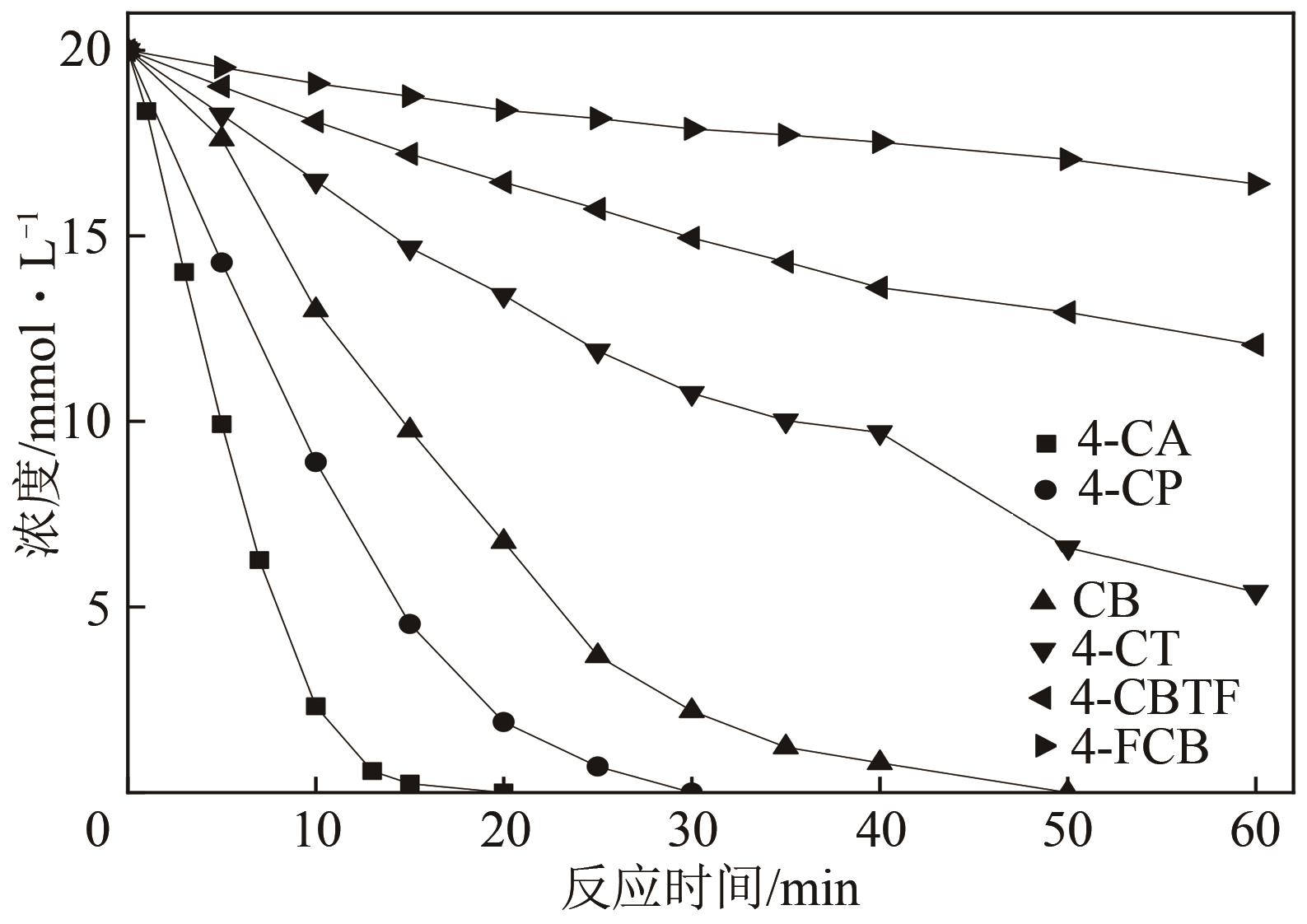
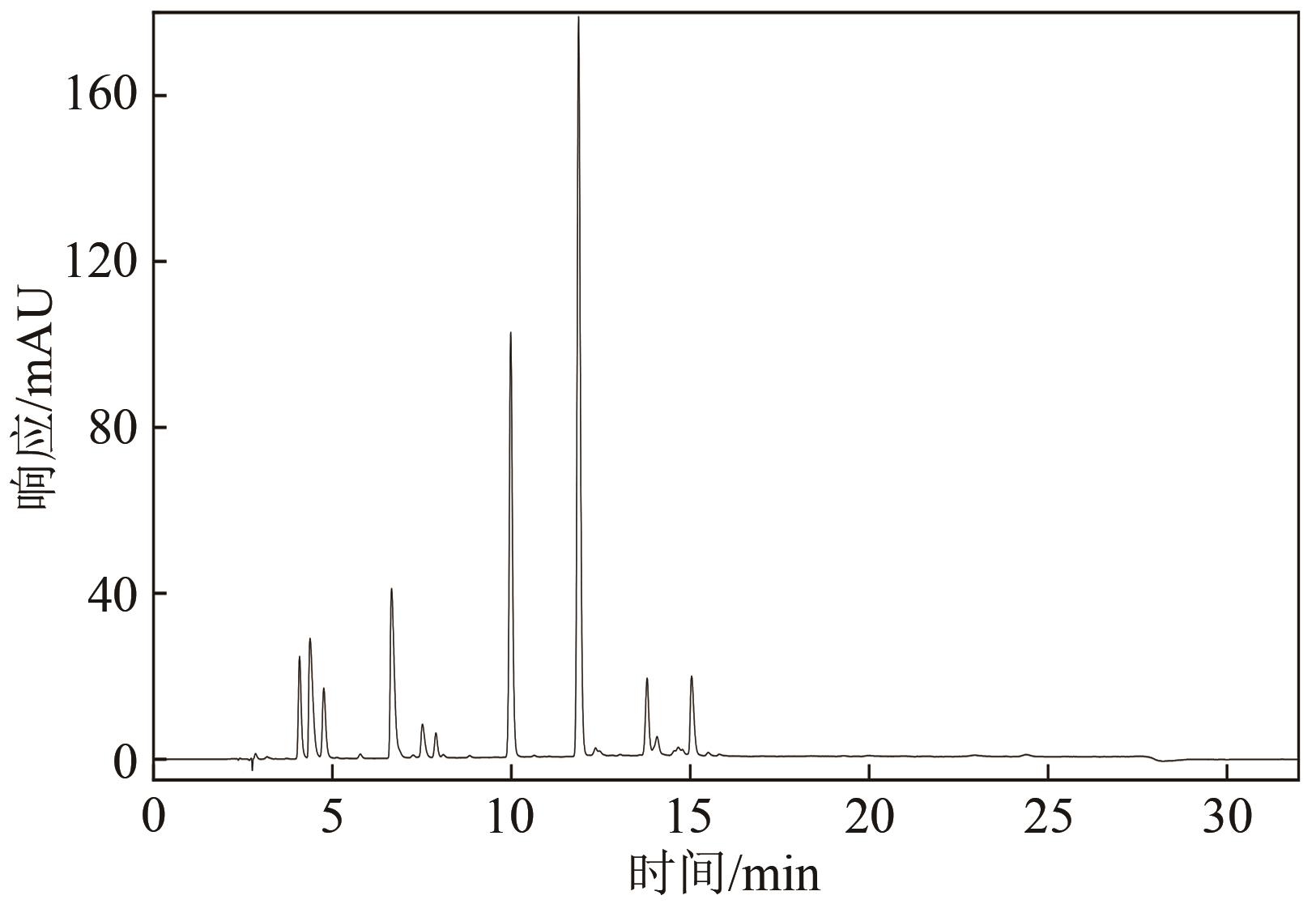
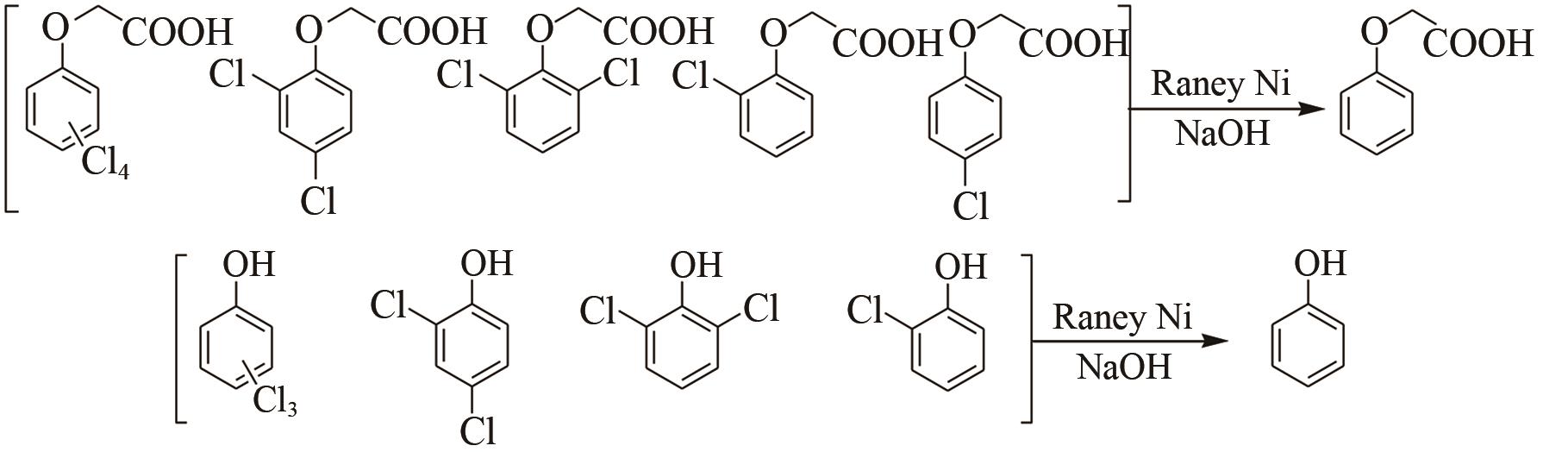
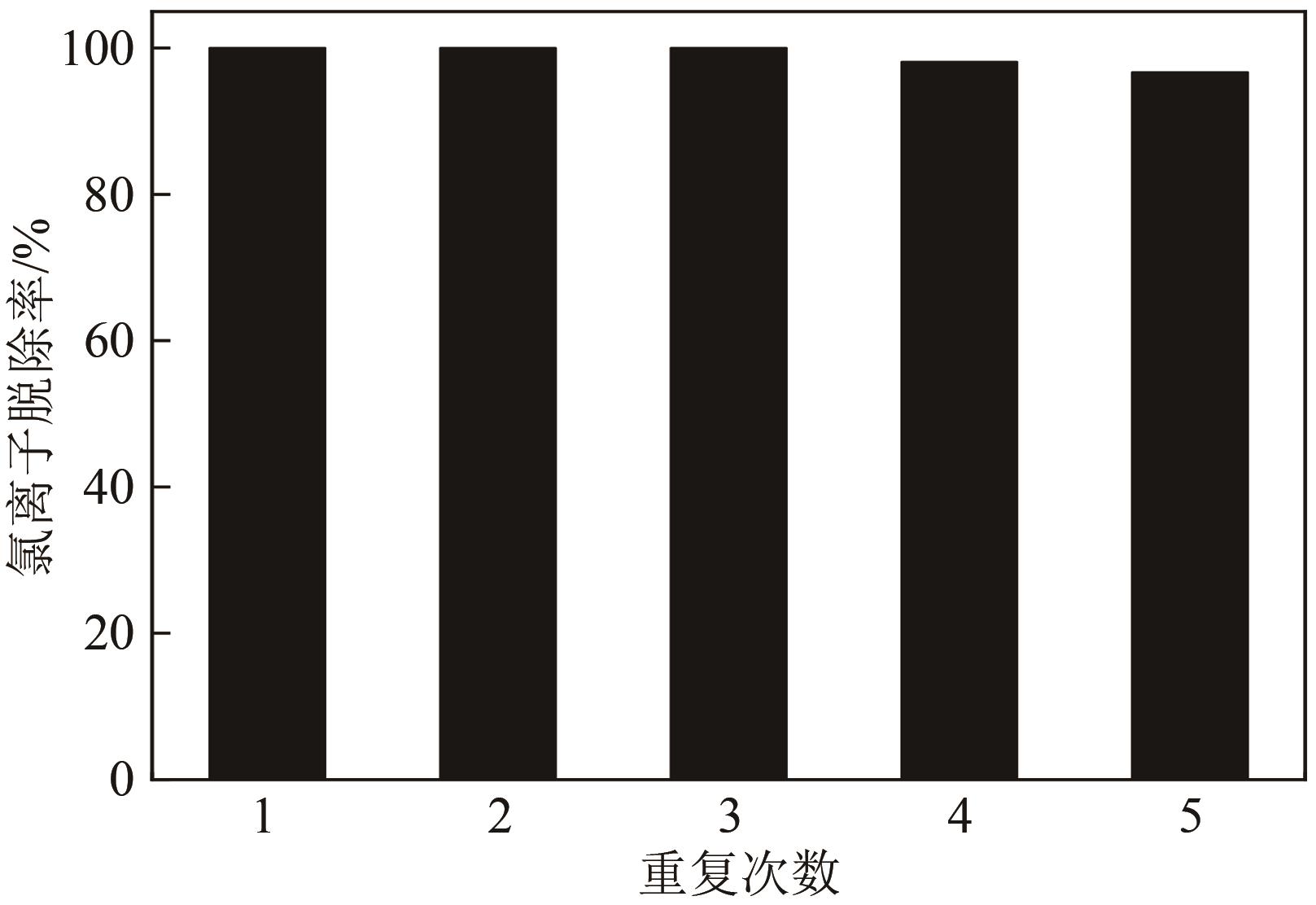
| 12 |
陈阳雯, 刘蕊, 姚洁, 等. La掺杂钛基氧化物电极降解水中2,4-二氯酚的研究[J]. 环境科学与技术, 2018, 41(1): 94-98.
|
|
CHEN Yangwen, LIU Rui, YAO Jie, et al. Degradation of 2,4-dichlorophenol by Ti-based oxide electrodes doped with La[J]. Environmental Science and Technology, 2018, 41(1): 94-98.
|
| 13 |
YANG Z, ZHANG X, PU S, et al. Novel Fenton-like system (Mg/Fe-O2) for degradation of 4-chlorophenol[J]. Environmental Pollution, 2019, 250: 906-913.
|
| 14 |
HADI S, TAHERI E, AMIN M M, et al. Synergistic degradation of 4-chlorophenol by persulfate and oxalic acid mixture with heterogeneous Fenton like system for wastewater treatment: adaptive neuro-fuzzy inference systems modeling[J]. Journal of Environmental Management, 2020, 268: 110678.
|
| 15 |
张云飞, 杨波, 张鸿, 等. 负载型催化剂用于卤代有机污染物氢解去除[J]. 化学进展, 2013, 25(12): 2159-2168.
|
|
ZHANG Yunfei, YANG Bo, ZHANG Hong, et al. Degradation of halogenated organic contaminants with hydrodehalogenation using supported catalysts[J]. Progress in Chemistry, 2013, 25(12): 2159-2168.
|
| 16 |
XIONG J, MA Y, YANG W, et al. Rapid, highly efficient and stable catalytic hydrodechlorination of chlorophenols over novel Pd/CNTs-Ni foam composite catalyst in continuous-flow[J]. Journal of Hazardous Materials, 2018, 355: 89-95.
|
| 17 |
XU F, DENG S, XU J, et al. Highly active and stable Ni-Fe bimetal prepared by ball milling for catalytic hydrodechlorination of 4-chlorophenol[J]. Environmental Science and Technology, 2012, 46: 4576-4582.
|
| 18 |
MA X, LIU Y, LI X, et al. Water: the most effective solvent for liquid-phase hydrodechlorination of chlorophenols over Raney Ni catalyst[J]. Applied Catalysis B: Environmental, 2015, 165: 351-359.
|
| 19 |
RAUT S S, SHETTY R, RAJU N M, et al. Screening of zero valent mono/bimetallic catalysts and recommendation of Raney Ni (without reducing agent) for dechlorination of 4-chlorophenol[J]. Chemosphere, 2020, 250: 126298.
|
| 20 |
WANG W, NIU J, YANG Z. An efficient reduction of unsaturated bonds and halogen-containing groups by nascent hydrogen over Raney Ni catalyst[J]. Journal of Hazardous Materials, 2020, 389: 121912.
|
| 21 |
URBANO F J, MARINAS J M. Hydrogenolysis of organohalogen compounds over palladium supported catalysts[J]. Journal of Molecular Catalysis A: Chemical, 2001, 173(1/2): 329-345.
|
| 22 |
CONCIBIDO N C, OKUDA T, NISHIJIMA W, et al. Deactivation and reactivation of Pd/C catalyst used in repeated batch hydrodechlorination of PCE[J]. Applied Catalysis B: Environmental, 2007, 71(1/2): 64-69.
|
| 23 |
XIA C, LIU Y, ZHOU S, et al. The Pd-catalyzed hydrodechlorination of chlorophenols in aqueous solutions under mild conditions: a promising approach to practical use in wastewater[J]. Journal of Hazardous Materials, 2009, 169(1/2/3): 1029-1033.
|
| 24 |
MA X, LIU Y, LIU S, et al. Water-promoted catalytic hydrodechlorination of transformer oil-contained PCBs in liquid system under mild conditions[J]. Applied Catalysis B: Environmental, 2014, 144: 580-587.
|
| 1 |
CHENG R, ZHOU W, WANG J, et al. Dechlorination of pentachlorophenol using nanoscale Fe/Ni particles: role of nano-Ni and its size effect[J]. Journal of Hazardous Materials, 2010, 180(1/2/3): 79-85.
|
| 2 |
GARBA Z N, ZHOU W, LAWAN I, et al. An overview of chlorophenols as contaminants and their removal from wastewater by adsorption: a review[J]. Journal of Environmental Management, 2019, 241: 59-75.
|
| 3 |
兰丽娟, 刘莺, 杜芳林. Pd/CNTs对4-氯苯酚的液相催化加氢去氯[J].化工进展, 2017, 36(6): 2171-2176.
|
| 25 |
马宣宣. Pd/C和Raney Ni催化有机卤代物的液相高效加氢脱卤研究[D]. 烟台: 中国科学院烟台海岸带研究所, 2015.
|
|
Ma Xuanxuan. Highly efficient liquid-phase hydrodehalogenation of halogenated aromatic compounds over Pd/C and Raney Ni catalysts[D]. Yantai: Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences, 2015.
|
| 26 |
MA X, ZHOU S, YANG C, et al. The influence of triethylamine on the hydrodechlorination reactivity of chlorophenols over Raney Ni catalyst[J]. Catalysis Communications, 2010, 12(4): 282-285.
|
| 27 |
YONEDA T, TAKIDO T, KONUMA K. Hydrodechlorination reactivity of para-substituted chlorobenzenes over platinum/carbon catalyst[J]. Journal of Molecular Catalysis A: Chemical, 2007, 265(1-2): 80-89.
|
| 28 |
AREMENDÍA M A, BORÁU V, GARCÍA I M, et al. Liquid-phase hydrodehalogenation of substituted chlorobenzenes over palladium supported catalysts[J]. Applied Catalysis B: Environmental, 2003, 43(1): 71-79.
|
| 29 |
KONUMA K, KAMEDA N. Effect of substituents on the hydrodechlorination reactivity of para-substituted chlorobenzenes[J]. Journal of Molecular Catalysis A: Chemical, 2002, 178(1-2): 239-251.
|
| 30 |
WU W H, XU J, OHNISHI R. Complete hydrodechlorination of chlorobenzene and its derivatives over supported nickel catalysts under liquid phase conditions[J]. Applied Catalysis B: Environmental, 2005, 60(1/2): 129-137.
|
| 31 |
GRYGLEWICZ S, PIECHOCKI W. Hydrodechlorination of dichlorobenzenes and their derivatives over Ni-Mo/C catalyst: kinetic analysis and effect of molecular structure of reactant[J]. Chemosphere, 2011, 83(3): 334-339.
|
| 3 |
LAN Lijuan, LIU Ying, DU Fanglin. Liquid-phase catalytic hydrodechlorination of 4-chlorophenol over Pd/CNTs[J]. Chemical Industry and Engineering Progress, 2017, 36(6): 2171-2176.
|
| 4 |
WEI D, ZHAO C, KHAN A, et al. Sorption mechanism and dynamic behavior of graphene oxide as an effective adsorbent for the removal of chlorophenol based environmental-hormones: a DFT and MD simulation study[J]. Chemical Engineering Journal, 2019, 375: 121964.
|
| 5 |
YOUNIS S A, MOTAWEA E A, MOUSTAFA Y M, et al. A strategy for the efficient removal of chlorophenols in petrochemical wastewater by organophilic and aminated silica@alginate microbeads: taguchi optimization and isotherm modeling based on partition coefficient[J]. Journal of Hazardous Materials, 2020, 397: 122792.
|
| 6 |
ADEYEMI I, SULAIMAN R, ALMAZROUI M, et al. Removal of chlorophenols from aqueous media with hydrophobic deep eutectic solvents: experimental study and COSMO RS evaluation[J]. Journal of Molecular Liquids, 2020, 311: 113180.
|
| 7 |
李燕妮, 陈泉源, 周娟, 等. 石墨烯杂氮载Pd催化剂对2,4-二氯酚的液相催化加氢脱氯[J]. 中国环境科学, 2017, 37(2): 577-583.
|
|
LI Yanni, CHEN Quanyuan, ZHOU Juan, et al. The liquid phase catalytic hydrogenation of 2,4-dichlorophenol over Pd catalyst supported on nitrogen-doped graphene[J]. China Environmental Science, 2017, 37(2): 577-583.
|
| 8 |
刘楚琛, 阎秀兰, 刘琼枝, 等. Fenton试剂和活化过硫酸钠氧化降解土壤中的二氯酚和三氯酚[J]. 环境工程学报, 2018, 12(6): 1749-1758.
|
|
LIU Chuchen, YAN Xiulan, LIU Qiongzhi, et al. Oxidative degradation of dichlorophenol and trichlorophenol in soils by Fenton reagent and activated persulfate[J]. Chinese Journal of Environmental Engineering, 2018, 12(6): 1749-1758.
|
| 9 |
阮霞, 刘红, 马驰, 等. 铜质量比对Fe/Cu还原2,4-二氯酚影响及还原途径[J]. 环境科学与技术, 2017, 40(5): 43-48.
|
|
RUAN Xia, LIU Hong, MA Chi, et al. Effects of mass ratio of copper on reduction of 2,4-dichlorophenol by Fe/Cu and reduction pathway[J]. Environmental Science and Technology, 2017, 40(5): 43-48.
|
| 10 |
MA X, LIU S, LIU Y, et al. Promoted liquid-phase hydrodechlorination of chlorophenol over Raney Ni via controlling base: performance, mechanism, and application[J]. Chemosphere, 2020, 242: 125202.
|
| 11 |
BAO T, DAMTIE M M, HOSSEINZADEH A, et al. Catalytic degradation of P-chlorophenol by muscovite-supported nano zero valent iron composite: synthesis, characterization, and mechanism studies[J]. Applied Clay Science, 2020, 195: 105735.
|
 ), 李晓涵2, 马宣宣2, 谢清明3, 刘莺2, 刘苏静2, 夏传海1,2(
), 李晓涵2, 马宣宣2, 谢清明3, 刘莺2, 刘苏静2, 夏传海1,2( )
)
 ), LI Xiaohan2, MA Xuanxuan2, XIE Qingming3, LIU Ying2, LIU Sujing2, XIA Chuanhai1,2(
), LI Xiaohan2, MA Xuanxuan2, XIE Qingming3, LIU Ying2, LIU Sujing2, XIA Chuanhai1,2( )
)