化工进展 ›› 2022, Vol. 41 ›› Issue (7): 3636-3647.DOI: 10.16085/j.issn.1000-6613.2021-1651
吸附式空气取水系统用吸湿材料研究进展
- 浙江理工大学建筑工程学院,浙江 杭州 310018
-
收稿日期:2021-08-03修回日期:2021-10-21出版日期:2022-07-25发布日期:2022-07-23 -
通讯作者:郑旭 -
作者简介:王胜楠(1998—),女,硕士研究生,研究方向为复合吸湿剂合成。E-mail:wangshengnan9843@163.com 。 -
基金资助:国家自然科学基金(51806195);中华人民共和国住房和城乡建设部科学技术项目(2018-K1-012);浙江省自然科学基金(LY22E060004)
Recent progress of moisture sorbent for adsorption-based atmospheric water harvesting
WANG Shengnan( ), CHEN Kang, ZHENG Xu(
), CHEN Kang, ZHENG Xu( )
)
- School of Civil Engineering and Architecture, Zhejiang Sci-Tech University, Hangzhou 310018, Zhejiang, China
-
Received:2021-08-03Revised:2021-10-21Online:2022-07-25Published:2022-07-23 -
Contact:ZHENG Xu
摘要:
吸附式空气取水技术因其适用环境范围广、低碳环保的特性,被认为是解决全球水资源短缺问题的重要技术之一。吸湿材料的特性决定着该技术的取水性能。本文对吸湿材料的最新研究进行了系统的归纳与总结。重点介绍了吸湿性聚合物和复合吸湿剂(多孔材料-盐、聚合物-盐、聚合物-聚合物和多孔材料-聚合物),并对各类吸湿剂的性能特点以及在吸附式空气取水系统的应用展开详细介绍。发现复合吸湿剂的吸湿性能更强,特别是聚合物类复合吸湿剂,满足宽领域吸附的要求,进而提高了在干旱地区的取水效果,在未来具有很好的应用前景。最后,指出了吸湿材料需要进一步研究和解决的相关问题,以期为推进吸附式空气取水技术早日实现从实验室研究到规模化工业应用提供有价值的借鉴和参考。
中图分类号:
引用本文
王胜楠, 陈康, 郑旭. 吸附式空气取水系统用吸湿材料研究进展[J]. 化工进展, 2022, 41(7): 3636-3647.
WANG Shengnan, CHEN Kang, ZHENG Xu. Recent progress of moisture sorbent for adsorption-based atmospheric water harvesting[J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3636-3647.
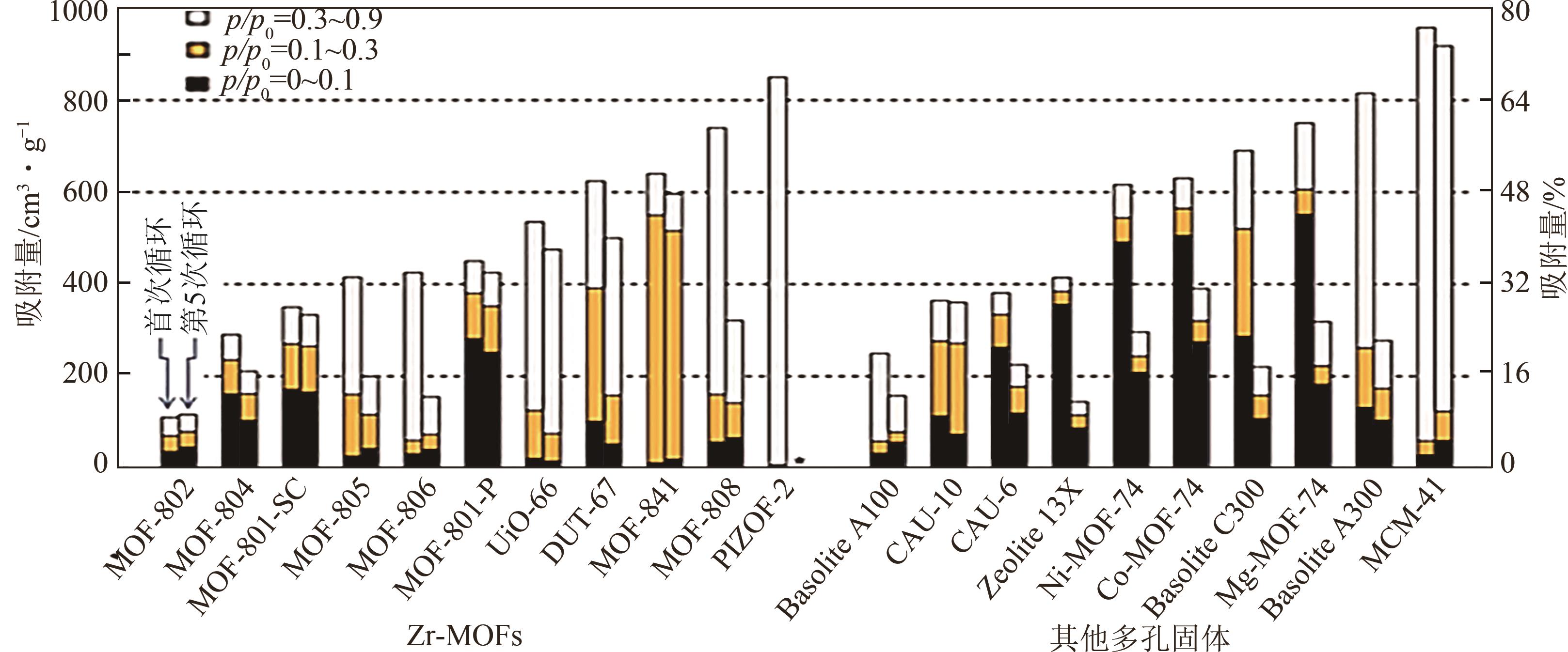
| 金属-有机骨架化合物 | 比表面积/m2·g-1 | 比孔容/cm3·g-1 | 吸附条件/℃,RH/% | 吸湿量/g·g-1 | 再生温度/℃ |
|---|---|---|---|---|---|
| Cr-soc-MOF-1[ | 4549 | 2.1 | 20,75 | 1.95 | — |
| CPO-27(Ni)[ | 1225.7 | 0.5 | 25,90 | 0.47 | 110 |
| 富马酸铝[ | 1211.5 | 0.436 | 25,90 | 0.53 | 70 |
| UiO-66[ | 1290 | 0.49 | 25,90 | 0.43 | — |
| MOF-801-SC[ | 690 | 0.27 | 25,90 | 0.28 | — |
| MOF-801-P[ | 990 | 0.45 | 25,90 | 0.36 | — |
| PIZOF-2[ | 2080 | 0.88 | 25,90 | 0.68 | — |
| DUT-67[ | 1560 | 0.60 | 25,90 | 0.50 | — |
| MOF-808[ | 2060 | 0.84 | 25,90 | 0.59 | — |
| CAU-10[ | 600 | 0.26 | 25,90 | 0.29 | — |
| Co-MOF-74[ | 1130 | 0.49 | 25,90 | 0.51 | — |
| Basolite-A300[ | 1660 | 0.82 | 25,90 | 0.65 | — |
| MIL-101(Cr)[ | 4150±100 | — | 30,60 | 1.5 | 70 |
| MIL-100(Fe)[ | 2300±100 | — | 30,60 | 0.84 | 70 |
| MIL-101[ | 3124 | 1.56 | 25,60 | 1.29 | 80 |
| MIL-101-NH2[ | 2146 | 1.22 | 25,60 | 0.94 | 80 |
| MIL-101-NO2[ | 2509 | 1.07 | 25,60 | 0.93 | 80 |
| MIL-101-SO3H[ | 1920 | 0.92 | 25,60 | 0.68 | 80 |
| Zn(NDI-H)[ | 1460 | — | 20,90 | 0.47 | — |
| Zn(NDI-NHET)[ | 1236 | — | 20,90 | 0.3 | — |
| Zn(NDI-SET)[ | 888 | — | 20,90 | 0.24 | — |
| H2N-UiO-66[ | — | — | 25,40 | 0.046 | 45~60 |
| HSO3-UiO-66[ | — | — | 25,40 | 0.06 | 45~60 |
表1 金属有机骨架化合物性能参数
| 金属-有机骨架化合物 | 比表面积/m2·g-1 | 比孔容/cm3·g-1 | 吸附条件/℃,RH/% | 吸湿量/g·g-1 | 再生温度/℃ |
|---|---|---|---|---|---|
| Cr-soc-MOF-1[ | 4549 | 2.1 | 20,75 | 1.95 | — |
| CPO-27(Ni)[ | 1225.7 | 0.5 | 25,90 | 0.47 | 110 |
| 富马酸铝[ | 1211.5 | 0.436 | 25,90 | 0.53 | 70 |
| UiO-66[ | 1290 | 0.49 | 25,90 | 0.43 | — |
| MOF-801-SC[ | 690 | 0.27 | 25,90 | 0.28 | — |
| MOF-801-P[ | 990 | 0.45 | 25,90 | 0.36 | — |
| PIZOF-2[ | 2080 | 0.88 | 25,90 | 0.68 | — |
| DUT-67[ | 1560 | 0.60 | 25,90 | 0.50 | — |
| MOF-808[ | 2060 | 0.84 | 25,90 | 0.59 | — |
| CAU-10[ | 600 | 0.26 | 25,90 | 0.29 | — |
| Co-MOF-74[ | 1130 | 0.49 | 25,90 | 0.51 | — |
| Basolite-A300[ | 1660 | 0.82 | 25,90 | 0.65 | — |
| MIL-101(Cr)[ | 4150±100 | — | 30,60 | 1.5 | 70 |
| MIL-100(Fe)[ | 2300±100 | — | 30,60 | 0.84 | 70 |
| MIL-101[ | 3124 | 1.56 | 25,60 | 1.29 | 80 |
| MIL-101-NH2[ | 2146 | 1.22 | 25,60 | 0.94 | 80 |
| MIL-101-NO2[ | 2509 | 1.07 | 25,60 | 0.93 | 80 |
| MIL-101-SO3H[ | 1920 | 0.92 | 25,60 | 0.68 | 80 |
| Zn(NDI-H)[ | 1460 | — | 20,90 | 0.47 | — |
| Zn(NDI-NHET)[ | 1236 | — | 20,90 | 0.3 | — |
| Zn(NDI-SET)[ | 888 | — | 20,90 | 0.24 | — |
| H2N-UiO-66[ | — | — | 25,40 | 0.046 | 45~60 |
| HSO3-UiO-66[ | — | — | 25,40 | 0.06 | 45~60 |
| 分类 | 多孔基质-盐 | 比表面积/m2·g-1 | 比孔容/cm3·g-1 | 吸附条件/℃,RH/% | 吸湿量/g·g-1 | 再生温度/℃ | |
|---|---|---|---|---|---|---|---|
| 硅胶-盐 | FNG硅胶/LiCl[ | — | — | 25,35 | 0.286 | 80 | |
| SWS-8L[ | 60 | 0.24 | 35,21 | 0.21 | 90~95 | ||
| 硅胶/CaCl2[ | — | — | 25,60 | 0.172 | 70 | ||
| 硅胶/LiCl[ | — | — | 25,60 | 0.168 | 70 | ||
| 分子筛-盐 | 3G-MZ[ | 276±3 | 0.16 | 25,60 | 0.587 | 80 | |
| MCM-41/CaCl2[ | — | — | 10~15,78~92 | 1.75 | 80 | ||
| 活性炭纤维-盐 | ACF/LiCl[ | 393 | 0.2 | 20,70 | 1.3 | — | |
| ACF/CaCl2[ | 75.62 | 0.04 | 20,70 | 1.6 | — | ||
| ACF/CaCl2[ | — | — | 25,60 | 0.38(集水量) | 90 | ||
| ACF/LiCl[ | — | — | 25,60 | 0.41(集水量) | 90 | ||
| ACF/硅溶胶/LiCl[ | 123.1 | 0.1 | 25,90 | 1.2 | 77 | ||
| HCS/LiCl[ | 620 | — | 25,80 | 2.2 | 80 | ||
| 双盐 | 活性炭/CaCl2/Na2SiO3[ | 82 | 0.0589 | 25,— | 0.85 | 55~100 | |
| CS6[ | 133 | 0.16 | 25,80 | 1.1 | — | ||
| 硅胶/CaCl2/MgCl2[ | — | — | —,— | 0.43 | 71.7 | ||
| ACF/LiCl/MgSO4[ | — | — | 20,70 | 2.2 | 80 |
表2 多孔基质-盐复合吸湿剂性能参数
| 分类 | 多孔基质-盐 | 比表面积/m2·g-1 | 比孔容/cm3·g-1 | 吸附条件/℃,RH/% | 吸湿量/g·g-1 | 再生温度/℃ | |
|---|---|---|---|---|---|---|---|
| 硅胶-盐 | FNG硅胶/LiCl[ | — | — | 25,35 | 0.286 | 80 | |
| SWS-8L[ | 60 | 0.24 | 35,21 | 0.21 | 90~95 | ||
| 硅胶/CaCl2[ | — | — | 25,60 | 0.172 | 70 | ||
| 硅胶/LiCl[ | — | — | 25,60 | 0.168 | 70 | ||
| 分子筛-盐 | 3G-MZ[ | 276±3 | 0.16 | 25,60 | 0.587 | 80 | |
| MCM-41/CaCl2[ | — | — | 10~15,78~92 | 1.75 | 80 | ||
| 活性炭纤维-盐 | ACF/LiCl[ | 393 | 0.2 | 20,70 | 1.3 | — | |
| ACF/CaCl2[ | 75.62 | 0.04 | 20,70 | 1.6 | — | ||
| ACF/CaCl2[ | — | — | 25,60 | 0.38(集水量) | 90 | ||
| ACF/LiCl[ | — | — | 25,60 | 0.41(集水量) | 90 | ||
| ACF/硅溶胶/LiCl[ | 123.1 | 0.1 | 25,90 | 1.2 | 77 | ||
| HCS/LiCl[ | 620 | — | 25,80 | 2.2 | 80 | ||
| 双盐 | 活性炭/CaCl2/Na2SiO3[ | 82 | 0.0589 | 25,— | 0.85 | 55~100 | |
| CS6[ | 133 | 0.16 | 25,80 | 1.1 | — | ||
| 硅胶/CaCl2/MgCl2[ | — | — | —,— | 0.43 | 71.7 | ||
| ACF/LiCl/MgSO4[ | — | — | 20,70 | 2.2 | 80 |
| 分类 | 聚合物-盐 | 比表面积/m2·g-1 | 比孔容/cm3·g-1 | 吸附条件/℃,RH/% | 吸湿量/g·g-1 | 再生温度/℃ |
|---|---|---|---|---|---|---|
| 聚合物电解质-盐 | SHC[ | 4.6±0.08 | — | 25,99 | 2.96 | 80 |
| PAM/CNT/CaCl2[ | — | — | 25,80 | 1.75 | 50~75 | |
| Alg-CaCl2[ | — | — | 28,26 | 1 | 100~150 | |
| Bina/FCNT[ | — | — | 25,70 | 5.6 | 80 | |
| L/FCNT[ | — | — | 25,70 | 3.7 | 80 | |
| MOFs-盐 | MIL-101(Cr)/CaCl2[ | 1876.01 | 0.99 | 25,30 | 0.65 | — |
| Mn2Cl2(BTDD)[ | — | — | 25,90 | 0.35 | — | |
| Co2Cl2(BTDD)[ | 1912 | — | 25,94 | 0.97 | 45 | |
| Ni2Cl2(BTDD)[ | 1762 | — | 25,90 | 0.75 | — | |
| CaCl2@UiO-66_38[ | 约70 | — | 20,90 | 1.93 | 57~100 | |
| CaCl2@UiO-66_50[ | — | — | 20,90 | 2.24 | 57~100 | |
| CaCl2@UiO-66_53[ | — | — | 20,90 | 2.59 | 57~100 | |
| LiCl/MIL-101(Cr)_51[ | 1179 | 0.69 | 30,30 | 0.77 | 70 |
表3 聚合物-盐复合吸湿剂性能参数
| 分类 | 聚合物-盐 | 比表面积/m2·g-1 | 比孔容/cm3·g-1 | 吸附条件/℃,RH/% | 吸湿量/g·g-1 | 再生温度/℃ |
|---|---|---|---|---|---|---|
| 聚合物电解质-盐 | SHC[ | 4.6±0.08 | — | 25,99 | 2.96 | 80 |
| PAM/CNT/CaCl2[ | — | — | 25,80 | 1.75 | 50~75 | |
| Alg-CaCl2[ | — | — | 28,26 | 1 | 100~150 | |
| Bina/FCNT[ | — | — | 25,70 | 5.6 | 80 | |
| L/FCNT[ | — | — | 25,70 | 3.7 | 80 | |
| MOFs-盐 | MIL-101(Cr)/CaCl2[ | 1876.01 | 0.99 | 25,30 | 0.65 | — |
| Mn2Cl2(BTDD)[ | — | — | 25,90 | 0.35 | — | |
| Co2Cl2(BTDD)[ | 1912 | — | 25,94 | 0.97 | 45 | |
| Ni2Cl2(BTDD)[ | 1762 | — | 25,90 | 0.75 | — | |
| CaCl2@UiO-66_38[ | 约70 | — | 20,90 | 1.93 | 57~100 | |
| CaCl2@UiO-66_50[ | — | — | 20,90 | 2.24 | 57~100 | |
| CaCl2@UiO-66_53[ | — | — | 20,90 | 2.59 | 57~100 | |
| LiCl/MIL-101(Cr)_51[ | 1179 | 0.69 | 30,30 | 0.77 | 70 |
| 吸湿剂 | 价格/CNY·kg-1 | 吸湿剂 | 价格/CNY·kg-1 | 吸湿剂 | 价格/CNY·kg-1 |
|---|---|---|---|---|---|
| 聚丙烯酸钠 | 276~460 | 聚丙烯酸型聚合物 | 1119.6~2180 | LiCl | 489 |
| PS-I/PS-II | 7980~17600 | UiO-66 | 5.6×105~9.6×105 | MgCl2 | 89 |
| MIL-101(Cr) | 1.44×106 | MOF-808 | 9×105~1.425×106 | MgSO4 | 51.6 |
| MIL-100(Fe) | 2.85×105 | MIL-101-NH2 | 9.84×105 | CaCl2 | 68 |
| MOF-801 | 8×105 | H2N-UiO-66 | 7.6×105~1.28×106 | Na2SiO3 | 117 |
| CAU-10 | 1×106 | Co-MOF-74 | 1.25×106 | LiBr | 261.6 |
| MIL-101-SO3H | 2.8×106 | MIL-101-NO2 | 2.5×106 | ACF | 850 |
| HSO3-UiO-66 | 2.8×106 | 3G-MZ | 2.04×104 | 活性炭 | 99.6 |
| FNG硅胶 | 168 | 13X分子筛 | 2400 | 硅胶 | 80 |
| 聚苯烯酰胺水凝胶 | 147.7 | MCM-41 | 2×104 | 海藻酸钠 | 446 |
表4 单一吸湿剂价格
| 吸湿剂 | 价格/CNY·kg-1 | 吸湿剂 | 价格/CNY·kg-1 | 吸湿剂 | 价格/CNY·kg-1 |
|---|---|---|---|---|---|
| 聚丙烯酸钠 | 276~460 | 聚丙烯酸型聚合物 | 1119.6~2180 | LiCl | 489 |
| PS-I/PS-II | 7980~17600 | UiO-66 | 5.6×105~9.6×105 | MgCl2 | 89 |
| MIL-101(Cr) | 1.44×106 | MOF-808 | 9×105~1.425×106 | MgSO4 | 51.6 |
| MIL-100(Fe) | 2.85×105 | MIL-101-NH2 | 9.84×105 | CaCl2 | 68 |
| MOF-801 | 8×105 | H2N-UiO-66 | 7.6×105~1.28×106 | Na2SiO3 | 117 |
| CAU-10 | 1×106 | Co-MOF-74 | 1.25×106 | LiBr | 261.6 |
| MIL-101-SO3H | 2.8×106 | MIL-101-NO2 | 2.5×106 | ACF | 850 |
| HSO3-UiO-66 | 2.8×106 | 3G-MZ | 2.04×104 | 活性炭 | 99.6 |
| FNG硅胶 | 168 | 13X分子筛 | 2400 | 硅胶 | 80 |
| 聚苯烯酰胺水凝胶 | 147.7 | MCM-41 | 2×104 | 海藻酸钠 | 446 |
| 复合吸湿剂 | 价格/CNY·kg-1 | 复合吸湿剂 | 价格/CNY·kg-1 | 复合吸湿剂 | 价格/CNY·kg-1 |
|---|---|---|---|---|---|
| 硅胶/LiCl/LiBr[ | 241 | ACF/LiCl/MgSO4[ | ACF/LiCl[ | 618 | |
| 硅胶/MgCl2[ | 86 | 活性炭/CaCl2/Na2SiO3[ | 94 | ACF/CaCl2[ | 348 |
| FNG硅胶/LiCl[ | 395 | 硅胶/CaCl2/MgCl2[ | 79 | CS6[ | 1009 |
| 硅胶/CaCl2[ | 72 | PAM/CNT/CaCl2[ | 91~125 | SHC[ | 468~480 |
| 硅胶/LiCl[ | 343 | MIL-101(Cr)/CaCl2[ | 3.6×105~9.6×105 | Alg-CaCl2[ | 136~283 |
| 13X分子筛/CaCl2[ | 901 | CaCl2@UiO-66[ | 2.4×105~6.4×105 | Bina/FCNT[ | 278~402 |
| 3G-MZ[ | 7600 | LiCl/MIL-101(Cr)_51[ | 3.5×105~9×105 | SMAG[ | 3.7×105~8×105 |
| MCM-41/CaCl2[ | 5930 | PNIPAM@MIL-101(Cr)[ | 8×105~1.44×106 | SAS[ | 1287~1379.6 |
| ACF/硅溶胶/LiCl[ | 672 | SC/PAA/PAAs[ | 1110~1200 |
表5 复合吸湿剂价格
| 复合吸湿剂 | 价格/CNY·kg-1 | 复合吸湿剂 | 价格/CNY·kg-1 | 复合吸湿剂 | 价格/CNY·kg-1 |
|---|---|---|---|---|---|
| 硅胶/LiCl/LiBr[ | 241 | ACF/LiCl/MgSO4[ | ACF/LiCl[ | 618 | |
| 硅胶/MgCl2[ | 86 | 活性炭/CaCl2/Na2SiO3[ | 94 | ACF/CaCl2[ | 348 |
| FNG硅胶/LiCl[ | 395 | 硅胶/CaCl2/MgCl2[ | 79 | CS6[ | 1009 |
| 硅胶/CaCl2[ | 72 | PAM/CNT/CaCl2[ | 91~125 | SHC[ | 468~480 |
| 硅胶/LiCl[ | 343 | MIL-101(Cr)/CaCl2[ | 3.6×105~9.6×105 | Alg-CaCl2[ | 136~283 |
| 13X分子筛/CaCl2[ | 901 | CaCl2@UiO-66[ | 2.4×105~6.4×105 | Bina/FCNT[ | 278~402 |
| 3G-MZ[ | 7600 | LiCl/MIL-101(Cr)_51[ | 3.5×105~9×105 | SMAG[ | 3.7×105~8×105 |
| MCM-41/CaCl2[ | 5930 | PNIPAM@MIL-101(Cr)[ | 8×105~1.44×106 | SAS[ | 1287~1379.6 |
| ACF/硅溶胶/LiCl[ | 672 | SC/PAA/PAAs[ | 1110~1200 |
| 1 | ZAREI Zahra, KARAMI Ezatollah, KESHAVARZ Marzieh. Co-production of knowledge and adaptation to water scarcity in developing countries[J]. Journal of Environmental Management, 2020, 262: 110283. |
| 2 | STEWART Gordon. The world's water: the biennial report on freshwater resources[J]. Taylor & Francis Group, 2012, 19(2): 134-135. |
| 3 | BEYSENS D. Estimating DEW yield worldwide from a few meteo data[J]. Atmospheric Research, 2016, 167: 146-155. |
| 4 | KIM Hyunho, RAO Sameer R, KAPUSTIN Eugene A, et al. Adsorption-based atmospheric water harvesting device for arid climates[J]. Nature Communications, 2018, 9: 1191. |
| 5 | 耿浩清, 石成君, 苏亚欣. 空气取水技术的研究进展[J]. 化工进展, 2011, 30(8): 1664-1669. |
| GENG Haoqing, SHI Chengjun, SU Yaxin. A review on water extraction from air[J]. Chemical Industry and Engineering Progress, 2011, 30(8): 1664-1669. | |
| 6 | LAPOTIN Alina, KIM Hyunho, RAO Sameer R, et al. Adsorption-based atmospheric water harvesting: impact of material and component properties on system-level performance[J]. Accounts of Chemical Research, 2019, 52(6): 1588-1597. |
| 7 | HUA Lingji, XU Jiaxing, WANG Ruzhu. Exergy-efficient boundary and design guidelines for atmospheric water harvesters with nano-porous sorbents[J]. Nano Energy, 2021, 85: 105977. |
| 8 | 赵惠忠, 雷敏, 黄天厚, 等. 太阳能吸附式空气取水研究进展[J]. 应用化工, 2020, 49(2): 414-419, 425. |
| ZHAO Huizhong, LEI Min, HUANG Tianhou, et al. A review on the development of water extraction from atmospheric air[J]. Applied Chemical Industry, 2020, 49(2): 414-419, 425. | |
| 9 | 姜海凤, 侯立安, 张林. 空气取水非常规技术及材料、装备研究进展[J]. 高校化学工程学报, 2018, 32(1): 1-7. |
| JIANG Haifeng, HOU Lian, ZHANG Lin. Review on unconventional techniques, materials and equipment for water extraction from air[J]. Journal of Chemical Engineering of Chinese Universities, 2018, 32(1): 1-7. | |
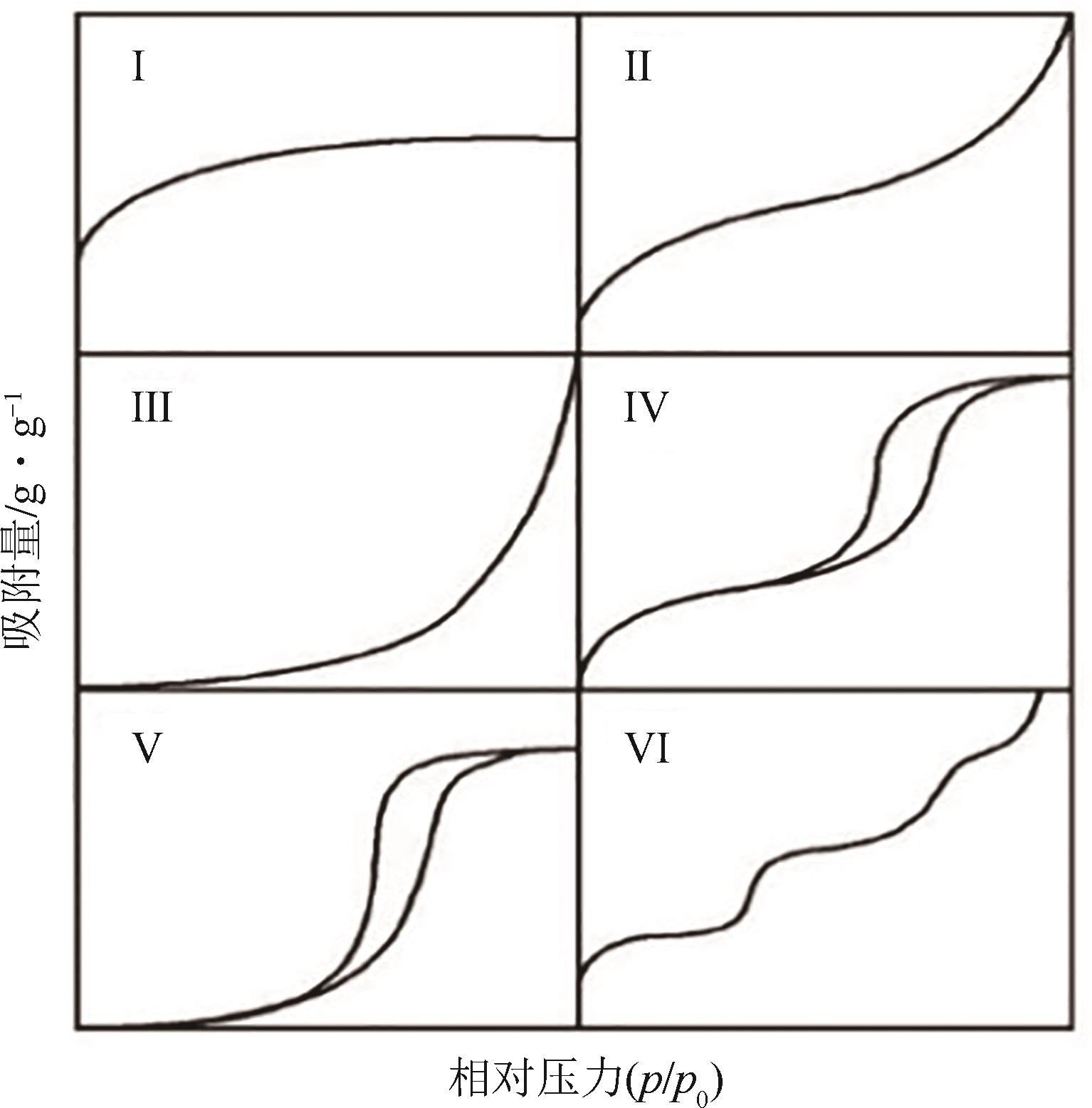
| 10 | DONOHUE M D, ARANOVICH G L. Classification of Gibbs adsorption isotherms[J]. Advances in Colloid and Interface Science, 1998, 76/77: 137-152. |
| 11 | ELSAYED Eman, Raya AL-DADAH, MAHMOUD Saad, et al. CPO-27(Ni), aluminium fumarate and MIL-101(Cr) MOF materials for adsorption water desalination[J]. Desalination, 2017, 406: 25-36. |
| 12 | Luis GARZÓN-TOVAR, Javier PÉREZ-CARVAJAL, IMAZ Inhar, et al. Composite salt in porous metal-organic frameworks for adsorption heat transformation[J]. Advanced Functional Materials, 2017, 27(21): 1606424. |
| 13 | HANIKEL Nikita, PRÉVOT Mathieu S, FATHIEH Farhad, et al. Rapid cycling and exceptional yield in a metal-organic framework water harvester[J]. ACS Central Science, 2019, 5(10): 1699-1706. |
| 14 | 于博, 郑旭. 离子改性的聚丙烯酸除湿换热器系统性能研究[J]. 制冷学报, 2020, 41(2): 63-69. |
| YU Bo, ZHENG Xu. Study on the system performance of desiccant coated heat exchangers using ion modified polyacrylic acid salt[J]. Journal of Refrigeration, 2020, 41(2): 63-69. | |
| 15 | TOWSIF ABTAB Sk Md, ALEZI Dalal, BHATT Prashant M, et al. Reticular chemistry in action: a hydrolytically stable MOF capturing twice its weight in adsorbed water[J]. Chem, 2018, 4(1): 94-105. |
| 16 | 郑旭. 小温差再生的干燥剂的优选及其在除湿换热器中的应用[D].上海:上海交通大学, 2016. |
| ZHENG Xu. Optimization and application of desiccant materials in desiccant coated heat exchanger[D]. Shanghai: Shanghai Jiao Tong University, 2016. | |
| 17 | 王佳韵. 基于复合活性炭纤维材料的吸附式空气取水原理与系统[D].上海: 上海交通大学, 2018. |
| WANG Jiayun. Research on principle and system of atmosphere water harvesting unit based on active carbon fiber composite material[D]. Shanghai: Shanghai Jiao Tong University, 2018. | |
| 18 | TU Yaodong, WANG Ruzhu, ZHANG Yannan, et al. Progress and expectation of atmospheric water harvesting[J]. Joule, 2018, 2(8): 1452-1475. |
| 19 | EJEIAN M, WANG R Z. Adsorption-based atmospheric water harvesting[J]. Joule, 2021, 5(7): 1678-1703. |
| 20 | ZHOU Xingyi, LU Hengyi, ZHAO Fei, et al. Atmospheric water harvesting: a review of material and structural designs[J]. ACS Materials Letters, 2020, 2(7): 671-684. |
| 21 | HANIKEL Nikita, PRÉVOT Mathieu S, YAGHI Omar M. MOF water harvesters[J]. Nature Nanotechnology, 2020, 15(5): 348-355. |
| 22 | 王雯雯, 葛天舒, 代彦军, 等. 太阳能吸附式空气取水研究现状[J]. 太阳能, 2020(1): 33-46. |
| WANG Wenwen, GE Tianshu, DAI Yanjun, et al. Status of solar-drivensorption-based atmosphere water harvesting[J]. Solar Energy, 2020(1): 33-46. | |
| 23 | FERAPONTOV Nikolai B, TOKMACHEV Mikhail G, GAGARIN Aleksander N, et al. Influence of the environment on swelling of hydrophilic polymers[J]. Reactive and Functional Polymers, 2013, 73(8): 1137-1143. |
| 24 | SULTAN Muhammad, EL-SHARKAWY Ibrahim I, MIYAZAKI Takahiko, et al. Insights of water vapor sorption onto polymer based sorbents[J]. Adsorption, 2015, 21(3): 205-215. |
| 25 | CHANG C C, LUO W J, LU C W, et al. Effects of process air conditions and switching cycle period on dehumidification performance of desiccant-coated heat exchangers[J]. Science and Technology for the Built Environment, 2017, 23(1): 81-90. |
| 26 | NANDAKUMAR Dilip Krishna, ZHANG Yaoxin, RAVI Sai Kishore, et al. Solar energy triggered clean water harvesting from humid air existing above sea surface enabled by a hydrogel with ultrahigh hygroscopicity[J]. Advanced Materials, 2019, 31(10): 1806730. |
| 27 | MCGUIRE Christina V, FORGAN Ross S. ChemInform abstract: the surface chemistry of metal-organic frameworks[J]. ChemInform, 2015, 46(21): no. |
| 28 | FURUKAWA Hiroyasu, Felipe GÁNDARA, ZHANG Yuebiao, et al. Water adsorption in porous metal-organic frameworks and related materials[J]. Journal of the American Chemical Society, 2014, 136(11): 4369-4381 |
| 29 | SEO You-Kyong, YOON Ji-Woong, LEE Ji-Sun, et al. Energy-efficient dehumidification over hierachically porous metal-organic frameworks as advanced water adsorbents[J]. Advanced Materials, 2012, 24(6): 806-810. |
| 30 | AKIYAMA George, MATSUDA Ryotaro, SATO Hiroshi, et al. Effect of functional groups in MIL-101 on water sorption behavior[J]. Microporous and Mesoporous Materials, 2012, 157: 89-93. |
| 31 | WADE Casey R, Tachmajal CORRALES-SANCHEZ, NARAYAN Tarun C, et al. Postsynthetic tuning of hydrophilicity in pyrazolate MOFs to modulate water adsorption properties[J]. Energy & Environmental Science, 2013, 6(7): 2172-2177. |
| 32 | TRAPANI F, POLYZOIDIS A, LOEBBECKE S, et al. On the general water harvesting capability of metal-organic frameworks under well-defined climatic conditions[J]. Microporous and Mesoporous Materials, 2016, 230: 20-24. |
| 33 | KIM Hyunho, YANG Sungwoo, RAO Sameer R, et al. Water harvesting from air with metal-organic frameworks powered by natural sunlight[J]. Science, 2017, 356(6336): 430-434. |
| 34 | YU N, WANG R Z, LU Z S, et al. Development and characterization of silica gel-LiCl composite sorbents for thermal energy storage[J]. Chemical Engineering Science, 2014, 111: 73-84. |
| 35 | GONG L X, WANG R Z, XIA Z Z, et al. Adsorption equilibrium of water on a composite adsorbent employing lithium chloride in silica gel[J]. Journal of Chemical & Engineering Data, 2010, 55(8): 2920-2923 |
| 36 | 刘林, 何兆红, 陈捷超, 等. 固体除湿复合干燥剂研究进展[J]. 新能源进展, 2017, 5(5): 377-385. |
| LIU Lin, HE Zhaohong, CHEN Jiechao, et al. Development on solid composite desiccants for desiccant cooling systems[J]. Advances in New and Renewable Energy, 2017, 5(5): 377-385. | |
| 37 | 程俊峰, 赵惠忠, 刘雪燕, 等. 新型空气取水用复合吸附剂的配制及吸附性能研究[J]. 化工新型材料, 2017, 45(10): 162-164. |
| CHENG Junfeng, ZHAO Huizhong, LIU Xueyan, et al. Research on the preparation and adsorption property of a new composite adsorbent for extracting water from air[J]. New Chemical Materials, 2017, 45(10): 162-164. | |
| 38 | 郝刘仓, 苏浩, 付英杰. 空气取水吸附剂的性能实验研究[J]. 舰船防化, 2012(3): 21-24. |
| HAO Liucang, SU Hao, FU Yingjie. Experimental study on performance of adsorbent for producing drinking water from air in arid area[J]. Chemical Protection of Ships, 2012(3):21-24. | |
| 39 | SIMONOVA IrinaA, FRENI Angelo, RESTUCCIA Giovanni, et al. Water sorption on composite “silica modified by calcium nitrate”[J]. Microporous and Mesoporous Materials, 2009, 122(1/2/3): 223-228. |
| 40 | 刘金亚, 王佳韵, 王丽伟, 等. 一种吸附式空气取水装置的性能实验[J]. 化工学报, 2016, 67(S2): 46-50. |
| LIU Jinya, WANG Jiayun, WANG Liwei, et al. Performance test of sorption air-to-water device[J]. CIESC Journal, 2016, 67(S2): 46-50. | |
| 41 | YANG Ralph T. Adsorbents: fundamentals and applications[M]. Hoboken, NJ, USA: John Wiley & Sons, Inc., 2003: 4-18. |
| 42 | KRESGE C T, LEONOWICZ M E, ROTH W J, et al. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism[J]. Nature, 1992, 359(6397): 710-712. |
| 43 | KATIYAR Amit, YADAV Santosh, SMIRNIOTIS Panagiotis G, et al. Synthesis of ordered large pore SBA-15 spherical particles for adsorption of biomolecules[J]. Journal of Chromatography A, 2006, 1122(1/2): 13-20. |
| 44 | 李军, 赵肃清, 朱冬生. 以沸石13X 和CaCl2组成的复合吸附储能材料[J]. 材料导报, 2005, 19(8): 109-110, 113. |
| LI Jun, ZHAO Suqing, ZHU Dongsheng. Composite adsorptive thermal energy storage material composed of zeolite 13X and calciumchloride[J]. Materials Review, 2005, 19(8): 109-110, 113. | |
| 45 | 赵惠忠, 刘涛, 黄天厚, 等. 石墨烯-13X/LiCl复合吸附剂开式吸附-解吸性能[J]. 化工进展, 2021, 40(2): 969-976. |
| ZHAO Huizhong, LIU Tao, HUANG Tianhou, et al. Open adsorption-desorption performance of graphene-13X/LiCl composite adsorbents[J]. Chemical Industry and Engineering Progress, 2021, 40(2): 969-976. | |
| 46 | JI J G, WANG R Z, LI L X. New composite adsorbent for solar-driven fresh water production from the atmosphere[J]. Desalination, 2007, 212(1/2/3): 176-182. |
| 47 | ZHENG Weihua, HU Jingtian, RAPPEPORT Sammuel, et al. Activated carbon fiber composites for gas phase ammonia adsorption[J]. Microporous and Mesoporous Materials, 2016, 234: 146-154. |
| 48 | ZHENG X, WANG R Z, GE T S. Experimental study and performance predication of carbon based composite desiccants for desiccant coated heat exchangers[J]. International Journal of Refrigeration, 2016, 72: 124-131. |
| 49 | WANG J Y, WANG R Z, WANG L W. Water vapor sorption performance of ACF-CaCl2 and silica gel-CaCl2 composite adsorbents[J]. Applied Thermal Engineering, 2016, 100: 893-901. |
| 50 | WANG J Y, LIU J Y, WANG R Z, et al. Experimental research of composite solid sorbents for fresh water production driven by solar energy[J]. Applied Thermal Engineering, 2017, 121: 941-950. |
| 51 | WANG J Y, WANG R Z, WANG L W, et al. A high efficient semi-open system for fresh water production from atmosphere[J]. Energy, 2017, 138: 542-551. |
| 52 | LI Renyuan, SHI Yusuf, WU Mengchun, et al. Improving atmospheric water production yield: enabling multiple water harvesting cycles with nano sorbent[J]. Nano Energy, 2020, 67: 104255. |
| 53 | EJEIAN M, ENTEZARI A, WANG R Z. Solar powered atmospheric water harvesting with enhanced LiCl /MgSO4/ACF composite[J]. Applied Thermal Engineering, 2020, 176: 115396. |
| 54 | GORDEEVA Larisa, GREKOVA Alexandra, KRIEGER Tamara, et al. Composites “binary salts in porous matrix” for adsorption heat transformation[J]. Applied Thermal Engineering, 2013, 50(2): 1633-1638. |
| 55 | TSO C Y, CHAO Christopher Y H. Activated carbon, silica-gel and calcium chloride composite adsorbents for energy efficient solar adsorption cooling and dehumidification systems[J]. International Journal of Refrigeration, 2012, 35(6): 1626-1638. |
| 56 | ZHAO Huizhong, WANG Zhaoyang, LI Qianwen, et al. Water sorption on composite material “zeolite 13X modified by LiCl and CaCl2 ”[J]. Microporous and Mesoporous Materials, 2020, 299: 110109. |
| 57 | 贺杨堃, 杨继萍, 付悍巍, 等. 新型硅胶复合干燥剂的制备与性能研究[J]. 高校化学工程学报, 2012, 26(6): 1054-1059. |
| HE Yangkun, YANG Jiping, FU Hanwei, et al. Research on a modified silica gel-based desiccant[J]. Journal of Chemical Engineering of Chinese Universities, 2012, 26(6): 1054-1059. | |
| 58 | YANG Yifan, RANA Dipak, LAN Christopher Q. Development of solid super desiccants based on a polymeric superabsorbent hydrogel composite[J]. RSC Advances, 2015, 5(73): 59583-59590. |
| 59 | LI Renyuan, SHI Yusuf, ALSAEDI Mossab, et al. Hybrid hydrogel with high water vapor harvesting capacity for deployable solar-driven atmospheric water generator[J]. Environmental Science & Technology, 2018, 52(19): 11367-11377. |
| 60 | KALLENBERGER Paul A, Michael FRÖBA. Water harvesting from air with a hygroscopic salt in a hydrogel-derived matrix[J]. Communications Chemistry, 2018, 1: 28. |
| 61 | ENTEZARI Akram, EJEIAN Mojtaba, WANG Ruzhu. Super atmospheric water harvesting hydrogel with alginate chains modified with binary salts[J]. ACS Materials Letters, 2020, 2(5): 471-477. |
| 62 | ELSAYED Eman, ANDERSON Paul, Raya AL-DADAH, et al. MIL-101(Cr)/calcium chloride composites for enhanced adsorption cooling and water desalination[J]. Journal of Solid State Chemistry, 2019, 277: 123-132. |
| 63 | RIETH Adam J, YANG Sungwoo, WANG Evelyn N, et al. Record atmospheric fresh water capture and heat transfer with a material operating at the water uptake reversibility limit[J]. ACS Central Science, 2017, 3(6): 668-672. |
| 64 | XU Jiaxing, LI Tingxian, CHAO Jingwei, et al. Efficient solar-driven water harvesting from arid air with metal-organic frameworks modified by hygroscopic salt[J]. Angewandte Chemie International Edition, 2020, 59(13): 5202-5210. |
| 65 | ZHAO Fei, ZHOU Xingyi, LIU Yi, et al. Super moisture-absorbent gels for all-weather atmospheric water harvesting[J]. Advanced Materials, 2019, 31(10): 1806446. |
| 66 | KARMAKAR Avishek, MILEO Paulo G M, Ivan BOK, et al. Thermo-responsive MOF/polymer composites for temperature-mediated water capture and release[J]. Angewandte Chemie, 2020, 132(27): 11096-11102. |
| 67 | 胡耀强, 权朝明, 刘海宁, 等. 温敏材料吸附研究进展[J]. 材料导报, 2016, 30(11): 126-130. |
| HU Yaoqiang, QUAN Chaoming, LIU Haining, et al. Review on thermo-responsive adsorbent material[J]. Materials Review, 2016, 30(11): 126-130. | |
| 68 | WANG Xikui, ZENG Jia, YU Xinquan, et al. Superamphiphobic coatings with polymer-wrapped particles: enhancing water harvesting[J]. Journal of Materials Chemistry A, 2019, 7(10): 5426-5433. |
| 69 | CHEN Chih Hao, HSU Chien Yeh, CHEN ChihChieh, et al. Silica gel polymer composite desiccants for air conditioning systems[J]. Energy and Buildings, 2015, 101: 122-132. |
| 70 | YAO Houz, ZHANG Panpan, HUANG Yaxin, et al. Highly efficient clean water production from contaminated air with a wide humidity range[J]. Advanced Materials, 2020, 32(6): 1905875. |
| [1] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [2] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [3] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [4] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [5] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [6] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [7] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [8] | 林晓鹏, 肖友华, 管奕琛, 鲁晓东, 宗文杰, 傅深渊. 离子聚合物-金属复合材料(IPMC)柔性电极的研究进展[J]. 化工进展, 2023, 42(9): 4770-4782. |
| [9] | 朱传强, 茹晋波, 孙亭亭, 谢兴旺, 李长明, 高士秋. 固体高分子脱硝剂选择性非催化还原NO x 特性[J]. 化工进展, 2023, 42(9): 4939-4946. |
| [10] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [11] | 李伯耿, 罗英武, 刘平伟. 聚合物产品工程研究内容与方法的思考[J]. 化工进展, 2023, 42(8): 3905-3909. |
| [12] | 王报英, 王皝莹, 闫军营, 汪耀明, 徐铜文. 聚合物包覆膜在金属分离回收中的研究进展[J]. 化工进展, 2023, 42(8): 3990-4004. |
| [13] | 姜晶, 陈霄宇, 张瑞妍, 盛光遥. 载锰生物炭制备及其在环境修复中应用研究进展[J]. 化工进展, 2023, 42(8): 4385-4397. |
| [14] | 张振, 李丹, 陈辰, 吴菁岚, 应汉杰, 乔浩. 吸附树脂对唾液酸的分离纯化[J]. 化工进展, 2023, 42(8): 4153-4158. |
| [15] | 于静文, 宋璐娜, 刘砚超, 吕瑞东, 武蒙蒙, 冯宇, 李忠, 米杰. 一种吲哚基超交联聚合物In-HCP对水中碘的吸附作用[J]. 化工进展, 2023, 42(7): 3674-3683. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||