化工进展 ›› 2021, Vol. 40 ›› Issue (2): 859-869.DOI: 10.16085/j.issn.1000-6613.2020-0685
常温下碳基活性材料催化氧化NO的研究进展
- 东华大学环境科学与工程学院,上海 201620
-
收稿日期:2020-04-27修回日期:2020-06-09出版日期:2021-02-05发布日期:2021-02-09 -
通讯作者:邓文义 -
作者简介:周易(1996—),男,硕士研究生。E-mail:zhouyi201508@163.com 。 -
基金资助:国家留学基金(201906635022);上海市自然科学基金(19ZR1400700);中央高校基本科研业务费专项资金(2232019D3-24)
Research progress in catalytic oxidation of NO by carbon-based active materials at room temperature
Yi ZHOU( ), Wenyi DENG(
), Wenyi DENG( ), Yaxin SU
), Yaxin SU
- School of Environmental Science and Engineering, Donghua University, Shanghai 201620
-
Received:2020-04-27Revised:2020-06-09Online:2021-02-05Published:2021-02-09 -
Contact:Wenyi DENG
摘要:
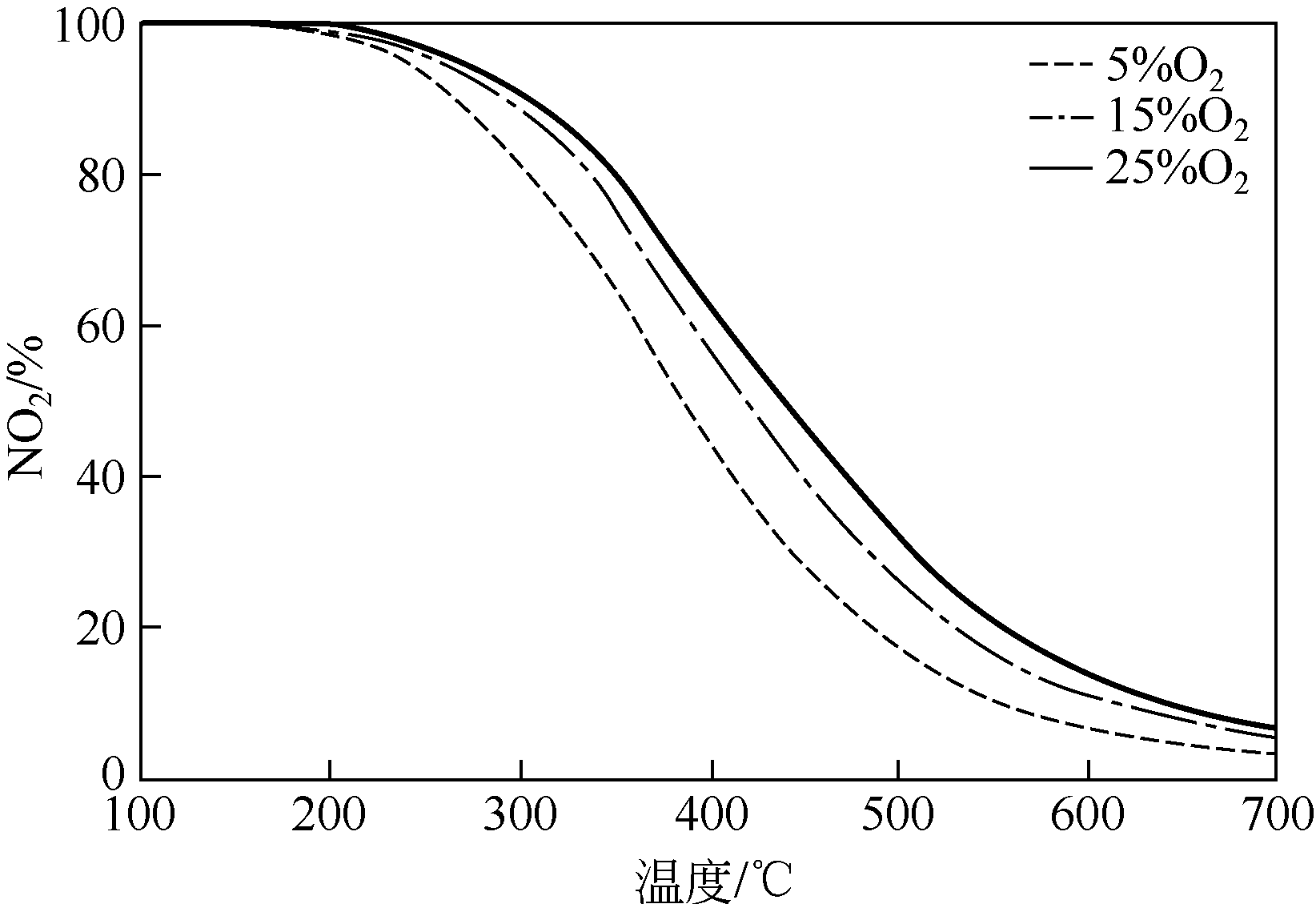
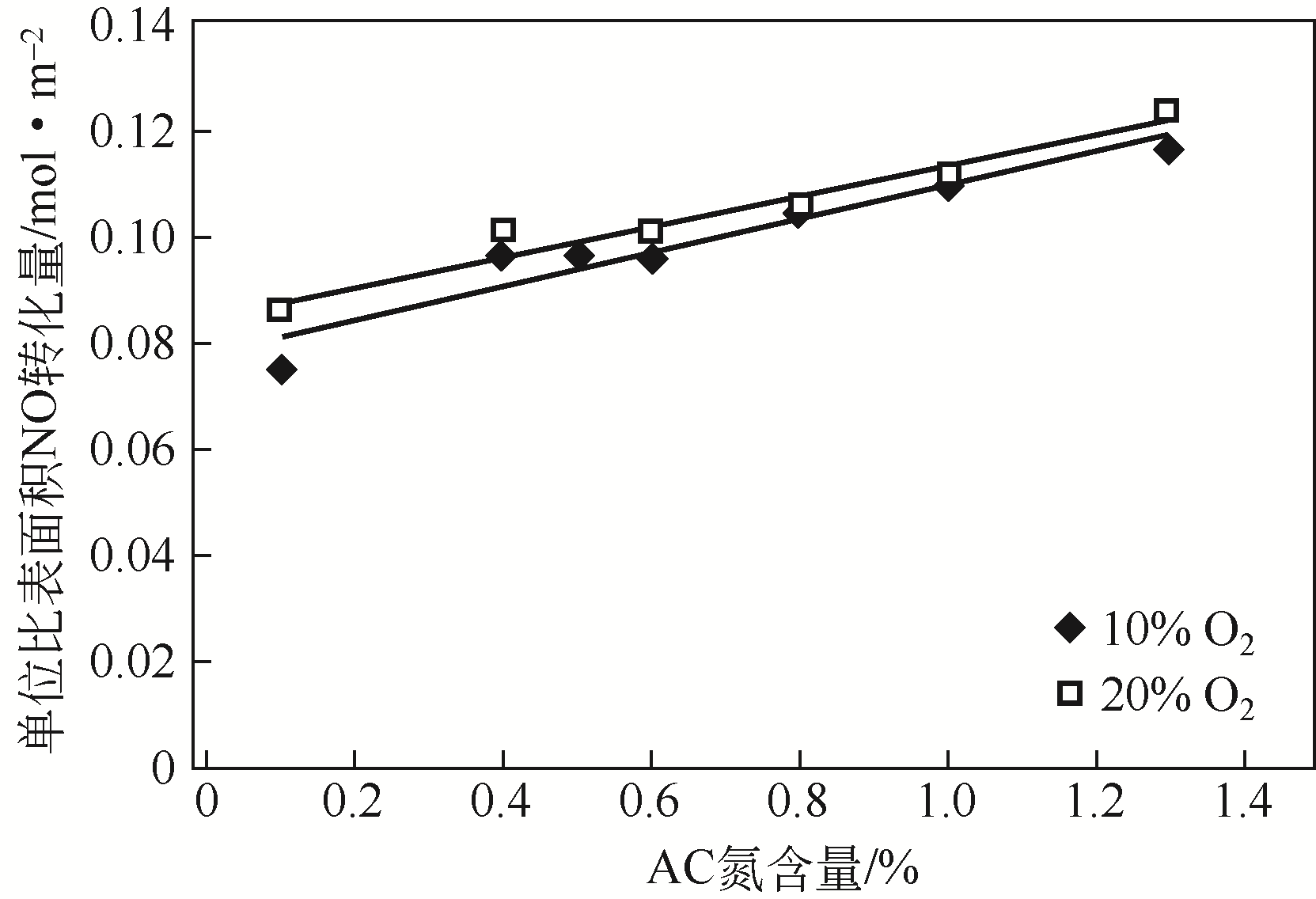
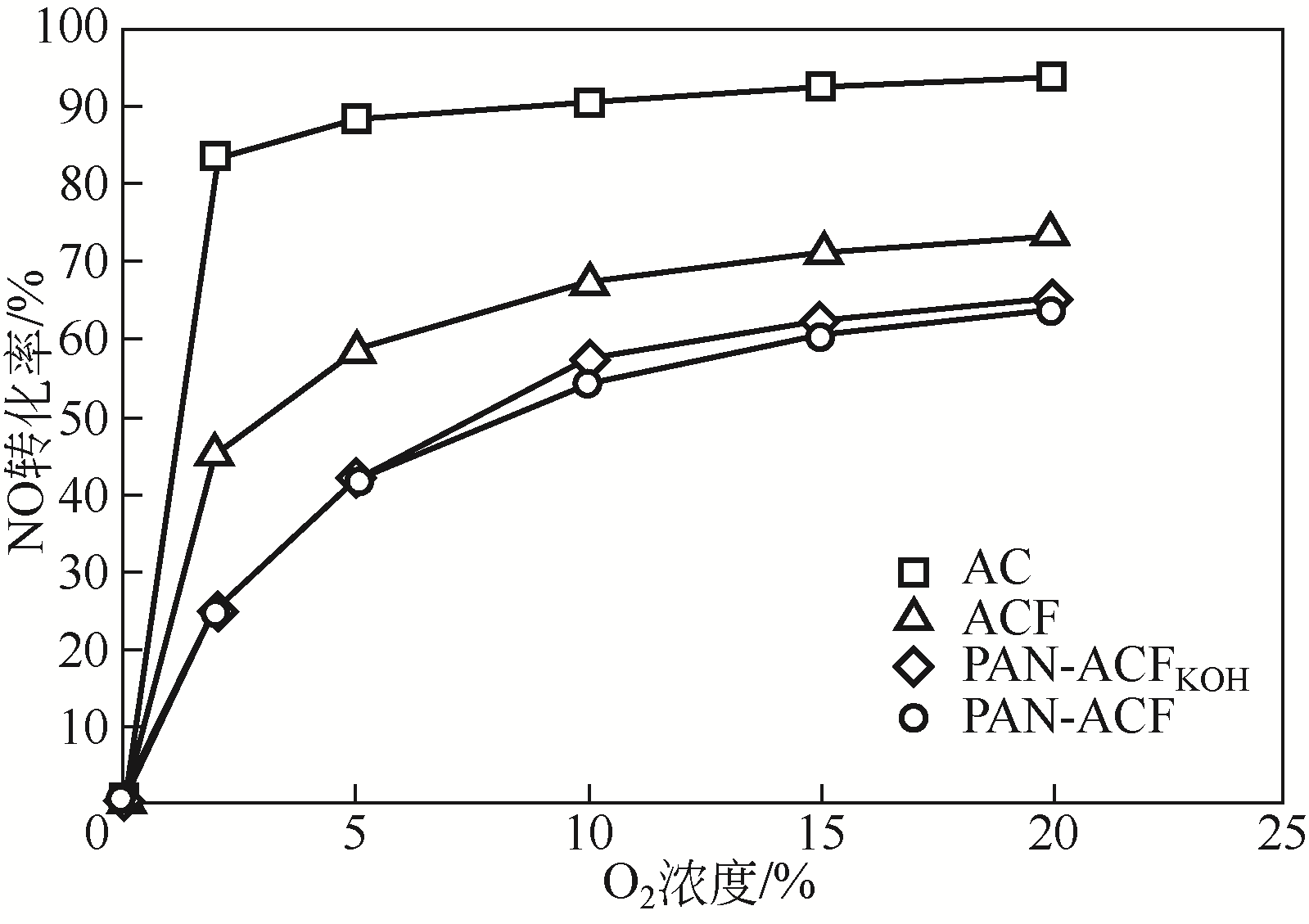
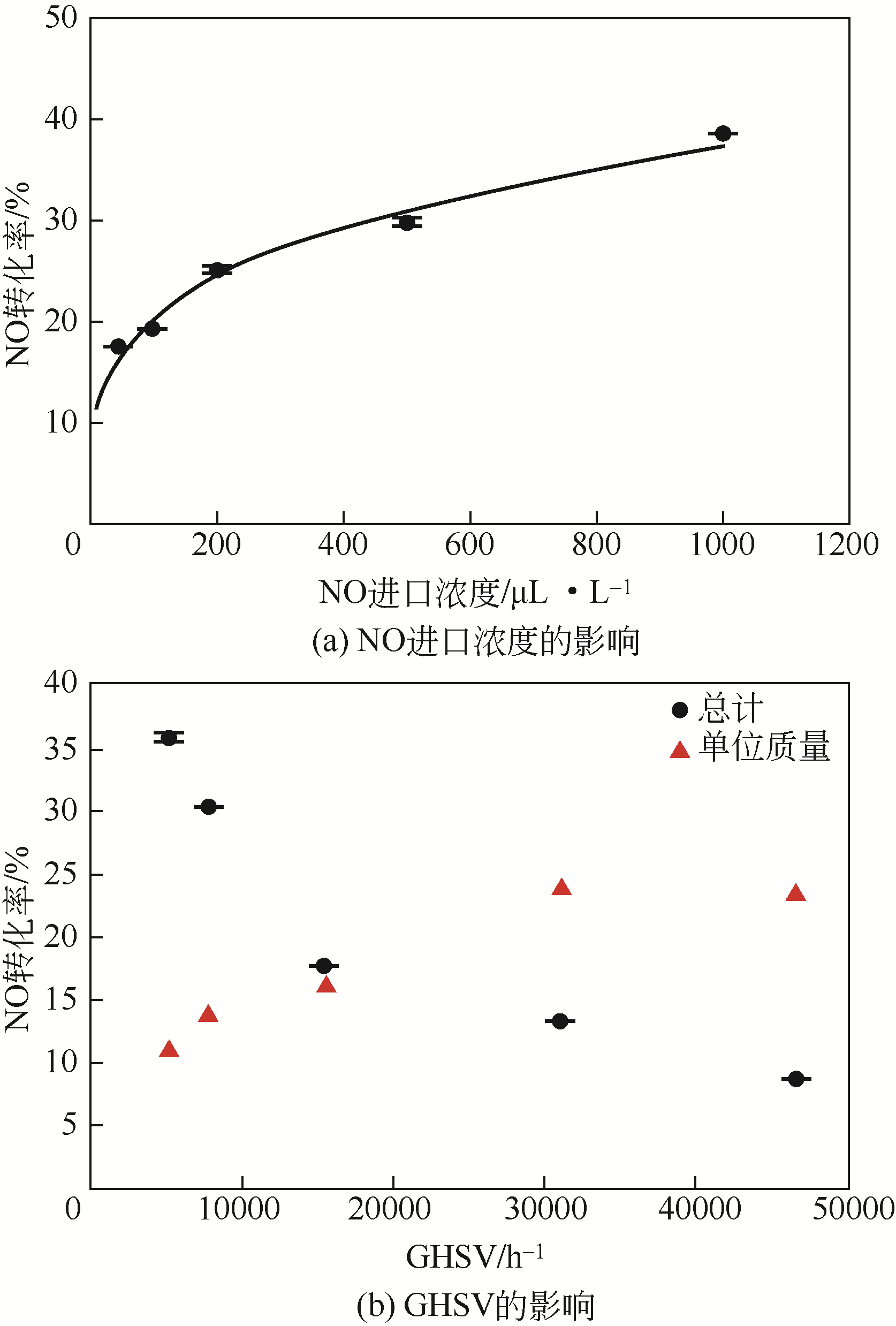
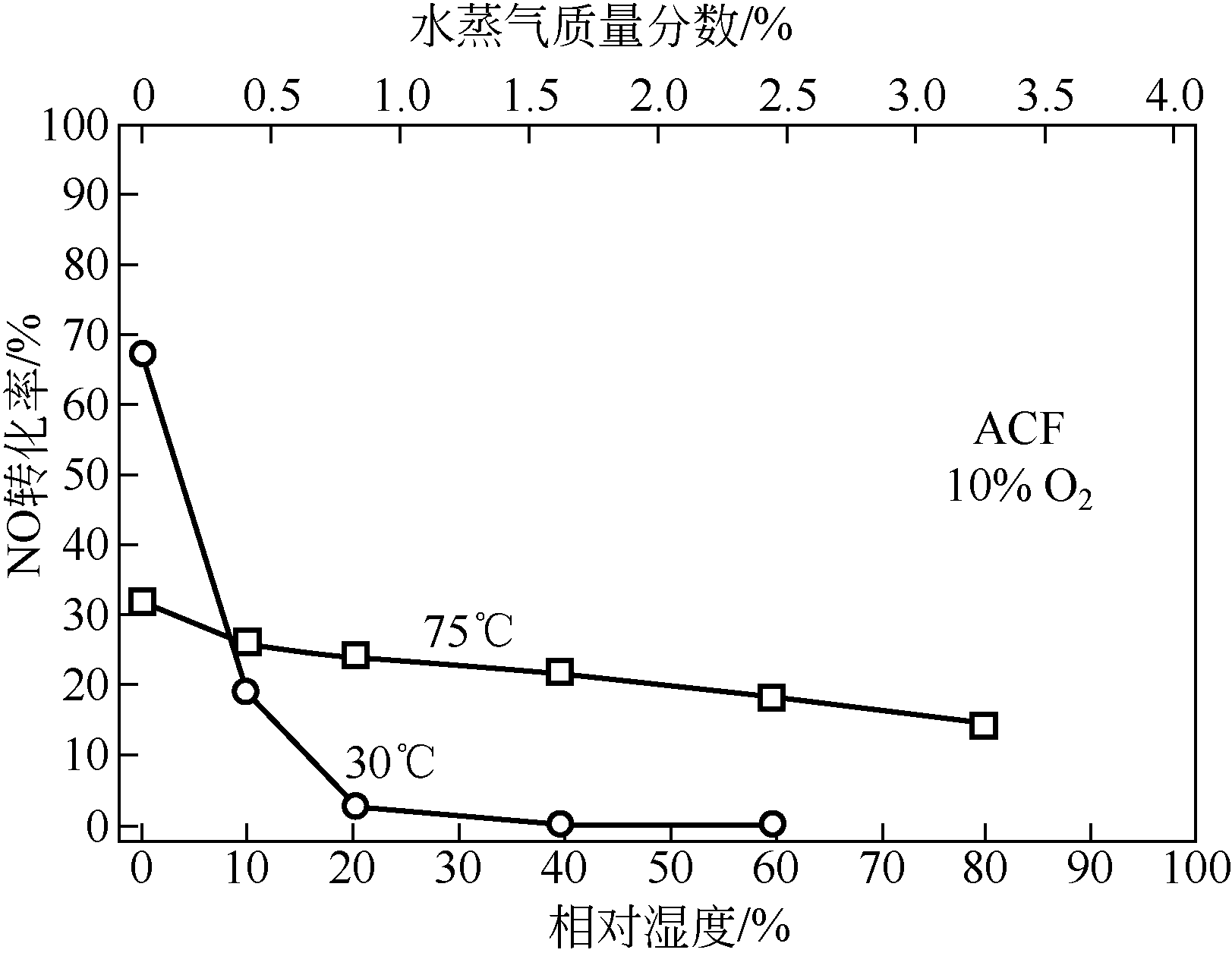
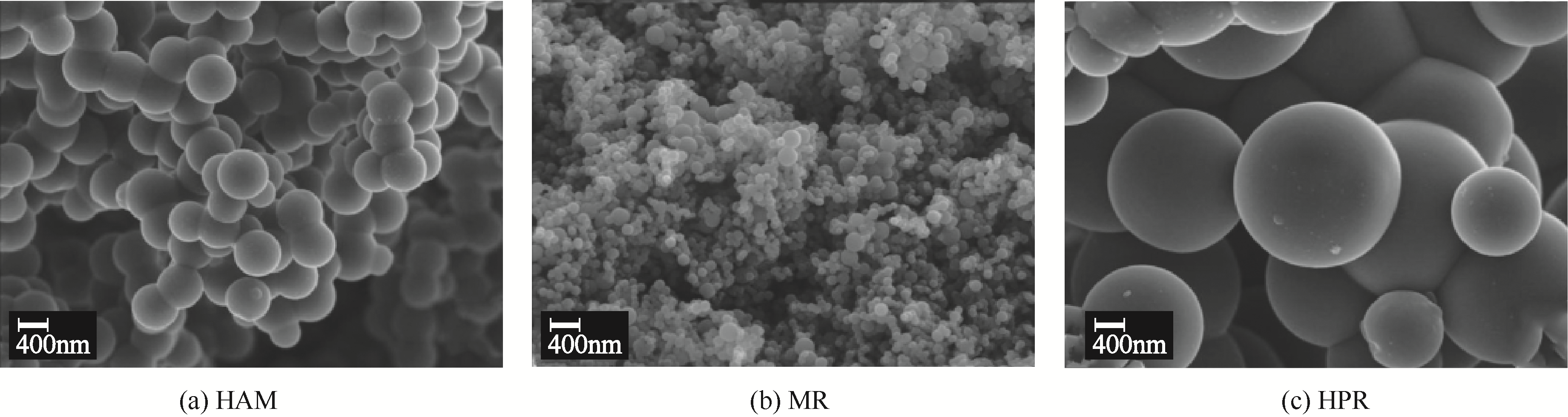
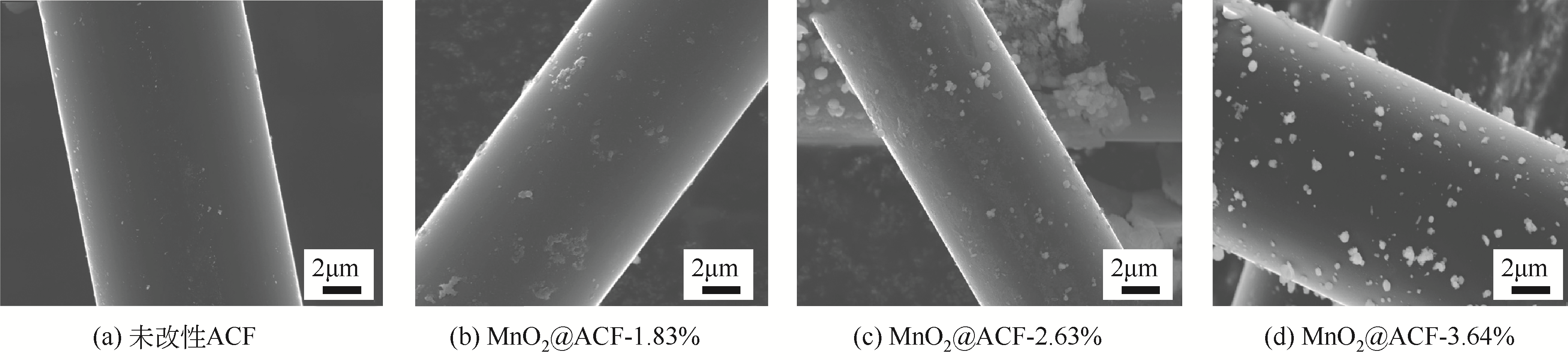
针对常温、含高浓度O2 的NO污染气体排放控制,典型的选择性催化还原(SCR)技术已不再适用。以碳基活性材料为催化剂的NO常温催化氧化技术得到了广泛关注,该技术在常温和高浓度O2条件下将NO氧化为NO2,并以硝酸或硝酸盐形式加以回收利用,因此具有环保和经济双重效益,应用前景广阔。本文简要综述了碳基活性材料常温催化氧化NO的研究进展,阐述了NO催化氧化机理,介绍了碳基活性材料的表面物化特性和反应条件(O2浓度、NO浓度、GHSV、反应温度、水蒸气和催化剂粒径等)对催化氧化NO的影响,以及活性炭、活性炭纤维、碳纳米纤维、炭干凝胶、金属负载碳基活性材料、炭化污泥等不同碳基活性材料的催化特性,总结并展望了未来碳基活性材料低温催化氧化NO的发展方向。
中图分类号:
引用本文
周易, 邓文义, 苏亚欣. 常温下碳基活性材料催化氧化NO的研究进展[J]. 化工进展, 2021, 40(2): 859-869.
Yi ZHOU, Wenyi DENG, Yaxin SU. Research progress in catalytic oxidation of NO by carbon-based active materials at room temperature[J]. Chemical Industry and Engineering Progress, 2021, 40(2): 859-869.
| L-H机理模型 | E-R机理模型 |
|---|---|
NO+Cf O2+2Cf C-NO+C-O C-NO2+C-NO2 C-NO3+C-NO C-NO-NO3 | NO+Cf 2C-NO+O2 C-NO2+C-NO2 C-NO3+C-NO C-NO-NO3 |
表1 AC材料表面催化氧化NO机理解释[41]
| L-H机理模型 | E-R机理模型 |
|---|---|
NO+Cf O2+2Cf C-NO+C-O C-NO2+C-NO2 C-NO3+C-NO C-NO-NO3 | NO+Cf 2C-NO+O2 C-NO2+C-NO2 C-NO3+C-NO C-NO-NO3 |
| AC种类 | V(0.5~0.8nm) /cm3 g-1 | V(0.9~1.5nm) /cm3 g-1 | 平均孔径 /nm | NO 转化率/% |
|---|---|---|---|---|
| SKC-AR | 0.12 | 0.04 | 0.57 | 22 |
| SKC-45 | 0.14 | 0.07 | 0.66 | 46 |
| SKC-90 | 0.23 | 0.14 | 0.72 | 52 |
| SKC-180 | 0.18 | 0.27 | 0.93 | 44 |
| SKC-360 | 0.17 | 0.37 | 1.22 | 36 |
| PICA | 0.12 | 0.17 | 1.11 | 31 |
| MSC-30 | 0.14 | 0.24 | 1.23 | 31 |
表2 AC的结构特性和NO转化率[43]
| AC种类 | V(0.5~0.8nm) /cm3 g-1 | V(0.9~1.5nm) /cm3 g-1 | 平均孔径 /nm | NO 转化率/% |
|---|---|---|---|---|
| SKC-AR | 0.12 | 0.04 | 0.57 | 22 |
| SKC-45 | 0.14 | 0.07 | 0.66 | 46 |
| SKC-90 | 0.23 | 0.14 | 0.72 | 52 |
| SKC-180 | 0.18 | 0.27 | 0.93 | 44 |
| SKC-360 | 0.17 | 0.37 | 1.22 | 36 |
| PICA | 0.12 | 0.17 | 1.11 | 31 |
| MSC-30 | 0.14 | 0.24 | 1.23 | 31 |
| 1 | ZAWADZKI J, WINIEWSKI M, SKOWROSK K. Heterogeneous reactions of NO2 and NO-O2 on the surface of carbons[J]. Carbon, 2003, 41(2): 235-246. |
| 2 | WAN X K, SHI H X, ZOU X Q, et al. Effect of nitrogen doping on the reduction of nitric oxide with activated carbon in the presence of oxygen[J]. Journal of Zhejiang University (Science A), 2008, 9(1): 113-117. |
| 3 | MATZNER S, BOEHM H P. Influence of nitrogen doping on the adsorption and reduction of nitric oxide by activated carbons[J]. Carbon, 1998, 36(11): 1697-1700. |
| 4 | SKALSKA K, MILLER J S, LEDAKOWICZ S. Trends in NOx abatement: a review[J]. Science of the Total Environment, 2010, 408(19): 3976-3989. |
| 5 | BARMAN S, PHILIP L. Integrated system for the treatment of oxides of nitrogen from flue gases[J]. Environmental Science & Technology, 2005, 40(3): 1035-1041. |
| 6 | WOJCIECHOWSKA M, LOMNICKI S. Nitrogen oxides removal by catalytic methods[J]. Clean Technologies and Environmental Policy, 1999, 1(4): 237-247. |
| 7 | TOPSOE N Y, TOPSOE H, DUMESIC J A. Vanadia/Titania catalysts for selective catalytic reduction (SCR) of nitric-oxide by ammonia[J]. Journal of Catalysis, 1995, 151: 226-240. |
| 8 | LI Q, YANG H S, NIE A, et al. Catalytic reduction of NO with NH3 over V2O5-MnOx/TiO2 carbon nanotube composites[J]. Catalysis Letters, 2011, 141(8): 1237-1242. |
| 9 | MUNIZ J, MARBAN G, FUERTES A B. Low temperature selective catalytic reduction of NO over modified activated carbon fibers[J]. Applied Catalysis. B: Environmental, 2000, 27(1): 27-36. |
| 10 | XU Q, FANG Z L, CHEN Y Y, et al. Titania-samarium-manganese composite oxide for the low-temperature selective catalytic reduction of NO with NH3[J]. Environmental Sciences and Technology, 2020, 54: 2530-2538. |
| 11 | LIU L J, XU K, SU S, et al. Efficient Sm modified Mn/TiO2 catalysts for selective catalytic reduction of NO with NH3 at low temperature[J]. Applied Catalysis A: General, 2020, 592: 117413. |
| 12 | HUSNAIN N, WANG E, LI K, et al. Iron oxide-based catalysts for low-temperature selective catalytic reduction of NOxwith NH3[J]. Reviews in Chemical Engineering, 2019, 35(2): 239-264. |
| 13 | ZHANG R D, LIU N, LEI Z G, et al. Selective transformation of various nitrogen-containing exhaust gases toward N2 over zeolite catalysts[J]. Chemical Reviews, 2016, 116(6): 3658-3721. |
| 14 | FU M F, LI C T, LU P, et al. A review on selective catalytic reduction of NOx by supported catalysts at 100—300℃catalysts mechanism, kinetics[J]. Catalysis Science & Technology, 2014, 4(1): 14-25. |
| 15 | WANG G H, ZHANG R Y, GOMEZ M E, et al. Persistent sulfate formation from London Fog to Chinese haze[J]. Proceeding of the National Academy of Sciences, 2016, 113(48): 13630-13635. |
| 16 | 刘华彦. NO的常温催化氧化及碱液吸收脱除NOx过程研究[D]. 杭州: 浙江大学, 2011. |
| LIU Huayan. Studies on NOx removal by catalytic oxidation and alkali solution absorption at ambient temperature[D]. Hangzhou: Zhejiang University, 2011. | |
| 17 | WANG J, ZHU J Z, ZHOU X X, et al. Nanoflower-like weak crystallization manganese oxide for efficient removal of low-concentration NO at room temperature[J]. Journal of Materials Chemistry A, 2015, 3(14): 7631-7638. |
| 18 | CHOI J, LEE K S, CHOI Y J, et al. Dry De-NOx process via gas-phase photochemical oxidation using an ultraviolet and aerosolized H2O/H2O2 hybrid system[J]. Energy Fuels, 2014, 28(8): 5270-5276. |
| 19 | CHAPPELL G A, JIMESON R. Aqueous scrubbing of nitrogen oxides from stack gases[J], Pollution Control and Energy Needs, 1974, 127: 206-217. |
| 20 | VERMA A A, BATES S A, ANGGARA T, et al. NO oxidation: a probe reaction on Cu-SSZ-13[J]. Journal of Catalysis, 2014, 312: 179-190. |
| 21 | KONG Y, CHA C Y. Reduction of NOx adsorbed on char with microwave energy[J]. Carbon, 1996, 34(8): 1035-1040. |
| 22 | ELLMERS I, VELEZ R P, BENTRUP U, et al. Oxidation and selective reduction of NO over Fe-ZSM-5 how related are these reactions[J]. Journal of Catalysis, 2014, 311: 199-211. |
| 23 | BODENSTEIN M. Die ermittlung des mechanismus chemischer reaktionen[J]. Helvetica Chimica Acta, 1935, 18(1): 743-759. |
| 24 | HAMAGUCHI T, TANAKA T, TAKAHASHI N, et al. Low-temperature NO-adsorption properties of manganese oxide octahedral molecular sieves with different potassium content[J]. Applied Catalysis B: Environmental, 2016, 193: 234-239. |
| 25 | LIU H Y, ZHANG Z K, XU Y Y, et al. Adsorption-oxidation reaction mechanism of NO on Na-ZSM-5 molecular sieves with a high Si/Al ratio at ambient temperature[J]. Chinese Journal of Catalysis, 2010, 31(10): 1233-1241. |
| 26 | CARLOS F Z. On the kinetics and mechanism of simultaneous CO and NO oxidations on polyoriented and Pt nanoparticles[J]. International Journal of Hydrogen Energy, 2020, 45(3):1453-1465. |
| 27 | AREVALO R L, OKA K, NAKANISHI H, et al. Oxidation of NO on Pt/M (M=Pt, Co, Fe, Mn): a first-principles density functional theory study[J]. Catalysis Science and Technology, 2015, 5(2): 882-886. |
| 28 | LUO S T, WU X D, JIN B F, et al. Size effect of Pt nanoparticles in acid-assisted soot oxidation in the presence of NO[J]. Journal of Environmental Sciences, 2020, 94: 64-71. |
| 29 | SHU Z, CHEN Y, HUANG W M, et al. Room-temperature catalytic removal of low-concentration NO over mesoporous Fe-Mn binary oxide synthesized using a template-free approach[J]. Applied Catalysis B: Environmental, 2013, 140/141: 42-50. |
| 30 | JIN J M, CHEN J F, WANG H F, et al. Insight into room-temperature catalytic oxidation of NO by CrO2(110): a DFT study[J]. Chinese Chemical Letters, 2019, 30(3): 618-623. |
| 31 | YUAN H, CHEN J, GUO Y, et al. Insight into the superior catalytic activity of MnO2 for low-content NO oxidation at room temperature[J]. Journal of Physical Chemistry C, 2018, 122(44): 25346-25373. |
| 32 | MIYWAKI J, SHIMOHARA T, SHIRAHAMA N, et al. Removal of NOx from air through cooperation of the TiO2 photocatalyst and urea on activated carbon fiber at room temperature[J]. Applied Catalysis B: Environmental, 2011, 110: 273-278. |
| 33 | WANG W, MCCOOL G, KAPUR N, et al. Mixed-phase oxide catalyst based on Mn-mullite (Sm, Gd) Mn2O5 for NO oxidation in diesel exhaust[J]. Science, 2012, 337: 832-835. |
| 34 | MANOCHA L M, WARRIER A, MANOCHA S, et al. Microstructure of carbon/carbon composites reinforced with pitch-based ribbon-shape carbon fibers[J]. Carbon, 2003, 41(7): 1425-1436. |
| 35 | WILLIAM S E, LARRY E C, ALEKSEY Y, et al. Overview of the fundamental reactions and degradation mechanisms of NOx storage/reduction catalysts[J]. Catalysis Reviews, 2004, 46(2): 163-245. |
| 36 | GALLIKER B, KISSNER R, NAUSER T, et al. Intermediates in the autoxidation of nitrogen monoxide[J]. Chemistry-A European Journal, 2009, 15(25): S6161(1-4. |
| 37 | MOCHIDA I, KISAMORI S, HIRONAKA M, et al. Oxidation of NO into NO2 over active carbon fibers[J]. Energy & Fuels, 1994, 8(6): 1341-1344. |
| 38 | AHMED S N, BALDWIN R, DERBYSHIRE F, et al. Catalytic reduction of nitric oxide over activated carbons[J]. Fuel, 1993, 72(3): 287-292. |
| 39 | MOCHIDA I, SHIRAHAMA N, KAWANO S, et al. NO oxidation over activated carbon fiber (ACF). Part 1. Extended kinetics over a pitch based ACF of very large surface area[J]. Fuel-Guildford, 2000, 79(14): 1713-1723. |
| 40 | CLAUDINO A, SOARES J L, MOREIRA R F P M, et al. Adsorption equilibrium and breakthrough analysis for NO adsorption on activated carbons at low temperatures[J]. Carbon, 2004, 42(8/9): 1483-1490. |
| 41 | ADAPA S, GAUR V, VERMA N. Catalytic oxidation of NO by activated carbon fiber (ACF)[J]. Chemical Engineering Journal, 2006, 116(1): 25-37. |
| 42 | ZHANG W J, BAGREEV A, RASOULI F, et al. Reaction of NO2 with activated carbon at ambient temperature[J]. Industrial and Engineering Chemistry Research, 2008, 47(13): 4358-4362. |
| 43 | ZHANG W J, RABIEA S, BAGREEV A, et al. Study of NO adsorption on activated carbons[J]. Applied Catalysis B: Environmental, 2008, 83(1/2): 63-71. |
| 44 | ATKINSON J D, ZHANG Z Q, YAN Z F, et al. Evolution and impact of acidic oxygen functional groups on activated carbon fiber cloth during NO oxidation[J]. Carbon, 2013, 54: 444-453. |
| 45 | YANG S J, LI H J, WANG C Z, et al. Fe-Ti spinel for the selective catalytic reduction of NO with NH3: mechanism and structure-activity relationship[J]. Applied Catalysis B: Environmental, 2012, 117/118: 73-80. |
| 46 | JOEL D, MARTIN E, MANFRED K, et al. Catalytic oxidation of nitrogen monoxide over Pt/SiO2[J]. Applied Catalysis B: Environmental, 2004, 50(2): 73-82. |
| 47 | ZHANG Z Q, ATKINSON J D, JIANG B Q, et al. Nitric oxide oxidation catalyzed by microporous activated carbon fiber cloth: an updated reaction mechanism[J]. Applied Catalysis B: Environmental, 2014, 148/149: 573-581. |
| 48 | GUO Z C, XIE Y S, IKPYO H, et al. Catalytic oxidation of NO to NO2 on activated carbon[J]. Energy Conversion and Management, 2001, 42(15/17): 2005-2018. |
| 49 | SHEN W Z, FAN W B. Nitrogen-containing porous carbons: synthesis and application[J]. Journal of Materials Chemistry A, 2013, 1(4): 999-1013. |
| 50 | KANEKO K, FUKUZAKI N, OZEKI S. The concentrated NO dimer in micropores above room temperature[J]. The Journal of Chemical Physics, 1987, 87(1): 776. |
| 51 | YOU F T, YU G W, XING Z J, et al. Enhancement of NO catalytic on activated carbon at room temperature by nitric acid hydrothermal treatment[J]. Applied Surface Science, 2019, 471: 633-644. |
| 52 | SHEN Y F, GE X L, CHEN M D, et al. Catalytic oxidation of nitric oxide (NO) with carbonaceous materials[J]. RSC Advances, 2016, 6(10): 8469-8482. |
| 53 | FIGUEIREDO J L, PEREIRA M F R, FREITAS M M A, et al. Modification of the surface chemistry of activated carbons[J]. Carbon, 1999, 37(9): 1379-1389. |
| 54 | BAGREEV A, MENENDEZ J A, DUKHNO I, et al. Bituminous coal based activated carbons modified with nitrogen as adsorbent of hydrogen sulfide[J]. Carbon, 2004, 42(3): 469-476. |
| 55 | PIETRZAK R. XPS study and physico-chemical properties of nitrogen-enriched microporous activated carbon from high volatile bituminous coal[J]. Fuel, 2009, 88(10): 1871-1877. |
| 56 | BUDAEVA A D, ZOLTOEV E V. Porous structure and sorption properties of nitrogen containing activated carbon[J]. Fuel, 2010, 89(9): 2623-2627. |
| 57 | GORGULHO H F, GONCALVES F, PEREIRA M F R, et al. Synthesis and characterization of nitrogen-doped carbon xerogels[J]. Carbon, 2009, 47(8): 2032-2039. |
| 58 | BOEHM H P. Catalytic properties of nitrogen-containing carbons[J]. Carbon Materials for Catalysis, 2008, 7: 219-265. |
| 59 | RATHORE R S, SRIVASTAVA D K, AGARWAL A K, et al. Development of surface functionalized activated carbon fiber for control of NO and particulate matter[J]. Journal of Hazardous Materials, 2010, 173(1/3): 211-222. |
| 60 | SOUSA J P S, PEREIRA M F R, FIGUEIREDO J L. Catalytic oxidation of NO to NO2 on N-doped activated carbons[J]. Catalysis Today, 2011, 176(1): 383-387. |
| 61 | SOUSA J P S, PEREIRA M F R, FIGUEIREDO J L. NO oxidation over nitrogen doped carbon xerogels[J]. Applied Catalysis B: Environmental, 2012, 125: 398-408. |
| 62 | STRELKO V V, THROWER P A, KUTS V S. On the mechanism of possible influence of heteroatoms of nitrogen, boron and phosphorus in a carbon matrix on the catalytic activity of carbons in electron transfer reactions[J]. Carbon, 2000, 38(10): 1499-1503. |
| 63 | YANG H, LIU H, ZHOU K, et al. Oxidation path analysis of NO in the adsorption and removal process using activated carbon fibers[J]. Journal of Fuel Chemistry and Technology, 2012, 40(8): 1002-1008. |
| 64 | YOU F T, YU G W, WANG Y, et al. Study of nitric oxide catalytic oxidation on manganese oxides-loaded activated carbon at low temperature[J]. Applied Surface Science, 2017, 413: 387-397. |
| 65 | MOCHIDA I, KAWABUCHI Y, KAWANO S, et al. High catalytic activity of pitch-based activated carbon fibres of moderate surface area for oxidation of NO to NO2 at room temperature[J]. Fuel, 1997, 76(6): 543-548. |
| 66 | MOHSEN G, ATKINSON J D. Catalytic NO oxidation in the presence of moisture using porous polymers and activated carbon[J]. Environmental Sciences and Technology, 2016, 50: 5189-5196. |
| 67 | 李兵, 张立强, 蒋海涛, 等. 粉末活性炭低温吸附氧化NO动力学研究[J]. 煤炭学报, 2011, 36(12): 2092-2096. |
| LI Bing, ZHANG Liqiang, JIANG Haitao, et al. Kinetics of NO adsorption and oxidation on powder activated carbon at low temperature[J]. Journal of China Coal Society, 2011, 36(12): 2092-2096. | |
| 68 | FANG Z Q, YU X H, TU S T. Catalytic oxidation of NO on activated carbons[J]. Energy Procedia, 2019, 158: 2366-2371. |
| 69 | SOUSA J P S, PEREIRA M F R, FIGUEIREDO J L. Modified activated carbon as catalyst for NO oxidation[J]. Fuel Processing Technology, 2013, 106: 727-733. |
| 70 | 金逸凡. 热处理改性活性炭纤维的NO催化氧化行为[D]. 上海:华东理工大学, 2018. |
| JIN Yifan. Catalytic oxidation behavior of NO over heat-treated ACFs[D]. Shanghai: East China University of Science and Technology, 2018. | |
| 71 | ESRAFILI M D, SAEIDI N. Si-embedded boron-nitride nanotubes as an efficient and metal-free catalyst for NO oxidation[J]. Superlattices and Microstructures, 2015, 81: 7-15. |
| 72 | GUO Z Y, HUANG Z H, WANG M X, et al. Graphene/carbon composite nanofibers for NO oxidation at room temperature[J]. Catalysis Science & Technology, 2014, 5(2): 827-829. |
| 73 | WANG M X, GUO Z Y, HUANG Z H, et al. Preparation of porous carbon nanofibers with controllable pore structures for low-concentration NO removal at room temperature[J]. Carbon, 2016, 110: 518-519. |
| 74 | YU Y, BU Y F, ZHONG Q, et al. Catalytic oxidation of NO by g-C3N4-assisted electrospun porous carbon nanofibers at room temperature: structure-activity relationship and mechanism study[J]. Catalysis Communications, 2016, 87: 62-65. |
| 75 | WANG M X, HUANG Z H, SHIMOHAR T, et al. NO removal by electrospun porous carbon nanofibers at room temperature[J]. Chemical Engineering Journal, 2011, 170(2/3): 505-511. |
| 76 | CADENAS M P, CASTILLA C M, FRANCISCO C M, et al. Surface chemistry, porous texture, and morphology of N-Doped carbon xerogels[J]. Langmuir, 2008, 25(1): 466-470. |
| 77 | MONGE J A, LOPEZ A B, RODENAS M A L, et al. NO adsorption on activated carbon fibers from iron-containing pitch[J]. Microporous and Mesoporous Materials, 2008, 108(1/3): 294-302. |
| 78 | NIKOLOV P, KHRISTOVA M, MEHANDJIEV D. Low-temperature NO removal over copper-containing activated carbon[J]. Colloids and Surfaces. A: Physicochemical and Engineering Aspects, 2007, 295(1/3): 239-245. |
| 79 | TALUKDAR P, BHADURI B, VERMA N. Catalytic oxidation of NO over CNF/ACF-supported CeO2 and Cu nanoparticles at room temperature[J]. Industrial & Engineering Chemistry Research, 2014, 53(31): 12537-12547. |
| 80 | WANG M X, LIU H N, HUANG Z H, et al. Activated carbon fibers loaded with MnO2 for removing NO at room temperature[J]. Chemical Engineering Journal, 2014, 256: 101-106. |
| 81 | BANDOSZ T J. On the adsorption/oxidation of hydrogen sulfide on activated carbons at ambient temperature[J]. Journal of Colloid and Interface Science, 2002, 246(1): 1-20. |
| 82 | DAS D, GAUR V, VERMA N. Removal of volatile organic compound by activated carbon fiber[J]. Carbon, 2004, 42(14): 2949-2962. |
| 83 | GAUR V, SHARMA A, VERMA N. Preparation and characterization of ACF for the adsorption of BTX and SO2[J]. Chemical Engineering and Processing: Process Intensification, 2006, 45(1): 1-13. |
| 84 | YAMAUCHI T, TANSURIYAVONG S, DOI K. Preparation of composite materials of polypyrrole and electroactive polymer gel using for actuating system[J]. Synthetic Metals, 2005, 152(1/3): 45-48. |
| 85 | LIU S H, ZHAO X W, PAN T S, et al. Template effect of hydrolysis of the catalyst precursor on growth of carbon nanotube arrays[J]. Journal of Colloid and Interface Science, 2012, 374(1): 34-39. |
| 86 | DEJONG K P, GEUS J W. Carbon nanofibers: catalytic synthesis and applications[J]. Catalysis Reviews, 2000, 42(4): 481-510. |
| 87 | JI L, LIN Z, MEDFORD A J, Porous carbon nanofibers from electrospun polyacrylonitrile/SiO2composites as an energy storage material[J]. Carbon, 2009, 47(14): 3346–3354. |
| 88 | KIM C, NGOC B T N, YANG K S, et al. Self-sustained thin webs consisting of porous carbon nanofibers for supercapacitors via the electrospinning of polyacrylonitrile solutions containing zinc chloride[J]. Advanced Materials (FRG), 2007, 19(17): 2341-2346. |
| 89 | ATRIBAK I, GUILLEN-HURTADO N, BUENO-LOPEZ, et al. Influence of the physico-chemical properties of CeO2-ZrO2 mixed oxides on the catalytic oxidation of NO to NO2[J]. Applied Surface Science, 2010, 256(24): 7706-7712. |
| 90 | LI L D, SHEN Q, CHENG J. Catalytic oxidation of NO over TiO2 supported platinum clusters I. Preparation, characterization and catalytic properties[J]. Applied Catalysis B: Environmental, 2010, 93(3/4): 259-266. |
| 91 | IRFAN M F, GOO J H, KIM S D. Co3O4 based catalysts for NO oxidation and NOx reduction in fast SCR process[J]. Applied Catalysis B: Environmental, 2008, 78(3/4): 267-274. |
| 92 | TANG X L, LI K, YI H H, et al. MnOx catalysts modified by nonthermal plasma for NO catalytic oxidation[J]. The Journal of Physical Chemistry C, 2012, 116(18): 10017-10028. |
| 93 | SHIRAHAMA N, MOON S H, CHOI K H, et al. Mechanistic study on adsorption and reduction of NO2 over activated carbon fibers[J]. Carbon, 2002, 40(14): 2605-2611. |
| 94 | DENG W Y, HU M H, MA J C, et al. Structural and functional relationships of activated char briquettes from pyrolysis of sewage sludge for methylene blue removal[J]. Journal of Cleaner Production, 2020, 259: 120907. |
| 95 | DENG W Y, YIN A D, MA J C, et al. Investigation of NO conversion by different types of sewage sludge chars under low temperature[J]. Journal of Environmental Management, 2018, 209: 236-244. |
| 96 | HADI P, XU M, NING C, et al. A critical review on preparation, characterization and utilization of sludge-derived activated carbons for wastewater treatment[J]. Chemical Engineering Journal, 2015, 260: 895-906. |
| 97 | MIAN M M, LIU G J, FU B, et al. Facile synthesis of sludge-derived MnOx-N-biochar as an efficient catalyst for peroxymonosulfate activation[J]. Applied Catalysis B: Environmental, 2019, 255: 117765. |
| 98 | 陶聪. 常温下炭化污泥催化氧化脱除NO的实验研究[D]. 南京:东华大学, 2020. |
| TAO Cong. Experimental study on catalytic oxidation of NO at ambient temperature over the chars from pyrolysis of sewage sludge[D]. Nanjing: Donghua University, 2020. | |
| 99 | DENG W Y, TAO C, COBB K, et al. Catalytic oxidation of NO at ambient temperature over the chars from pyrolysis of sewage sludge[J]. Chemosphere, 2020, 251: 126429. |
| [1] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [2] | 王科菊, 赵成, 胡晓玫, 云军阁, 魏凝涵, 姜雪迎, 邹昀, 陈志航. 金属氧化物低温催化氧化VOCs的研究进展[J]. 化工进展, 2023, 42(5): 2402-2412. |
| [3] | 周皞, 张恒, 温妮妮, 王旭瑞, 徐璐, 李玮, 苏亚欣. Cu-SAPO-44分子筛的制备及其C3H6-SCR脱硝性能[J]. 化工进展, 2023, 42(3): 1373-1382. |
| [4] | 郭晓宇, 李冬晨, 赵炜, 杜朕屹, 李晓良. Au-Pd/MnO2催化剂的制备及其苯甲醇氧化性能[J]. 化工进展, 2023, 42(10): 5223-5231. |
| [5] | 张铭, 高永康, 纪德龙, 刘福杰, 朱文帅, 李华明. 多酸材料在燃油脱硫中的研究进展[J]. 化工进展, 2022, 41(9): 4782-4789. |
| [6] | 朱飞飞, 马磊, 龙慧敏. PdxSy材料的制备及其在催化领域的研究进展[J]. 化工进展, 2022, 41(2): 740-749. |
| [7] | 高天, 张伊黎, 熊卓, 赵永椿, 张军营. 改性氧化钛光催化氧化单质汞性能及其影响因素研究进展[J]. 化工进展, 2022, 41(2): 690-700. |
| [8] | 聂紫萌, 杨点, 熊玉路, 李英杰, 田森林, 宁平. 电解锰渣浆液烟气脱硫性能及机制[J]. 化工进展, 2022, 41(2): 1063-1072. |
| [9] | 翟重渊, 赵丹荻, 何亚鹏, 黄惠, 陈步明, 郭忠诚. 掺硼金刚石阳极电催化降解新兴抗生素类污染物研究进展[J]. 化工进展, 2022, 41(12): 6615-6626. |
| [10] | 钟丽蓉, 何飞强, 董贝贝, 刘紫薇, 丁健桦. 湿法烟气脱硝Fe Ⅱ EDTA-NO液的NO真空解吸[J]. 化工进展, 2022, 41(11): 6176-6184. |
| [11] | 王吉坤, 李阳, 陈贵锋, 刘敏, 寇丽红, 王琦, 何毅聪. 臭氧催化氧化降解煤化工高盐废水有机物的机理[J]. 化工进展, 2022, 41(1): 493-502. |
| [12] | 张轩, 宋小三, 赵珀, 董元华, 刘云. 高级氧化技术处理1,4-二 烷污染研究进展[J]. 化工进展, 2021, 40(S2): 380-388. 烷污染研究进展[J]. 化工进展, 2021, 40(S2): 380-388. |
| [13] | 苏碧云, 冉良涛, 胡雅和, 张翱, 韩巧巧, 武晋娣, 刘伊婷, 孟祖超. 分子氧化及光电催化氧化对石油Pickering乳液的破乳研究进展[J]. 化工进展, 2021, 40(7): 3995-4002. |
| [14] | 孙浩, 何雪英, 胡一超, 刘哲艺, 张瑛洁. 铁锰氧化膜同步除微污染地表水铁锰氨氮研究进展[J]. 化工进展, 2021, 40(3): 1634-1642. |
| [15] | 黄建雄, 郭英明, 杨靖, 许伟, 王旭, 张瑞峰. 铁锰复合氧化膜对水中双酚A的去除及影响因素[J]. 化工进展, 2021, 40(3): 1551-1557. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||