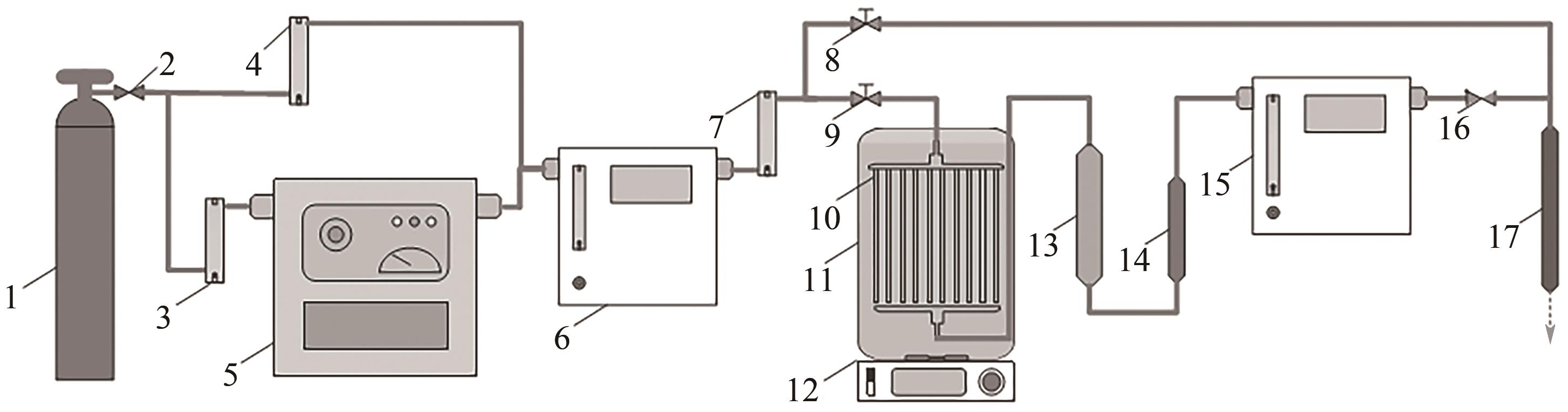
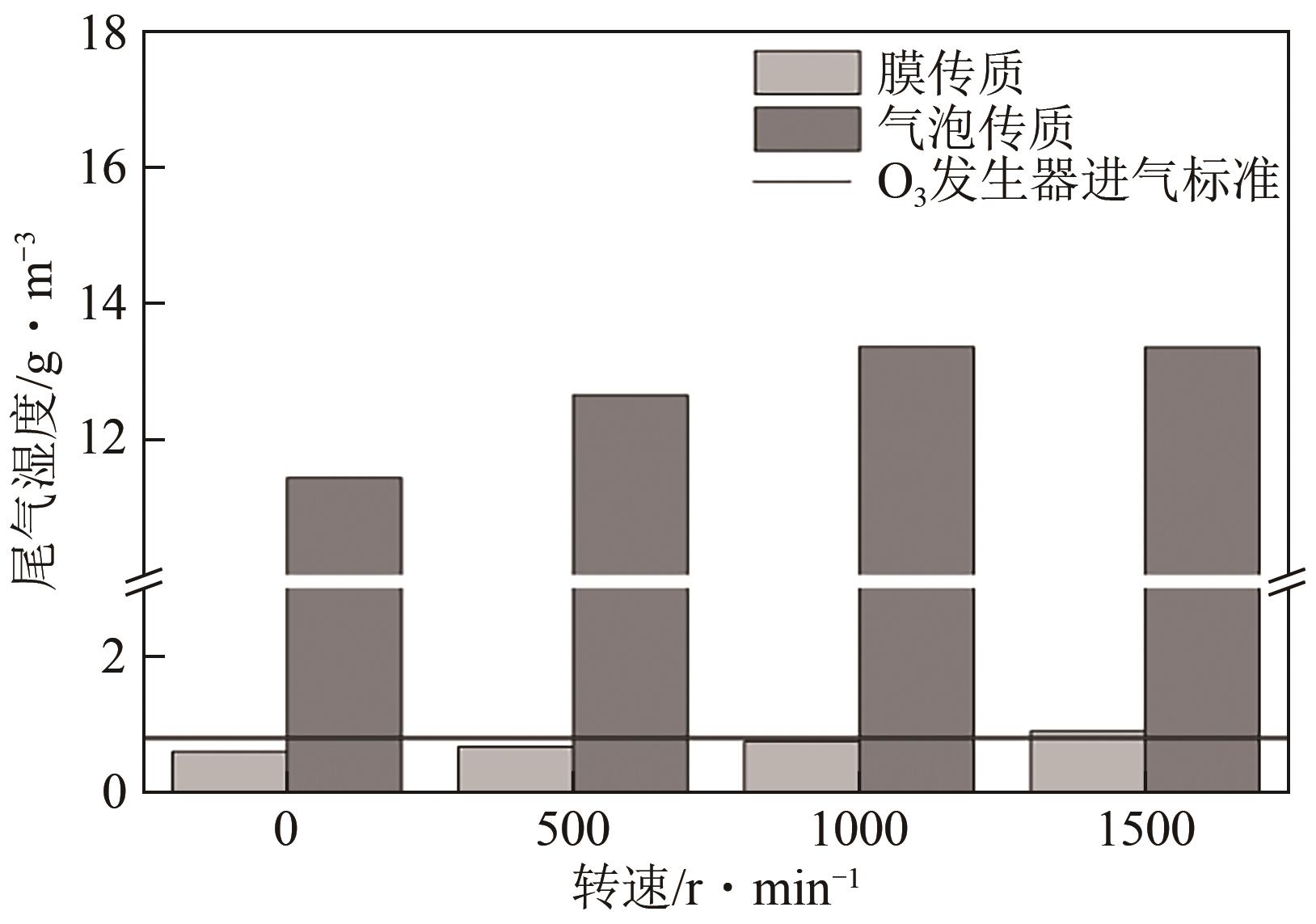
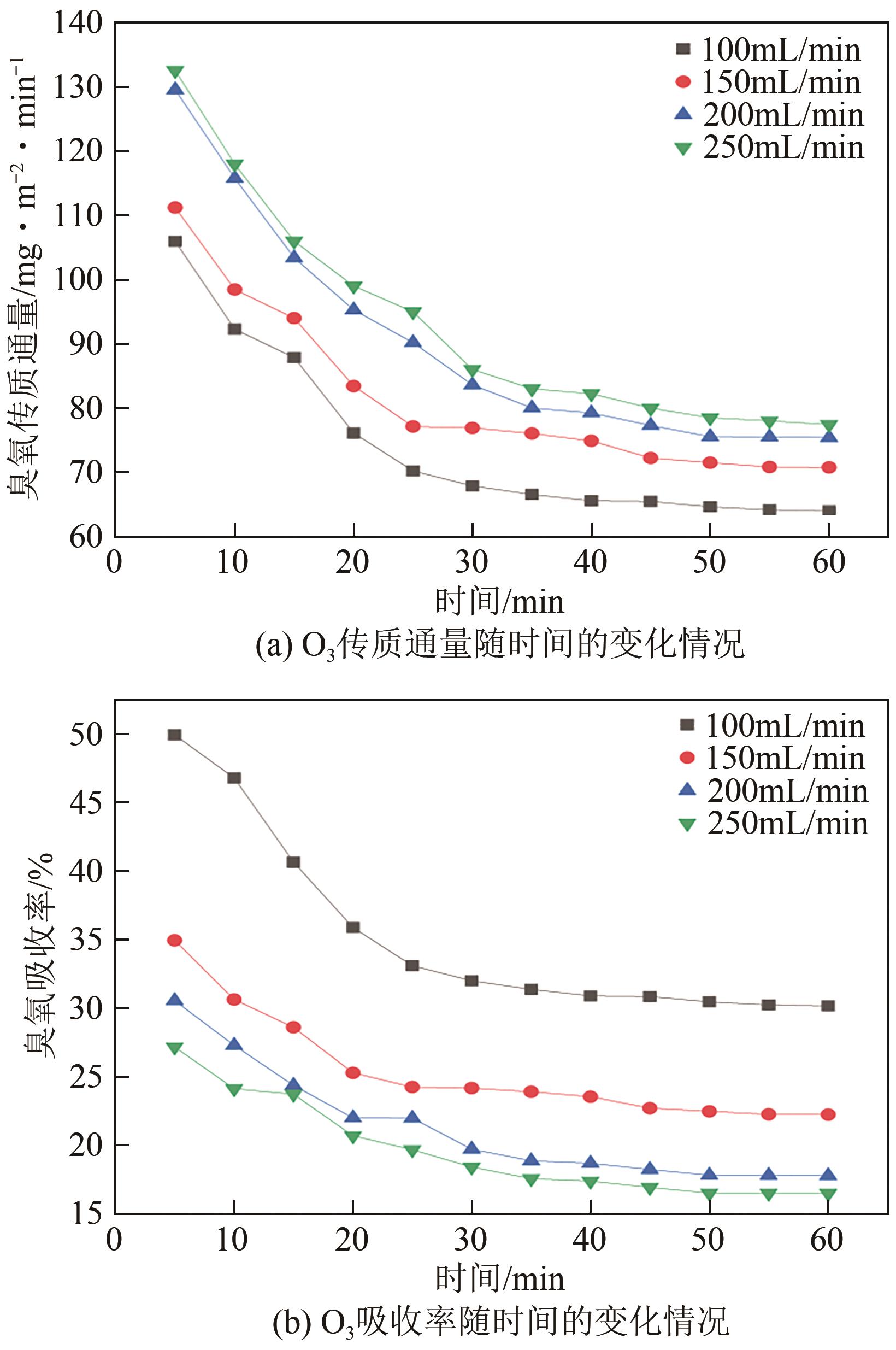
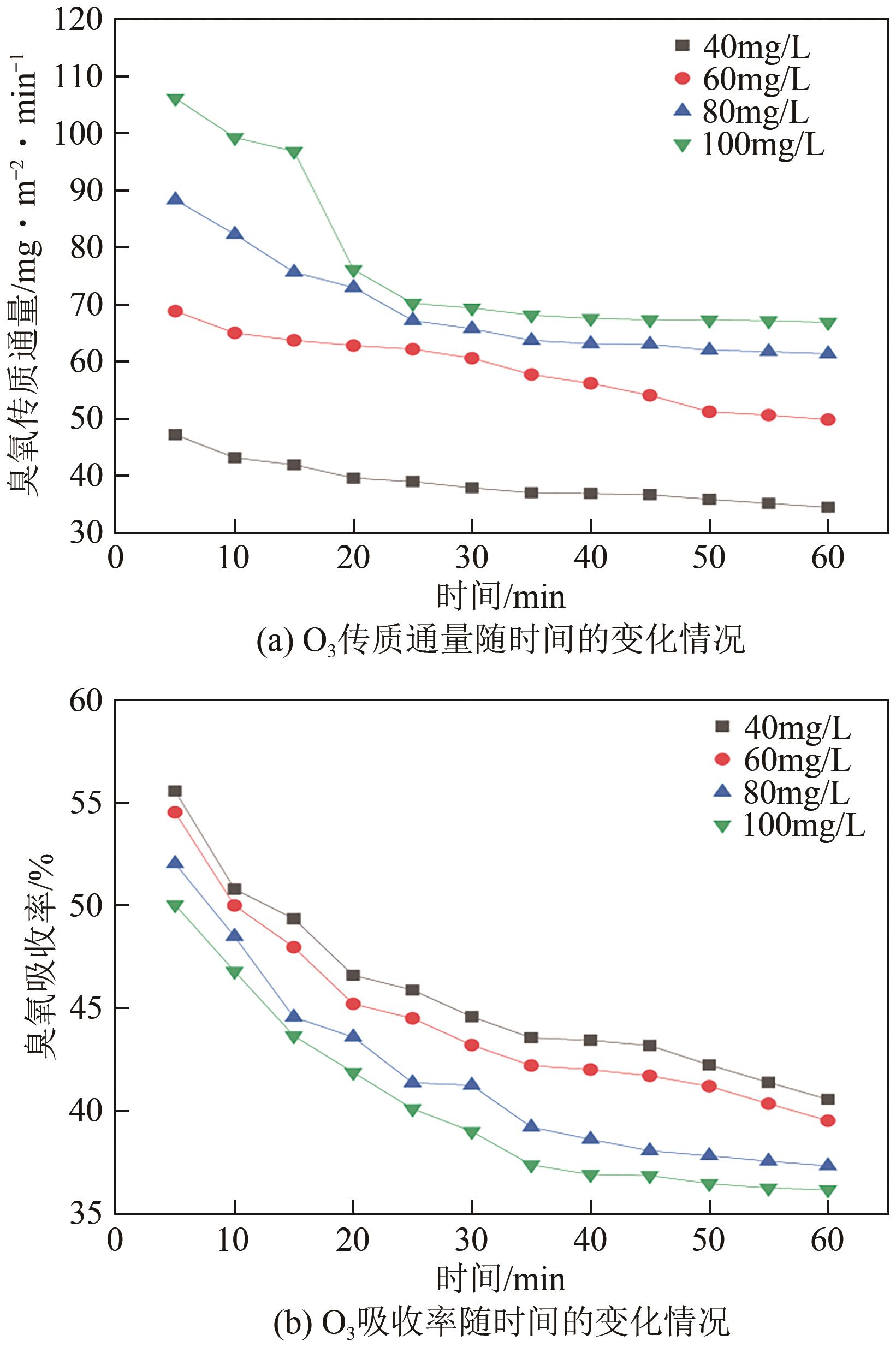
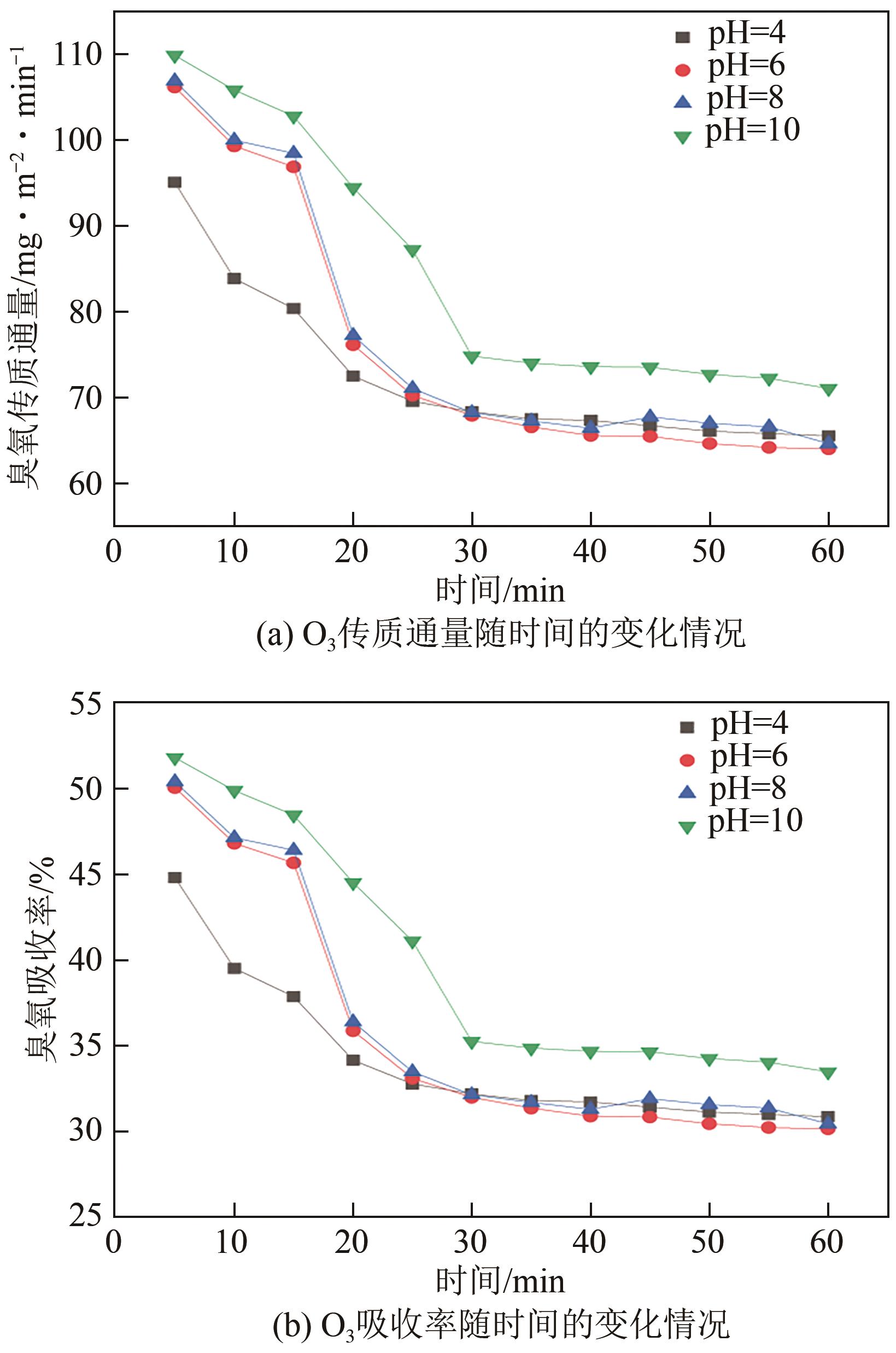
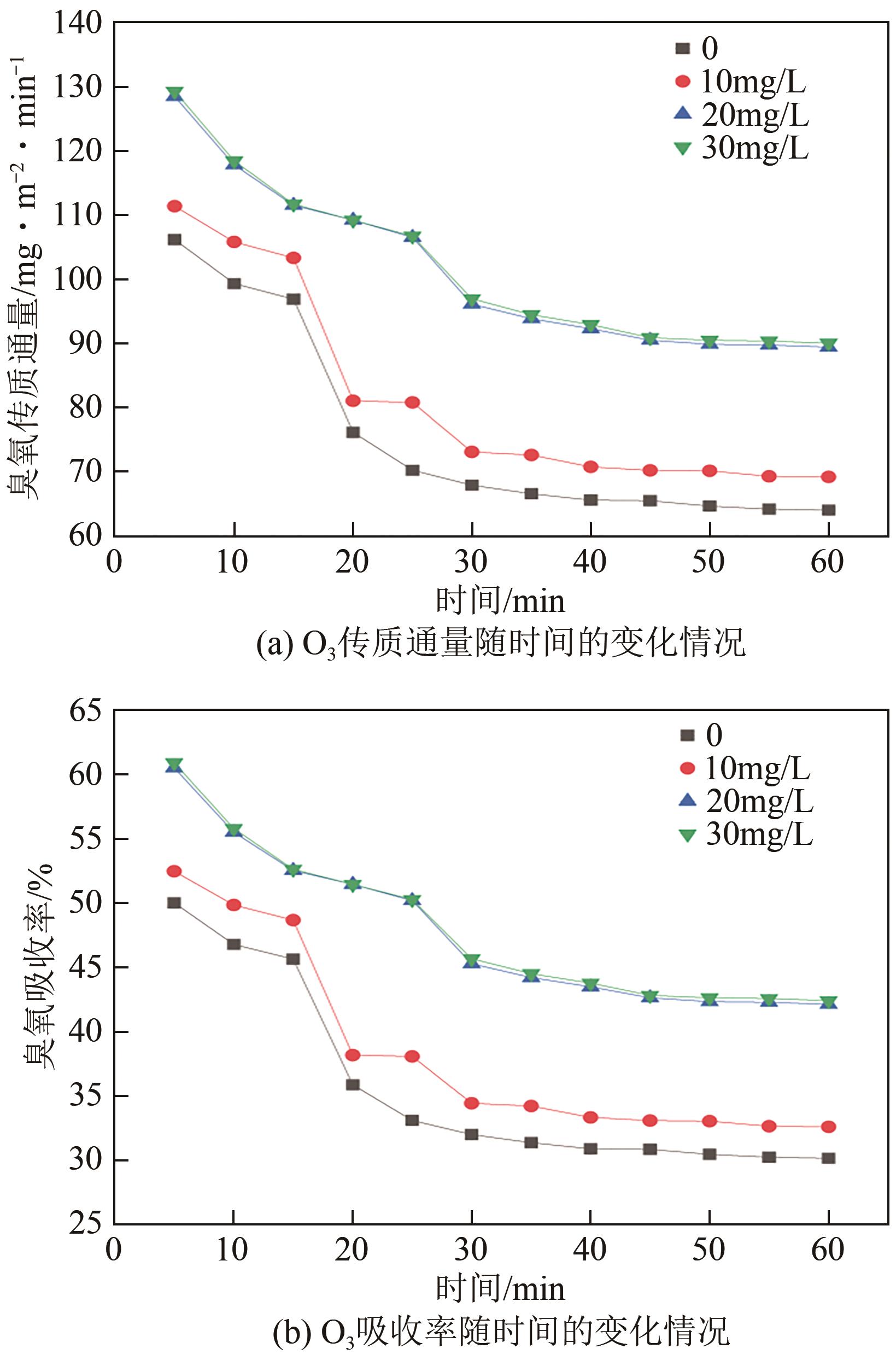
将疏水性聚四氟乙烯(PTFE)中空纤维膜组装成膜接触式反应器,开发了膜接触式臭氧(O3)无气泡传质技术。当搅拌速度达到1000r/min时,膜传质的传质情况(表观传质系数KLa=0.0807min-1)与气泡传质(KLa=0.0791min-1)相当。进行了单因素实验及L16(44)正交实验,研究了进气流量、O3进气浓度、pH、苯酚浓度等对中空纤维膜接触式反应器O3传质情况的影响;采用极差分析、方差分析、综合平衡分析及权矩阵分析进行数据处理,优化膜接触式O3无气泡传质技术的传质条件。结果表明,四种实验因素均对O3无气泡传质效果产生了显著性的影响;优化后的传质条件为:进气流量100mL/min,O3进气浓度100mg/L、初始pH为10、污染物浓度30mg/L。优化条件下三次重复实验O3传质通量均保持在170mg/(m2·min)以上,O3吸收率均达到了80%以上。
中图分类号:
引用本文
姚福春, 毕莹莹, 刘超, 唐晨, 李泽莹, 张耀宗, 孙晓明. 矩阵分析法优化臭氧膜接触传质技术[J]. 化工进展, 2024, 43(11): 6553-6562.
YAO Fuchun, BI Yingying, LIU Chao, TANG Chen, LI Zeying, ZHANG Yaozong, SUN Xiaoming. Matrix analysis method to optimize the ozone membrane contact mass transfer technology[J]. Chemical Industry and Engineering Progress, 2024, 43(11): 6553-6562.
| 参数 | 数值 |
|---|---|
| 膜 | |
| 内径/mm | 0.5 |
| 外径/mm | 1.5 |
| 膜厚/mm | 0.5 |
| 表面孔径分布/μm | 2~10 |
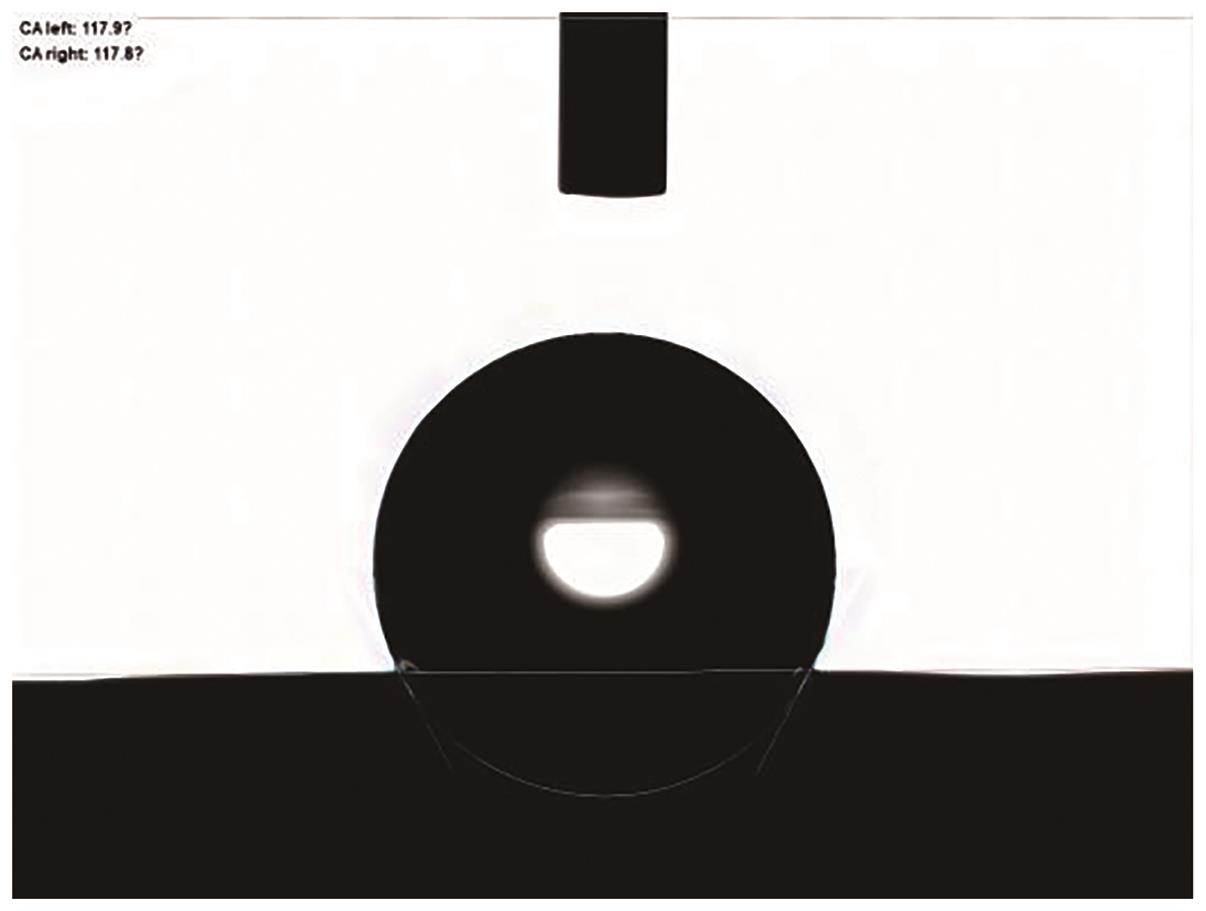
| 接触角/(°) | 117.85 |
| 起泡压力/MPa | 0.025 |
| 膜接触反应器 | |
| 膜丝数量/根 | 20 |
| 有效长度/mm | 500 |
| 有效接触面积/mm2 | 47123.9 |
表1 疏水性PTFE中空纤维膜参数表
| 参数 | 数值 |
|---|---|
| 膜 | |
| 内径/mm | 0.5 |
| 外径/mm | 1.5 |
| 膜厚/mm | 0.5 |
| 表面孔径分布/μm | 2~10 |
| 接触角/(°) | 117.85 |
| 起泡压力/MPa | 0.025 |
| 膜接触反应器 | |
| 膜丝数量/根 | 20 |
| 有效长度/mm | 500 |
| 有效接触面积/mm2 | 47123.9 |
| 序号 | A/mL∙min-1 | B/mg∙L-1 | C | D/mg∙L-1 |
|---|---|---|---|---|
| 1 | 100 | 40 | 4 | 0 |
| 2 | 150 | 60 | 6 | 10 |
| 3 | 200 | 80 | 8 | 20 |
| 4 | 250 | 100 | 10 | 30 |
表2 中空纤维膜臭氧无气泡传质正交实验因素及水平表
| 序号 | A/mL∙min-1 | B/mg∙L-1 | C | D/mg∙L-1 |
|---|---|---|---|---|
| 1 | 100 | 40 | 4 | 0 |
| 2 | 150 | 60 | 6 | 10 |
| 3 | 200 | 80 | 8 | 20 |
| 4 | 250 | 100 | 10 | 30 |
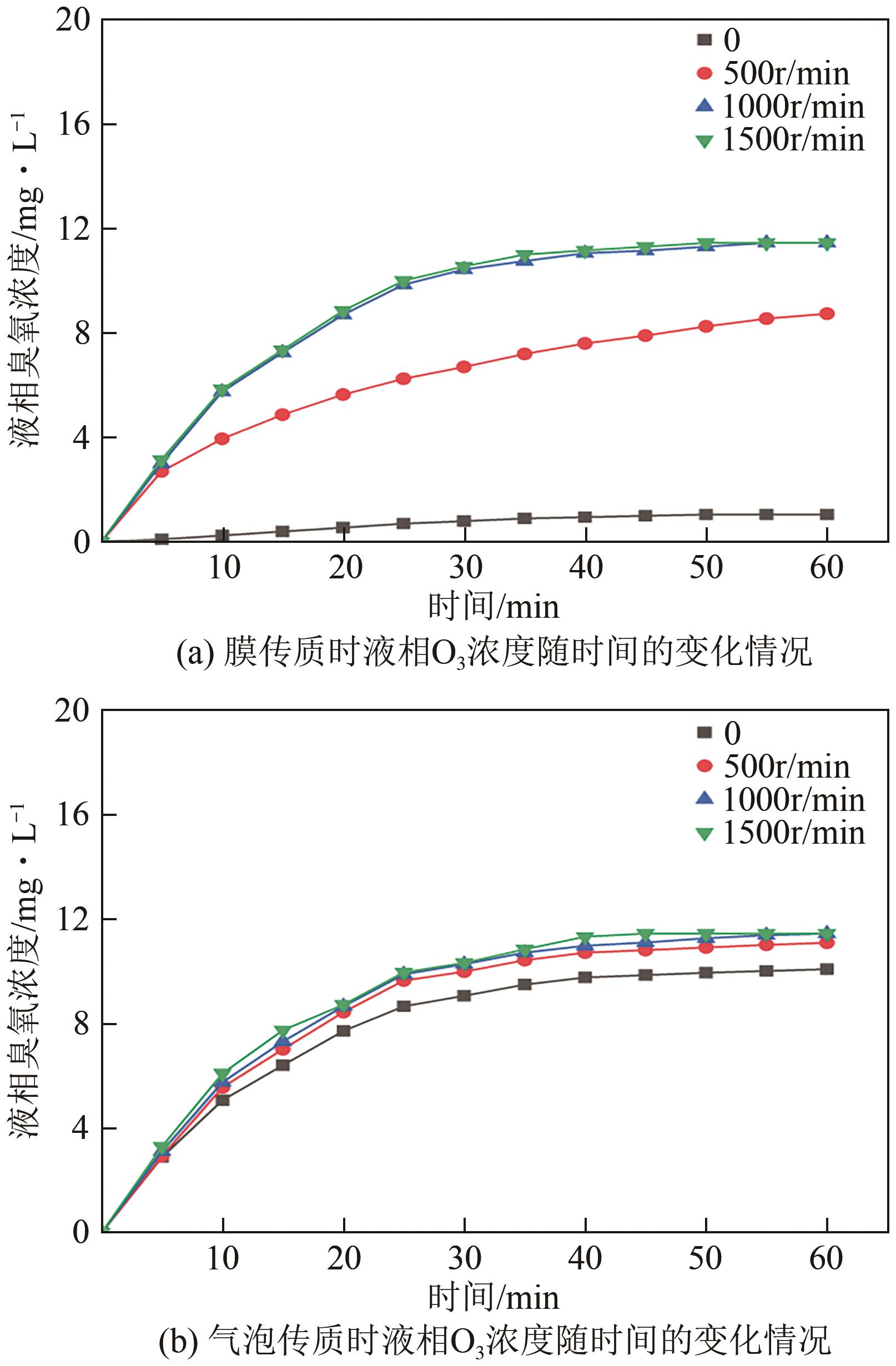
| 搅拌速度/r·min-1 | 膜传质 | 气泡传质 | ||
|---|---|---|---|---|
| KLa/min-1 | R2 | KLa/min-1 | R2 | |
| 0 | 0.0024 | 0.9952 | 0.0533 | 0.9915 |
| 500 | 0.0287 | 0.9939 | 0.0677 | 0.9931 |
| 1000 | 0.0807 | 0.9903 | 0.0791 | 0.9939 |
| 1500 | 0.0806 | 0.9908 | 0.0808 | 0.9954 |
表3 基于传质的拟合结果
| 搅拌速度/r·min-1 | 膜传质 | 气泡传质 | ||
|---|---|---|---|---|
| KLa/min-1 | R2 | KLa/min-1 | R2 | |
| 0 | 0.0024 | 0.9952 | 0.0533 | 0.9915 |
| 500 | 0.0287 | 0.9939 | 0.0677 | 0.9931 |
| 1000 | 0.0807 | 0.9903 | 0.0791 | 0.9939 |
| 1500 | 0.0806 | 0.9908 | 0.0808 | 0.9954 |
| 编号 | A | B | C | D | O3传质通量(E)/mg∙m-2∙min-1 | O3吸收率(F)/% |
|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 33.40 | 39.35 |
| 2 | 1 | 2 | 2 | 2 | 59.82 | 46.98 |
| 3 | 1 | 3 | 3 | 3 | 91.57 | 53.94 |
| 4 | 1 | 4 | 4 | 4 | 124.61 | 58.72 |
| 5 | 2 | 1 | 2 | 3 | 67.80 | 53.25 |
| 6 | 2 | 2 | 1 | 4 | 94.73 | 49.60 |
| 7 | 2 | 3 | 4 | 1 | 71.49 | 28.08 |
| 8 | 2 | 4 | 3 | 2 | 105.23 | 33.06 |
| 9 | 3 | 1 | 3 | 4 | 85.69 | 50.48 |
| 10 | 3 | 2 | 4 | 3 | 113.45 | 44.55 |
| 11 | 3 | 3 | 1 | 2 | 100.29 | 29.54 |
| 12 | 3 | 4 | 2 | 1 | 107.84 | 25.41 |
| 13 | 4 | 1 | 4 | 2 | 78.62 | 37.05 |
| 14 | 4 | 2 | 3 | 1 | 60.85 | 19.12 |
| 15 | 4 | 3 | 2 | 4 | 133.00 | 31.34 |
| 16 | 4 | 4 | 1 | 3 | 120.43 | 22.70 |
表4 正交实验结果
| 编号 | A | B | C | D | O3传质通量(E)/mg∙m-2∙min-1 | O3吸收率(F)/% |
|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 33.40 | 39.35 |
| 2 | 1 | 2 | 2 | 2 | 59.82 | 46.98 |
| 3 | 1 | 3 | 3 | 3 | 91.57 | 53.94 |
| 4 | 1 | 4 | 4 | 4 | 124.61 | 58.72 |
| 5 | 2 | 1 | 2 | 3 | 67.80 | 53.25 |
| 6 | 2 | 2 | 1 | 4 | 94.73 | 49.60 |
| 7 | 2 | 3 | 4 | 1 | 71.49 | 28.08 |
| 8 | 2 | 4 | 3 | 2 | 105.23 | 33.06 |
| 9 | 3 | 1 | 3 | 4 | 85.69 | 50.48 |
| 10 | 3 | 2 | 4 | 3 | 113.45 | 44.55 |
| 11 | 3 | 3 | 1 | 2 | 100.29 | 29.54 |
| 12 | 3 | 4 | 2 | 1 | 107.84 | 25.41 |
| 13 | 4 | 1 | 4 | 2 | 78.62 | 37.05 |
| 14 | 4 | 2 | 3 | 1 | 60.85 | 19.12 |
| 15 | 4 | 3 | 2 | 4 | 133.00 | 31.34 |
| 16 | 4 | 4 | 1 | 3 | 120.43 | 22.70 |
| 参数 | A | B | C | D |
|---|---|---|---|---|
| O3传质通量 | ||||
| K1 | 309.4 | 265.5 | 348.8 | 273.6 |
| K2 | 339.3 | 328.8 | 368.5 | 344.0 |
| K3 | 407.3 | 396.3 | 343.3 | 393.2 |
| K4 | 392.9 | 458.1 | 388.2 | 438.0 |
| k1 | 77.3 | 66.4 | 87.2 | 68.4 |
| k2 | 84.8 | 82.2 | 92.1 | 86.0 |
| k3 | 101.8 | 99.1 | 85.8 | 98.3 |
| k4 | 98.2 | 114.5 | 97.0 | 109.5 |
| R1 | 24.5 | 48.1 | 11.2 | 41.1 |
| O3吸收率 | ||||
| Z1 | 1.9899 | 1.8013 | 1.4119 | 1.1195 |
| Z2 | 1.6399 | 1.6025 | 1.5698 | 1.4663 |
| Z3 | 1.4997 | 1.4289 | 1.5659 | 1.7444 |
| Z4 | 1.1020 | 1.3989 | 1.6840 | 1.9013 |
| z1 | 0.4975 | 0.4503 | 0.3530 | 0.2799 |
| z2 | 0.4100 | 0.4006 | 0.3925 | 0.3666 |
| z3 | 0.3749 | 0.3572 | 0.3915 | 0.4361 |
| z4 | 0.2755 | 0.3497 | 0.4210 | 0.4753 |
| R2 | 0.2220 | 0.1006 | 0.0680 | 0.1955 |
表5 正交实验极差分析表
| 参数 | A | B | C | D |
|---|---|---|---|---|
| O3传质通量 | ||||
| K1 | 309.4 | 265.5 | 348.8 | 273.6 |
| K2 | 339.3 | 328.8 | 368.5 | 344.0 |
| K3 | 407.3 | 396.3 | 343.3 | 393.2 |
| K4 | 392.9 | 458.1 | 388.2 | 438.0 |
| k1 | 77.3 | 66.4 | 87.2 | 68.4 |
| k2 | 84.8 | 82.2 | 92.1 | 86.0 |
| k3 | 101.8 | 99.1 | 85.8 | 98.3 |
| k4 | 98.2 | 114.5 | 97.0 | 109.5 |
| R1 | 24.5 | 48.1 | 11.2 | 41.1 |
| O3吸收率 | ||||
| Z1 | 1.9899 | 1.8013 | 1.4119 | 1.1195 |
| Z2 | 1.6399 | 1.6025 | 1.5698 | 1.4663 |
| Z3 | 1.4997 | 1.4289 | 1.5659 | 1.7444 |
| Z4 | 1.1020 | 1.3989 | 1.6840 | 1.9013 |
| z1 | 0.4975 | 0.4503 | 0.3530 | 0.2799 |
| z2 | 0.4100 | 0.4006 | 0.3925 | 0.3666 |
| z3 | 0.3749 | 0.3572 | 0.3915 | 0.4361 |
| z4 | 0.2755 | 0.3497 | 0.4210 | 0.4753 |
| R2 | 0.2220 | 0.1006 | 0.0680 | 0.1955 |
| 指标 | 实验因素 | 平方和 | 自由度 | 均方 | F | 显著性 |
|---|---|---|---|---|---|---|
| 臭氧传质 通量 | A | 1572.042 | 3 | 524.014 | 4.751 | 0.116 |
| B | 5206.517 | 3 | 1735.506 | 15.735 | 0.024 | |
| C | 311.908 | 3 | 103.969 | 0.943 | 0.519 | |
| D | 3724.449 | 3 | 1241.483 | 11.256 | 0.039 | |
| 臭氧吸收率 | A | 0.101 | 3 | 0.034 | 16.799 | 0.022 |
| B | 0.026 | 3 | 0.009 | 4.283 | 0.132 | |
| C | 0.009 | 3 | 0.003 | 1.554 | 0.363 | |
| D | 0.088 | 3 | 0.029 | 14.672 | 0.027 |
表6 正交实验方差分析表
| 指标 | 实验因素 | 平方和 | 自由度 | 均方 | F | 显著性 |
|---|---|---|---|---|---|---|
| 臭氧传质 通量 | A | 1572.042 | 3 | 524.014 | 4.751 | 0.116 |
| B | 5206.517 | 3 | 1735.506 | 15.735 | 0.024 | |
| C | 311.908 | 3 | 103.969 | 0.943 | 0.519 | |
| D | 3724.449 | 3 | 1241.483 | 11.256 | 0.039 | |
| 臭氧吸收率 | A | 0.101 | 3 | 0.034 | 16.799 | 0.022 |
| B | 0.026 | 3 | 0.009 | 4.283 | 0.132 | |
| C | 0.009 | 3 | 0.003 | 1.554 | 0.363 | |
| D | 0.088 | 3 | 0.029 | 14.672 | 0.027 |
| 条件 | 进气流量/mL∙min-1 | O3进气浓度/mg∙L-1 | 初始pH | 污染物浓度/mg∙L-1 |
|---|---|---|---|---|
| 臭氧传质通量最优单因素 | A3 | B4 | C4 | D4 |
| 臭氧吸收率最优单因素 | A1 | B1 | C4 | D4 |
| 臭氧传质通量k值偏差率/% | 24.07 | 42.01 | 0 | 0 |
| 臭氧吸收率z值偏差率/% | 24.64 | 22.34 | 0 | 0 |
| 最优传质条件 | A1B4C4D4 | |||
表7 综合平衡法优化工艺参数
| 条件 | 进气流量/mL∙min-1 | O3进气浓度/mg∙L-1 | 初始pH | 污染物浓度/mg∙L-1 |
|---|---|---|---|---|
| 臭氧传质通量最优单因素 | A3 | B4 | C4 | D4 |
| 臭氧吸收率最优单因素 | A1 | B1 | C4 | D4 |
| 臭氧传质通量k值偏差率/% | 24.07 | 42.01 | 0 | 0 |
| 臭氧吸收率z值偏差率/% | 24.64 | 22.34 | 0 | 0 |
| 最优传质条件 | A1B4C4D4 | |||
| 指标 | 实验 1 | 实验 2 | 实验 3 | 均值 |
|---|---|---|---|---|
| O3传质通量/mg∙m-2∙min-1 | 171.2 | 170.7 | 172.0 | 171.3 |
| O3吸收率/% | 80.66 | 80.43 | 81.03 | 80.71 |
表8 优化参数条件下实验结果
| 指标 | 实验 1 | 实验 2 | 实验 3 | 均值 |
|---|---|---|---|---|
| O3传质通量/mg∙m-2∙min-1 | 171.2 | 170.7 | 172.0 | 171.3 |
| O3吸收率/% | 80.66 | 80.43 | 81.03 | 80.71 |
| 1 | VAN AKEN P, LAMBERT N, VAN DEN BROECK R, et al. Advances in ozonation and biodegradation processes to enhance chlorophenol abatement in multisubstrate wastewaters: A review[J]. Environmental Science: Water Research & Technology, 2019, 5(3): 444-481. |
| 2 | 年国伟. 压力式臭氧催化氧化苯酚废水研究[D]. 扬州: 扬州大学, 2022. |
| NIAN Guowei. Catalytic oxidation of phenol wastewater by pressure ozonation[D]. Yangzhou: Yangzhou University, 2022. | |
| 3 | JABESA Abdisa, GHOSH Pallab. A comparative study on the removal of dimethyl sulfoxide from water using microbubbles and millibubbles of ozone[J]. Journal of Water Process Engineering, 2021, 40: 101937. |
| 4 | MAO Yuqin, QI Shengqi, GUO Xianfen, et al. Optimization of ozone dosage in an ozone contact tank using a numerical model[J]. Environmental Science and Pollution Research, 2021, 28(33): 44987-44997. |
| 5 | ROBEY Nicole M, SILVA Bianca F DA, ANNABLE Michael D, et al. Concentrating per- and polyfluoroalkyl substances (PFAS) in municipal solid waste landfill leachate using foam separation[J]. Environmental Science & Technology, 2020, 54(19): 12550-12559. |
| 6 | JANKNECHT P, WILDERER P A, PICARD C, et al. Bubble-free ozone contacting with ceramic membranes for wet oxidative treatment[J]. Chemical Engineering & Technology, 2000, 23(8): 674-677. |
| 7 | JANKNECHT P, WILDERER P A, PICARD C, et al. Ozone-water contacting by ceramic membranes[J]. Separation and Purification Technology, 2001, 25(1/2/3): 341-346. |
| 8 | SOHAIB Qazi, MUHAMMAD Amir, YOUNAS Mohammad, et al. Modeling pre-combustion CO2 capture with tubular membrane contactor using ionic liquids at elevated temperatures[J]. Separation and Purification Technology, 2020, 241: 116677. |
| 9 | ROSLI Aishah, AHMAD Abdul Latif, Siew Chun LOW. Enhancing membrane hydrophobicity using silica end-capped with organosilicon for CO2 absorption in membrane contactor[J]. Separation and Purification Technology, 2020, 251: 117429. |
| 10 | JU Jingge, FEJJARI Kaouthar, CHENG Yi, et al. Engineering hierarchically structured superhydrophobic PTFE/POSS nanofibrous membranes for membrane distillation[J]. Desalination, 2020, 486: 114481. |
| 11 | SCHMITT Alice, MENDRET Julie, BROSILLON Stephan. Evaluation of an ozone diffusion process using a hollow fiber membrane contactor[J]. Chemical Engineering Research and Design, 2022, 177: 291-303. |
| 12 | BEIN Emil, ZUCKER Ines, DREWES Jörg E, et al. Ozone membrane contactors for water and wastewater treatment: A critical review on materials selection, mass transfer and process design[J]. Chemical Engineering Journal, 2021, 413: 127393. |
| 13 | LI Kuiling, ZHANG Yong, XU Lili, et al. Mass transfer and interfacial reaction mechanisms in a novel electro-catalytic membrane contactor for wastewater treatment by O3 [J]. Applied Catalysis B: Environmental, 2020, 264: 118512. |
| 14 | CONG Menglong, ZHANG Shanshan, SUN Dandan, et al. Optimization of preparation of foamed concrete based on orthogonal experiment and range analysis[J]. Frontiers in Materials, 2021, 8: 778173. |
| 15 | HARVEY Philip, BIGDELI Tim, LI Yuli, et al. Tu72. Genomic analyses of schizophrenia and bipolar patients with very poor outcomes[J]. European Neuropsychopharmacology, 2021, 51: e134-e135. |
| 16 | WANG Xing, LIU Xiaomin, ZHANG Chuhua. Parametric optimization and range analysis of Organic Rankine Cycle for binary-cycle geothermal plant[J]. Energy Conversion and Management, 2014, 80: 256-265. |
| 17 | VELINA Mara, VALEINIS Janis, GRECO Luca, et al. Empirical likelihood-based ANOVA for trimmed means[J]. International Journal of Environmental Research and Public Health, 2016, 13(10): 953. |
| 18 | DE MELO Márcio Braga, Dimitri DALDEGAN-BUENO, OLIVEIRA Maria Gabriela Menezes, et al. Beyond ANOVA and MANOVA for repeated measures: Advantages of generalized estimated equations and generalized linear mixed models and its use in neuroscience research[J]. The European journal of neuroscience, 2022, 56(12): 6089-6098. |
| 19 | 蒋凌燕, 成金曦, 夏东, 等. 矩阵分析法优化花椒树脂提取工艺研究[J]. 中国油脂, 2020, 45(10): 106-114. |
| JIANG Lingyan, CHENG Jinxi, XIA Dong, et al. Optimization of extracting Zanthoxylum bungeanum resin by matrix analysis method[J]. China Oils and Fats, 2020, 45(10): 106-114. | |
| 20 | 赵风文, 胡建华, 曾平平, 等. 基于正交试验的碱基-磷石膏胶结充填体配比优化[J]. 中国有色金属学报, 2021, 31(4): 1096-1105. |
| ZHAO Fengwen, HU Jianhua, ZENG Pingping, et al. Optimization research of base-phosphogypsum cemented backfill ratio based on orthogonal test[J]. The Chinese Journal of Nonferrous Metals, 2021, 31(4): 1096-1105. | |
| 21 | 闫红杰, 夏韬, 刘柳, 等. 高铅渣还原炉内气液两相流的数值模拟与结构优化[J]. 中国有色金属学报, 2014, 24(10): 2642-2651. |
| YAN Hongjie, XIA Tao, LIU Liu, et al. Numerical simulation and structural optimization of gas-liquid two-phase flow in reduction furnace of lead-rich slag[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(10): 2642-2651. | |
| 22 | BELTRÁN Fernando J. 水和废水的臭氧反应动力学[M]. 周云瑞,译. 北京: 中国建筑工业出版社, 2007. |
| BELTRÁN Fernando J. Ozone reaction kinetics for water and wastewater systems[M]. ZHOU Yunduan, trans. Beijing: China Architecture & Building Press, 2007. | |
| 23 | WANG Bing, SHI Wen, ZHANG Huan, et al. Promoting the ozone-liquid mass transfer through external physical fields and their applications in wastewater treatment: A review[J]. Journal of Environmental Chemical Engineering, 2021, 9(5): 106115. |
| 24 | WEAVERS Linda K, HOFFMANN Michael R. Sonolytic decomposition of ozone in aqueous solution: mass transfer effects[J]. Environmental Science & Technology, 1998, 32(2): 3941-3947. |
| 25 | CUONG Le Cao, NGHI Nguyen Hoang, DIEU Tran Vinh, et al. Influence of oxygen concentration, feed gas flow rate and air humidity on the output of ozone produced by corona discharge[J]. Vietnam Journal of Chemistry, 2019, 57(5): 604-608. |
| 26 | YULIANTO E, RESTIWIJAYA M, SASMITA E, et al. Power analysis of ozone generator for high capacity production[J]. Journal of Physics: Conference Series, 2019, 1170: 012013. |
| 27 | ZHANG Yong, LI Kuiling, WANG Jun, et al. Ozone mass transfer behaviors on physical and chemical absorption for hollow fiber membrane contactors[J]. Water Science and Technology, 2017, 76(6): 1360-1369. |
| 28 | XIONG Wei, CUI Weihua, LI Rui, et al. Mineralization of phenol by ozone combined with activated carbon: Performance and mechanism under different pH levels[J]. Environmental Science and Ecotechnology, 2020, 1: 100005. |
| [1] | 刘青晨, 王华伟, 刘荣稳, 邹融雪, 占美丽, 王亚楠, 孙英杰, 夏正启, 单斌. 混凝-臭氧氧化对渗滤液生化出水有机微污染物的去除效果[J]. 化工进展, 2024, 43(S1): 545-554. |
| [2] | 梁永琪, 汤健, 夏恒, 陈佳昆, 乔俊飞. 基于耦合数值仿真的基准工况下焚烧炉内颗粒物浓度建模与分析[J]. 化工进展, 2024, 43(S1): 106-120. |
| [3] | 胡君杰, 黄兴俊, 雷成, 杨敏, 兰元宵, 罗建洪. 页岩气采出水中小分子有机物的深度处理[J]. 化工进展, 2024, 43(8): 4674-4680. |
| [4] | 宋占龙, 汤涛, 潘蔚, 赵希强, 孙静, 毛岩鹏, 王文龙. 微纳米气泡强化臭氧氧化降解含酚废水[J]. 化工进展, 2024, 43(8): 4614-4623. |
| [5] | 姚福春, 毕莹莹, 唐晨, 杜明辉, 李泽莹, 张耀宗, 孙晓明. 中空纤维膜臭氧接触式反应器传质机理分析[J]. 化工进展, 2024, 43(2): 1089-1097. |
| [6] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [7] | 詹咏, 王慧, 韦婷婷, 朱星宇, 王先恺, 陈思思, 董滨. Mn2+强化臭氧调理对生物处理工艺的污泥原位减量效果[J]. 化工进展, 2023, 42(6): 3253-3260. |
| [8] | 朱昊, 刘汉飞, 高源, 白蓉蓉, 倪嵩波, 黄益平, 李庆同, 李小东, 韩卫清. 催化臭氧化体系射流曝气系统参数优化及苯酚阶段氧化分析[J]. 化工进展, 2023, 42(5): 2717-2723. |
| [9] | 陈佳昆, 汤健, 夏恒, 乔俊飞. 城市固废炉排炉焚烧过程二𫫇英排放浓度数值仿真[J]. 化工进展, 2023, 42(2): 1061-1072. |
| [10] | 尹晓云, 付林浩, 李佳忆, 程思杰, 敬加强, MASTOBAEV Boris N, 孙杰. 稠油水环输送管道再启动压降特性分析[J]. 化工进展, 2023, 42(11): 5669-5679. |
| [11] | 马云飞, 王建兵, 贾超敏, 邢懿心, 柯述, 张先. 臭氧氧化动力学模型及反应器建模研究进展[J]. 化工进展, 2022, 41(S1): 556-570. |
| [12] | 朱昊, 刘汉飞, 季雨凡, 李双涛, 黄益平, 高源, 魏振浩, 主凯, 韩卫清, 魏卡佳. 催化臭氧化处理酚类化合物的研究进展与机理解析[J]. 化工进展, 2022, 41(S1): 545-555. |
| [13] | 刘汉飞, 朱昊, 李双涛, 季雨凡, 黄益平, 黄晶晶, 倪嵩波, 倪泽雨. 凹凸棒负载型催化剂的制备及处理低浓度有机物效能[J]. 化工进展, 2022, 41(9): 5103-5108. |
| [14] | 郑瑾, 韩瑞瑞, 李丹丹, 王馨妤, 高春阳, 杜显元, 张晓飞, 邹德勋. 过氧化尿素与微生物联合修复石油污染土壤[J]. 化工进展, 2022, 41(9): 5085-5093. |
| [15] | 曹冬冬, 李兴春, 薛明. 石化中间储罐挥发性有机物排放特征与反应活性[J]. 化工进展, 2022, 41(7): 3974-3982. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||