化工进展 ›› 2022, Vol. 41 ›› Issue (12): 6245-6254.DOI: 10.16085/j.issn.1000-6613.2022-0337
松木屑颗粒热解过程中的热/质传递规律
- 太原理工大学省部共建煤基能源清洁高效利用国家重点实验室,山西 太原 030024
-
收稿日期:2022-03-07修回日期:2022-05-18出版日期:2022-12-20发布日期:2022-12-29 -
通讯作者:宋云彩,冯杰 -
作者简介:薛小慧(1996—),女,硕士研究生,研究方向为有机固废热转化利用。E-mail:xuexiaohui0550@link.tyut.edu.cn。 -
基金资助:国家重点研发计划(2019YFC1906802)
Rule of heat and mass transfer during the pyrolysis process of pine sawdust particles
XUE Xiaohui( ), YUAN Mengli, SONG Yuncai(
), YUAN Mengli, SONG Yuncai( ), FENG Jie(
), FENG Jie( )
)
- State Key Laboratory of Clean and Efficient Coal Utilization, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
-
Received:2022-03-07Revised:2022-05-18Online:2022-12-20Published:2022-12-29 -
Contact:SONG Yuncai, FENG Jie
摘要:
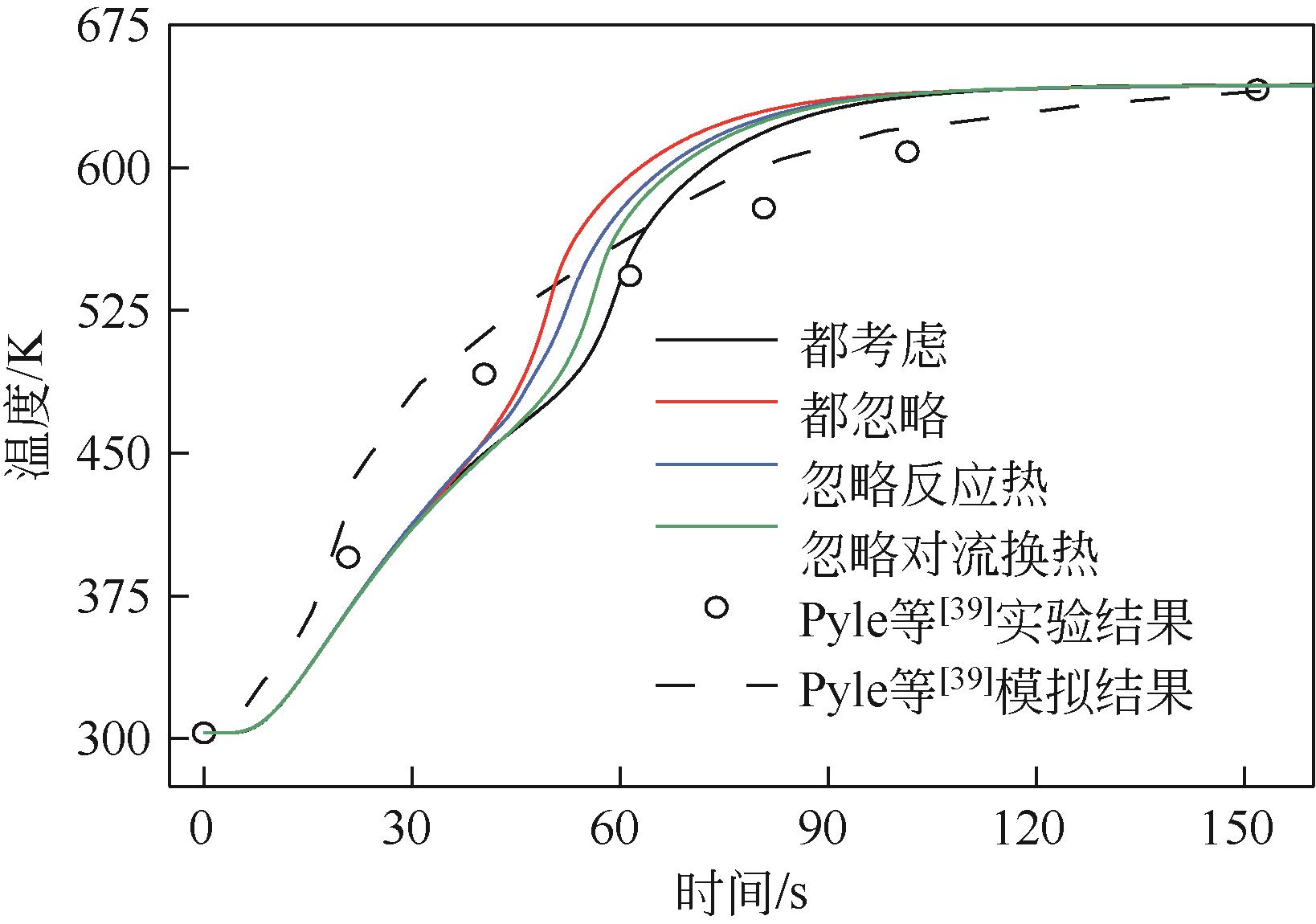
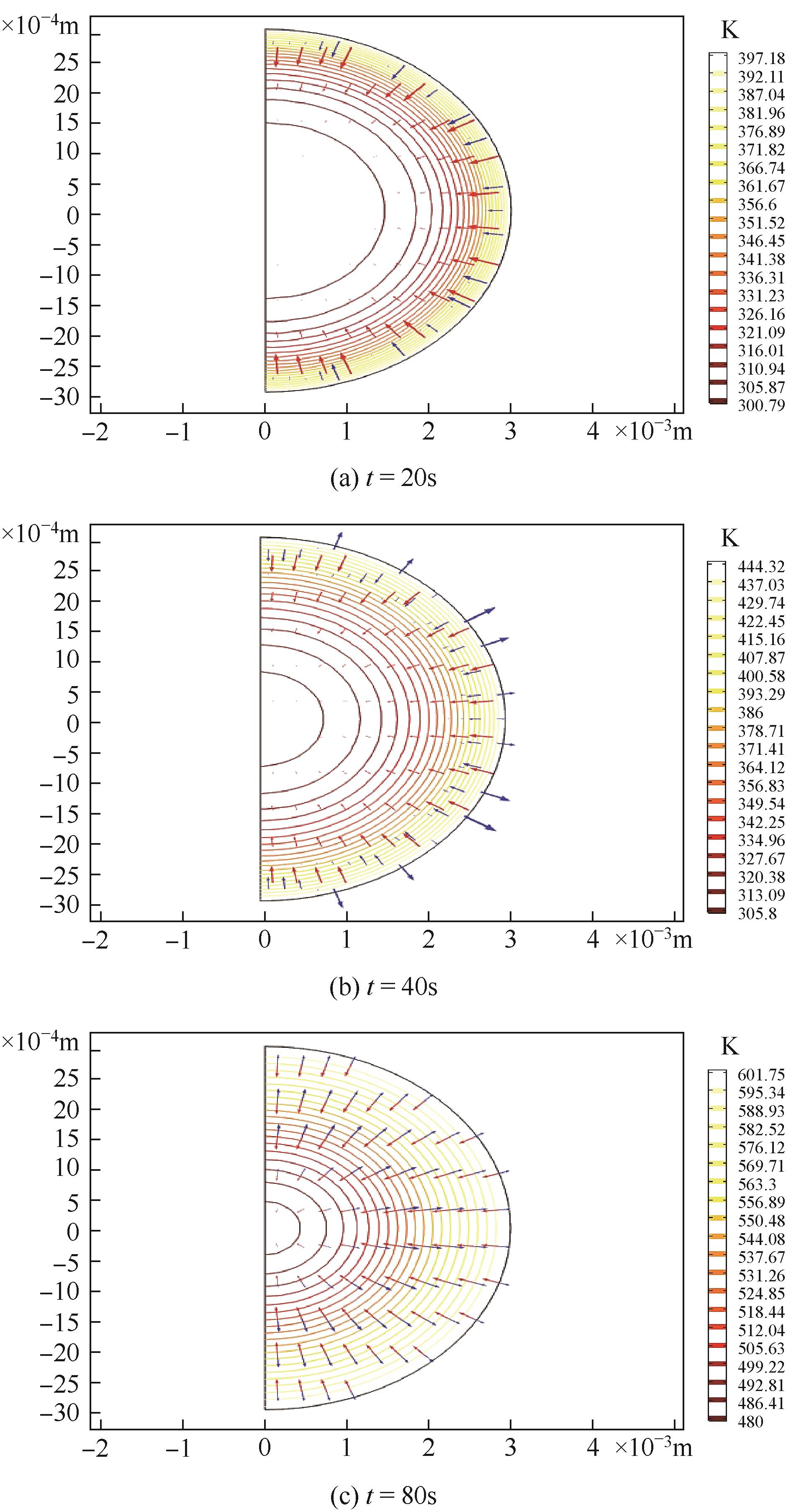
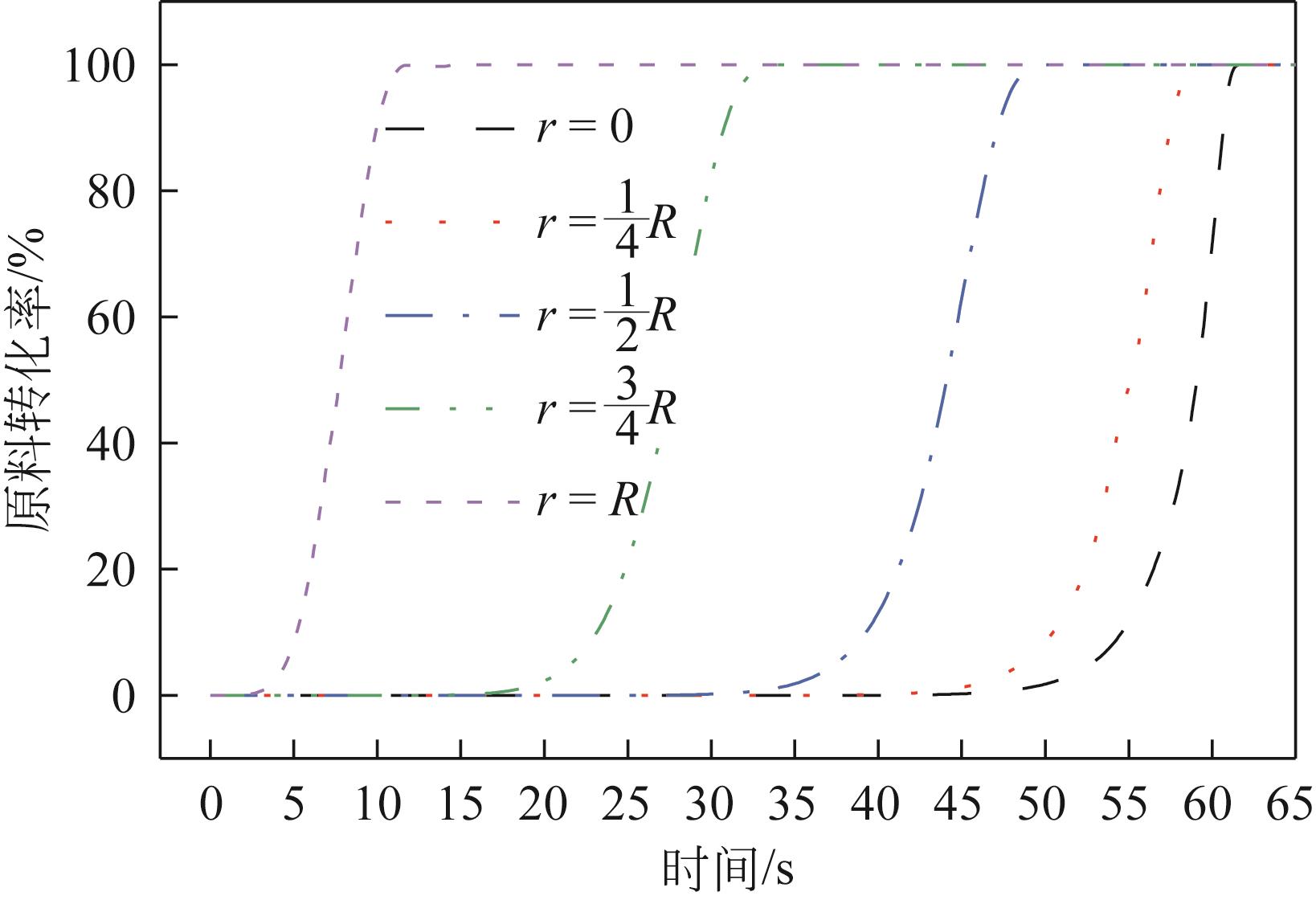
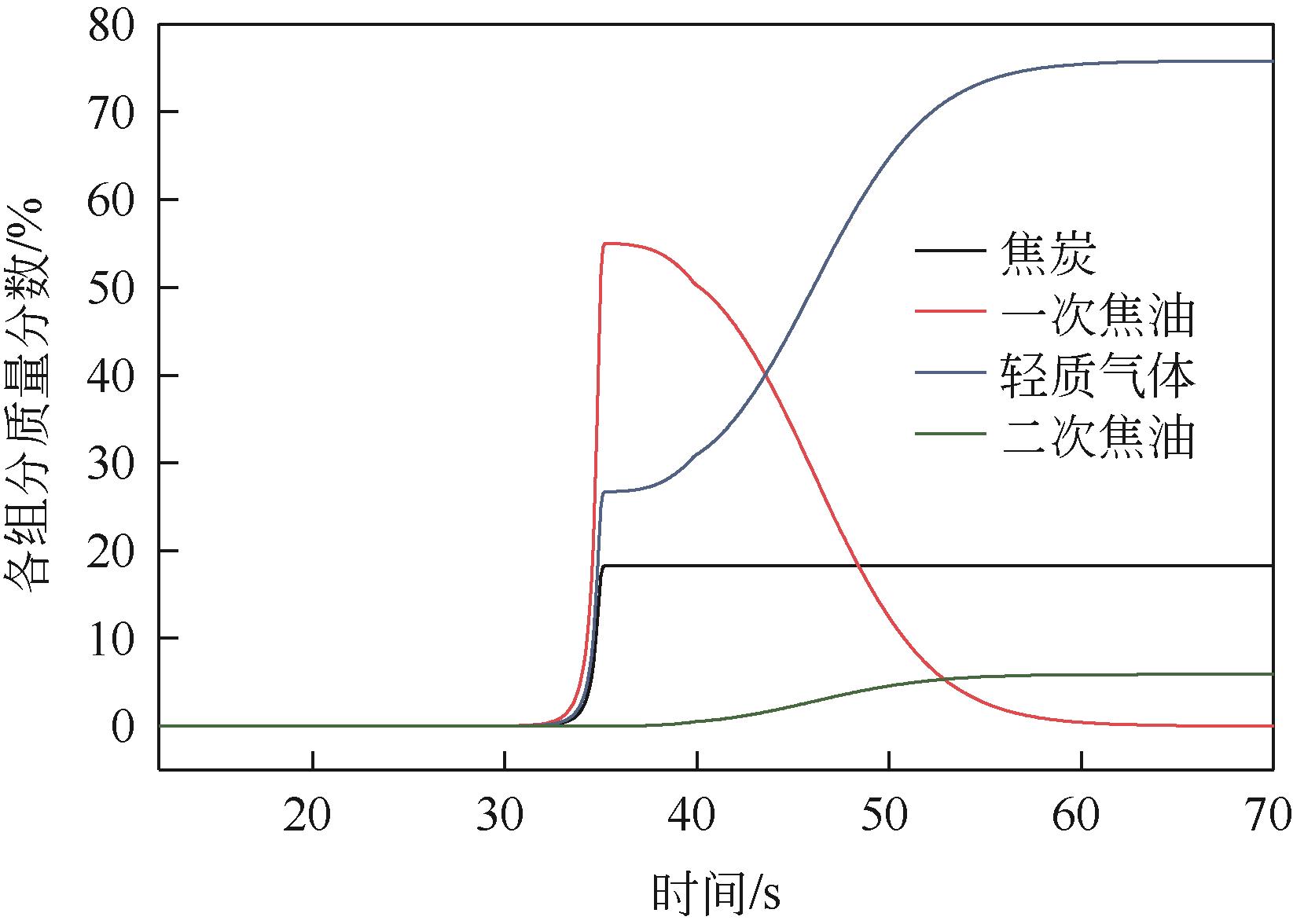
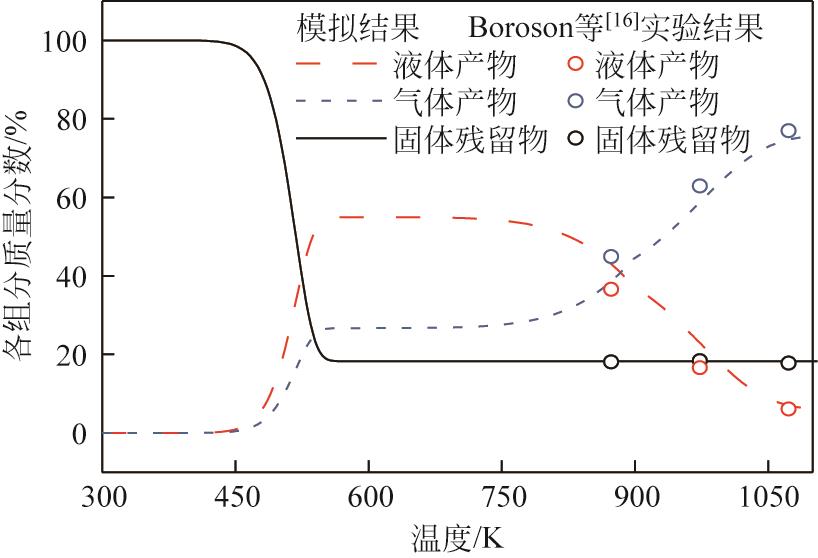
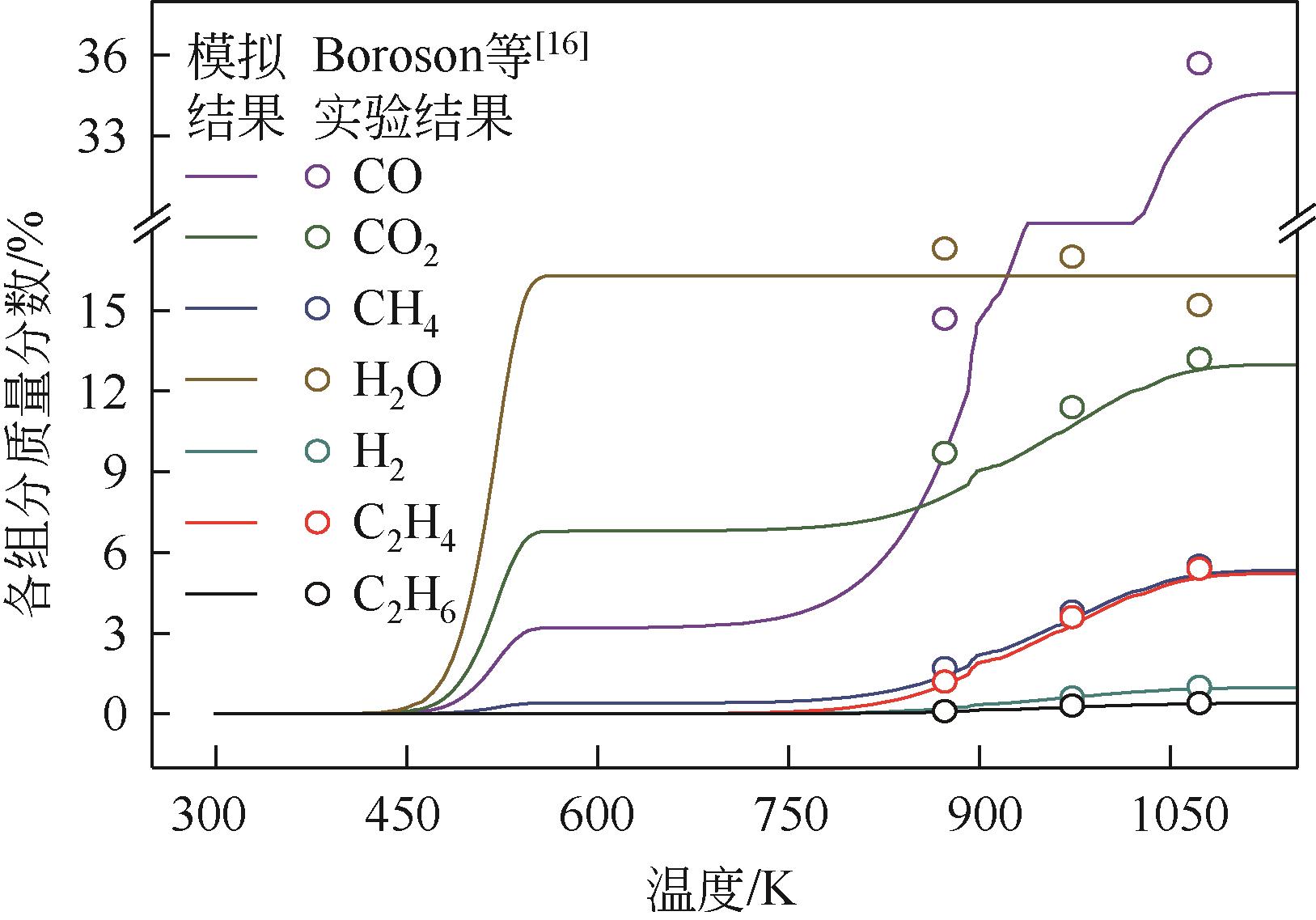
为探索在固定床反应器中有机固废颗粒热解过程中的热量、质量传递机理,本研究从颗粒尺度上对有机固废松木屑颗粒热解过程建模分析,模型中考虑了焦油的二次裂解反应及挥发分在颗粒孔隙中的质量、动量传递过程,并采用达西定律模拟了挥发分在颗粒孔隙内的流动现象,对颗粒热解过程的吸热反应以及挥发分逸出时的对流换热对颗粒温度的影响进行考察。基于两步反应动力学模型,探讨了不同颗粒尺寸、热解温度对有机固废松木屑颗粒热解过程的影响。结果表明,热解吸热反应和挥发分的对流换热阻碍了热量向颗粒中心的传递,延长了颗粒达到均温的时间;松木屑颗粒热解时,颗粒内会存在明显的温度梯度,在颗粒表面主要受化学反应动力学限制,在颗粒内部则主要受热量传递过程限制。此外,热解温度越低,粒径越大,颗粒内部的传热阻力越大。松木屑颗粒完全热解所需时间会随着颗粒粒径的增大而增加,但当颗粒粒径在10mm以上时,随着颗粒粒径的增大,颗粒完全热解所需时间的增量要大于10mm以下颗粒。
中图分类号:
引用本文
薛小慧, 袁梦丽, 宋云彩, 冯杰. 松木屑颗粒热解过程中的热/质传递规律[J]. 化工进展, 2022, 41(12): 6245-6254.
XUE Xiaohui, YUAN Mengli, SONG Yuncai, FENG Jie. Rule of heat and mass transfer during the pyrolysis process of pine sawdust particles[J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6245-6254.
| 工业分析/% | 元素分析(daf)/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vad | FCad | C | H | N | S | O* | |
| 5.76 | 1.03 | 65.72 | 27.49 | 52.41 | 6.58 | 0.09 | 0.14 | 40.78 | |
表1 原料的工业分析与元素分析
| 工业分析/% | 元素分析(daf)/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vad | FCad | C | H | N | S | O* | |
| 5.76 | 1.03 | 65.72 | 27.49 | 52.41 | 6.58 | 0.09 | 0.14 | 40.78 | |
| 组分 | w/% | ξ/% | 组分 | w/% | ξ/% |
|---|---|---|---|---|---|
| T* | 55 | 0 | H2O | 16.3 | 0 |
| C | 18.3 | 0 | T2 | 0 | 5.4 |
| CH4 | 0.4 | 5.43 | C2H4 | 0 | 5.17 |
| CO | 3.2 | 31.13 | C2H6 | 0 | 0.38 |
| CO2 | 6.8 | 6.4 | H2 | 0 | 1.09 |
表2 原料一次裂解和焦油二次裂解的最终产率
| 组分 | w/% | ξ/% | 组分 | w/% | ξ/% |
|---|---|---|---|---|---|
| T* | 55 | 0 | H2O | 16.3 | 0 |
| C | 18.3 | 0 | T2 | 0 | 5.4 |
| CH4 | 0.4 | 5.43 | C2H4 | 0 | 5.17 |
| CO | 3.2 | 31.13 | C2H6 | 0 | 0.38 |
| CO2 | 6.8 | 6.4 | H2 | 0 | 1.09 |
| 反应 | 指前因子A/s-1 | 活化能E/kJ·mol-1 | 反应热∆H/kJ·kg-1 |
|---|---|---|---|
| 1 | 2.66×1010 | 106.5 | 64 |
| 2 | 104.98 | 93.3 | -42 |
表3 热解反应的动力学参数和反应热[16,18-19]
| 反应 | 指前因子A/s-1 | 活化能E/kJ·mol-1 | 反应热∆H/kJ·kg-1 |
|---|---|---|---|
| 1 | 2.66×1010 | 106.5 | 64 |
| 2 | 104.98 | 93.3 | -42 |
| 物性参数 | 值或表达式 |
|---|---|
| 比热容/J·kg-1·K-1 | |
| 热导率/W·m-1·K-1 | |
| 摩尔质量/g·mol-1 | |
颗粒的初始密度 /kg·m-3 | |
| 黏度/Pa·s | |
| 环境压力/Pa | |
| 特征孔径/m | |
| 黑度 |
表4 模型中使用的物性参数[13,19,31]
| 物性参数 | 值或表达式 |
|---|---|
| 比热容/J·kg-1·K-1 | |
| 热导率/W·m-1·K-1 | |
| 摩尔质量/g·mol-1 | |
颗粒的初始密度 /kg·m-3 | |
| 黏度/Pa·s | |
| 环境压力/Pa | |
| 特征孔径/m | |
| 黑度 |
| 位置 | 最大热通量/W·m-2 |
|---|---|
| r=0 | 772.26 |
| r=1/4R | 5189.2 |
| r=1/2R | 8575.9 |
| r=3/4R | 11648 |
| r=R | 22114 |
表5 颗粒内部各位置的最大热通量值
| 位置 | 最大热通量/W·m-2 |
|---|---|
| r=0 | 772.26 |
| r=1/4R | 5189.2 |
| r=1/2R | 8575.9 |
| r=3/4R | 11648 |
| r=R | 22114 |
| 指标参数 | 2mm | 4mm | 6mm | 8mm | 10mm | 12mm | 14mm | 16mm |
|---|---|---|---|---|---|---|---|---|
颗粒表面最大 温升速率/K·s-1 | 246 | 232 | 220 | 186 | 172 | 154 | 136 | 115 |
| 最大温差/K | 130 | 170 | 257 | 292 | 330 | 338 | 353 | 366 |
| 均温/K | 868 | 871 | 870 | 870 | 870 | 871 | 871 | 871 |
| 达均温时间/s | 17 | 38 | 62 | 92 | 127 | 153 | 184 | 215 |
| 完全热解时间/s | 9 | 20 | 32 | 46 | 61 | 77 | 94 | 110 |
表6 不同粒径下颗粒内部热历程和反应的指标参数
| 指标参数 | 2mm | 4mm | 6mm | 8mm | 10mm | 12mm | 14mm | 16mm |
|---|---|---|---|---|---|---|---|---|
颗粒表面最大 温升速率/K·s-1 | 246 | 232 | 220 | 186 | 172 | 154 | 136 | 115 |
| 最大温差/K | 130 | 170 | 257 | 292 | 330 | 338 | 353 | 366 |
| 均温/K | 868 | 871 | 870 | 870 | 870 | 871 | 871 | 871 |
| 达均温时间/s | 17 | 38 | 62 | 92 | 127 | 153 | 184 | 215 |
| 完全热解时间/s | 9 | 20 | 32 | 46 | 61 | 77 | 94 | 110 |
| 指标参数 | 773.15K | 823.15K | 873.15K |
|---|---|---|---|
| 颗粒表面最大温升速率/K·s-1 | 124 | 153 | 172 |
| 最大温差/K | 244 | 275 | 330 |
| 均温/K | 771 | 820 | 870 |
| 达均温时间/s | 135 | 132 | 127 |
| 完全热解时间/s | 77 | 69 | 61 |
表7 不同热解温度下颗粒内部热历程和反应的指标参数
| 指标参数 | 773.15K | 823.15K | 873.15K |
|---|---|---|---|
| 颗粒表面最大温升速率/K·s-1 | 124 | 153 | 172 |
| 最大温差/K | 244 | 275 | 330 |
| 均温/K | 771 | 820 | 870 |
| 达均温时间/s | 135 | 132 | 127 |
| 完全热解时间/s | 77 | 69 | 61 |
| 1 | 季江云. 2019全国有机固废处理与资源化利用研讨会召开 有机固废资源化有多少妙招[J]. 环境与生活, 2019(5): 40-42. |
| JI Jiangyun. The 2019 national organic solid waste treatment and resource utilization seminar was held-organic solid waste resource utilization has a number of clever tricks[J]. Green Living, 2019(5): 40-42. | |
| 2 | 张腾. 厌氧消化技术在有机固体废弃物处理中的应用[J]. 四川化工, 2019, 22(5): 37-40, 54. |
| ZHANG Teng. Application of anaerobic digestion technology in organic solid wastes treatment[J]. Sichuan Chemical Industry, 2019, 22(5): 37-40, 54. | |
| 3 | HU Xun, GHOLIZADEH Mortaza. Biomass pyrolysis: a review of the process development and challenges from initial researches up to the commercialisation stage[J]. Journal of Energy Chemistry, 2019, 39: 109-143. |
| 4 | 陈江, 章旭明. 城郊乡村生活垃圾衍生燃料热解特性研究[J]. 环境污染与防治, 2012, 34(2): 45-49. |
| CHEN Jiang, ZHANG Xuming. Study on the pyrolysis characteristics of refuse derived fuel from village solid waste[J]. Environmental Pollution & Control, 2012, 34(2): 45-49. | |
| 5 | 蒋黎阳, 周臻, 田红, 等. 碱金属对生物质热解特性的影响[J]. 林产化学与工业, 2020, 40(4): 114-122. |
| JIANG Liyang, ZHOU Zhen, TIAN Hong, et al. Effect of alkali metal impregnation on pyrolysis characteristics of biomass[J]. Chemistry and Industry of Forest Products, 2020, 40(4): 114-122. | |
| 6 | 王文燕, 张光义, 孟辉波, 等. 糠醛渣热解特性及热解挥发产物对其燃烧烟气原位控氮作用[J]. 化工学报, 2021, 72(11): 5770-5778. |
| WANG Wenyan, ZHANG Guangyi, MENG Huibo, et al. Furfural residue pyrolysis characteristics and the effect of its pyrolysis products on in-situ control of NO x emission from its combustion flue gas[J]. CIESC Journal, 2021, 72(11): 5770-5778. | |
| 7 | REZAEI Hamid, SOKHANSANJ Shahab, BI Xiaotao, et al. A numerical and experimental study on fast pyrolysis of single woody biomass particles[J]. Applied Energy, 2017, 198: 320-331. |
| 8 | CHERN Jyuung Shauu, HAYHURST Allan N. A simple theoretical analysis of the pyrolysis of an isothermal particle of coal[J]. Combustion and Flame, 2010, 157(5): 925-933. |
| 9 | SOLOMON P R, FLETCHER T H, PUGMIRE R J. Progress in coal pyrolysis[J]. Fuel, 1993, 72(5): 587-597. |
| 10 | HABERLE Inge, Øyvind SKREIBERG, Joanna ŁAZAR, et al. Numerical models for thermochemical degradation of thermally thick woody biomass, and their application in domestic wood heating appliances and grate furnaces[J]. Progress in Energy and Combustion Science, 2017, 63: 204-252. |
| 11 | CALONACI Matteo, GRANA Roberto, BARKER HEMINGS Emma, et al. Comprehensive kinetic modeling study of bio-oil formation from fast pyrolysis of biomass[J]. Energy & Fuels, 2010, 24(10): 5727-5734. |
| 12 | CHEN Tao, KU Xiaoke, LI Tian, et al. High-temperature pyrolysis modeling of a thermally thick biomass particle based on an MD-derived tar cracking model[J]. Chemical Engineering Journal, 2021, 417: 127923. |
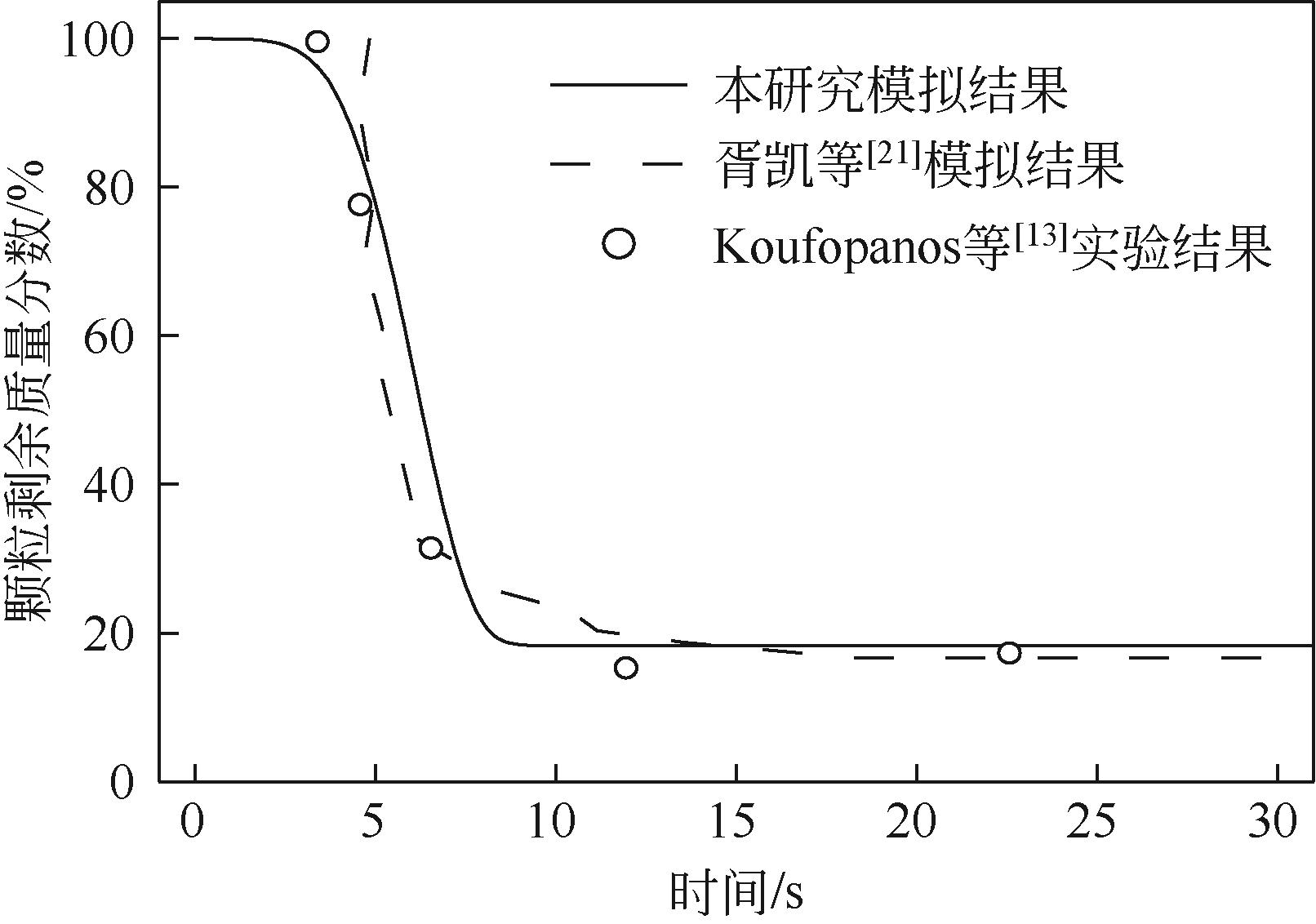
| 13 | KOUFOPANOS C A, PAPAYANNAKOS N, MASCHIO G, et al. Modelling of the pyrolysis of biomass particles. Studies on kinetics, thermal and heat transfer effects[J]. The Canadian Journal of Chemical Engineering, 1991, 69(4): 907-915. |
| 14 | XU Yao, ZHAI Ming, GUO Hongkun, et al. High-temperature pyrolysis characteristics for a single biomass particle[J]. Energy & Fuels, 2019, 33(11): 11153-11162. |
| 15 | CHAN Wai Chun R, KELBON Marcia, KRIEGER Barbara B. Modelling and experimental verification of physical and chemical processes during pyrolysis of a large biomass particle[J]. Fuel, 1985, 64(11): 1505-1513. |
| 16 | BOROSON Michael L, HOWARD Jack B, LONGWELL John P, et al. Product yields and kinetics from the vapor phase cracking of wood pyrolysis tars[J]. AIChE Journal, 1989, 35(1): 120-128. |
| 17 | ARRHENIUS Svante. Über die reaktionsgeschwindigkeit bei der inversion von rohrzucker durch säuren[J]. Zeitschrift für Physikalische Chemie, 1889, 4(1): 226-248. |
| 18 | THURNER Franz, MANN Uzi. Kinetic investigation of wood pyrolysis[J]. Industrial & Engineering Chemistry Process Design and Development, 1981, 20(3): 482-488. |
| 19 | GRØNLI Morten G, MELAAEN Morten C. Mathematical model for wood pyrolysis-comparison of experimental measurements with model predictions[J]. Energy & Fuels, 2000, 14(4): 791-800. |
| 20 | BLASI Colomba Di. Heat, momentum and mass transport through a shrinking biomass particle exposed to thermal radiation[J]. Chemical Engineering Science, 1996, 51(7): 1121-1132. |
| 21 | 胥凯, 卢文强. 单个球形生物质颗粒热解过程的数值模拟[J]. 工程热物理学报, 2006, 27(6): 981-983. |
| XU Kai, LU Wenqiang. Numerical simulation for the pyrolysis of a single globular biomass particle[J]. Journal of Engineering Thermophysics, 2006, 27(6): 981-983. | |
| 22 | MERMOUD F, GOLFIER F, SALVADOR S, et al. Experimental and numerical study of steam gasification of a single charcoal particle[J]. Combustion and Flame, 2006, 145(1-2): 59-79. |
| 23 | LI Shunan, CAO Bingyang. Vortex characteristics in Fourier and non-Fourier heat conduction based on heat flux rotation[J]. International Journal of Heat and Mass Transfer, 2017, 108: 2403-2407. |
| 24 | 刘永忠, 王乐. 多孔介质中超临界流体可调特性的模拟与分析——温度梯度的作用[J]. 高校化学工程学报, 2008, 22(6): 915-920. |
| LIU Yongzhong, WANG Le. Simulation and analysis of tunable performances of supercritical fluid flow in porous medium——effect of temperature gradients[J]. Journal of Chemical Engineering of Chinese Universities, 2008, 22(6): 915-920. | |
| 25 | ATANGANA A. Fractional operators with constant and variable order with application to geo-hydrology[M]. Pittsburgh: Academic Press, 2018: 221-243. |
| 26 | DALLAVALLE J M. Book reviews: flow of gases through porous media[J]. Science, 1956, 124: 1254-1255. |
| 27 | 徐鹏, 邱淑霞, 姜舟婷, 等. 各向同性多孔介质中Kozeny-Carman常数的分形分析[J]. 重庆大学学报, 2011, 34(4): 78-82. |
| XU Peng, QIU Shuxia, JIANG Zhouting, et al. Fractal analysis of Kozeny-Carman constant in the homogenous porous media[J]. Journal of Chongqing University, 2011, 34(4): 78-82. | |
| 28 | 曾子粤, 杨建森, 魏永起. 脱硫石膏灌芯墙脱水过程的仿真模拟与分析[J]. 材料导报, 2022, 36(5): 85-90. |
| ZENG Ziyue, YANG Jiansen, WEI Yongqi. The simulation and analysis of dehydration process of FGD gypsum grouted wall[J]. Materials Reports, 2022, 36(5): 85-90. | |
| 29 | FLORES Romeo M. Coal and coalbed gas[M]. Waltham: Fueling the Future, 2014: 587-614. |
| 30 | 李位位, 黄戒介, 王志青, 等. 煤焦CO2气化反应动力学及内扩散对气化过程的影响分析[J]. 燃料化学学报, 2016, 44(12): 1216-1221. |
| LI Weiwei, HUANG Jiejie, WANG Zhiqing, et al. Reaction kinetics of coal char gasification with CO2 of internal diffusion on the gasification[J]. Journal of Fuel Chemistry and Technology, 2016, 44(12): 1216-1221. | |
| 31 | XU Qixiang, PANG Shusheng, LEVI Tana. Reaction kinetics and producer gas compositions of steam gasification of coal and biomass blend chars, Part 2: Mathematical modelling and model validation[J]. Chemical Engineering Science, 2011, 66(10): 2232-2240. |
| 32 | KRISHNA Rajamani, VAN BATEN Jasper M. Investigating the validity of the Bosanquet formula for estimation of diffusivities in mesopores[J]. Chemical Engineering Science, 2012, 69(1): 684-688. |
| 33 | FULLER Edward N, SCHETTLER paul D, Calvin GIDDINGS J. New method for prediction of binary gas-phase diffusion coefficients[J]. Industrial & Engineering Chemistry, 1966, 58(5): 18-27. |
| 34 | 李方舟, 李文英, 冯杰. 固体热载体法褐煤热解过程中的传质传热特性[J]. 化工学报, 2016, 67(4): 1136-1144. |
| LI Fangzhou, LI Wenying, FENG Jie. Characteristics of mass and heat transfer in lignite pyrolysis with solid heat carrier[J]. CIESC Journal, 2016, 67(4): 1136-1144. | |
| 35 | ADESANYA Babafemi A, PHAM Hoanh N. Mathematical modelling of devolatilization of large coal particles in a convective environment[J]. Fuel, 1995, 74(6): 896-902. |
| 36 | WAKAO N, KAGUEI S, FUNAZKRI T. Effect of fluid dispersion coefficients on particle-to-fluid heat transfer coefficients in packed beds: correlation of nusselt numbers[J]. Chemical Engineering Science, 1979, 34(3): 325-336. |
| 37 | GUNN D J. Transfer of heat or mass to particles in fixed and fluidised beds[J]. International Journal of Heat and Mass Transfer, 1978, 21(4): 467-476. |
| 38 | 唐洪波, 陈永杰, 唐洪涛. 振动参数对床层空隙率的影响[J]. 沈阳工业大学学报, 2001, 23(3): 258-260. |
| TANG Hongbo, CHEN Yongjie, TANG Hongtao. Effect of vibrational parameter on bed voidage[J]. Journal of Shenyang University of Technology, 2001, 23(3): 258-260. | |
| 39 | PYLE D L, ZAROR C A. Heat transfer and kinetics in the low temperature pyrolysis of solids[J]. Chemical Engineering Science, 1984, 39(1): 147-158. |
| [1] | 肖辉, 张显均, 兰治科, 王苏豪, 王盛. 液态金属绕流管束流动传热进展[J]. 化工进展, 2023, 42(S1): 10-20. |
| [2] | 王太, 苏硕, 李晟瑞, 马小龙, 刘春涛. 交流电场中贴壁气泡的动力学行为[J]. 化工进展, 2023, 42(S1): 133-141. |
| [3] | 盛维武, 程永攀, 陈强, 李小婷, 魏嘉, 李琳鸽, 陈险峰. 微气泡和微液滴双强化脱硫反应器操作分析[J]. 化工进展, 2023, 42(S1): 142-147. |
| [4] | 赵晨, 苗天泽, 张朝阳, 洪芳军, 汪大海. 负压状态窄缝通道乙二醇水溶液传热特性[J]. 化工进展, 2023, 42(S1): 148-157. |
| [5] | 黄益平, 李婷, 郑龙云, 戚傲, 陈政霖, 史天昊, 张新宇, 郭凯, 胡猛, 倪泽雨, 刘辉, 夏苗, 主凯, 刘春江. 三级环流反应器中气液流动与传质规律[J]. 化工进展, 2023, 42(S1): 175-188. |
| [6] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [7] | 陈匡胤, 李蕊兰, 童杨, 沈建华. 质子交换膜燃料电池气体扩散层结构与设计研究进展[J]. 化工进展, 2023, 42(S1): 246-259. |
| [8] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [9] | 邵博识, 谭宏博. 锯齿波纹板对挥发性有机物低温脱除过程强化模拟分析[J]. 化工进展, 2023, 42(S1): 84-93. |
| [10] | 陈林, 徐培渊, 张晓慧, 陈杰, 徐振军, 陈嘉祥, 密晓光, 冯永昌, 梅德清. 液化天然气绕管式换热器壳侧混合工质流动及传热特性[J]. 化工进展, 2023, 42(9): 4496-4503. |
| [11] | 刘炫麟, 王驿凯, 戴苏洲, 殷勇高. 热泵中氨基甲酸铵分解反应特性及反应器结构优化[J]. 化工进展, 2023, 42(9): 4522-4530. |
| [12] | 赵曦, 马浩然, 李平, 黄爱玲. 错位碰撞型微混合器混合性能的模拟分析与优化设计[J]. 化工进展, 2023, 42(9): 4559-4572. |
| [13] | 卜治丞, 焦波, 林海花, 孙洪源. 脉动热管计算流体力学模型与研究进展[J]. 化工进展, 2023, 42(8): 4167-4181. |
| [14] | 汪健生, 张辉鹏, 刘雪玲, 傅煜郭, 朱剑啸. 多孔介质结构对储层内流动和换热特性的影响[J]. 化工进展, 2023, 42(8): 4212-4220. |
| [15] | 王云刚, 焦健, 邓世丰, 赵钦新, 邵怀爽. 冷凝换热与协同脱硫性能实验分析[J]. 化工进展, 2023, 42(8): 4230-4237. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||