化工进展 ›› 2022, Vol. 41 ›› Issue (10): 5669-5676.DOI: 10.16085/j.issn.1000-6613.2022-0015
基于酸度系数(pKa)模型的胺法SO2捕集过程多目标效能分析
王东亮1,2( ), 谢江鹏1,2, 孟文亮1,2, 李婧玮1,2, 周怀荣1,2(
), 谢江鹏1,2, 孟文亮1,2, 李婧玮1,2, 周怀荣1,2( )
)
- 1.兰州理工大学石油化工学院,甘肃 兰州 730050
2.甘肃省低碳能源化工重点实验室,甘肃 兰州 730050
-
收稿日期:2022-01-04修回日期:2022-06-06出版日期:2022-10-20发布日期:2022-10-21 -
通讯作者:周怀荣 -
作者简介:王东亮(1982—),男,博士,副教授,研究方向为化工过程集成与资源综合利用。E-mail:wangdl@lut.edu.cn。 -
基金资助:甘肃省科技重大专项(19ZD2G1D001)
Multi-objective capacity and heat analysis of amine-based SO2 capture process from acidity coefficient (pKa) model
WANG Dongliang1,2( ), XIE Jiangpeng1,2, MENG Wenliang1,2, LI Jingwei1,2, ZHOU Huairong1,2(
), XIE Jiangpeng1,2, MENG Wenliang1,2, LI Jingwei1,2, ZHOU Huairong1,2( )
)
- 1.School of Petrochemical Engineering, Lanzhou University of Technology, Lanzhou 730050, Gansu, China
2.Key Laboratory of Low Carbon Energy and Chemical Engineering of Gansu Province, Lanzhou 730050, Gansu, China
-
Received:2022-01-04Revised:2022-06-06Online:2022-10-20Published:2022-10-21 -
Contact:ZHOU Huairong
摘要:
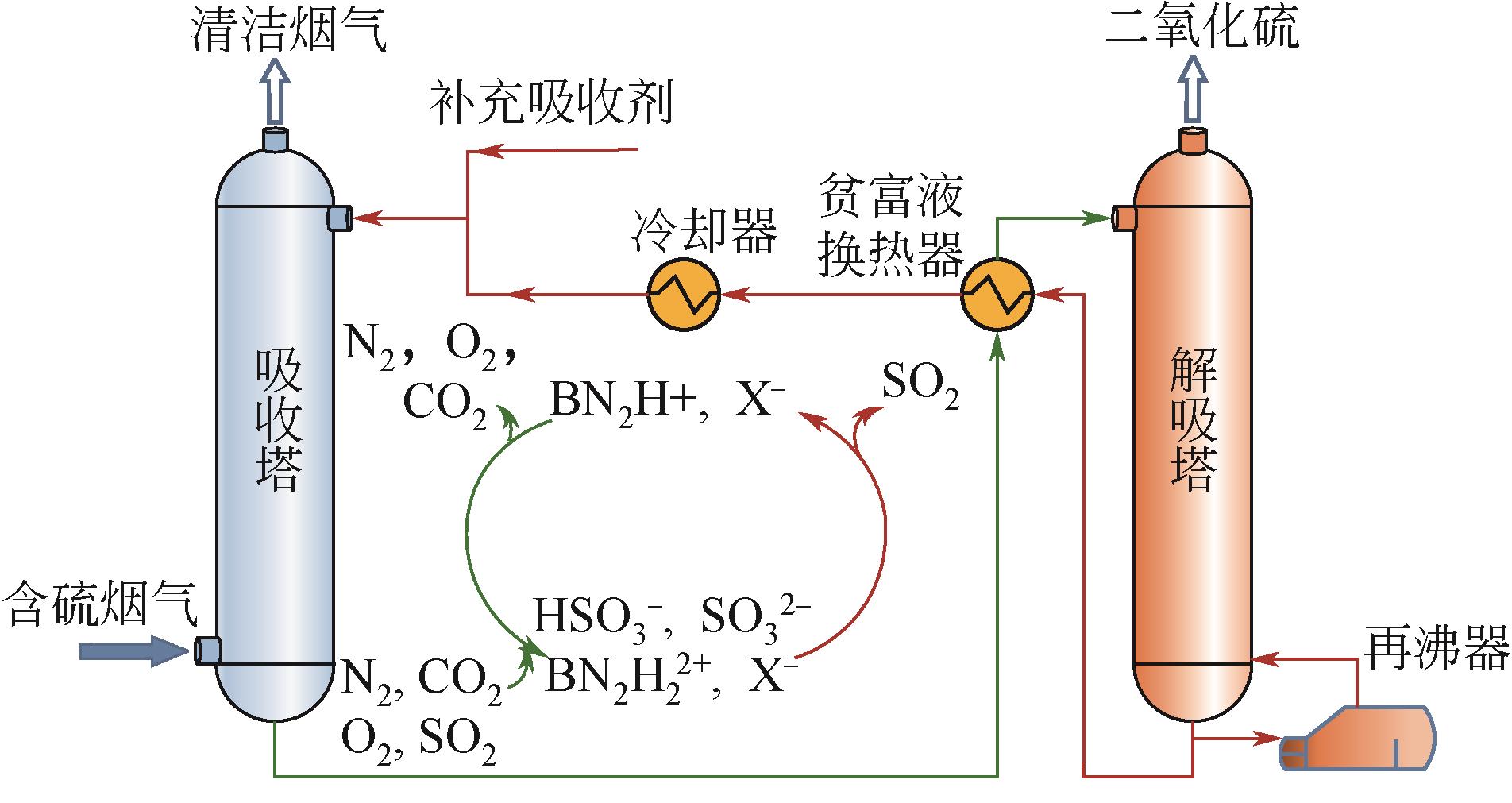
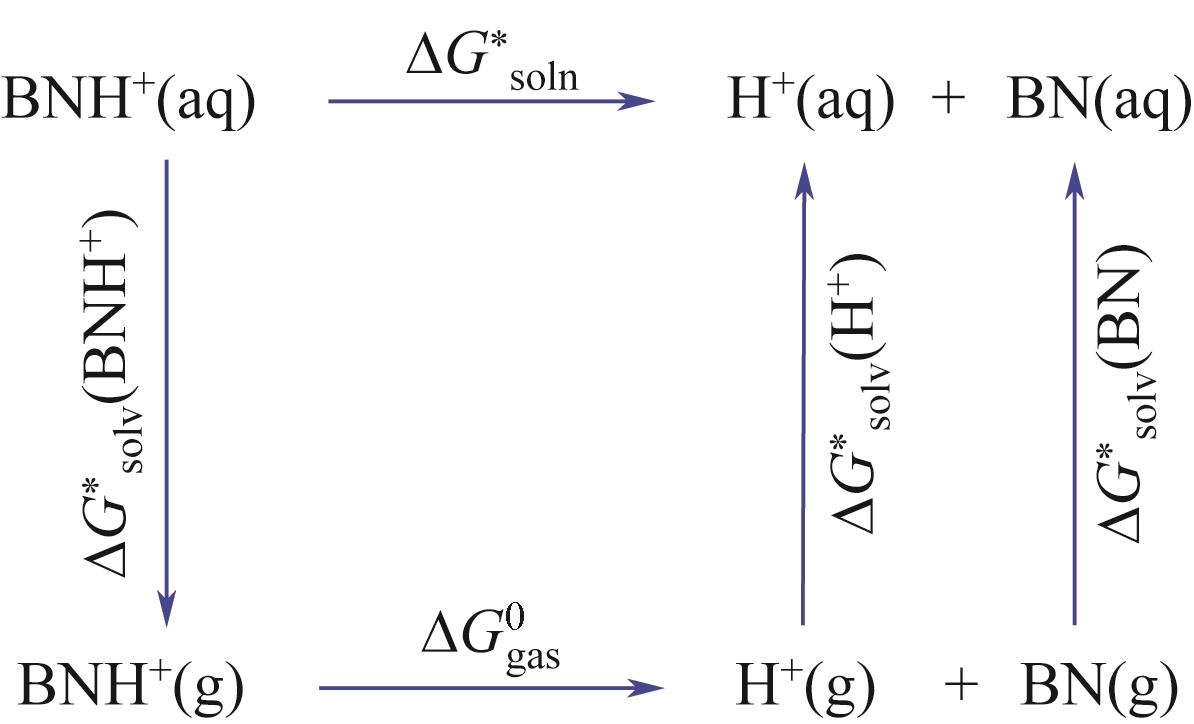
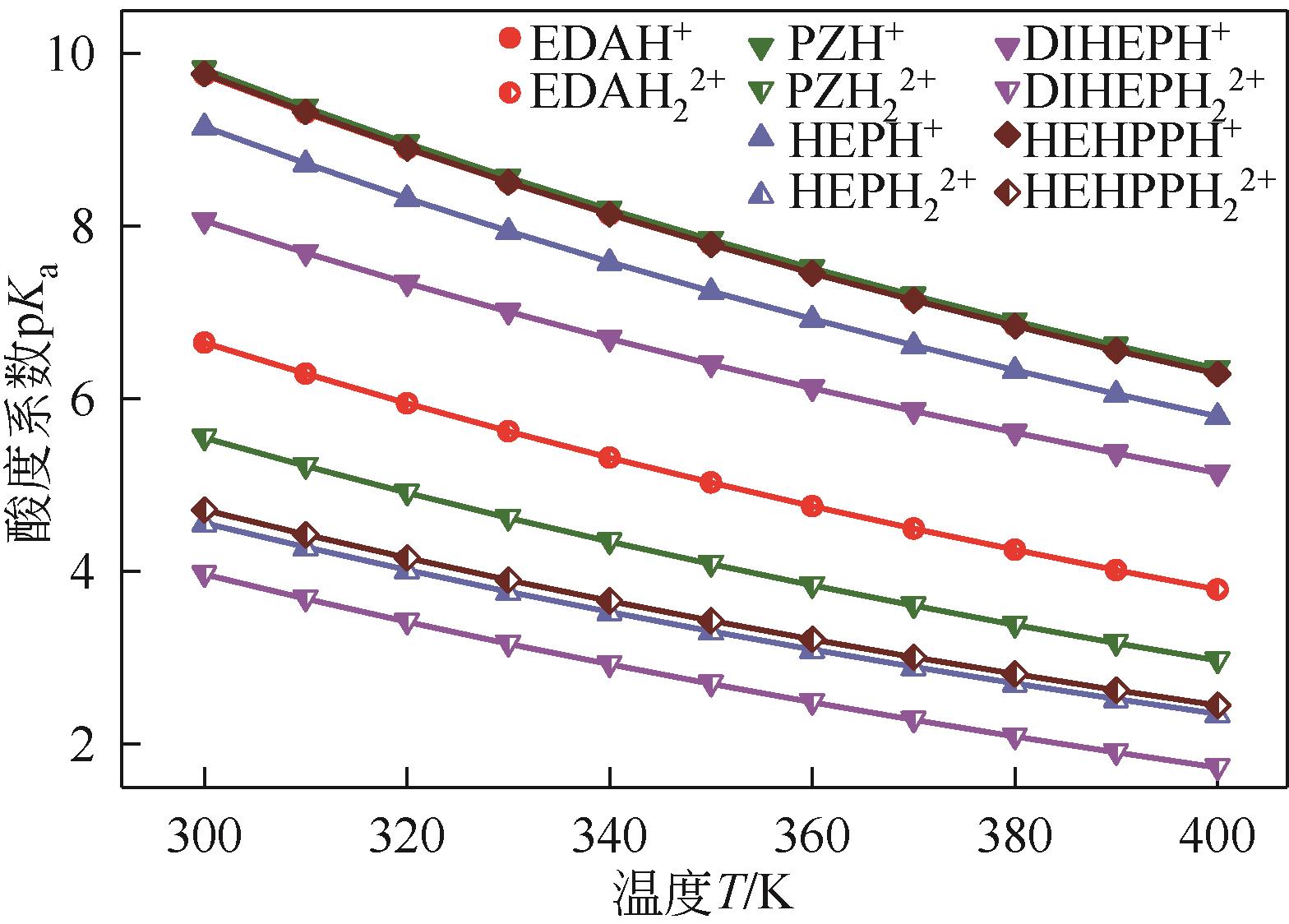
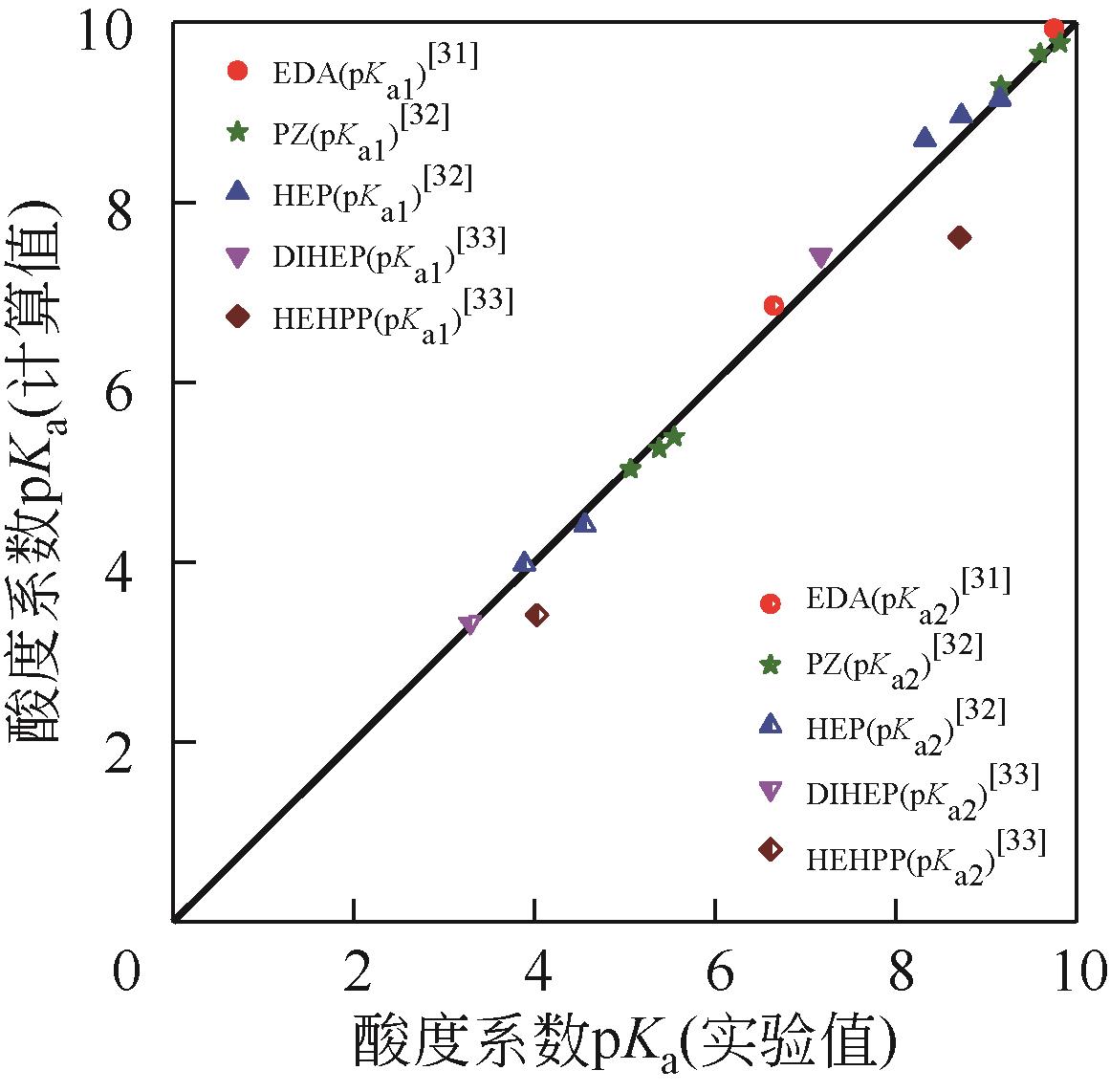
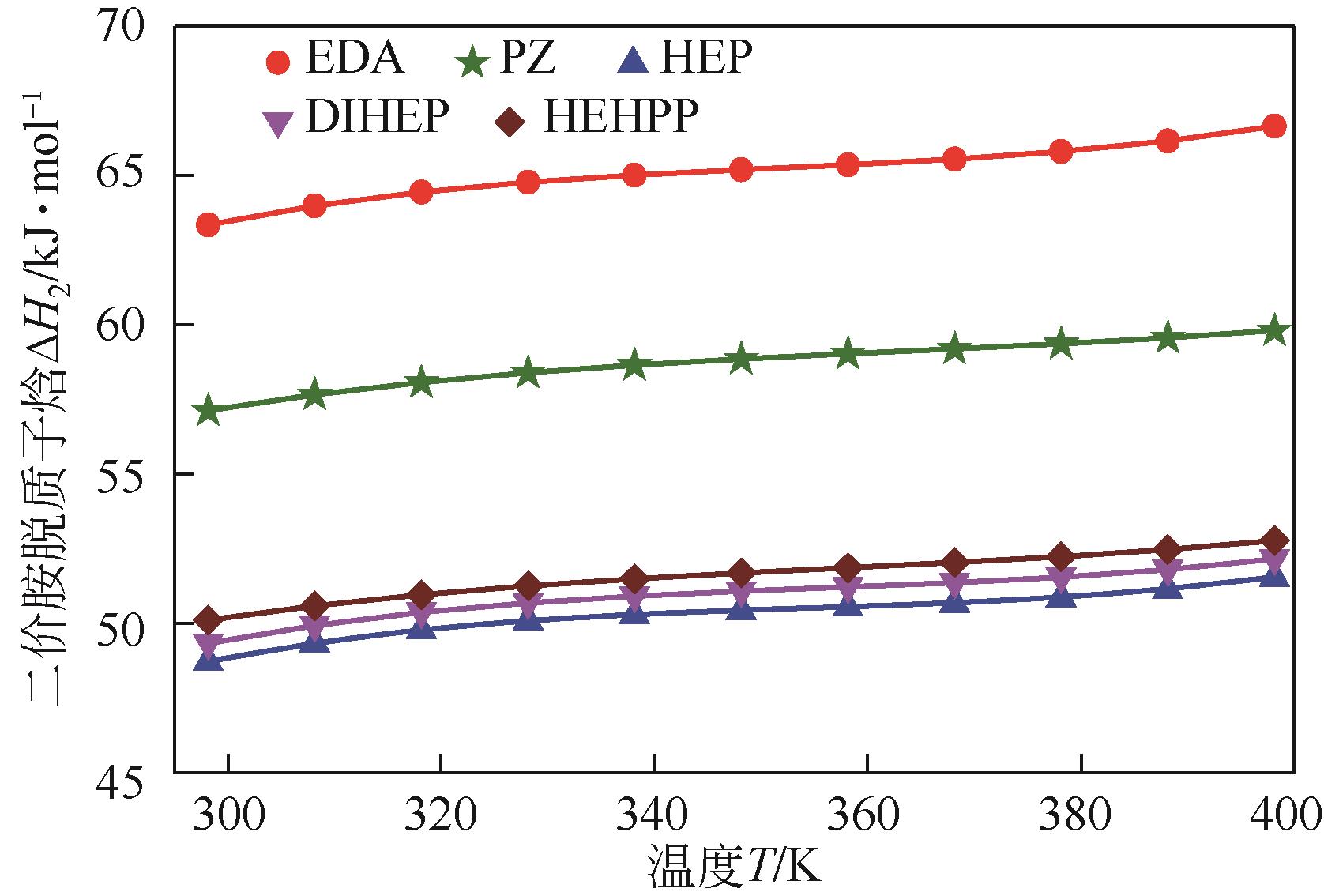
筛选高SO2吸收容量、低解吸能耗的吸收剂是提高胺基烟气SO2捕集工艺应用潜力的重要途径。本研究采用SMD连续溶剂化模型和密度泛函理论在M05-2X/6-31G*基组水平上预测了5种有机二胺类物质的pKa,基于预测的pKa建立起吸收剂对SO2吸收容量和解吸反应热的数学模型,以探讨有机二胺-酸-水三元体系吸收剂捕集SO2过程中的效能关系。结果表明,数学模型符合工程精度要求。二胺的SO2吸收容量和脱质子反应焓都随pKa增大而增加,筛选有机胺吸收剂展现出对SO2吸收容量和反应焓的多目标矛盾性特征;量化了5种二胺的SO2循环吸收容量和解吸反应热的数值关系,在相同SO2循环容量条件下,5种二胺中羟乙基哌嗪(HEP)的解吸热最小,HEP为有机二胺-酸-水三元体系中的一种潜力二胺类吸收剂。
中图分类号:
引用本文
王东亮, 谢江鹏, 孟文亮, 李婧玮, 周怀荣. 基于酸度系数(pKa)模型的胺法SO2捕集过程多目标效能分析[J]. 化工进展, 2022, 41(10): 5669-5676.
WANG Dongliang, XIE Jiangpeng, MENG Wenliang, LI Jingwei, ZHOU Huairong. Multi-objective capacity and heat analysis of amine-based SO2 capture process from acidity coefficient (pKa) model[J]. Chemical Industry and Engineering Progress, 2022, 41(10): 5669-5676.
| 类别 | A | B | C | D | 温度区间/K | 参考 |
|---|---|---|---|---|---|---|
| lnKs1(SO2) | -10.9670 | 1972.5 | — | — | — | Rabe等[ |
| lnKs1(HSO | -357.5700 | 5477.1 | 65.31 | -0.1624 | — | Millero等[ |
| lnK(H2SO4) | >10 | — | — | — | — | Sippola等[ |
| lnK(HSO | -49.7086 | 178.1142 | 10.9523 | -0.0598 | — | Sippola等[ |
| lnK(EDAH+) | -79.7113 | -6508.6363 | 14.4126 | -0.0169 | 298.15~398.15 | 拟合 |
| lnK(PZH+) | -62.6234 | -7025.0400 | 11.8682 | -0.0136 | 298.15~398.15 | 拟合 |
| lnK(HEPH+) | -69.6302 | -6496.8100 | 13.1533 | -0.0154 | 298.15~398.15 | 拟合 |
| lnK(DIHEPH+) | -56.7455 | -5761.7300 | 10.7373 | -0.0123 | 298.15~398.15 | 拟合 |
| lnK(HEHPPH+) | -75.0596 | -6708.4600 | 14.0755 | -0.0172 | 298.15~398.15 | 拟合 |
| lnK(EDAH | -66.4837 | -5110.9300 | 12.7213 | -0.0140 | 298.15~398.15 | 拟合 |
| lnK(PZH | -92.5319 | -3609.9200 | 17.2128 | -0.0208 | 298.15~398.15 | 拟合 |
| lnK(HEPH | -84.1281 | -2928.1100 | 15.6410 | -0.0190 | 298.15~398.15 | 拟合 |
| lnK(DIHEPH | -87.4632 | -2832.4700 | 16.4588 | -0.0199 | 298.15~398.15 | 拟合 |
| lnK(HEHPPH | -66.4512 | -3557.3000 | 12.5968 | -0.0142 | 298.15~398.15 | 拟合 |
表1 lnK-T关系参数
| 类别 | A | B | C | D | 温度区间/K | 参考 |
|---|---|---|---|---|---|---|
| lnKs1(SO2) | -10.9670 | 1972.5 | — | — | — | Rabe等[ |
| lnKs1(HSO | -357.5700 | 5477.1 | 65.31 | -0.1624 | — | Millero等[ |
| lnK(H2SO4) | >10 | — | — | — | — | Sippola等[ |
| lnK(HSO | -49.7086 | 178.1142 | 10.9523 | -0.0598 | — | Sippola等[ |
| lnK(EDAH+) | -79.7113 | -6508.6363 | 14.4126 | -0.0169 | 298.15~398.15 | 拟合 |
| lnK(PZH+) | -62.6234 | -7025.0400 | 11.8682 | -0.0136 | 298.15~398.15 | 拟合 |
| lnK(HEPH+) | -69.6302 | -6496.8100 | 13.1533 | -0.0154 | 298.15~398.15 | 拟合 |
| lnK(DIHEPH+) | -56.7455 | -5761.7300 | 10.7373 | -0.0123 | 298.15~398.15 | 拟合 |
| lnK(HEHPPH+) | -75.0596 | -6708.4600 | 14.0755 | -0.0172 | 298.15~398.15 | 拟合 |
| lnK(EDAH | -66.4837 | -5110.9300 | 12.7213 | -0.0140 | 298.15~398.15 | 拟合 |
| lnK(PZH | -92.5319 | -3609.9200 | 17.2128 | -0.0208 | 298.15~398.15 | 拟合 |
| lnK(HEPH | -84.1281 | -2928.1100 | 15.6410 | -0.0190 | 298.15~398.15 | 拟合 |
| lnK(DIHEPH | -87.4632 | -2832.4700 | 16.4588 | -0.0199 | 298.15~398.15 | 拟合 |
| lnK(HEHPPH | -66.4512 | -3557.3000 | 12.5968 | -0.0142 | 298.15~398.15 | 拟合 |
| 温度T/K | 解离自由能ΔG1/kJ·mol-1 | 解离自由能∆G2/kJ·mol-1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EDAH+ | PZH+ | HEPH+ | DIHEPH+ | HEHPPH+ | EDAH | PZH | HEPH | DIHEPH | HEHPPH | ||
| 298.15 | 55.6187 | 55.9831 | 52.2001 | 45.973 | 47.1383 | 37.9241 | 31.6248 | 25.9967 | 22.6101 | 23.8055 | |
| 308.15 | 54.628 | 54.9944 | 51.1718 | 45.0618 | 46.1351 | 36.8039 | 30.5023 | 24.9643 | 21.4439 | 22.7563 | |
| 318.15 | 53.3262 | 53.6688 | 49.7306 | 43.657 | 44.6075 | 35.9446 | 29.6664 | 24.2946 | 20.7295 | 22.1856 | |
| 328.15 | 52.5966 | 52.9567 | 49.0525 | 43.1891 | 44.066 | 34.4923 | 28.1971 | 22.8366 | 19.0488 | 20.612 | |
| 338.15 | 51.5683 | 51.9323 | 47.9866 | 42.2486 | 43.0294 | 33.3303 | 27.0363 | 21.7666 | 17.8491 | 19.5294 | |
| 348.15 | 50.5358 | 50.904 | 46.9165 | 41.2998 | 41.9844 | 32.1641 | 25.8701 | 20.6923 | 16.6369 | 18.4426 | |
| 358.15 | 49.495 | 49.8674 | 45.8381 | 40.3467 | 40.931 | 30.9853 | 24.6913 | 19.6097 | 15.4205 | 17.3474 | |
| 368.15 | 48.4542 | 48.8224 | 44.7597 | 39.3895 | 39.8819 | 29.8024 | 23.5126 | 18.5187 | 14.2 | 16.2439 | |
| 378.15 | 47.405 | 47.7816 | 43.6729 | 38.4323 | 38.8243 | 28.6152 | 22.3255 | 17.4319 | 12.9711 | 15.1404 | |
| 388.15 | 46.3475 | 46.7282 | 42.5777 | 37.4625 | 37.7584 | 27.4156 | 21.1342 | 16.3367 | 11.738 | 14.0285 | |
| 398.15 | 45.2899 | 45.6707 | 41.4825 | 36.4928 | 36.6967 | 26.2159 | 19.9429 | 15.2332 | 10.5049 | 12.9124 | |
表2 一价质子胺(BN2H+)解离自由能(ΔG1)和二价质子胺(BN2H2+)解离自由能(ΔG2)
| 温度T/K | 解离自由能ΔG1/kJ·mol-1 | 解离自由能∆G2/kJ·mol-1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EDAH+ | PZH+ | HEPH+ | DIHEPH+ | HEHPPH+ | EDAH | PZH | HEPH | DIHEPH | HEHPPH | ||
| 298.15 | 55.6187 | 55.9831 | 52.2001 | 45.973 | 47.1383 | 37.9241 | 31.6248 | 25.9967 | 22.6101 | 23.8055 | |
| 308.15 | 54.628 | 54.9944 | 51.1718 | 45.0618 | 46.1351 | 36.8039 | 30.5023 | 24.9643 | 21.4439 | 22.7563 | |
| 318.15 | 53.3262 | 53.6688 | 49.7306 | 43.657 | 44.6075 | 35.9446 | 29.6664 | 24.2946 | 20.7295 | 22.1856 | |
| 328.15 | 52.5966 | 52.9567 | 49.0525 | 43.1891 | 44.066 | 34.4923 | 28.1971 | 22.8366 | 19.0488 | 20.612 | |
| 338.15 | 51.5683 | 51.9323 | 47.9866 | 42.2486 | 43.0294 | 33.3303 | 27.0363 | 21.7666 | 17.8491 | 19.5294 | |
| 348.15 | 50.5358 | 50.904 | 46.9165 | 41.2998 | 41.9844 | 32.1641 | 25.8701 | 20.6923 | 16.6369 | 18.4426 | |
| 358.15 | 49.495 | 49.8674 | 45.8381 | 40.3467 | 40.931 | 30.9853 | 24.6913 | 19.6097 | 15.4205 | 17.3474 | |
| 368.15 | 48.4542 | 48.8224 | 44.7597 | 39.3895 | 39.8819 | 29.8024 | 23.5126 | 18.5187 | 14.2 | 16.2439 | |
| 378.15 | 47.405 | 47.7816 | 43.6729 | 38.4323 | 38.8243 | 28.6152 | 22.3255 | 17.4319 | 12.9711 | 15.1404 | |
| 388.15 | 46.3475 | 46.7282 | 42.5777 | 37.4625 | 37.7584 | 27.4156 | 21.1342 | 16.3367 | 11.738 | 14.0285 | |
| 398.15 | 45.2899 | 45.6707 | 41.4825 | 36.4928 | 36.6967 | 26.2159 | 19.9429 | 15.2332 | 10.5049 | 12.9124 | |
SO2分压p(SO2) /Pa | 浓度 | 相对误差 /% | |||
|---|---|---|---|---|---|
c(EDA) /mol·L-1 | c(H3PO4) /mol·L-1 | cs(exp) /mol·L-1 | cs(cal) /mol·L-1 | ||
| 143 | 0.3 | 0 | 0.5610 | 0.5945 | -5.6350 |
| 204 | 0.3 | 0 | 0.5630 | 0.5970 | -5.6951 |
| 294 | 0.3 | 0 | 0.5820 | 0.5992 | -2.8705 |
| 112 | 0.3 | 0.13 | 0.4820 | 0.4653 | 3.5891 |
| 232 | 0.3 | 0.13 | 0.4660 | 0.4700 | -0.8511 |
| 301 | 0.3 | 0.13 | 0.4680 | 0.4715 | -0.7423 |
| 108 | 0.3 | 0.26 | 0.3325 | 0.3380 | -1.6272 |
| 298 | 0.3 | 0.26 | 0.3450 | 0.3444 | 0.1742 |
| 373 | 0.3 | 0.26 | 0.3525 | 0.3462 | 1.8198 |
| 118 | 0.3 | 0.39 | 0.2235 | 0.2122 | 5.3252 |
| 252 | 0.3 | 0.39 | 0.2288 | 0.2180 | 4.9541 |
| 337 | 0.3 | 0.39 | 0.2341 | 0.2211 | 5.8797 |
表3 EDA-H3PO3-H2O三元体系吸收剂的SO2溶解度实验值与计算值比较
SO2分压p(SO2) /Pa | 浓度 | 相对误差 /% | |||
|---|---|---|---|---|---|
c(EDA) /mol·L-1 | c(H3PO4) /mol·L-1 | cs(exp) /mol·L-1 | cs(cal) /mol·L-1 | ||
| 143 | 0.3 | 0 | 0.5610 | 0.5945 | -5.6350 |
| 204 | 0.3 | 0 | 0.5630 | 0.5970 | -5.6951 |
| 294 | 0.3 | 0 | 0.5820 | 0.5992 | -2.8705 |
| 112 | 0.3 | 0.13 | 0.4820 | 0.4653 | 3.5891 |
| 232 | 0.3 | 0.13 | 0.4660 | 0.4700 | -0.8511 |
| 301 | 0.3 | 0.13 | 0.4680 | 0.4715 | -0.7423 |
| 108 | 0.3 | 0.26 | 0.3325 | 0.3380 | -1.6272 |
| 298 | 0.3 | 0.26 | 0.3450 | 0.3444 | 0.1742 |
| 373 | 0.3 | 0.26 | 0.3525 | 0.3462 | 1.8198 |
| 118 | 0.3 | 0.39 | 0.2235 | 0.2122 | 5.3252 |
| 252 | 0.3 | 0.39 | 0.2288 | 0.2180 | 4.9541 |
| 337 | 0.3 | 0.39 | 0.2341 | 0.2211 | 5.8797 |
| 有机胺 | a | b | c | d |
|---|---|---|---|---|
| EDA | -14.2062 | 21.6396 | -10.8574 | 83.3219 |
| PZ | -8.1086 | 11.8772 | -6.9219 | 76.1474 |
| HEP | -23.8924 | 25.9303 | -10.7243 | 67.6164 |
| DIHEP | -91.1772 | 66.9002 | -18.0294 | 68.1945 |
| HEHPP | -19.6444 | 24.5696 | -11.1515 | 68.9849 |
表4 解吸反应焓ΔHdes与SO2吸收容量α关系的参数
| 有机胺 | a | b | c | d |
|---|---|---|---|---|
| EDA | -14.2062 | 21.6396 | -10.8574 | 83.3219 |
| PZ | -8.1086 | 11.8772 | -6.9219 | 76.1474 |
| HEP | -23.8924 | 25.9303 | -10.7243 | 67.6164 |
| DIHEP | -91.1772 | 66.9002 | -18.0294 | 68.1945 |
| HEHPP | -19.6444 | 24.5696 | -11.1515 | 68.9849 |
| 1 | LIU H L, GAO H X, IDEM R, et al. Analysis of CO2 solubility and absorption heat into 1-dimethylamino-2-propanol solution[J]. Chemical Engineering Science, 2017, 170: 3-15. |
| 2 | BEYAD Y, PUXTY G, WEI S, et al. An SO2 tolerant process for CO2 capture[J]. International Journal of Greenhouse Gas Control, 2014, 31: 205-213. |
| 3 | UYANGA I J, IDEM R O. Studies of SO2- and O2-induced degradation of aqueous MEA during CO2 capture from power plant flue gas streams[J]. Industrial & Engineering Chemistry Research, 2007, 46(8): 2558-2566. |
| 4 | GARG B, VERHEYEN T V, PEARSON P, et al. A technology review for regeneration of sulfur rich amine systems[J]. International Journal of Greenhouse Gas Control, 2018, 75: 243-253. |
| 5 | HANIF M A, IBRAHIM N, ABDUL JALIL A. Sulfur dioxide removal: an overview of regenerative flue gas desulfurization and factors affecting desulfurization capacity and sorbent regeneration[J]. Environmental Science and Pollution Research International, 2020, 27(22): 27515-27540. |
| 6 | SHAW D. Cansolv CO2 capture: the value of integration[J]. Energy Procedia, 2009, 1(1): 237-246. |
| 7 | WALKER R J, WILDMAN D J, GASIOR S J. Evaluation of some regenerate sulfur dioxide absorbents for flue gas desulfurization[J]. Journal of the Air Pollution Control Association, 1983, 33(11): 1061-1067. |
| 8 | TANG Z G, ZHOU C C, CHEN C. Studies on flue gas desulfurization by chemical absorption using an ethylenediamine-phosphoric acid solution[J]. Industrial & Engineering Chemistry Research, 2004, 43(21): 6714-6722. |
| 9 | 汤志刚, 周长城, 陈成. 乙二胺/磷酸溶液化学吸收法烟气脱硫的研究[J]. 高校化学工程学报, 2005, 19(3): 285-291. |
| TANG Zhigang, ZHOU Changcheng, CHEN Cheng. Chemical absorption of SO2 by using ethylene diamine/phosphoric acid solution[J]. Journal of Chemical Engineering of Chinese Universities, 2005, 19(3): 285-291. | |
| 10 | 周长城, 汤志刚. 乙二胺/磷酸溶液吸收SO2的实验研究[J]. 精细化工, 2003, 20(8): 509-512. |
| ZHOU Changcheng, TANG Zhigang. Experimental research on flue gas desulfurization by ethylenediamine/phosphoric acid solution[J]. Fine Chemicals, 2003, 20(8): 509-512. | |
| 11 | WANGLER A, SIEDER G, INGRAM T, et al. Prediction of CO2 and H2S solubility and enthalpy of absorption in reacting N-methyldiethanolamine/water systems with ePC-SAFT[J]. Fluid Phase Equilibria, 2018, 461: 15-27. |
| 12 | KHALILI F, HENNI A, EAST A L L. pKa values of some piperazines at (298, 303, 313, and 323) K[J]. Journal of Chemical & Engineering Data, 2009, 54(10): 2914-2917. |
| 13 | REIJENGA J, VAN HOOF A, VAN LOON A, et al. Development of methods for the determination of pKa values[J]. Analytical Chemistry Insights, 2013, 8: 53-71. |
| 14 | PEREIRA R W, RAMABHADRAN R O. pK-yay: a black-box method using density functional theory and implicit solvation models to compute aqueous pKa values of weak and strong acids[J]. The Journal of Physical Chemistry A, 2020, 124(43): 9061-9074. |
| 15 | SANDER R. Compilation of Henry's law constants (version 4.0) for water as solvent[J]. Atmospheric Chemistry and Physics, 2015, 15(8): 4399-4981. |
| 16 | RABE A E, HARRIS J F. Vapor liquid equilibrium data for the binary system sulfur dioxide and water[J]. Journal of Chemical & Engineering Data, 1963, 8(3): 333-336. |
| 17 | MILLERO F J, HERSHEY J P, JOHNSON G, et al. The solubility of SO2 and the dissociation of H2SO3 in NaCl solutions[J]. Journal of Atmospheric Chemistry, 1989, 8(4): 377-389. |
| 18 | SIPPOLA H. Critical evaluation of the 2nd dissociation constants for aqueous sulfuric acid[J]. Thermochimica Acta, 2012, 532: 65-77. |
| 19 | GUPTA M, SILVA E F DA, HARTONO A, et al. Theoretical study of differential enthalpy of absorption of CO2 with MEA and MDEA as a function of temperature[J]. The Journal of Physical Chemistry B, 2013, 117(32): 9457-9468. |
| 20 | WEILAND R H, CHAKRAVARTY T, MATHER A E. Solubility of carbon dioxide and hydrogen sulfide in aqueous alkanolamines[J]. Industrial & Engineering Chemistry Research, 1993, 32(7): 1419-1430. |
| 21 | KIM H, HWANG S J, LEE K S. Novel shortcut estimation method for regeneration energy of amine solvents in an absorption-based carbon capture process[J]. Environmental Science & Technology, 2015, 49(3):1478-1485. |
| 22 | SUMON K Z, HENNI A, EAST A L L. Predicting pKa of amines for CO2 capture: computer versus pencil-and-paper[J]. Industrial & Engineering Chemistry Research, 2012, 51(37): 11924-11930. |
| 23 | GUPTA M, SILVA E F DA, SVENDSEN H F. Modeling temperature dependency of amine basicity using PCM and SM8T implicit solvation models[J]. The Journal of Physical Chemistry B, 2012, 116(6): 1865-1875. |
| 24 | OCHTERSKI J W, PETERSSON G A, MONTGOMERY J A. A complete basis set model chemistry (Ⅴ): extensions to six or more heavy atoms[J]. The Journal of Chemical Physics, 1996, 104(7): 2598-2619. |
| 25 | MONTGOMERY J A, FRISCH M J, OCHTERSKI J W, et al. A complete basis set model chemistry ( Ⅶ ): use of the minimum population localization method[J]. The Journal of Chemical Physics, 2000, 112(15): 6532-6542. |
| 26 | MARENICH A V, CRAMER C J, TRUHLAR D G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions[J]. The Journal of Physical Chemistry B, 2009, 113(18): 6378-6396. |
| 27 | LU T, CHEN Q X. Shermo: a general code for calculating molecular thermochemistry properties[J]. Computational and Theoretical Chemistry, 2021, 1200: 113249. |
| 28 | ZHAO Y, TRUHLAR D G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals[J]. Theoretical Chemistry Accounts, 2008, 120(1/2/3): 215-241. |
| 29 | PETERSSON G A, BENNETT A, TENSFELDT T G, et al. A complete basis set model chemistry. Ⅰ. The total energies of closed-shell atoms and hydrides of the first-row elements[J]. Journal of Chemical Physics, 1988, 89(4): 2193-2218. |
| 30 | PETERSSON G A, AL-LAHAM M A. A complete basis set model chemistry (Ⅱ): Open-shell systems and the total energies of the first-row atoms[J]. The Journal of Chemical Physics, 1991, 94(9): 6081-6090. |
| 31 | HO J, COOTE M L. A universal approach for continuum solvent pKa calculations: are we there yet?[J]. Theoretical Chemistry Accounts, 2009, 125(1/2): 3-21. |
| 32 | 张向宇. 实用化学手册[M]. 北京: 国防工业出版社, 1986. |
| ZHANG Xiangyu. Handbook of practical chemistry[M]. Beijing: National Defense Industry Press, 1986. | |
| 33 | TAGIURI A, MOHAMEDALI M, HENNI A. Dissociation constant (pKa) and thermodynamic properties of some tertiary and cyclic amines from (298 to 333) K[J]. Journal of Chemical & Engineering Data, 2015, 61(1), 247-254. |
| 34 | 孙志豪, 郭子东, 陈俊, 等. 哌嗪类有机胺脱除二氧化硫性能及机理探讨[J]. 化工进展, 2019, 38(S1): 46-51. |
| SUN Zhihao, GUO Zidong, CHEN Jun, et al. Performances and mechanism of piperazine-based organic amines removal of SO2 [J]. Chemical Industry and Engineering Progress, 2019, 38(S1): 46-51. |
| [1] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [2] | 欧阳素芳, 周道伟, 黄伟, 贾凤. 新型耐迁移橡胶防老剂的研究进展[J]. 化工进展, 2023, 42(7): 3708-3719. |
| [3] | 李瑞东, 黄辉, 同国虎, 王跃社. 原油精馏塔中铵盐吸湿特性及其腐蚀行为[J]. 化工进展, 2023, 42(6): 2809-2818. |
| [4] | 杨发容, 顾丽莉, 刘洋, 李伟雪, 蔡洁云, 王惠平. 计算机模拟辅助特丁津分子印迹聚合物的制备及应用[J]. 化工进展, 2023, 42(6): 3157-3166. |
| [5] | 赵毅, 杨臻, 张新为, 王刚, 杨旋. 不同裂缝损伤和愈合温度条件下沥青自愈合行为的分子模拟[J]. 化工进展, 2023, 42(6): 3147-3156. |
| [6] | 宋超, 叶学民, 李春曦. 纳米颗粒与表面活性剂的自组装行为对硅油-水界面性质影响的分子动力学[J]. 化工进展, 2022, 41(S1): 366-375. |
| [7] | 冯颖, 赵孟杰, 崔倩, 解玉鞠, 张建伟, 董鑫. 分子模拟技术在壳聚糖功能材料开发和应用中的研究进展[J]. 化工进展, 2022, 41(8): 4241-4253. |
| [8] | 李艳平, 严大洲, 杨涛, 温国胜, 韩治成. 硅基电子气去除甲基氯硅烷的分子动力学模拟[J]. 化工进展, 2022, 41(8): 4375-4385. |
| [9] | 张辛铖, 何林, 隋红, 李鑫钢. 重质油包水乳液破乳过程及降黏强化机制[J]. 化工进展, 2022, 41(7): 3534-3544. |
| [10] | 秦丽, 李东东, 田景升, 韩俊华, 张寅, 李建文, 高文惠. 融合多种技术的隐色孔雀石绿印迹传感器的制备及其应用[J]. 化工进展, 2022, 41(11): 6018-6028. |
| [11] | 马云波, 王彦斐, 李鹏, 刘欢, 武海洋, 苏衡, 马宣宣, 夏传海. 有机胺对Pd/C催化氯酚加氢脱氯反应的影响[J]. 化工进展, 2022, 41(11): 6185-6194. |
| [12] | 李秉繁, 刘刚, 陈雷. CH4在原油体系中溶解规律及影响机理[J]. 化工进展, 2021, 40(8): 4205-4222. |
| [13] | 王傢俊, 邓帅, 赵睿恺, 赵力. 电子级HF吸附法回收的节能降耗潜力分析[J]. 化工进展, 2021, 40(7): 3645-3655. |
| [14] | 王晓天, 罗海燕, 徐俊波, 杨超, 刘会洲, 李英波. AHT型磷酸铝分子筛的合成及表征[J]. 化工进展, 2021, 40(11): 6219-6227. |
| [15] | 孔令云, 全秀洁, 李朝波, 余苗. 乳化剂在集料化学成分表面吸附行为的分子模拟与试验论证[J]. 化工进展, 2020, 39(8): 3196-3204. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||