化工进展 ›› 2022, Vol. 41 ›› Issue (6): 3010-3021.DOI: 10.16085/j.issn.1000-6613.2021-1339
基于分子动力学模拟的轮胎橡胶催化热解制氢机理
陶礼( ), 杨启容(
), 杨启容( ), 李昭莹(
), 李昭莹( ), 亓昊, 王力伟, 马欣如
), 亓昊, 王力伟, 马欣如
- 青岛大学机电工程学院,山东 青岛 266071
-
收稿日期:2021-06-25修回日期:2021-09-01出版日期:2022-06-10发布日期:2022-06-21 -
通讯作者:杨启容,李昭莹 -
作者简介:陶礼(1997—),女,硕士研究生,研究方向为轮胎橡胶热解制氢。E-mail:2256713985@qq.com 。 -
基金资助:青岛大学青年卓越人才科研启动经费(QDPYHT-5-065)
Mechanism of hydrogen production by catalytic pyrolysis of tire rubber based on molecular dynamics simulation
TAO Li( ), YANG Qirong(
), YANG Qirong( ), LI Zhaoying(
), LI Zhaoying( ), QI Hao, WANG Liwei, MA Xinru
), QI Hao, WANG Liwei, MA Xinru
- College of Mechanical and Electrical Engineering, Qingdao University, Qingdao 266071, Shandong, China
-
Received:2021-06-25Revised:2021-09-01Online:2022-06-10Published:2022-06-21 -
Contact:YANG Qirong,LI Zhaoying
摘要:
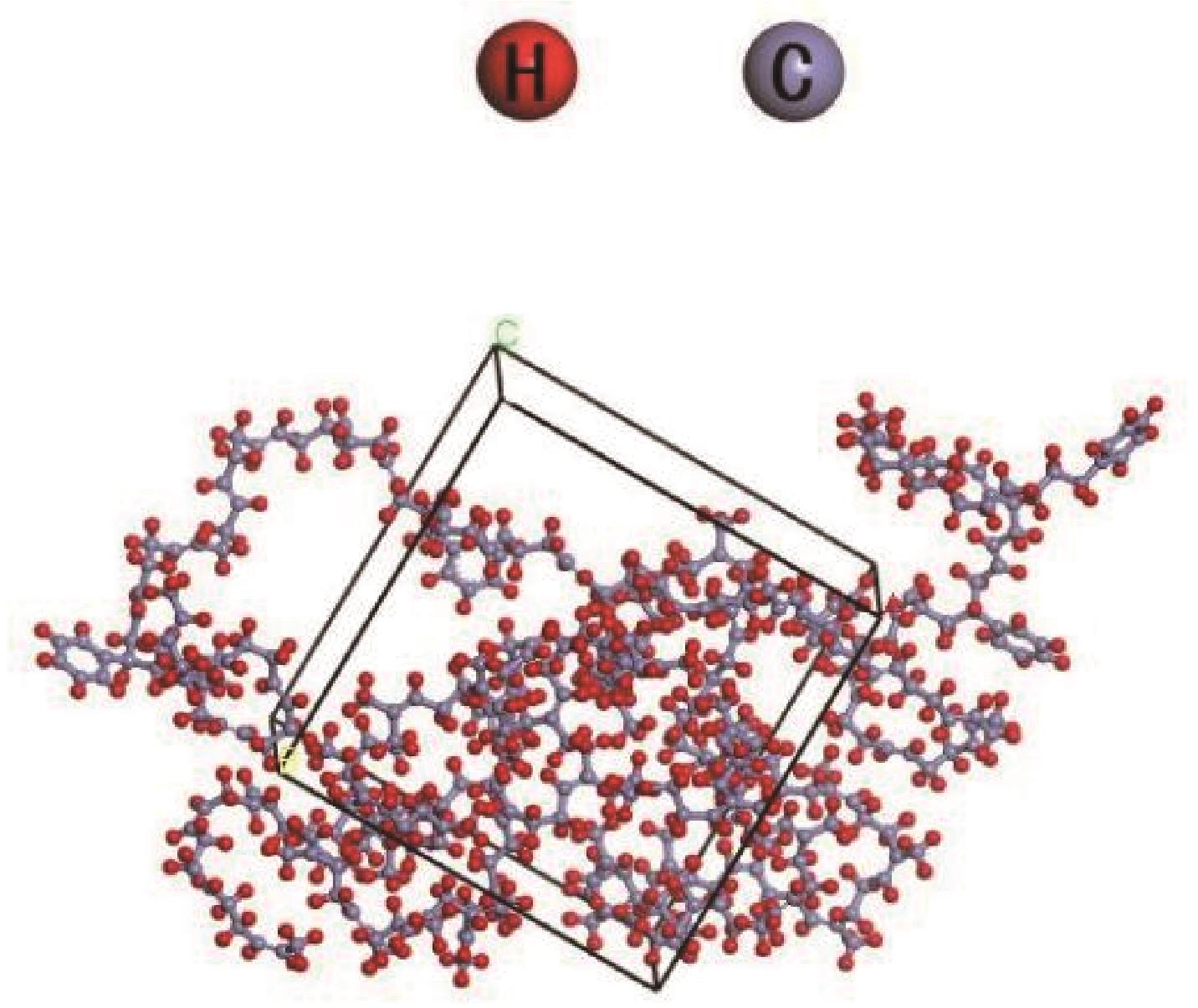
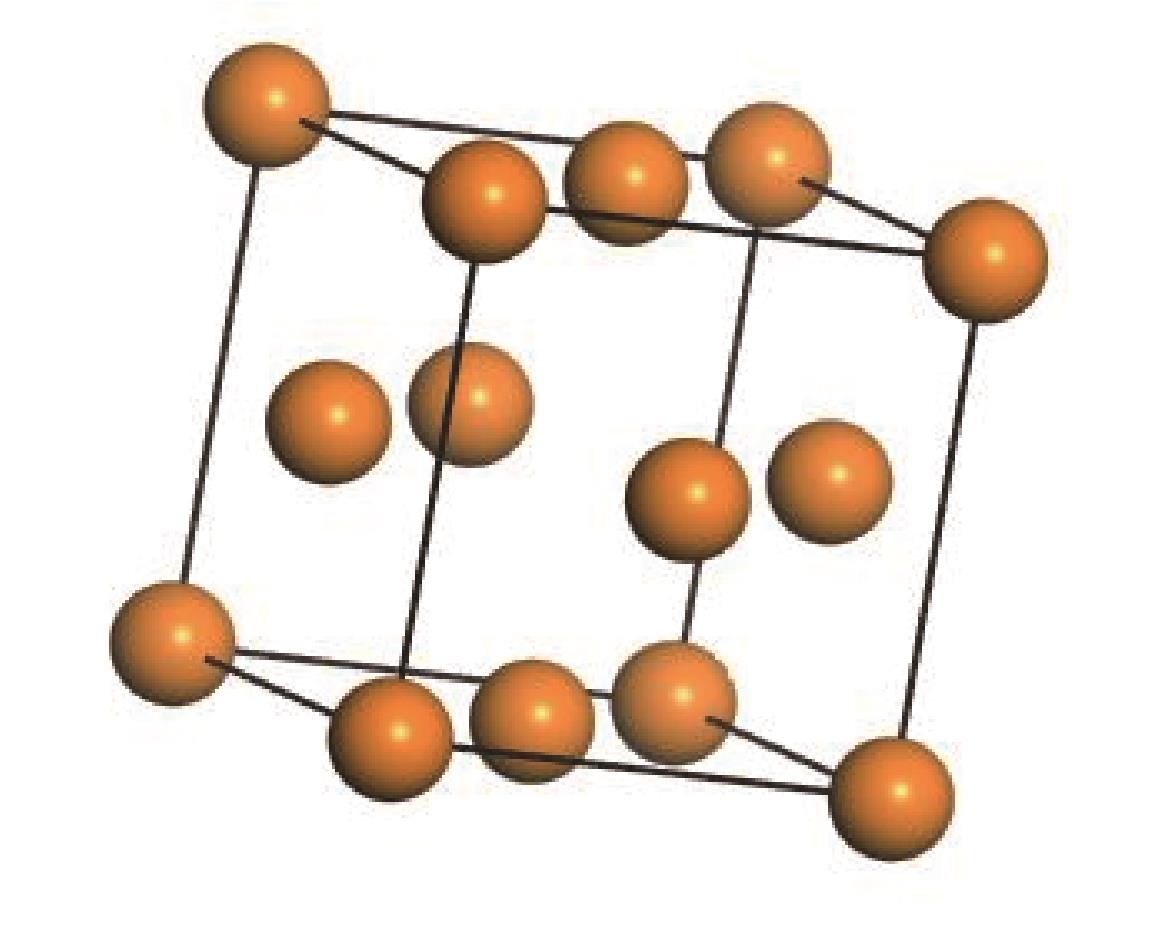
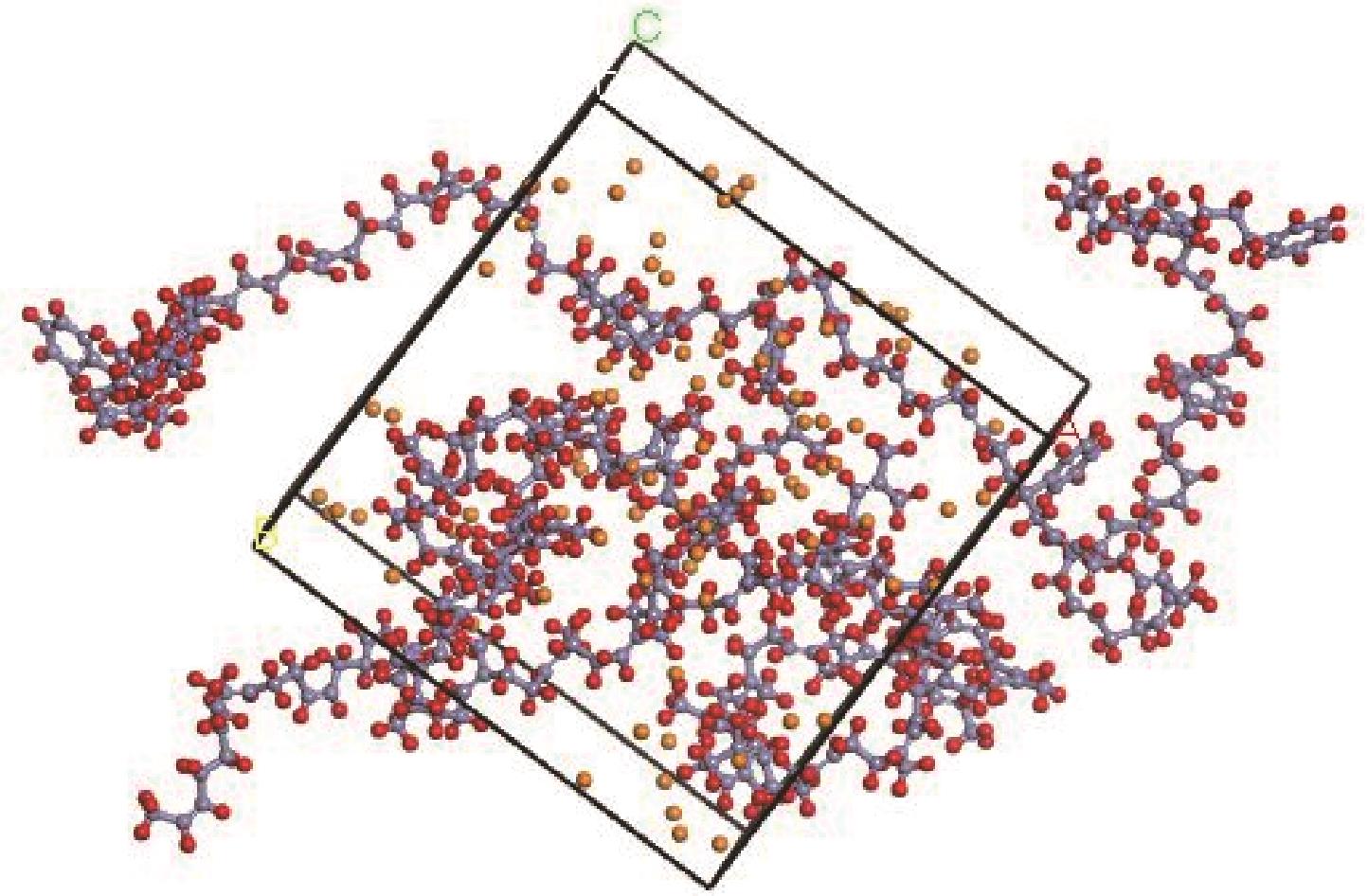
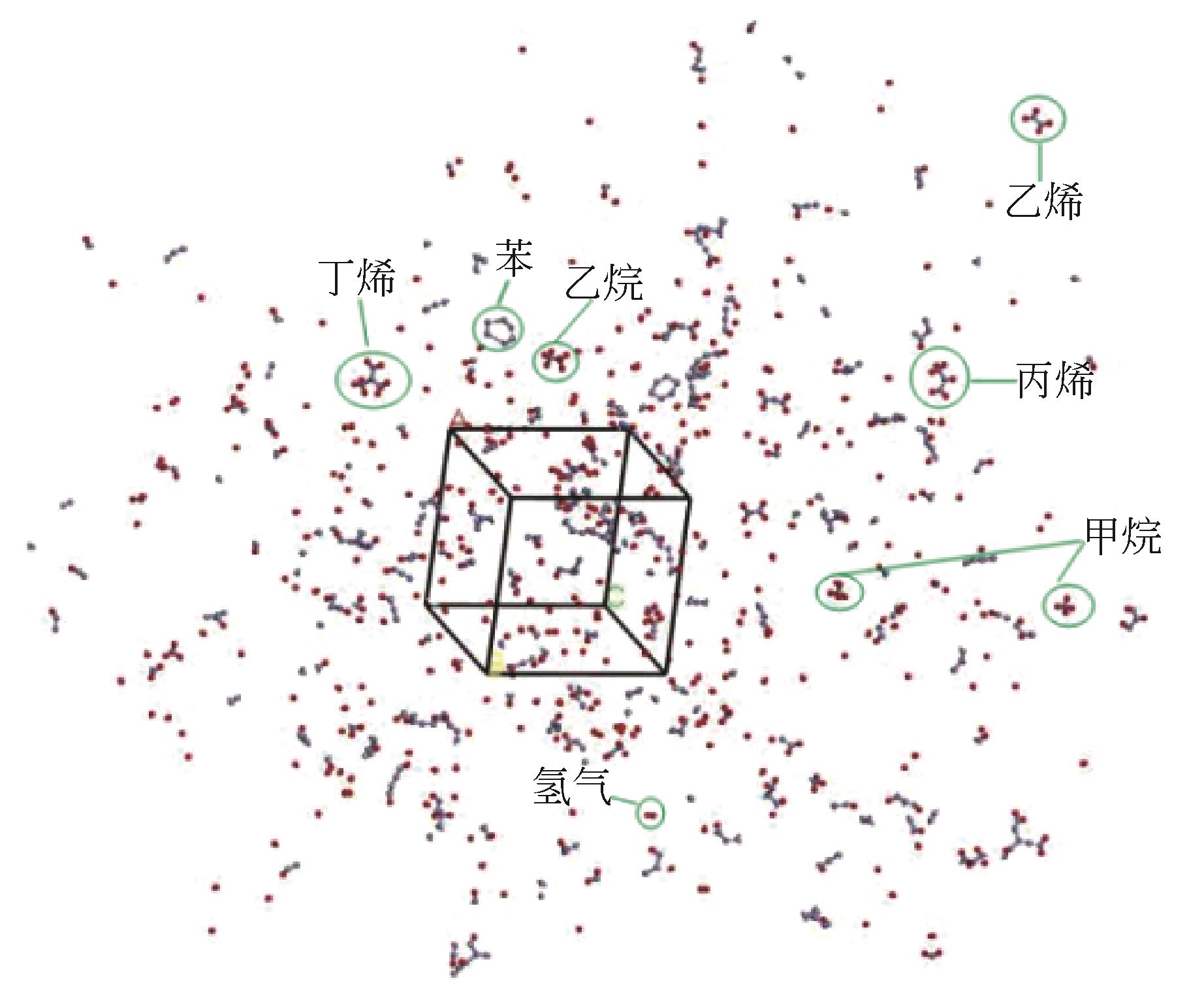
采用分子动力学模拟方法,选取Ni、ZSM-5以及Ni/ZSM-5催化剂,对轮胎橡胶热解制氢的机理进行探究,并同时与前人做过的实验研究进行对比验证模拟计算。文中利用Material Studio建立轮胎橡胶模型,DMol3模块对生成氢气路径进行过渡态搜索,CULP模块对其加入Ni催化剂的热解过程进行模拟。模拟结果表明,制氢催化效果顺序为Ni>Ni/ZSM-5>ZSM-5。催化热解大致分为两个阶段:①低温阶段长链裂解成单体化合物,单体主要是异戊二烯、苯乙烯以及1,3-丁二烯;②高温阶段自由基攻击单体生成小分子物质。加入Ni催化剂后降低了热解终止温度。催化剂的加入在低温阶段主要表现在加快热解进程,增加低温阶段时单体数量。在高温阶段主要表现在改变了气体产物分布,Ni的加入降低了轮胎热解温度,并且使氢比例增加。
中图分类号:
引用本文
陶礼, 杨启容, 李昭莹, 亓昊, 王力伟, 马欣如. 基于分子动力学模拟的轮胎橡胶催化热解制氢机理[J]. 化工进展, 2022, 41(6): 3010-3021.
TAO Li, YANG Qirong, LI Zhaoying, QI Hao, WANG Liwei, MA Xinru. Mechanism of hydrogen production by catalytic pyrolysis of tire rubber based on molecular dynamics simulation[J]. Chemical Industry and Engineering Progress, 2022, 41(6): 3010-3021.
| 橡胶 | 主要成分分子式 | 单链模型 | 长链模型 |
|---|---|---|---|
天然 橡胶 |
顺-1,4-聚异戊二烯 |
单链 |
长链 |
丁苯 橡胶 |
苯乙烯 1,3-丁二烯 |
单链 |
长链 |
顺丁 橡胶 |
顺-1,4-聚丁二烯 |
单链 |
长链 |
表1 橡胶主要成分分子式、单链及长链模型
| 橡胶 | 主要成分分子式 | 单链模型 | 长链模型 |
|---|---|---|---|
天然 橡胶 |
顺-1,4-聚异戊二烯 |
单链 |
长链 |
丁苯 橡胶 |
苯乙烯 1,3-丁二烯 |
单链 |
长链 |
顺丁 橡胶 |
顺-1,4-聚丁二烯 |
单链 |
长链 |
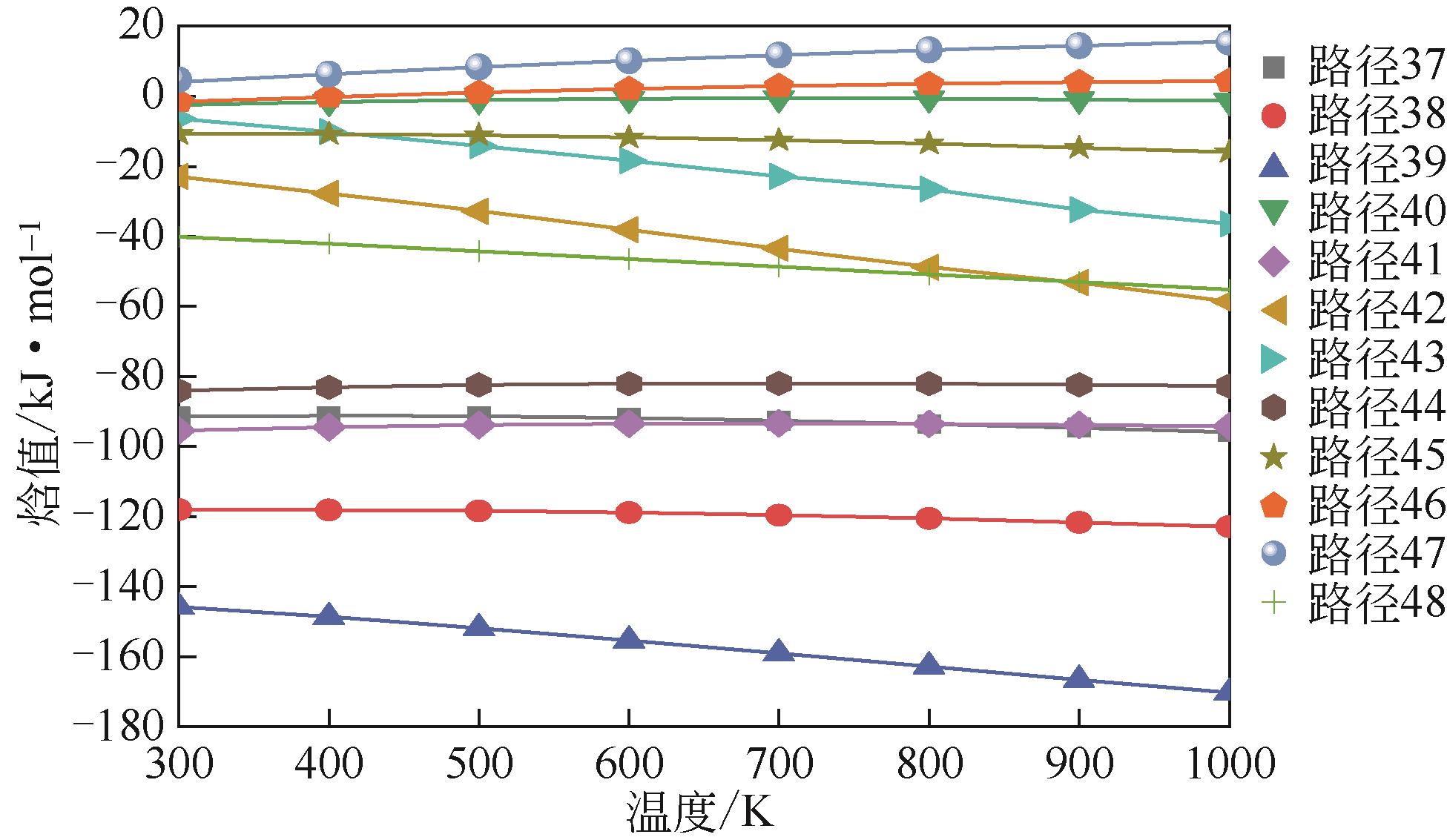
催化前路径→ 催化后路径 | 反应式 | 催化前能垒E /kJ·mol-1 | 催化后能垒E /kJ·mol-1 | 差值(前-后)?E /kJ·mol-1 |
|---|---|---|---|---|
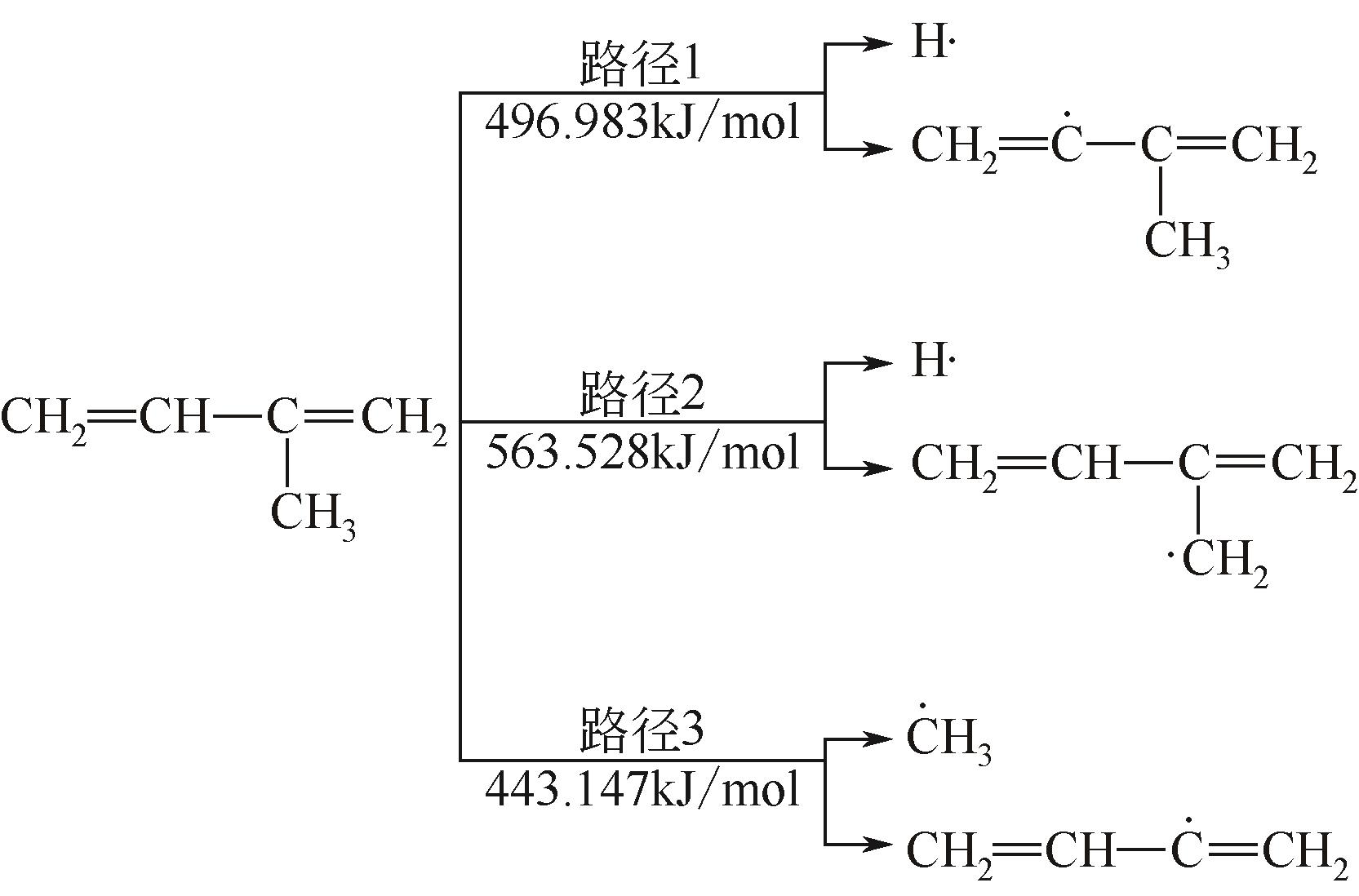
| 1→25 | CH2CHC(CH3)CH2 | 496.983 | 489.913 | 7.070 |
| 2→26 | CH2CHC(CH3)CH2 | 563.528 | 471.695 | 91.833 |
| 3→27 | CH2CHC(CH3)CH2 | 443.147 | 426.989 | 16.158 |
| 4→28 | CH2CHCHCH2 | 557.935 | 540.672 | 17.263 |
| 5→29 | CH2CHCHCH2 | 472.465 | 422.380 | 50.085 |
| 6→30 | CH2CHCHCH2 | 568.622 | 551.242 | 17.380 |
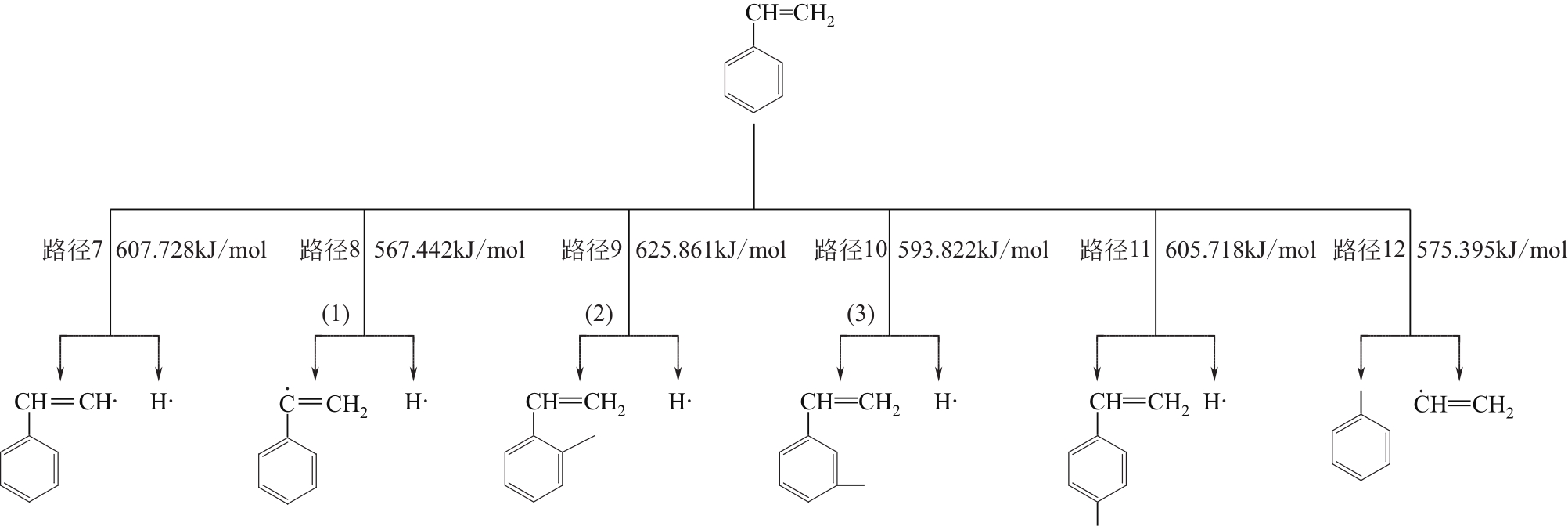
| 7→31 | C6H5CHCH2 | 607.728 | 588.120 | 19.608 |
| 8→32 | C6H5CHCH2 | 567.442 | 548.374 | 19.068 |
| 9→33 | (1) C6H5CHCH2 | 625.861 | 571.079 | 54.782 |
| 10→34 | (2) C6H5CHCH2 | 593.822 | 589.774 | 4.048 |
| 11→35 | (3) C6H5CHCH2 | 605.718 | 597.183 | 8.535 |
| 12→36 | C6H5CHCH2 | 575.395 | 555.164 | 20.231 |
表2 加入Ni催化剂前后自由基生成能垒
催化前路径→ 催化后路径 | 反应式 | 催化前能垒E /kJ·mol-1 | 催化后能垒E /kJ·mol-1 | 差值(前-后)?E /kJ·mol-1 |
|---|---|---|---|---|
| 1→25 | CH2CHC(CH3)CH2 | 496.983 | 489.913 | 7.070 |
| 2→26 | CH2CHC(CH3)CH2 | 563.528 | 471.695 | 91.833 |
| 3→27 | CH2CHC(CH3)CH2 | 443.147 | 426.989 | 16.158 |
| 4→28 | CH2CHCHCH2 | 557.935 | 540.672 | 17.263 |
| 5→29 | CH2CHCHCH2 | 472.465 | 422.380 | 50.085 |
| 6→30 | CH2CHCHCH2 | 568.622 | 551.242 | 17.380 |
| 7→31 | C6H5CHCH2 | 607.728 | 588.120 | 19.608 |
| 8→32 | C6H5CHCH2 | 567.442 | 548.374 | 19.068 |
| 9→33 | (1) C6H5CHCH2 | 625.861 | 571.079 | 54.782 |
| 10→34 | (2) C6H5CHCH2 | 593.822 | 589.774 | 4.048 |
| 11→35 | (3) C6H5CHCH2 | 605.718 | 597.183 | 8.535 |
| 12→36 | C6H5CHCH2 | 575.395 | 555.164 | 20.231 |
催化前路径→ 催化后路径 | 反应式 | 催化前能垒E /kJ·mol-1 | 催化后能垒E /kJ·mol-1 | 差值(前-后)?E /kJ·mol-1 |
|---|---|---|---|---|
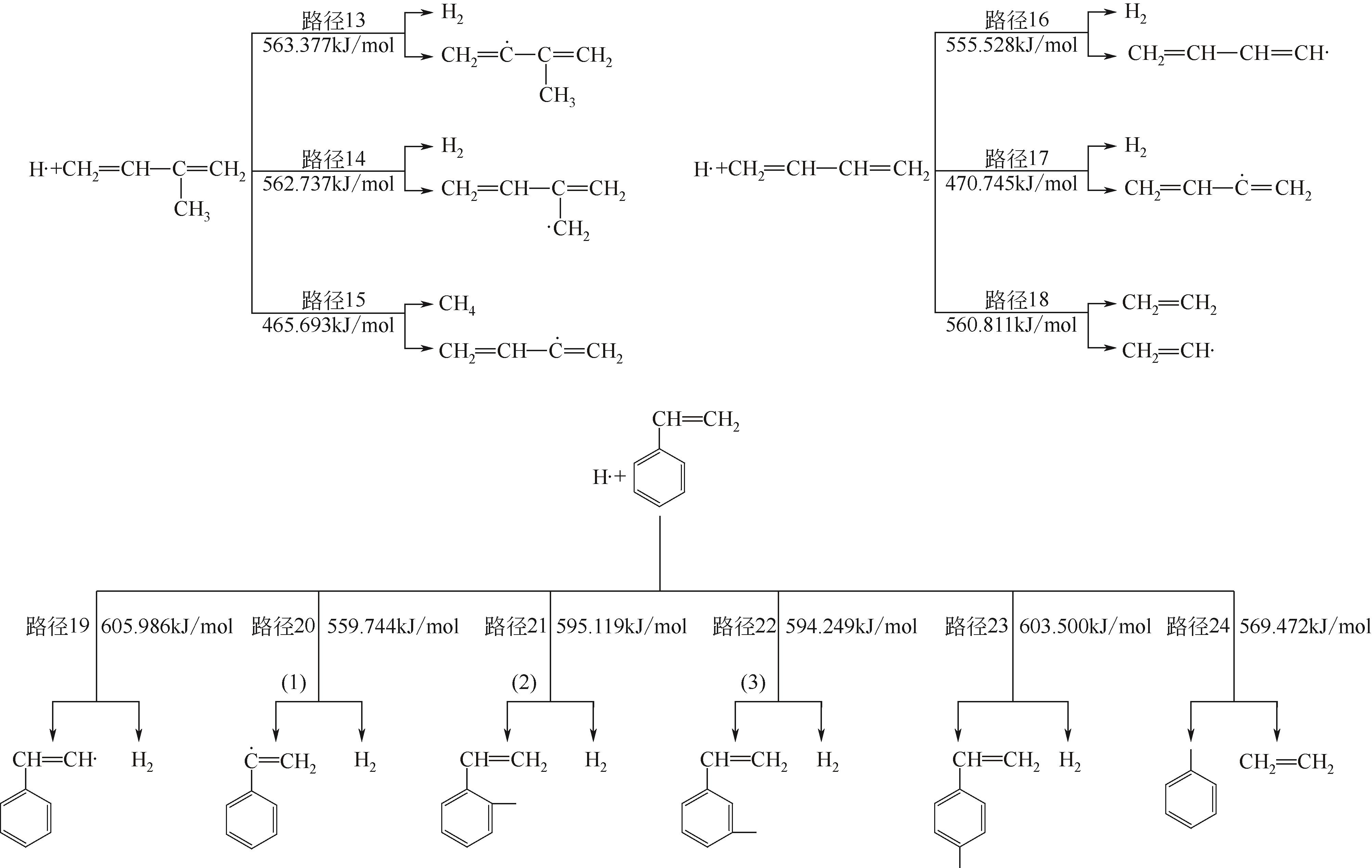
| 13→37 | CH2CHC(CH3)CH2+H· | 563.377 | 558.412 | 4.965 |
| 14→38 | CH2CHC(CH3)CH2+H· | 562.737 | 466.852 | 95.885 |
| 15→39 | CH2CHC(CH3)CH2+H· | 465.693 | 534.293 | -68.600 |
| 16→40 | CH2CHCHCH2+H· | 555.528 | 548.718 | 6.810 |
| 17→41 | CH2CHCHCH2+H· | 470.745 | 424.791 | 45.954 |
| 18→42 | CH2CHCHCH2+H· | 560.811 | 552.665 | 8.146 |
| 19→43 | C6H5CHCH2+H· | 605.986 | 596.668 | 9.318 |
| 20→44 | C6H5CHCH2+H· | 559.744 | 550.074 | 9.670 |
| 21→45 | (1) C6H5CHCH2+H· | 595.119 | 574.616 | 20.503 |
| 22→46 | (2) C6H5CHCH2+H· | 594.249 | 585.621 | 8.628 |
| 23→47 | (3) C6H5CHCH2+H· | 603.500 | 598.502 | 4.998 |
| 24→48 | C6H5CHCH2+H· | 569.472 | 555.126 | 14.346 |
表3 加入Ni催化剂前后H基攻击单体能垒
催化前路径→ 催化后路径 | 反应式 | 催化前能垒E /kJ·mol-1 | 催化后能垒E /kJ·mol-1 | 差值(前-后)?E /kJ·mol-1 |
|---|---|---|---|---|
| 13→37 | CH2CHC(CH3)CH2+H· | 563.377 | 558.412 | 4.965 |
| 14→38 | CH2CHC(CH3)CH2+H· | 562.737 | 466.852 | 95.885 |
| 15→39 | CH2CHC(CH3)CH2+H· | 465.693 | 534.293 | -68.600 |
| 16→40 | CH2CHCHCH2+H· | 555.528 | 548.718 | 6.810 |
| 17→41 | CH2CHCHCH2+H· | 470.745 | 424.791 | 45.954 |
| 18→42 | CH2CHCHCH2+H· | 560.811 | 552.665 | 8.146 |
| 19→43 | C6H5CHCH2+H· | 605.986 | 596.668 | 9.318 |
| 20→44 | C6H5CHCH2+H· | 559.744 | 550.074 | 9.670 |
| 21→45 | (1) C6H5CHCH2+H· | 595.119 | 574.616 | 20.503 |
| 22→46 | (2) C6H5CHCH2+H· | 594.249 | 585.621 | 8.628 |
| 23→47 | (3) C6H5CHCH2+H· | 603.500 | 598.502 | 4.998 |
| 24→48 | C6H5CHCH2+H· | 569.472 | 555.126 | 14.346 |
| 路径 | ZSM-5催化前 能垒E/kJ·mol-1 | ZSM-5催化后 能垒E/kJ·mol-1 | ZSM-5差值?E(前-后) /kJ·mol-1 | Ni/ZSM-5催化前 能垒E/kJ·mol-1 | Ni/ZSM-5催化后 能垒E/kJ·mol-1 | Ni/ZSM-5差值?E(前-后) /kJ·mol-1 |
|---|---|---|---|---|---|---|
| 1 | 496.983 | 538.039 | -41.056 | 496.983 | 514.535 | -17.552 |
| 2 | 563.528 | 563.264 | 0.264 | 563.528 | 557.316 | 6.212 |
| 3 | 443.147 | 516.820 | -73.673 | 443.147 | 501.211 | -58.064 |
| 4 | 557.935 | 560.108 | -2.173 | 557.935 | 554.662 | 3.273 |
| 5 | 472.465 | 483.454 | -10.988 | 472.465 | 468.531 | 3.934 |
| 6 | 568.622 | 547.395 | 21.227 | 568.622 | 543.058 | 25.564 |
| 7 | 607.728 | 595.726 | 12.002 | 607.728 | 593.713 | 14.015 |
| 8 | 567.442 | 568.036 | -0.594 | 567.442 | 560.853 | 6.589 |
| 9 | 625.861 | 585.312 | 40.549 | 625.861 | 577.266 | 48.595 |
| 10 | 593.822 | 586.593 | 7.229 | 593.822 | 586.341 | 7.481 |
| 11 | 605.718 | 595.927 | 9.791 | 605.718 | 595.153 | 10.565 |
| 12 | 575.395 | 573.566 | 1.829 | 575.395 | 567.689 | 7.706 |
表4 加入ZSM-5、Ni/ZSM-5催化剂前后自由基生成能垒
| 路径 | ZSM-5催化前 能垒E/kJ·mol-1 | ZSM-5催化后 能垒E/kJ·mol-1 | ZSM-5差值?E(前-后) /kJ·mol-1 | Ni/ZSM-5催化前 能垒E/kJ·mol-1 | Ni/ZSM-5催化后 能垒E/kJ·mol-1 | Ni/ZSM-5差值?E(前-后) /kJ·mol-1 |
|---|---|---|---|---|---|---|
| 1 | 496.983 | 538.039 | -41.056 | 496.983 | 514.535 | -17.552 |
| 2 | 563.528 | 563.264 | 0.264 | 563.528 | 557.316 | 6.212 |
| 3 | 443.147 | 516.820 | -73.673 | 443.147 | 501.211 | -58.064 |
| 4 | 557.935 | 560.108 | -2.173 | 557.935 | 554.662 | 3.273 |
| 5 | 472.465 | 483.454 | -10.988 | 472.465 | 468.531 | 3.934 |
| 6 | 568.622 | 547.395 | 21.227 | 568.622 | 543.058 | 25.564 |
| 7 | 607.728 | 595.726 | 12.002 | 607.728 | 593.713 | 14.015 |
| 8 | 567.442 | 568.036 | -0.594 | 567.442 | 560.853 | 6.589 |
| 9 | 625.861 | 585.312 | 40.549 | 625.861 | 577.266 | 48.595 |
| 10 | 593.822 | 586.593 | 7.229 | 593.822 | 586.341 | 7.481 |
| 11 | 605.718 | 595.927 | 9.791 | 605.718 | 595.153 | 10.565 |
| 12 | 575.395 | 573.566 | 1.829 | 575.395 | 567.689 | 7.706 |
| 路径 | ZSM-5催化前 能垒E/kJ·mol-1 | ZSM-5催化后 能垒E/kJ·mol-1 | ZSM-5差值?E(前-后) /kJ·mol-1 | Ni/ZSM-5催化前 能垒E /kJ·mol-1 | Ni/ZSM-5催化后 能垒E /kJ·mol-1 | Ni/ZSM-5差值?E(前-后) /kJ·mol-1 |
|---|---|---|---|---|---|---|
| 13 | 563.377 | 551.610 | 11.767 | 563.377 | 512.982 | 50.395 |
| 14 | 562.737 | 485.404 | 77.333 | 562.737 | 563.570 | -0.833 |
| 15 | 465.693 | 429.513 | 36.180 | 465.693 | 431.711 | 33.982 |
| 16 | 555.528 | 561.711 | -6.183 | 555.528 | 553.184 | 2.344 |
| 17 | 470.745 | 458.099 | 12.646 | 470.745 | 441.238 | 29.507 |
| 18 | 560.811 | 537.675 | 23.136 | 560.811 | 546.240 | 14.571 |
| 19 | 605.986 | 599.984 | 6.002 | 605.986 | 606.070 | -0.084 |
| 20 | 559.744 | 579.891 | -20.147 | 559.744 | 550.765 | 8.979 |
| 21 | 595.119 | 598.029 | -2.910 | 595.119 | 590.540 | 4.579 |
| 22 | 594.249 | 588.556 | 5.693 | 594.249 | 586.053 | 8.196 |
| 23 | 603.500 | 602.583 | 0.917 | 603.500 | 598.740 | 4.760 |
| 24 | 569.472 | 585.647 | -16.175 | 569.472 | 567.295 | 2.177 |
表5 加入ZSM-5、Ni/ZSM-5催化剂前后H基攻击单体能垒
| 路径 | ZSM-5催化前 能垒E/kJ·mol-1 | ZSM-5催化后 能垒E/kJ·mol-1 | ZSM-5差值?E(前-后) /kJ·mol-1 | Ni/ZSM-5催化前 能垒E /kJ·mol-1 | Ni/ZSM-5催化后 能垒E /kJ·mol-1 | Ni/ZSM-5差值?E(前-后) /kJ·mol-1 |
|---|---|---|---|---|---|---|
| 13 | 563.377 | 551.610 | 11.767 | 563.377 | 512.982 | 50.395 |
| 14 | 562.737 | 485.404 | 77.333 | 562.737 | 563.570 | -0.833 |
| 15 | 465.693 | 429.513 | 36.180 | 465.693 | 431.711 | 33.982 |
| 16 | 555.528 | 561.711 | -6.183 | 555.528 | 553.184 | 2.344 |
| 17 | 470.745 | 458.099 | 12.646 | 470.745 | 441.238 | 29.507 |
| 18 | 560.811 | 537.675 | 23.136 | 560.811 | 546.240 | 14.571 |
| 19 | 605.986 | 599.984 | 6.002 | 605.986 | 606.070 | -0.084 |
| 20 | 559.744 | 579.891 | -20.147 | 559.744 | 550.765 | 8.979 |
| 21 | 595.119 | 598.029 | -2.910 | 595.119 | 590.540 | 4.579 |
| 22 | 594.249 | 588.556 | 5.693 | 594.249 | 586.053 | 8.196 |
| 23 | 603.500 | 602.583 | 0.917 | 603.500 | 598.740 | 4.760 |
| 24 | 569.472 | 585.647 | -16.175 | 569.472 | 567.295 | 2.177 |
不同比例 (橡胶∶催化剂) | 1100K时热解情况 | 1500K时热解情况 |
|---|---|---|
| 1∶0 |  |  |
| 5∶1 |  |  |
| 3∶1 |  |  |
| 1∶1 |  |  |
表6 低温阶段热解情况
不同比例 (橡胶∶催化剂) | 1100K时热解情况 | 1500K时热解情况 |
|---|---|---|
| 1∶0 |  |  |
| 5∶1 |  |  |
| 3∶1 |  |  |
| 1∶1 |  |  |
| 1 | 张立民, 李志学. 我国新能源经济发展现状分析[J]. 现代商业, 2021(7): 25-27. |
| ZHANG Limin, LI Zhixue. Analysis of the present situation of the development of new energy economy in China[J]. Modern Business, 2021 (7): 25-27. | |
| 2 | LI G, ZHANG K, YANG B, et al. Life cycle analysis of a coal to hydrogen process based on ash agglomerating fluidized bed gasification[J]. Energy, 2019, 174: 638-646. |
| 3 | TOUILI S, ALAMI MERROUNI A, HASSOUANI Y EL, et al. Analysis of the yield and production cost of large-scale electrolytic hydrogen from different solar technologies and under several Moroccan climate zones[J]. International Journal of Hydrogen Energy, 2020, 45(51): 26785-26799. |
| 4 | LI W C, REN X S, DING S M, et al. A multi-criterion decision making for sustainability assessment of hydrogen production technologies based on objective grey relational analysis[J]. International Journal of Hydrogen Energy, 2020, 45(59): 34385-34395. |
| 5 | 郭博文, 罗聃, 周红军. 可再生能源电解制氢技术及催化剂的研究进展[J]. 化工进展, 2021, 40(6): 2933-2951. |
| GUO Bowen, LUO Dan, ZHOU Hongjun. Recent advances in renewable energy electrolysis hydrogen production technology and related electrocatalysts[J]. Chemical Industry and Engineering Progress, 2021, 40(6): 2933-2951. | |
| 6 | 国家统计局. 2019年7—12月我国合成橡胶、轮胎和汽车产量[J]. 橡胶科技, 2020, 18(3): 178. |
| National Bureau of Statistics. Output of synthetic rubber, tires and cars in China from July to December 2019[J]. Rubber Science and Technology, 2020, 18(3): 178. | |
| 7 | CHOI G G, OH S J, KIM J S, et al. Non-catalytic pyrolysis of scrap tires using a newly developed two-stage pyrolyzer for the production of a pyrolysis oil with a low sulfur content[J]. Applied Energy, 2016, 170: 140-147. |
| 8 | 狄伟强. 废轮胎热解炭特性及其对废轮胎催化性能研究[D]. 大连: 大连理工大学, 2019. |
| DI Weiqiang. Study on the characteristics of waste tire pyrolysis char and its catalytic pyrolysis effect on waste tires[D]. Dalian: Dalian University of Technology, 2019. | |
| 9 | LI W P, WEI M M, LIU Y Q, et al. Catalysts evaluation for production of hydrogen gas and carbon nanotubes from the pyrolysis-catalysis of waste tyres[J]. International Journal of Hydrogen Energy, 2019, 44(36): 19563-19572. |
| 10 | Williams P T, Brindle A J.Catalytic pyrolysis of tyres: influence of catalyst temperature[J]. Fuel, 2002, 81(18): 2425-2434. |
| 11 | YU J, LIU S, CARDOSO A, et al. Catalytic pyrolysis of rubbers and vulcanized rubbers using modified zeolites and mesoporous catalysts with Zn and Cu[J]. Energy, 2019, 188: 116117. |
| 12 | 李秉繁, 刘刚, 陈雷. 基于分子动力学模拟的CH4溶解对原油分子间作用的影响机制研究[J]. 化工学报, 2021, 72(3): 1253-1263. |
| LI Bingfan, LIU Gang, CHEN Lei. Study on the influence mechanism of CH4 dissolution on the intermolecular interaction between crude oil molecules based on molecular dynamics simulation[J]. CIESC Journal, 2021, 72(3): 1253-1263. | |
| 13 | LIU Y L, DING J X, HAN K L. Molecular dynamics simulation of the high-temperature pyrolysis of methylcyclohexane[J]. Fuel, 2018, 217: 185-192. |
| 14 | 魏鑫. 天然橡胶热解气相产物生成机理研究[D]. 青岛: 青岛大学, 2019. |
| WEI Xin. Study on the formation mechanism of gas phase products of natural rubber pyrolysis[D]. Qingdao: Qingdao University, 2019. | |
| 15 | YANG Q R, YU S P, ZHONG H W, et al. Gas products generation mechanism during co-pyrolysis of styrene-butadiene rubber and natural rubber[J]. Journal of Hazardous Materials, 2021, 401: 123302. |
| 16 | 于双鹏, 杨启容, 陶礼, 等. 基于分子动力学模拟的轮胎橡胶气相热解产物反应机理[J]. 化工进展, 2021, 40(6): 3119-3131. |
| YU Shuangpeng, YANG Qirong, TAO Li, et al. Gas phase pyrolysis products of tire rubber based on molecular dynamics simulation[J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3119-3131. | |
| 17 | 潘旗. 基于分子动力学模拟的变压器油纸绝缘老化特性基础研究[D]. 徐州: 中国矿业大学, 2020. |
| PAN Qi. Basic research on aging characteristics of transformer oil paper insulation based on molecular dynamics simulation[D]. Xuzhou: China University of Mining and Technology, 2020. | |
| 18 | HU S D, SUN W G, FU J, et al. Initiation mechanisms and kinetic analysis of the isothermal decomposition of poly(α-methylstyrene): a ReaxFF molecular dynamics study[J]. RSC Advances, 2018, 8(7): 3423-3432. |
| 19 | KWON H, LELE A, ZHU J Q, et al. ReaxFF-based molecular dynamics study of bio-derived polycyclic alkanes as potential alternative jet fuels[J]. Fuel, 2020, 279: 118548. |
| 20 | 张贻亮. 原子-键电负性均衡方法及其在理论化学中的应用[D]. 长春: 吉林大学, 2002. |
| ZHANG Yiliang. Atom-bond electronegativity equalization method and its application in theoretical chemistry[D]. Changchun: Jilin University, 2002. | |
| 21 | ZHENG M, WANG Z, LI X X, et al. Initial reaction mechanisms of cellulose pyrolysis revealed by ReaxFF molecular dynamics[J]. Fuel, 2016, 177: 130-141. |
| 22 | 杜鸟锋, 甯红波, 李泽荣, 等. 1,3-丁二烯热裂解的动力学计算与模型研究[J]. 物理化学学报, 2016, 32(2): 453-464. |
| DU Niaofeng, NING Hongbo, LI Zerong, et al. Kinetic calculation and modeling study of 1,3-butadiene pyrolysis[J]. Acta Physico-Chimica Sinica, 2016, 32(2): 453-464. | |
| 23 | 徐宗平, 郭庆民. 废轮胎热解回收中的废气综合利用[J]. 中国轮胎资源综合利用, 2018(11): 36-39. |
| XU Zongping, GUO Qingmin. The comprehensive utilization of waste gas in waste tire pyrolysis recycling[J]. China Tire Resources Recycling, 2018(11): 36-39. | |
| 24 | 张会亮, 范晓旭, 刘彦丰, 等. 块状废轮胎固定床热解特性实验研究[J]. 可再生能源, 2015, 33(1): 149-153. |
| ZHANG Huiliang, FAN Xiaoxu, LIU Yanfeng, et al. Experimental study on pyrolysis of blocky tires in a fixed-bed reactor[J]. Renewable Energy Resources, 2015, 33(1): 149-153. | |
| 25 | 张冰, 付琦, 梁畅. 废轮胎橡胶热解技术研究进展[J]. 橡塑技术与装备, 2018, 44(15): 19-23. |
| ZHANG Bing, FU Qi, LIANG Chang. Research progress on waste tire rubber pyrolysis technology[J]. China Rubber/Plastics Technology and Equipment, 2018, 44(15): 19-23. | |
| 26 | ELBABA I F, WILLIAMS P T. Two stage pyrolysis-catalytic gasification of waste tyres: influence of process parameters[J]. Applied Catalysis B: Environmental, 2012, 125: 136-143. |
| 27 | ELBABA I F, WU C F, WILLIAMS P T. Hydrogen production from the pyrolysis-gasification of waste tyres with a nickel/cerium catalyst[J]. International Journal of Hydrogen Energy, 2011, 36(11): 6628-6637. |
| 28 | ZHANG Y S, WU C F, NAHIL M A, et al. Pyrolysis-catalytic reforming/gasification of waste tires for production of carbon nanotubes and hydrogen[J]. Energy & Fuels, 2015, 29(5): 3328-3334. |
| [1] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [2] | 王家庆, 宋广伟, 李强, 郭帅成, DAI Qingli. 橡胶混凝土界面改性方法及性能提升路径[J]. 化工进展, 2023, 42(S1): 328-343. |
| [3] | 张亚娟, 徐惠, 胡贝, 史星伟. 化学镀法制备NiCoP/rGO/NF高效电解水析氢催化剂[J]. 化工进展, 2023, 42(8): 4275-4282. |
| [4] | 王蕴青, 杨国锐, 延卫. 过渡金属磷化物的改性方法及其在电化学析氢中的应用[J]. 化工进展, 2023, 42(7): 3532-3549. |
| [5] | 符淑瑢, 王丽娜, 王东伟, 刘蕊, 张晓慧, 马占伟. 析氧助催化剂增强光阳极光电催化分解水性能研究进展[J]. 化工进展, 2023, 42(5): 2353-2370. |
| [6] | 郑云武, 裴涛, 李冬华, 王继大, 李继容, 郑志锋. 金属氧化物活化P/HZSM-5催化生物质热解气重整制备富烃生物油[J]. 化工进展, 2023, 42(3): 1353-1364. |
| [7] | 赵毅, 杨臻, 王佳, 李静雯, 郑煜. 沥青胶结料自愈合行为分子动力学模拟研究进展[J]. 化工进展, 2023, 42(2): 803-813. |
| [8] | 李攀, 王彪, 徐骏浩, 王贤华, 胡俊豪, 宋建德, 白净, 常春. 生物质热解催化剂积炭问题的研究进展[J]. 化工进展, 2023, 42(1): 236-246. |
| [9] | 肖周荣, 李国柱, 王涖, 张香文, 谷建民, 王德松. 液体碳氢燃料蒸汽重整制氢催化剂研究进展[J]. 化工进展, 2022, 41(S1): 97-107. |
| [10] | 孙宪航, 任铸, 张国军, 孙媛, 范开峰, 黄维秋. 超临界CO2作用下甲苯在活性炭中的脱附机理[J]. 化工进展, 2022, 41(S1): 631-636. |
| [11] | 胡兵, 徐立军, 何山, 苏昕, 汪继伟. 碳达峰与碳中和目标下PEM电解水制氢研究进展[J]. 化工进展, 2022, 41(9): 4595-4604. |
| [12] | 李玉峰, 王绍庆, 张安东, 毕冬梅, 李志合, 高亮, 万震. 催化型多孔陶瓷球制备及催化玉米秸秆热解[J]. 化工进展, 2022, 41(7): 3597-3607. |
| [13] | 闫鹏, 程易. 用于分布式制氢的甲烷蒸汽重整膜反应器的数值模拟[J]. 化工进展, 2022, 41(7): 3446-3454. |
| [14] | 张雷, 王海英, 韩洪晶, 陈彦广, 王程昊. 木质素催化热解用催化剂的研究进展[J]. 化工进展, 2022, 41(5): 2429-2440. |
| [15] | 张轩, 樊昕晔, 吴振宇, 郑丽君. 氢能供应链成本分析及建议[J]. 化工进展, 2022, 41(5): 2364-2371. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||