化工进展 ›› 2021, Vol. 40 ›› Issue (8): 4117-4126.DOI: 10.16085/j.issn.1000-6613.2020-1938
基于二维纳米材料的水处理功能膜研究进展
李胄彦1( ), 戴若彬1, 李洋1, 王雪野1, 王志伟1,2(
), 戴若彬1, 李洋1, 王雪野1, 王志伟1,2( )
)
- 1.同济大学环境科学与工程学院,上海 200092
2.同济大学污染控制与资源化研究国家重点实验室,上海 200092
-
收稿日期:2020-09-22出版日期:2021-08-05发布日期:2021-08-12 -
通讯作者:王志伟 -
作者简介:李胄彦(1996—),男,博士研究生,研究方向为膜法污水处理与资源化技术。E-mail:zhouyan_li@tongji.edu.cn 。 -
基金资助:上海市科委科技攻关项目(20dz1207700)
Research progress of functional membranes based on two-dimensional nanomaterials for water treatment
LI Zhouyan1( ), DAI Ruobin1, LI Yang1, WANG Xueye1, WANG Zhiwei1,2(
), DAI Ruobin1, LI Yang1, WANG Xueye1, WANG Zhiwei1,2( )
)
- 1.College of Environmental Science and Engineering, Tongji University, Shanghai 200092, China
2.State Key Laboratory of Pollution Control and Resource Reuse, Tongji University, Shanghai 200092, China
-
Received:2020-09-22Online:2021-08-05Published:2021-08-12 -
Contact:WANG Zhiwei
摘要:
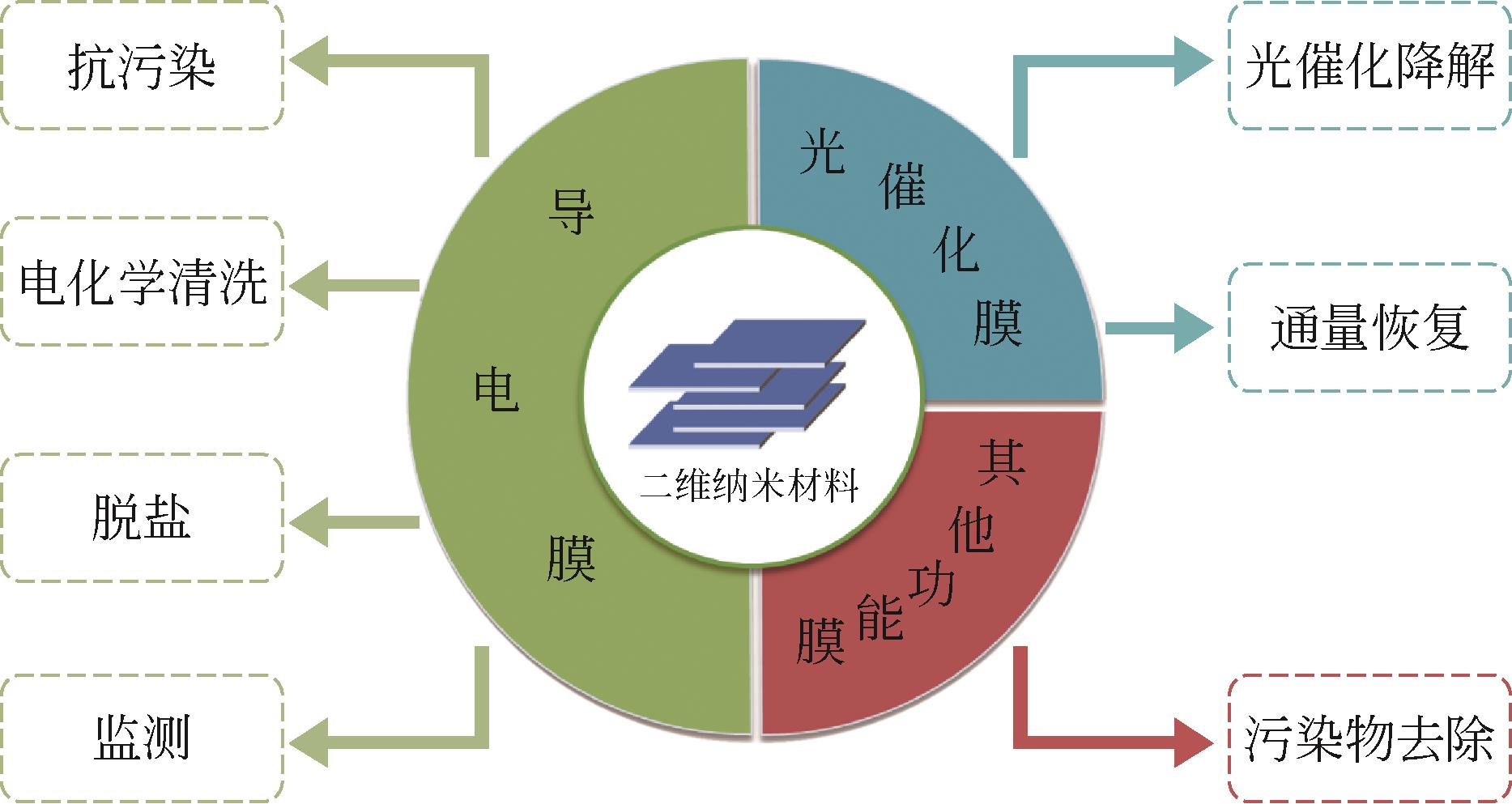
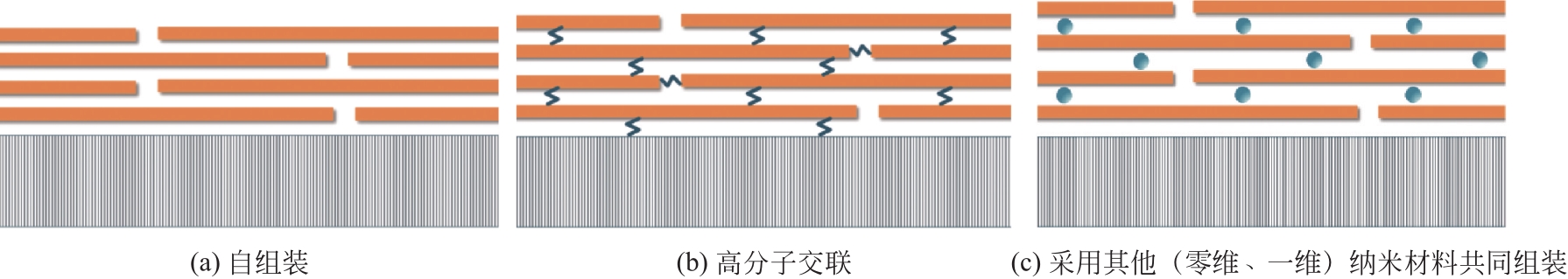
二维纳米材料是制备膜材料中一类重要的掺杂材料或膜构筑单元,也是新型水处理功能膜的研究热点。已有许多研究报道了二维纳米材料通过有序的堆叠和自组装在膜内构建出规整的水通道,可以赋予膜可调控的分离性能,进而实现trade-off效应的突破,被认为是“下一代膜材料”(next-generation membranes)。同时,二维纳米材料的独特片层结构、催化性能及可修饰性可使膜材料获得新的功能,如导电性能、光/电催化性能等。本文综述了近年来基于二维纳米材料的水处理功能膜研究进展,重点介绍了共混法、自组装等制备方法,并总结了此类功能膜在抗污染、膜通量恢复、强化污染物去除、调控盐截留及污染物监测领域的应用。最后对基于二维纳米材料的水处理功能膜发展方向,如限域催化、调控盐分离、监测传感等新兴领域进行了分析和展望。
中图分类号:
引用本文
李胄彦, 戴若彬, 李洋, 王雪野, 王志伟. 基于二维纳米材料的水处理功能膜研究进展[J]. 化工进展, 2021, 40(8): 4117-4126.
LI Zhouyan, DAI Ruobin, LI Yang, WANG Xueye, WANG Zhiwei. Research progress of functional membranes based on two-dimensional nanomaterials for water treatment[J]. Chemical Industry and Engineering Progress, 2021, 40(8): 4117-4126.
二维 纳米材料 | 其他分离层组分 | 制备方法 | 分类 | 应用场景 | 最优条件 | 抗污染效果 | 参考 文献 |
|---|---|---|---|---|---|---|---|
| GO | 激光诱导石墨烯(LIG),戊二醛 | VAF自组装 | 导电膜 | 微(超)滤 | 3V,作为阳极 | 在混菌的错流过滤实验中,导电膜通量较普通超滤膜提升11% | [ |
| Gr | PANI | PAF自组装 | 导电膜 | 正渗透 | 2V,作为阳极 | 在海藻酸钠污染实验中,污染速率降低40%~45%,通量恢复率提升20%~30% | [ |
| rGO | PDVF,PDAAQ | 相转化法 | 导电膜 | 膜生物 反应器 | 1.0V·cm-1,作为阴极 | 牛血清蛋白(BSA)的污染速率降低63.5% | [ |
| GO | 导电高分子聚吡咯(PPy) | 气相聚合法 | 导电膜 | 膜生物 反应器 | 1.0V·cm-1,作为阴极 | 在酵母菌污染实验中,较对照组通量提升20% | [ |
| GO | 导电高分子聚吡咯(PPy) | 气相聚合法 | 导电膜 | 膜生物 反应器 | 2V·cm-1,作为阴极,并投加群体感应淬灭细菌 | 在MBR中污染速率仅为其他对照组的34%~55% | [ |
| Gr | 基膜为镍基导电膜 | 化学气相沉积 | 导电膜 | 厌氧膜生物 反应器 | 0.7V或0.9V,作为阴极,阴阳极间距约1.5cm的矩形反应器 | 运行50余天后,跨膜压力仅为0.10bar(0.7V)和0.05bar(0.9V),远低于管式反应器(0.7V)的0.46bar(1bar=105Pa) | [ |
表1 基于二维纳米材料功能膜的抗污染研究总结
二维 纳米材料 | 其他分离层组分 | 制备方法 | 分类 | 应用场景 | 最优条件 | 抗污染效果 | 参考 文献 |
|---|---|---|---|---|---|---|---|
| GO | 激光诱导石墨烯(LIG),戊二醛 | VAF自组装 | 导电膜 | 微(超)滤 | 3V,作为阳极 | 在混菌的错流过滤实验中,导电膜通量较普通超滤膜提升11% | [ |
| Gr | PANI | PAF自组装 | 导电膜 | 正渗透 | 2V,作为阳极 | 在海藻酸钠污染实验中,污染速率降低40%~45%,通量恢复率提升20%~30% | [ |
| rGO | PDVF,PDAAQ | 相转化法 | 导电膜 | 膜生物 反应器 | 1.0V·cm-1,作为阴极 | 牛血清蛋白(BSA)的污染速率降低63.5% | [ |
| GO | 导电高分子聚吡咯(PPy) | 气相聚合法 | 导电膜 | 膜生物 反应器 | 1.0V·cm-1,作为阴极 | 在酵母菌污染实验中,较对照组通量提升20% | [ |
| GO | 导电高分子聚吡咯(PPy) | 气相聚合法 | 导电膜 | 膜生物 反应器 | 2V·cm-1,作为阴极,并投加群体感应淬灭细菌 | 在MBR中污染速率仅为其他对照组的34%~55% | [ |
| Gr | 基膜为镍基导电膜 | 化学气相沉积 | 导电膜 | 厌氧膜生物 反应器 | 0.7V或0.9V,作为阴极,阴阳极间距约1.5cm的矩形反应器 | 运行50余天后,跨膜压力仅为0.10bar(0.7V)和0.05bar(0.9V),远低于管式反应器(0.7V)的0.46bar(1bar=105Pa) | [ |
二维 纳米材料 | 其他分离层组分 | 制备方法 | 分类 | 应用场景 | 实验参数 | 通量恢复效果 | 参考文献 |
|---|---|---|---|---|---|---|---|
| rGO | PANI | PAF自组装 | 导电膜 | 微(超)滤 | 3~9V作为阳极进行清洗,阴阳极间距0.5cm | 清洗实验中通量恢复率最高达到97% | [ |
| rGO | 有机主体为PES、PANI(HCSA) 或PANI(DBSA) | 相转化法 | 导电膜 | 微(超)滤 | 电压5V,清洗时间10min | 在腐殖酸(HA)和BSA污染实验中,通量恢复率较超纯水清洗提升6%~8%,较普通PES膜提升48%~68% | [ |
| rGO | 二维TiO2 | 溶剂热诱导组装 | 光催化膜 | 染料分离 | 可见光照射一定时间 | 通量恢复率约为95% | [ |
| GO | 一维TiO2纳米棒 | VAF自组装 | 光催化膜 | 染料分离 | 可见光照射24h,功率500W,照明距离35cm | 3轮实验后,通量恢复率均大于83% | [ |
| GO,g-C3N4 | 零维TiO2 | VAF自组装 | 光催化膜 | 油水分离 | 去离子水清洗20min,模拟光照1h | 通量为普通GO膜的45倍,10轮油水分离试验后自清洁的通量恢复率仍高于95% | [ |
| GO,g-C3N4 | 一维坡缕石(PG)、光催化剂Bi2O2CO3 | VAF自组装 | 光催化膜 | 油水分离 | 模拟光照射1h | 通量为普通GO膜的46倍,自清洁的通量恢复率高于95%(最高接近100%) | [ |
| g-C3N4 | 芬顿催化剂 Fe-POMs | 氢键作用辅助的VAF自组装 | 光催化膜 | 染料分离 | 100mW·cm-2模拟太阳光照,连续流实验中添加30mmol·L-1 H2O2 | 5轮试验后,通量恢复率均接近100%;连续流实验中通量在12h内保持稳定 | [ |
表2 基于二维纳米材料功能膜的膜通量恢复研究总结
二维 纳米材料 | 其他分离层组分 | 制备方法 | 分类 | 应用场景 | 实验参数 | 通量恢复效果 | 参考文献 |
|---|---|---|---|---|---|---|---|
| rGO | PANI | PAF自组装 | 导电膜 | 微(超)滤 | 3~9V作为阳极进行清洗,阴阳极间距0.5cm | 清洗实验中通量恢复率最高达到97% | [ |
| rGO | 有机主体为PES、PANI(HCSA) 或PANI(DBSA) | 相转化法 | 导电膜 | 微(超)滤 | 电压5V,清洗时间10min | 在腐殖酸(HA)和BSA污染实验中,通量恢复率较超纯水清洗提升6%~8%,较普通PES膜提升48%~68% | [ |
| rGO | 二维TiO2 | 溶剂热诱导组装 | 光催化膜 | 染料分离 | 可见光照射一定时间 | 通量恢复率约为95% | [ |
| GO | 一维TiO2纳米棒 | VAF自组装 | 光催化膜 | 染料分离 | 可见光照射24h,功率500W,照明距离35cm | 3轮实验后,通量恢复率均大于83% | [ |
| GO,g-C3N4 | 零维TiO2 | VAF自组装 | 光催化膜 | 油水分离 | 去离子水清洗20min,模拟光照1h | 通量为普通GO膜的45倍,10轮油水分离试验后自清洁的通量恢复率仍高于95% | [ |
| GO,g-C3N4 | 一维坡缕石(PG)、光催化剂Bi2O2CO3 | VAF自组装 | 光催化膜 | 油水分离 | 模拟光照射1h | 通量为普通GO膜的46倍,自清洁的通量恢复率高于95%(最高接近100%) | [ |
| g-C3N4 | 芬顿催化剂 Fe-POMs | 氢键作用辅助的VAF自组装 | 光催化膜 | 染料分离 | 100mW·cm-2模拟太阳光照,连续流实验中添加30mmol·L-1 H2O2 | 5轮试验后,通量恢复率均接近100%;连续流实验中通量在12h内保持稳定 | [ |
二维 纳米材料 | 其他分离层组分 | 制备方法 | 分类 | 应用场景 | 关键参数 | 去除效果 | 参考文献 |
|---|---|---|---|---|---|---|---|
| MoS2 | Fe(OH)3作为模板,成膜后刻蚀掉 | VAF自组装/ 模板刻蚀 | 其他功能膜 | 污染物降解 | 过一硫酸盐浓度为50ppm(1ppm=1mg/kg),pH为4,过滤性能为154L·m-2·h-1·bar-1 | 接触时间为60.4ms,BPA(2ppm)的去除率大于90% | [ |
| MoS2 | 无 | VAF自组装 | 其他功能膜 | 重金属去除 | pH为6,反应时间为1天,MoS2膜未经真空干燥 | 还原去除容量约为4000mg Ag/g MoS2,远高于干燥后的MoS2膜 | [ |
| Gr | TiO2 | 溶胶凝胶法 | 导电膜/ 光催化膜 | 微(超)滤 | pH为5.4,模拟太阳光照,4V作为阳极 | 30min光电协同对100mL罗丹明B(10mg·L-1)的去除效率达97.8%,高于300min的光催化去除效率(87.6%) | [ |
| GO | 一维TiO2纳米线,聚多巴胺 | VAF自组装 | 光催化膜 | 油水/染料分离 | 可见光照射条件下,过滤性能为273L·m-2·h-1·bar-1 | 对油水混合液中MB(10ppm)的去除率大于98% | [ |
| GO | 零维TiO2、Co3O4 | VAF自组装 | 光催化膜 | 油水分离 | 光源功率为250W | 100mL刚果红/油水混合液,刚果红(20ppm)降解效率为82% | [ |
| GO | 零维纳米Ag、一维钛纳米管 | VAF自组装 | 光催化膜 | 染料分离 | 可见光照射条件下,通量为34.7L·m-2·h-1 | 对MB(10mg·L-1)的去除率大于65%,且通量约为无光照条件下的两倍 | [ |
| rGO | 一维g-C3N4 纳米管 | VAF自组装 | 光催化膜 | 染料分离 | 300W可见光照射条件下,过滤性能为4.77L·m-2·h-1·bar-1 | 对罗丹明B(5mg·L-1)的去除率大于98% | [ |
GO, g-C3N4 | 一维CNTs | VAF自组装 | 光催化膜 | 污染物降解 | 可见光照射条件下,过滤性能为14.35L·m-2·h-1·bar-1 | 罗丹明B和盐酸四环素去除率分别为98.31%、84.81% | [ |
| GO | 光Fenton催化剂:一维MOF (MIL-88A) | VAF自组装 | 光催化膜 | 染料分离 | 模拟太阳光104mW·cm-2,10mmol·L-1 H2O2 | 对50mL MB和BPA(10mg·L-1)去除率分别为98.8%和97.3% | [ |
表3 基于二维纳米材料功能膜的强化污染物去除研究总结
二维 纳米材料 | 其他分离层组分 | 制备方法 | 分类 | 应用场景 | 关键参数 | 去除效果 | 参考文献 |
|---|---|---|---|---|---|---|---|
| MoS2 | Fe(OH)3作为模板,成膜后刻蚀掉 | VAF自组装/ 模板刻蚀 | 其他功能膜 | 污染物降解 | 过一硫酸盐浓度为50ppm(1ppm=1mg/kg),pH为4,过滤性能为154L·m-2·h-1·bar-1 | 接触时间为60.4ms,BPA(2ppm)的去除率大于90% | [ |
| MoS2 | 无 | VAF自组装 | 其他功能膜 | 重金属去除 | pH为6,反应时间为1天,MoS2膜未经真空干燥 | 还原去除容量约为4000mg Ag/g MoS2,远高于干燥后的MoS2膜 | [ |
| Gr | TiO2 | 溶胶凝胶法 | 导电膜/ 光催化膜 | 微(超)滤 | pH为5.4,模拟太阳光照,4V作为阳极 | 30min光电协同对100mL罗丹明B(10mg·L-1)的去除效率达97.8%,高于300min的光催化去除效率(87.6%) | [ |
| GO | 一维TiO2纳米线,聚多巴胺 | VAF自组装 | 光催化膜 | 油水/染料分离 | 可见光照射条件下,过滤性能为273L·m-2·h-1·bar-1 | 对油水混合液中MB(10ppm)的去除率大于98% | [ |
| GO | 零维TiO2、Co3O4 | VAF自组装 | 光催化膜 | 油水分离 | 光源功率为250W | 100mL刚果红/油水混合液,刚果红(20ppm)降解效率为82% | [ |
| GO | 零维纳米Ag、一维钛纳米管 | VAF自组装 | 光催化膜 | 染料分离 | 可见光照射条件下,通量为34.7L·m-2·h-1 | 对MB(10mg·L-1)的去除率大于65%,且通量约为无光照条件下的两倍 | [ |
| rGO | 一维g-C3N4 纳米管 | VAF自组装 | 光催化膜 | 染料分离 | 300W可见光照射条件下,过滤性能为4.77L·m-2·h-1·bar-1 | 对罗丹明B(5mg·L-1)的去除率大于98% | [ |
GO, g-C3N4 | 一维CNTs | VAF自组装 | 光催化膜 | 污染物降解 | 可见光照射条件下,过滤性能为14.35L·m-2·h-1·bar-1 | 罗丹明B和盐酸四环素去除率分别为98.31%、84.81% | [ |
| GO | 光Fenton催化剂:一维MOF (MIL-88A) | VAF自组装 | 光催化膜 | 染料分离 | 模拟太阳光104mW·cm-2,10mmol·L-1 H2O2 | 对50mL MB和BPA(10mg·L-1)去除率分别为98.8%和97.3% | [ |
| 1 | WANG Z W, MA J X, TANG C Y, et al. Membrane cleaning in membrane bioreactors: a review[J]. Journal of Membrane Science, 2014, 468: 276-307. |
| 2 | LEE K P, ARNOT T C, DE MATTIA D. A review of reverse osmosis membrane materials for desalination—Development to date and future potential[J]. Journal of Membrane Science, 2011, 370(1/2): 1-22. |
| 3 | ZHANG R, LIU Y, HE M, et al. Antifouling membranes for sustainable water purification: strategies and mechanisms[J]. Chemical Society Reviews, 2016, 45(21): 5888-5924. |
| 4 | SHE Q H, WANG R, FANE A G, et al. Membrane fouling in osmotically driven membrane processes: a review[J]. Journal of Membrane Science, 2016, 499: 201-233. |
| 5 | PARK H B, KAMCEV J, ROBESON L M, et al. Maximizing the right stuff: the trade-off between membrane permeability and selectivity[J]. Science, 2017, 356(6343): eaab0530. |
| 6 | WANG Z Y, MI B X. Environmental applications of 2D molybdenum disulfide (MoS2) nanosheets[J]. Environmental Science & Technology, 2017, 51(15): 8229-8244. |
| 7 | JUN B M, KIM S, HEO J, et al. Review of MXenes as new nanomaterials for energy storage/delivery and selected environmental applications[J]. Nano Research, 2019, 12(3): 471-487. |
| 8 | WANG S F, YANG L X, HE G W, et al. Two-dimensional nanochannel membranes for molecular and ionic separations[J]. Chemical Society Reviews, 2020, 49(4): 1071-1089. |
| 9 | ZHANG Y, GONG S, ZHANG Q, et al. Graphene-based artificial nacre nanocomposites[J]. Chemical Society Reviews, 2016, 45(9): 2378-2395. |
| 10 | GAO J, FENG Y, GUO W, et al. Nanofluidics in two-dimensional layered materials: inspirations from nature[J]. Chemical Society Reviews, 2017, 46(17): 5400-5424. |
| 11 | NUNES S P, CULFAZ-EMECEN P Z, RAMON G Z, et al. Thinking the future of membranes: perspectives for advanced and new membrane materials and manufacturing processes[J]. Journal of Membrane Science, 2020, 598: 117761. |
| 12 | MAHMOUD K A, MANSOOR B, MANSOUR A, et al. Functional graphene nanosheets: the next generation membranes for water desalination[J]. Desalination, 2015, 356: 208-225. |
| 13 | WERBER J R, OSUJI C O, ELIMELECH M. Materials for next-generation desalination and water purification membranes[J]. Nature Reviews Materials, 2016, 1: 16018. |
| 14 | DIMIEV A M, TOUR J M. Mechanism of graphene oxide formation[J]. ACS Nano, 2014, 8(3): 3060-3068. |
| 15 | NAIR R R, WU H A, JAYARAM P N, et al. Unimpeded permeation of water through helium-leak-tight graphene-based membranes[J]. Science, 2012, 335(6067): 442-444. |
| 16 | HEGAB H M, ZOU L D. Graphene oxide-assisted membranes: fabrication and potential applications in desalination and water purification[J]. Journal of Membrane Science, 2015, 484: 95-106. |
| 17 | JOSHI R K, ALWARAPPAN S, YOSHIMURA M, et al. Graphene oxide: the new membrane material[J]. Applied Materials Today, 2015, 1(1): 1-12. |
| 18 | LI Y, YANG S S, ZHANG K S, et al. Thin film nanocomposite reverse osmosis membrane modified by two dimensional laminar MoS2 with improved desalination performance and fouling-resistant characteristics[J]. Desalination, 2019, 454: 48-58. |
| 19 | LIU L F, ZHAO F, LIU J D, et al. Preparation of highly conductive cathodic membrane with graphene (oxide)/PPy and the membrane antifouling property in filtrating yeast suspensions in EMBR[J]. Journal of Membrane Science, 2013, 437: 99-107. |
| 20 | DONG L X, HUANG X C, WANG Z, et al. A thin-film nanocomposite nanofiltration membrane prepared on a support with in situ embedded zeolite nanoparticles[J]. Separation and Purification Technology, 2016, 166: 230-239. |
| 21 | SUBTIL E L, GONÇALVES J, LEMOS H G, et al. Preparation and characterization of a new composite conductive polyethersulfone membrane using polyaniline (PANI) and reduced graphene oxide (rGO)[J]. Chemical Engineering Journal, 2020, 390: 124612. |
| 22 | 樊江, 汪唯, 蔡佳浩, 等. 二维膜的精密构筑和结构调控策略综述[J]. 化工进展, 2020, 39(12): 4823-4836. |
| FAN Jiang, WANG Wei, CAI Jiahao, et al. A review of structural design and tuning methods of two-dimensional membranes[J]. Chemical Industry and Engineering Progress, 2020, 39(12): 4823-4836. | |
| 23 | JIANG B, ZENG Q Z, HOU Y, et al. Quorum quenching bacteria bioaugmented GO/PPy modified membrane in EMBR for membrane antifouling[J]. Science of the Total Environment, 2020, 718: 137412. |
| 24 | REN M, LIU H Y, QU J, et al. The different paths and potential risks of photo(-electro)-catalytic degradation for rhodamine B in water by graphene/TiO2 membrane[J]. Environmental Science and Pollution Research, 2018, 25(14): 13988-13999. |
| 25 | WANG Y Q, ZHANG H C, KANG Y, et al. Voltage-gated ion transport in two-dimensional sub-1-nanometer nanofluidic channels[J]. ACS Nano, 2019, 13(10): 11793-11799. |
| 26 | REN C, ALHABEB M, BYLES B W, et al. Voltage-gated ions sieving through 2D MXene Ti3C2Tx membranes[J]. ACS Applied Nano Materials, 2018, 1(7): 3644-3652. |
| 27 | LAO J C, LV R, GAO J, et al. Aqueous stable Ti3C2 MXene membrane with fast and photoswitchable nanofluidic transport[J]. ACS Nano, 2018, 12(12): 12464-12471. |
| 28 | WANG Z Y, SIM A, URBAN J J, et al. Removal and recovery of heavy metal ions by two-dimensional MoS2 nanosheets: performance and mechanisms[J]. Environmental Science & Technology, 2018, 52(17): 9741-9748. |
| 29 | KONG M, LI M J, SHANG R X, et al. Nacre-templated synthesis of highly dispersible carbon nanomeshes for layered membranes with high-flux filtration and sensing properties[J]. ACS Applied Materials & Interfaces, 2018, 10(3): 2850-2858. |
| 30 | THAKUR A K, SINGH S P, THAMARAISELVAN C, et al. Graphene oxide on laser-induced graphene filters for antifouling, electrically conductive ultrafiltration membranes[J]. Journal of Membrane Science, 2019, 591: 117322. |
| 31 | YU Z X, ZENG H J, MIN X, et al. High-performance composite photocatalytic membrane based on titanium dioxide nanowire/graphene oxide for water treatment[J]. Journal of Applied Polymer Science, 2020, 137(12): 48488. |
| 32 | KARKOOTI A, RASTGAR M, NAZEMIFARD N, et al. Graphene-based electro-conductive anti-fouling membranes for the treatment of oil sands produced water[J]. Science of the Total Environment, 2020, 704: 135365. |
| 33 | SHAKERI A, SALEHI H, RASTGAR M. Antifouling electrically conductive membrane for forward osmosis prepared by polyaniline/graphene nanocomposite[J]. Journal of Water Process Engineering, 2019, 32: 100932. |
| 34 | HU F, LI C N, YANG R Z, et al. Two-dimensional nanoconfined channels for a heavy metal sensor[J]. ACS Applied Electronic Materials, 2020, 2(1): 41-47. |
| 35 | DUAN W Y, RONEN A, WALKER S, et al. Polyaniline-coated carbon nanotube ultrafiltration membranes: enhanced anodic stability for in situ cleaning and electro-oxidation processes[J]. ACS Applied Materials & Interfaces, 2016, 8(34): 22574-22584. |
| 36 | LIU Y N, SU Y L, GUAN J Y, et al. 2D heterostructure membranes with sunlight-driven self-cleaning ability for highly efficient oil-water separation[J]. Advanced Functional Materials, 2018, 28(13): 1706545. |
| 37 | BAO Z, CHEN D Y, LI N J, et al. Superamphiphilic and underwater superoleophobic membrane for oil/water emulsion separation and organic dye degradation[J]. Journal of Membrane Science, 2020, 598: 117804. |
| 38 | LIU G G, HAN K, ZHOU Y H, et al. Facile synthesis of highly dispersed Ag doped graphene oxide/titanate nanotubes as a visible light photocatalytic membrane for water treatment[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(5): 6256-6263. |
| 39 | SHI Y H, WAN D J, HUANG J H, et al. Stable LBL self-assembly coating porous membrane with 3D heterostructure for enhanced water treatment under visible light irradiation[J]. Chemosphere, 2020, 252: 126581. |
| 40 | CAI Y H, CHEN D Y, LI N J, et al. A self-cleaning heterostructured membrane for efficient oil-in-water emulsion separation with stable flux[J]. Advanced Materials, 2020, 32(25): 2001265. |
| 41 | WEI Y B, ZHU Y X, JIANG Y J. Photocatalytic self-cleaning carbon nitride nanotube intercalated reduced graphene oxide membranes for enhanced water purification[J]. Chemical Engineering Journal, 2019, 356: 915-925. |
| 42 | XIE A, CUI J Y, YANG J, et al. Graphene oxide/Fe(Ⅲ)-based metal-organic framework membrane for enhanced water purification based on synergistic separation and photo-Fenton processes[J]. Applied Catalysis B: Environmental, 2020, 264: 118548. |
| 43 | LIU Y C, YU Z X, PENG Y X, et al. A novel photocatalytic self-cleaning TiO2 nanorods inserted graphene oxide-based nanofiltration membrane[J]. Chemical Physics Letters, 2020, 749: 137424. |
| 44 | WEN Q, JIA P, CAO L, et al. Electric-field-induced ionic sieving at planar graphene oxide heterojunctions for miniaturized water desalination[J]. Advanced Materials, 2020, 32(16): e1903954. |
| 45 | CHEN Y, ZHANG G, LIU H J, et al. Confining free radicals in close vicinity to contaminants enables ultrafast Fenton-like processes in the interspacing of MoS2 membranes[J]. Angewandte Chemie, 2019, 58(24): 8134-8138. |
| 46 | LAN H C, WANG F, LAN M, et al. Hydrogen-bond-mediated self-assembly of carbon-nitride-based photo-Fenton-like membranes for wastewater treatment[J]. Environmental Science & Technology, 2019, 53(12): 6981-6988. |
| 47 | YU J, ZHANG Y, CHEN J H, et al. Solvothermal-induced assembly of 2D-2D rGO-TiO2 nanocomposite for the construction of nanochannel membrane[J]. Journal of Membrane Science, 2020, 600: 117870. |
| 48 | WERNER C M, KATURI K P, HARI A R, et al. Graphene-coated hollow fiber membrane as the cathode in anaerobic electrochemical membrane bioreactors - effect of configuration and applied voltage on performance and membrane fouling[J]. Environmental Science & Technology, 2016, 50(8): 4439-4447. |
| 49 | ZHANG J, JI Q H, LAN H C, et al. Synchronous reduction-oxidation process for efficient removal of trichloroacetic acid: H* initiates dechlorination and ·OH is responsible for removal efficiency[J]. Environmental Science & Technology, 2019, 53(24): 14586-14594. |
| 50 | MAO R, LI N, LAN H C, et al. Dechlorination of trichloroacetic acid using a noble metal-free graphene-Cu foam electrode via direct cathodic reduction and atomic H[J]. Environmental Science & Technology, 2016, 50(7): 3829-3837. |
| 51 | ZHENG J J, WANG Z W, MA J X, et al. Development of an electrochemical ceramic membrane filtration system for efficient contaminant removal from waters[J]. Environmental Science & Technology, 2018, 52(7): 4117-4126. |
| 52 | RADJENOVIC J, SEDLAK D L. Challenges and opportunities for electrochemical processes as next-generation technologies for the treatment of contaminated water[J]. Environmental Science & Technology, 2015, 49(19): 11292-11302. |
| 53 | FANG Y Z, HU R, LIU B Y, et al. MXene-derived TiO2/reduced graphene oxide composite with an enhanced capacitive capacity for Li-ion and K-ion batteries[J]. Journal of Materials Chemistry A, 2019, 7(10): 5363-5372. |
| 54 | SHI Y H, HUANG J H, ZENG G M, et al. Photocatalytic membrane in water purification: is it stepping closer to be driven by visible light?[J]. Journal of Membrane Science, 2019, 584: 364-392. |
| 55 | 阿伦·J·巴德, 拉里·R·福克纳. 电化学方法原理和应用[M]. 邵元华等, 译. 2版. 北京: 化学工业出版社, 2005. |
| BARD A J, FAULKNER L R. Electrochemical methods fundamentals and applications[M]. SHAO Y H et al,trans. 2nd ed. Beijing: Chemical Industry Press, 2005. | |
| 56 | ALAM I, GUINEY L M, HERSAM M C, et al. Application of external voltage for fouling mitigation from graphene oxide, reduced graphene oxide and molybdenum disulfide functionalized surfaces[J]. Environmental Science: Nano, 2019, 6(3): 925-936. |
| 57 | YANG Y, QIAO S, JIN R F, et al. A novel aerobic electrochemical membrane bioreactor with CNTs hollow fiber membrane by electrochemical oxidation to improve water quality and mitigate membrane fouling[J]. Water Research, 2019, 151: 54-63. |
| 58 | HU C Z, LIU Z T, LU X L, et al. Enhancement of the Donnan effect through capacitive ion increase using an electroconductive rGO-CNT nanofiltration membrane[J]. Journal of Materials Chemistry A, 2018, 6(11): 4737-4745. |
| 59 | SUN J, HU C, WU B, et al. Improving ion rejection of graphene oxide conductive membranes by applying electric field[J]. Journal of Membrane Science, 2020, 604: 118077. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [6] | 张祚群, 高扬, 白超杰, 薛立新. 二次界面聚合同步反扩散原位生长ZIF-8纳米粒子制备聚酰胺混合基质反渗透(RO)膜[J]. 化工进展, 2023, 42(S1): 364-373. |
| [7] | 王正坤, 黎四芳. 双子表面活性剂癸炔二醇的绿色合成[J]. 化工进展, 2023, 42(S1): 400-410. |
| [8] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [9] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [10] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [11] | 赵景超, 谭明. 表面活性剂对电渗析减量化工业含盐废水的影响[J]. 化工进展, 2023, 42(S1): 529-535. |
| [12] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [13] | 许友好, 王维, 鲁波娜, 徐惠, 何鸣元. 中国炼油创新技术MIP的开发策略及启示[J]. 化工进展, 2023, 42(9): 4465-4470. |
| [14] | 耿源泽, 周俊虎, 张天佑, 朱晓宇, 杨卫娟. 部分填充床燃烧器中庚烷均相/异相耦合燃烧[J]. 化工进展, 2023, 42(9): 4514-4521. |
| [15] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||