| 1 |
陈成, 何欢, 杨绍贵, 等. 微波诱导NiO催化降解水中邻苯二甲酸二甲酯[J]. 中国环境科学, 2018, 38(7): 2512-2519.
|
|
CHEN Cheng, HE Huan, YANG Shaogui, et al. Degradation of dimethyl phthalate in microwave-induced NiO catalytic system[J]. China Environmental Science, 2018, 38(7): 2512-2519.
|
| 2 |
NET S, SEMPÉRÉ R, DELMONT A, et al. Occurrence, fate, behavior and ecotoxicological state of phthalates in different environmental matrices[J]. Environmental Science & Technology, 2015, 49(7): 4019-4035.
|
| 3 |
WANG J, CHEN G, CHRISTIE P, et al. Occurrence and risk assessment of phthalate esters (PAEs) in vegetables and soils of suburban plastic film greenhou[J]. Science of the Total Environment, 2015, 523: 129-137.
|
| 4 |
翟斌, 王朝万, 张巍, 等. 环境介质中邻苯二甲酸酯类化合物的环境行为及生态风险研究进展[J]. 环境与健康杂志, 2016, 33(11): 1030-1034.
|
|
ZHAI Bin, WANG Chaowan, ZHANG Wei, et al. Environmental behavior and ecotoxicological risk of phthalic acid esters: a review of recent studies[J]. Journal of Environment and Health, 2016, 33(11): 1030-1034.
|
| 5 |
LIU Y, GUAN Y T, YANG Z H, et al. Toxicity of seven phthalate esters to embryonic development of the abalone halitosis diverticular supertexta[J]. Ecotoxicology, 2018, 18(3): 293-303.
|
| 6 |
BUI T T, GIOVANOULIS G, COUSINS A P, et al. Human exposure, hazard and risk of alternative plasticizers to phthalate esters[J]. Science of the Total Environment, 2016, 541: 451-467.
|
| 7 |
胡晓宇, 张克荣, 孙俊红, 等. 中国环境中邻苯二甲酸酯类化合物污染的研究[J]. 中国卫生检验杂志, 2003, 13(1): 9-14.
|
|
HU Xiaoyu, ZHANG Kerong, SUN Junhong, et al. Study on the pollution of phthalate esters in the environment of China[J]. Chinese Journal of Health Laboratory Technology, 2003, 13(1): 9-14.
|
| 8 |
张颖, 董俊伟, 王蕾, 等. 邻苯二甲酸二甲酯(DMP)对黄瓜抗氧化代谢及果实品质的影响[J]. 农业环境科学学报, 2017, 36(5): 847-854.
|
|
ZHANG Ying, DONG Junwei, WANG Lei, et al. Toxic effects of dimethyl phthalate on cucumber at the growth stage[J]. Journal of Agro-Environment Science, 2017, 36(5): 847-854.
|
| 9 |
WANG W L, WU Q Y, WANG C, et al. Health risk assessment of phthalate esters (PAEs) in drinking water sources of China[J]. Environmental Science and Pollution Research, 2015, 22(5): 3620-3630.
|
| 10 |
BAJT O, MAILHOT G, BOLTE M. Degradation of dibutyl phthalate by homogeneous photocatalysis with Fe( ) in aqueous solution[J]. Applied Catalysis B: Environmental, 2001, 33(3): 239-248. ) in aqueous solution[J]. Applied Catalysis B: Environmental, 2001, 33(3): 239-248.
|
| 11 |
李章良, 黄建辉, 肖尚忠, 等. 活性炭负载Fe3+催化H2O2氧化邻苯二甲酸二甲酯[J]. 环境工程学报, 2014, 8(3): 827-833.
|
|
LI Zhangliang, HUANG Jianhui, XIAO Shangzhong, et al. Degradation of dimethyl phthalate solution catalyzed by Fe3+ immobilized activated carbon in the presence of H2O2[J]. Chinese Journal of Environmental Engineering, 2014, 8(3): 827-833.
|
| 12 |
YIN J Y, CAI J J, YIN C, et al. Degradation performance of crystal violet over CuO@AC and CeO2-CuO@AC catalysts using microwave catalytic oxidation degradation method[J]. Journal of Environmental Chemical Engineering, 2016(4): 958-964.
|
| 13 |
LIU Z L, MENT H L, ZHANG H, et al. Highly efficient degradation of phenol wastewater by microwave induced H2O2-CuOx/GAC catalytic oxidation process[J]. Separation and Purification Technology, 2018, 193: 49-57.
|
| 14 |
刘再亮, 孟海玲, 周科, 等. 微波-载铜活性炭催化氧化降解腐殖酸[J]. 过程工程学报, 2018, 18(4): 886-892.
|
|
LIU Zailiang, MENG Hailing, ZHOU Ke, et al. Degradation of humic acid by microwave-Cu loaded activated carbon catalytic oxidation[J]. The Chinese Journal of Process Engineering, 2018, 18(4): 886-892.
|
| 15 |
李章良, 饶艳英, 赵晓旭, 等. 微波诱导改性活性炭催化降解邻苯二甲酸二甲酯[J]. 环境工程学报, 2019, 13(2): 341-347.
|
|
LI Zhangliang, RAO Yanying, ZHAO Xiaoxu, et al. Degredation of dimethyl phthalate in aqueous solution by microwave-induced catalytic oxidation with modified activated carbon[J]. Chinese Journal of Environmental Engineering, 2019, 13(2): 341-347.
|
| 16 |
杨全, 张浩, 卜龙利. 复合载体负载型催化剂制备及其微波辅助催化氧化甲苯性能试验[J]. 西安建筑科技大学学报(自然科学版), 2014, 46(1): 131-136.
|
|
YANG Quan,ZHANG Hao,BO Longli. Preparation of composite carrier loaded catalyst and microwave assisted catalytic oxidation of gaseous toluene[J]. Journal Xi’an University of Architecture and Technology (Natural Science Edition), 2014, 46(1): 131-136.
|
| 17 |
REMYA N, LIN J G. Microwave-granular activated carbon (MW-GAC) system for carbofuran degradation: analysis of characteristics and recyclability of the spent GAC[J]. Desalination & Water Treatment, 2015, 53(6): 1621-1631.
|
| 18 |
LEE C L, JOU C J G, PAUL H. Enhanced degradation of chlorobenzene in aqueous solution using microwave-induced zero-valent iron and copper particles[J]. Water Environment Research, 2010, 82(7): 642-647.
|
| 19 |
LIU S Y, MEI L F, LIANG X L, et al. Anchoring Fe3O4 nanoparticles on carbon nanotubes for microwave-induced catalytic degradation of antibiotics[J]. ACS Applied Materials & Interfaces, 2018, 10: 29467-29475.
|
| 20 |
SHI W, LIU X Y, ZHANG T T, et al. Magnetic nano-sized cadmium ferrite as an efficient catalyst for the degradation of Congo red in the presence of microwave irradiation[J]. RSC Advances, 2015, 5: 51027-51034.
|
| 21 |
YIN C, CAI J J, GAO L F, et al. Highly efficient degradation of 4-nitrophenol over the catalyst of Mn2O3/AC by microwave catalytic oxidation degradation method[J]. Journal of Hazardous Materials, 2016, 305: 15-20.
|
| 22 |
XU P, MA W C, HOU B L, et al. A novel integration of microwave catalytic oxidation and MBBR process and its application in advanced treatment of biologically pretreated Lurgi coal gasification wastewater[J]. Separation and Purification Technology, 2017, 177: 233-238.
|
| 23 |
郑庆福, 王志民, 陈保国, 等. 制备生物炭的结构特征及炭化机理的XRD光谱分析[J]. 光谱学与光谱分析, 2016, 36(10): 3355-3359.
|
|
ZHENG Qingfu, WANG Zhimin, CHEN Baoguo, et al. Analysis of XRD spectral structure and carbonization of the biochar preparation[J]. Spectroscopy and Spectral Analysis, 2016, 36(10): 3355-3359.
|
| 24 |
宋小宝, 何世颖, 冯彦房, 等. 载镧磁性水热生物炭的制备及其除磷性能[J]. 环境科学, 2020, 41(2): 773-783.
|
|
SONG Xiaobao, HE Shiying, FENG Yanfang, et al. Fabrication of La-MHTC composites for phosphate removal: adsorption behavior and mechanism[J]. Environmental Science, 2020, 41(2): 773-783.
|
| 25 |
朱荣, 江雯, 曾晓波, 等. 不同方法制备的Fe3O4磁性纳米粒表面性能研究[J]. 四川大学学报(自然科学版), 2011, 48(1): 133-138.
|
|
ZHU Rong, JIANG Wen, ZENG Xiaobo, et al. The surface property investigation of Fe3O4 nanoparticles synthesized via different methods[J]. Journal of Sichuan University(Natural Science Edition), 2011, 48(1): 133-138.
|
| 26 |
LU J, JIAO X L, CHEN D R, et al. Solvothermal synthesis and characterization of Fe3O4 and γ-Fe2O3 nanoplates[J]. The Journal of Physical Chemistry C, 2009, 113: 4012-4017.
|
| 27 |
谷麟, 陈旭, 吴思帆, 等. 微量1,6-己二胺改性纳米Fe3O4对PVDF共混膜性能的影响[J]. 环境工程学报, 2019, 13(2): 291-301.
|
|
GU Lin, CHEN Xu, WU Sifan, et al. Effect of trace 1,6-hexanediamine-modified nano-Fe3O4 on physiochemical properties of PVDF composite membrane[J]. Chinese Journal of Environmental Engineering, 2019, 13(2): 291-301.
|
| 28 |
师艳婷, 乔生莉, 张巧玲, 等. 磁性光催化剂Fe3O4/SiO2/TiO2的制备及光催化降解苯酚[J]. 化工进展, 2018, 37(11): 4322-4329.
|
|
SHI Yanting, QIAO Shengli, ZHANG Qiaoling, et al. Preparation of Fe3O4/SiO2/TiO2 magnetic photocatalyst and the photocatalytic degradation of phenol[J]. Chemical Industry and Engineering Progress, 2018, 37(11): 4322-4329.
|
| 29 |
HE J, YANG X F, ZhANG W J, et al. Catalyzed oxidation of catechol by the heterogeneous Fenton-like reaction of nano-Fe3O4-H2O2 system[J]. Environmental Science, 2013, 34(5): 1773-1781.
|
| 30 |
张依含, 史静, 杜琼, 等. 磁性生物炭非均相类Fenton体系去除水中四环素[J]. 工业水处理, 2020, 40(2): 32-35.
|
|
ZhANG Yihan, SHI Jing, DU Qiong, et al. Tetracycline removal in the magnetic biochar heterogeneous Fenton-like system[J]. Industrial Water Treatment, 2020, 40(2): 32-35.
|
| 31 |
殷诚, 周继承, 尹静雅, 等. 微波催化剂CuO/AC微波催化氧化降解废水中的苯酚[J]. 环境工程学报, 2015, 9(11): 5329-5335.
|
|
YIN Cheng, ZHOU Jicheng, YIN Jingya, et al. Microwave catalytic oxidation degradation of phenol in wastewater over CuO/AC microwave catalyst[J]. Chinese Journal of Environmental Engineering, 2015, 9(11): 5329-5335.
|
| 32 |
REMYA N, LIN J G. Current status of microwave application in wastewater treatment—A review[J]. Chemical Engineering Journal, 2011, 166(3): 797-813.
|
| 33 |
宋振宇, 童张法, 张寒冰, 等. 微波辅助制备纳米ZnO及其光催化降解染料的性能[J]. 化工进展, 2015, 34(12): 4310-4314.
|
|
SONG Zhenyu, TONG Zhangfa, ZHANG Hanbing, et al. Photocatalytic degradation of dyes by nano-ZnO prepared with microwave assistance[J]. Chemical Industry and Engineering Progress, 2015, 34(12): 4310-4314.
|
 ), 赵晓旭1,2,3, 黄建辉1,2,3, 王侯琼1, 李萍1
), 赵晓旭1,2,3, 黄建辉1,2,3, 王侯琼1, 李萍1
 ), Xiaoxu ZHAO1,2,3, Jianhui HUANG1,2,3, Houqiong WANG1, Ping LI1
), Xiaoxu ZHAO1,2,3, Jianhui HUANG1,2,3, Houqiong WANG1, Ping LI1
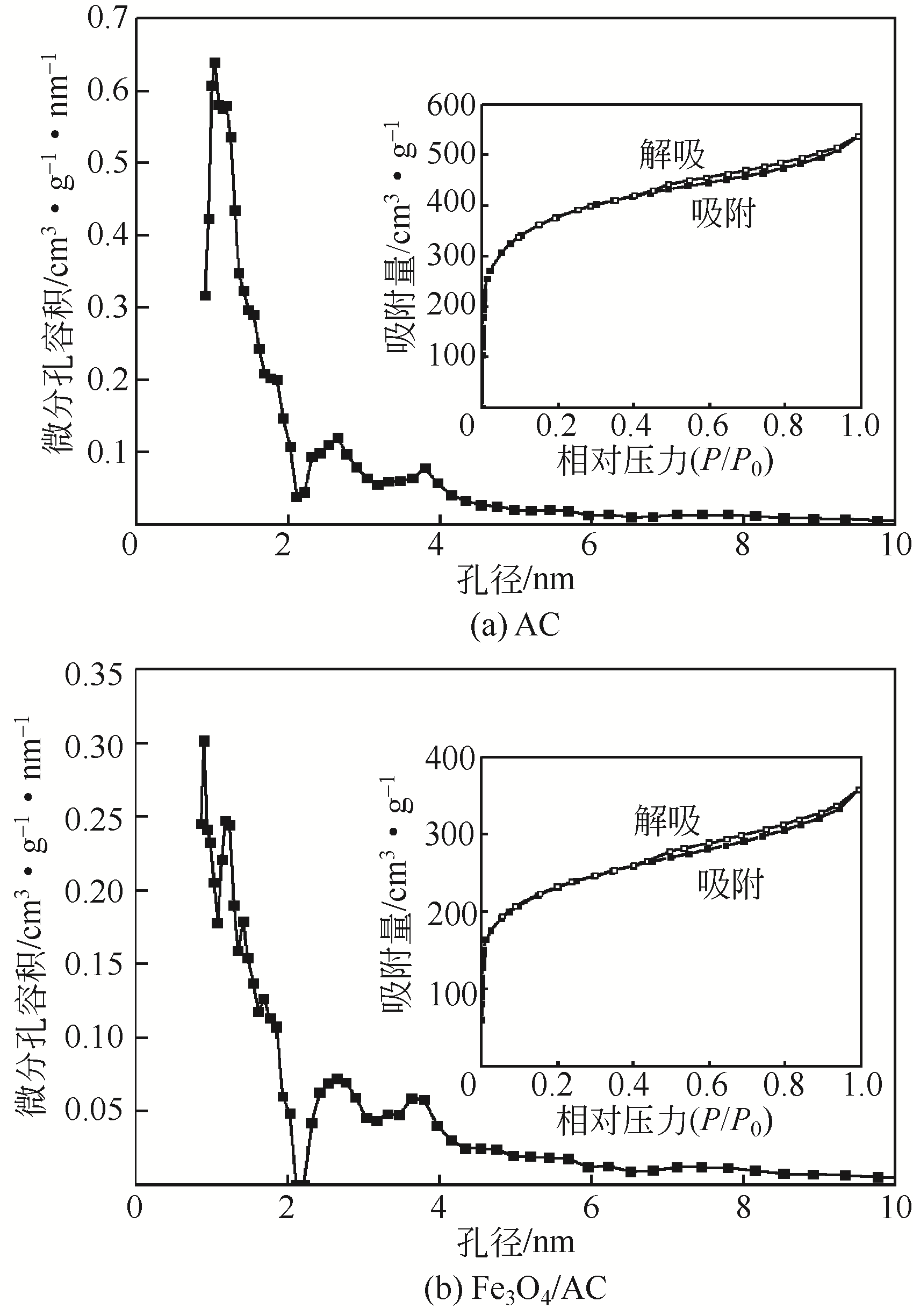
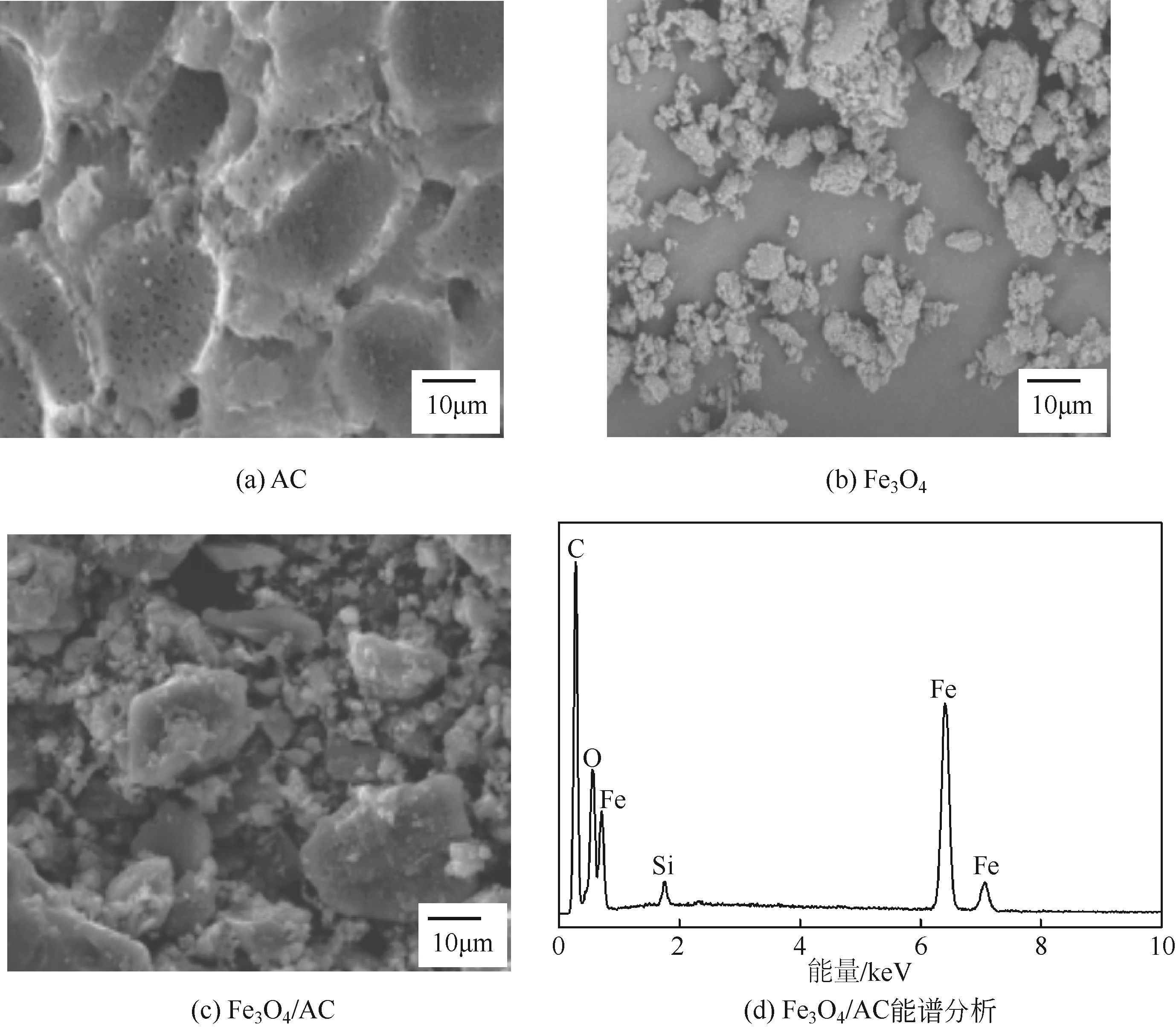

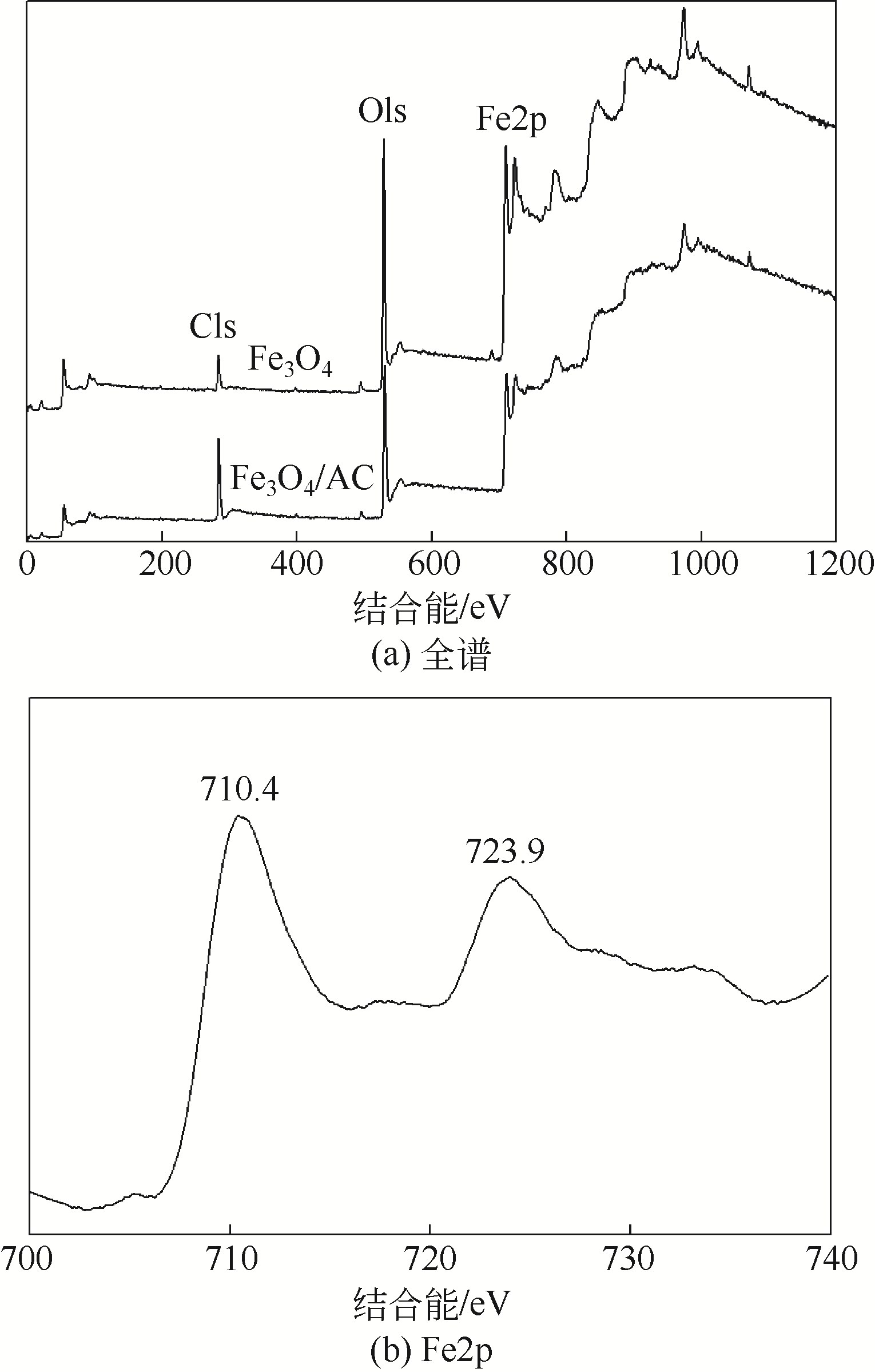
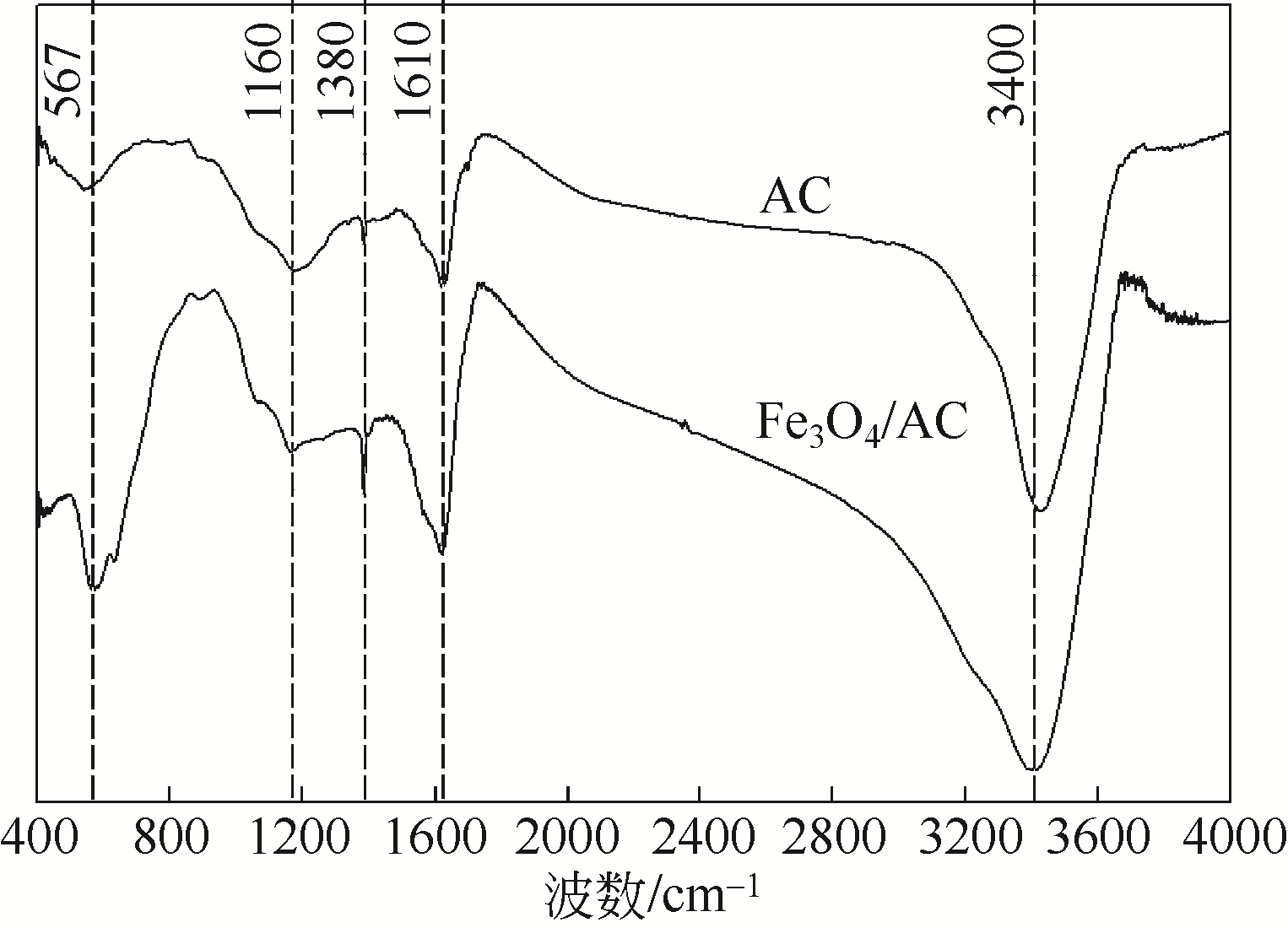
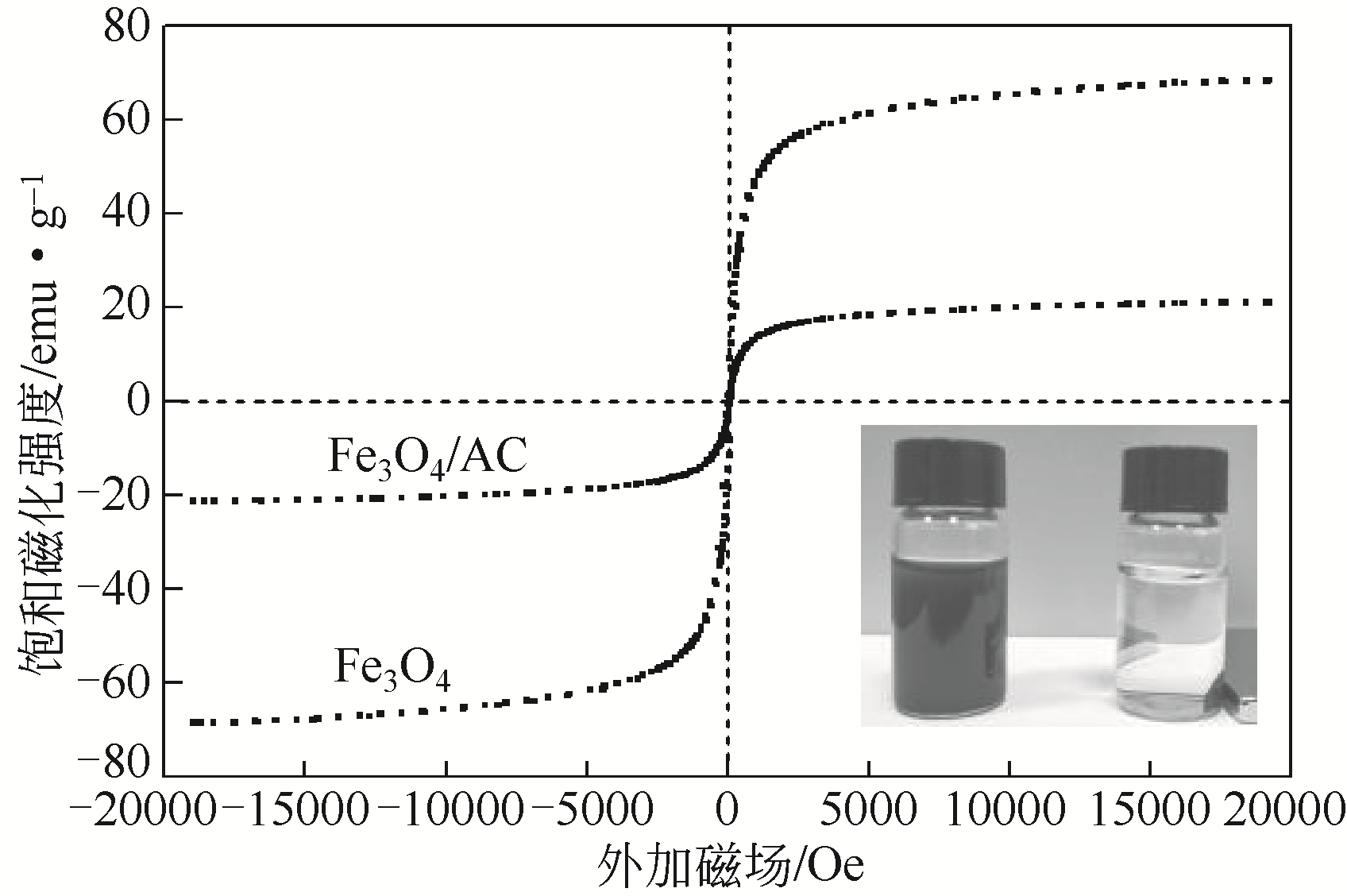
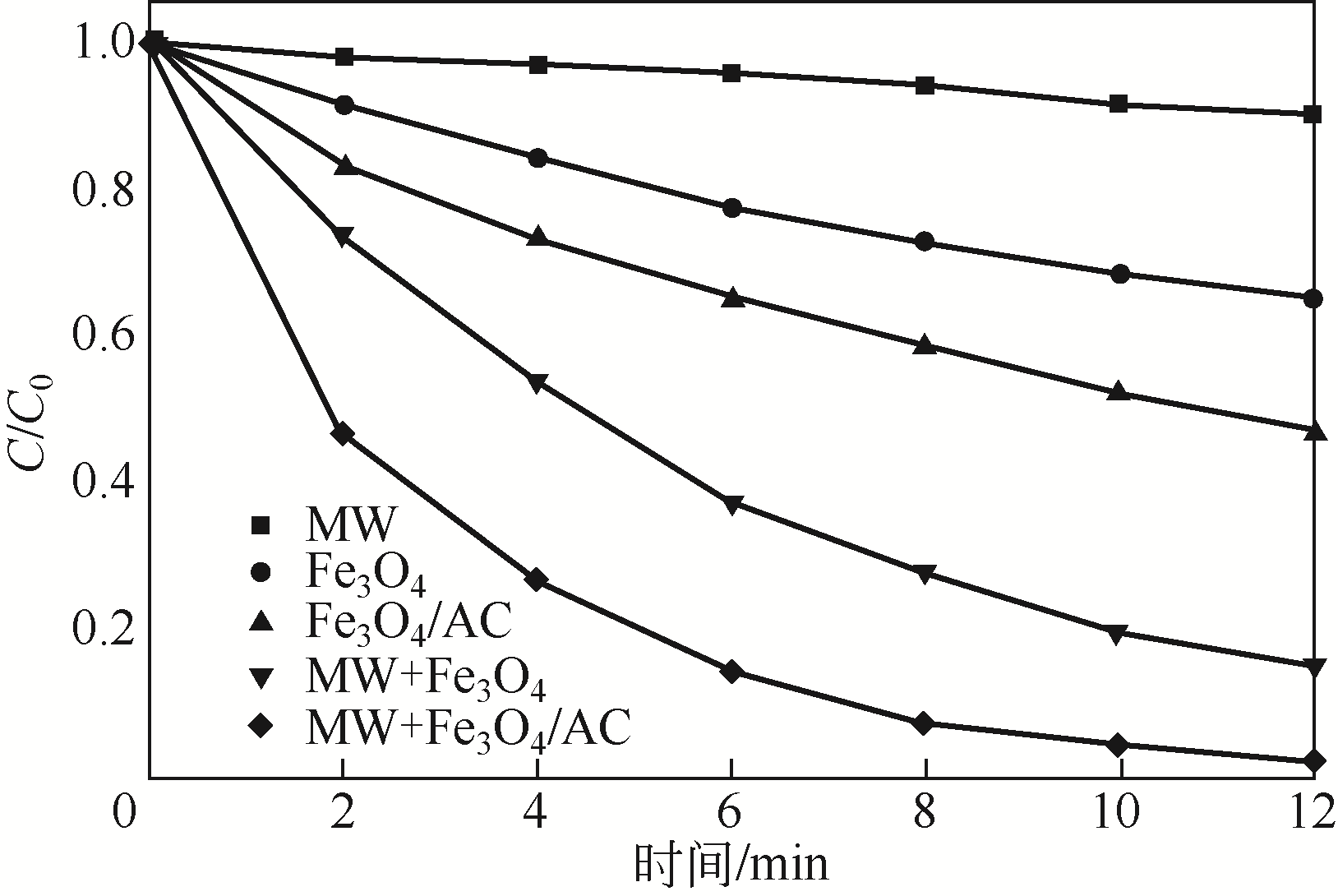

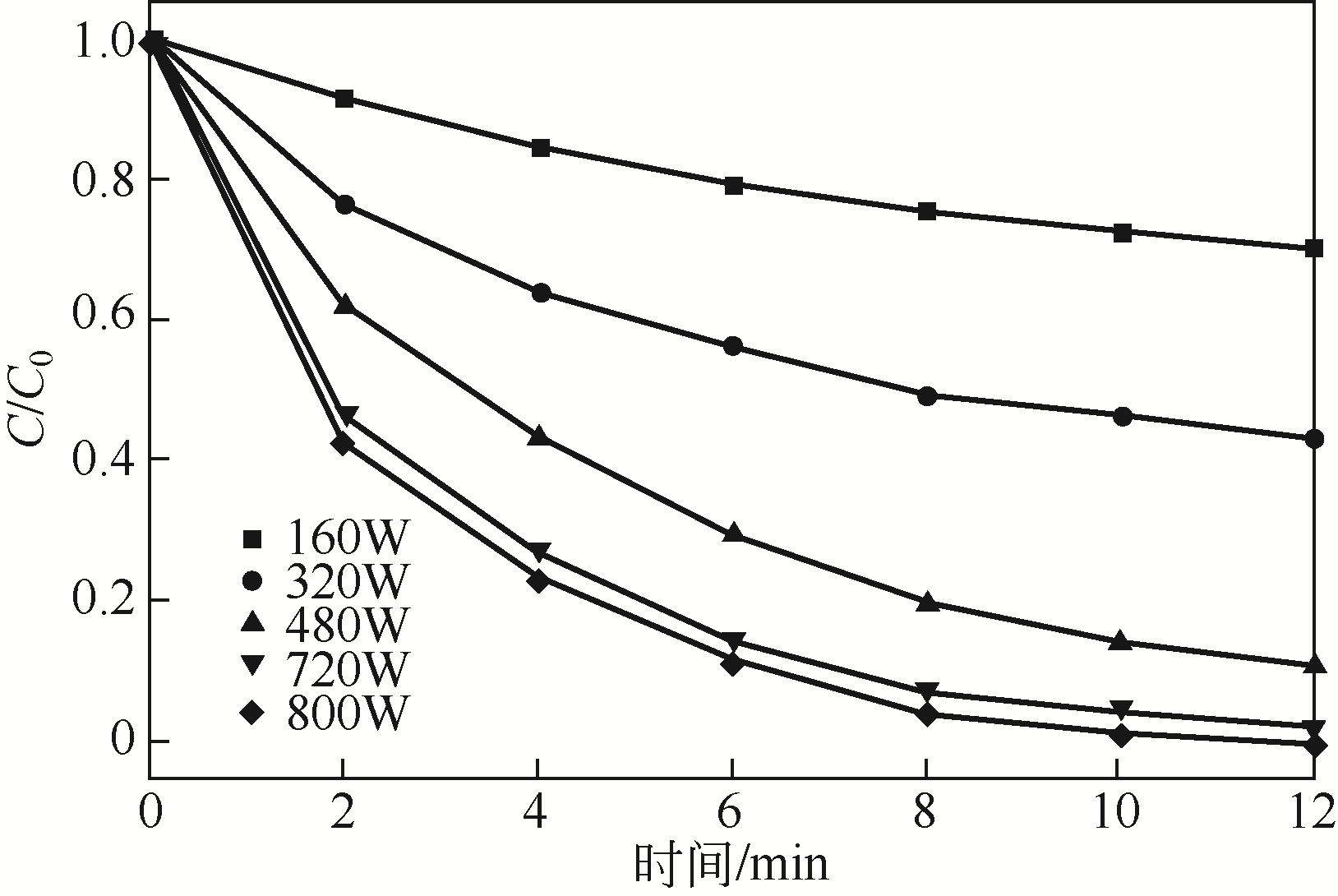
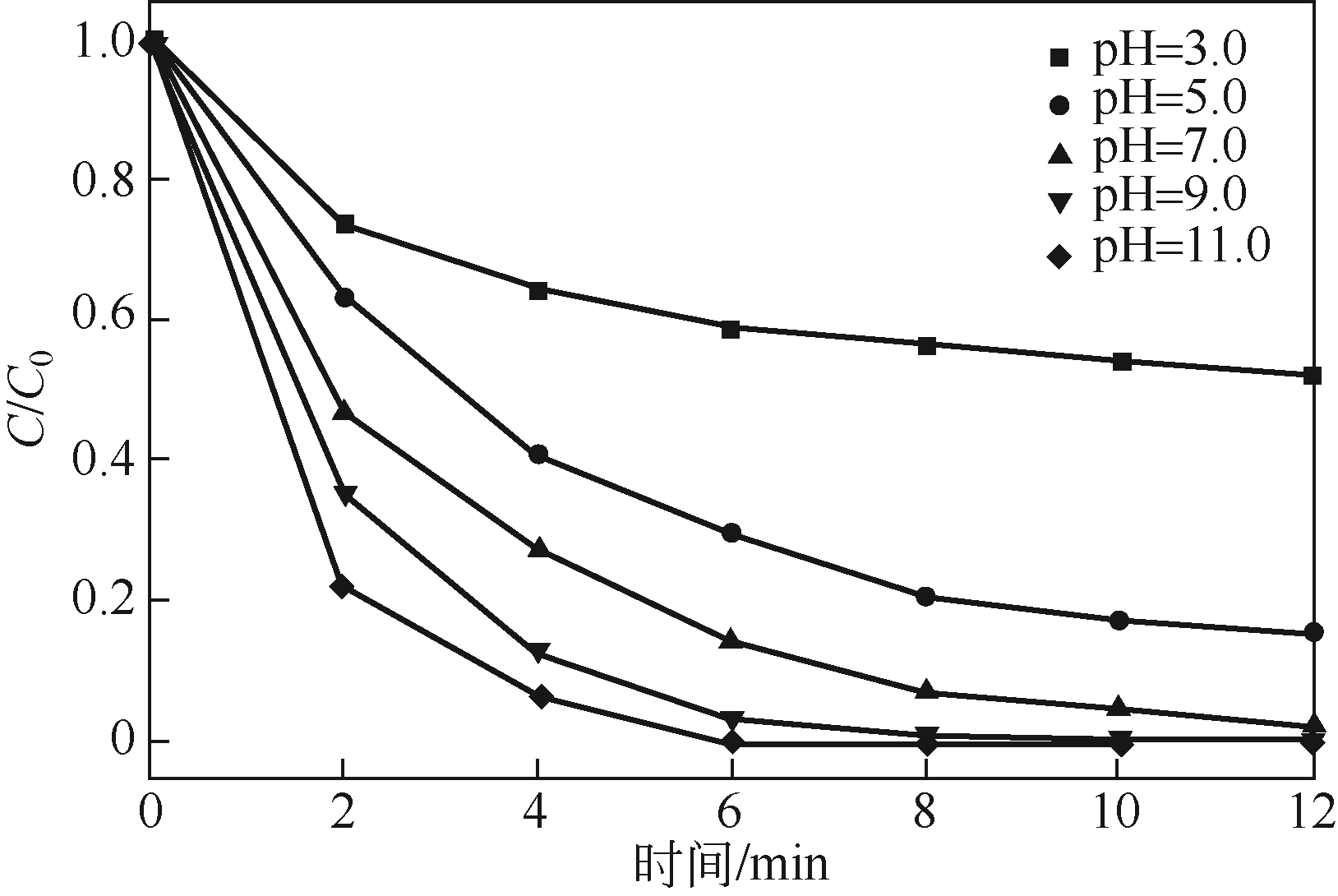
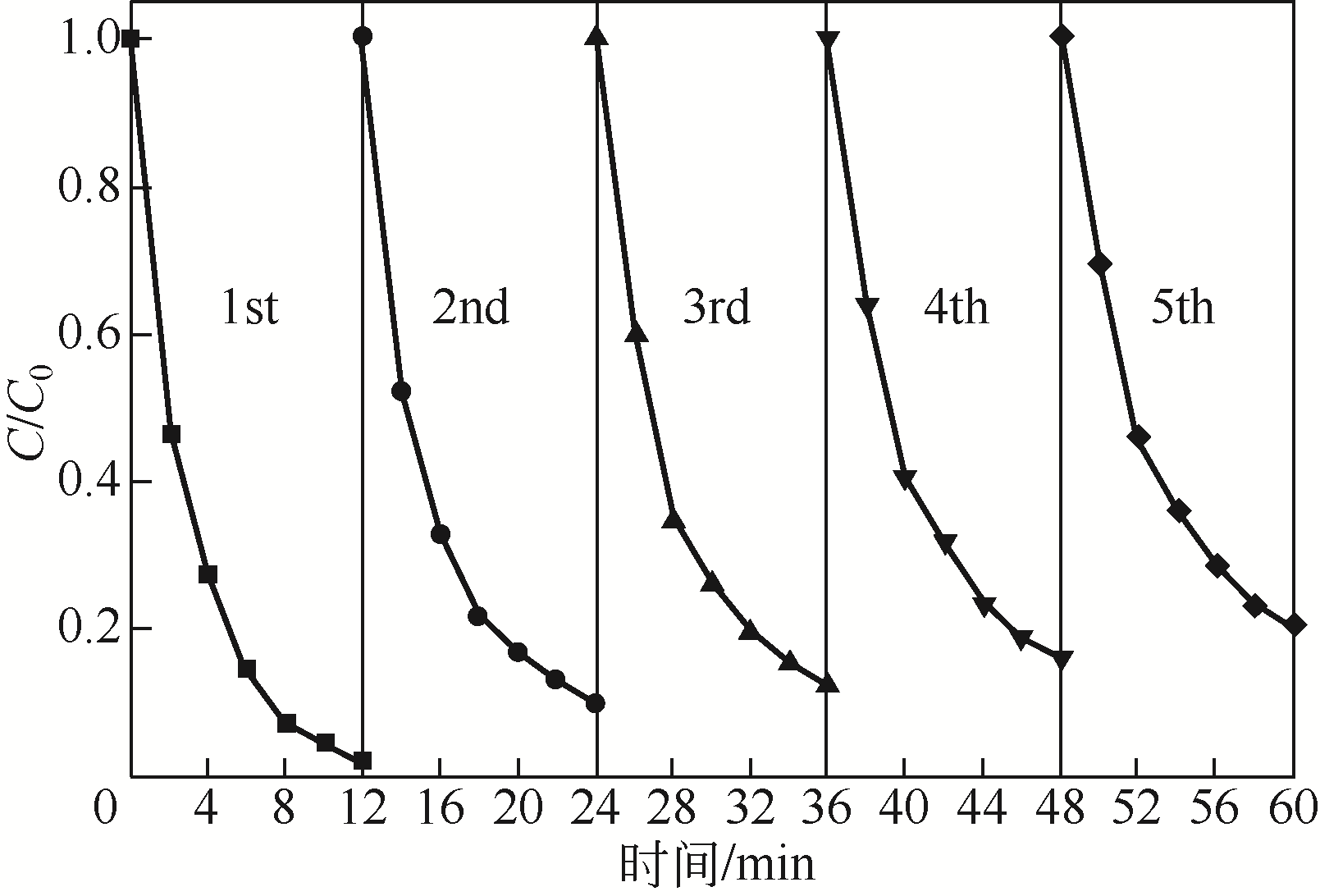
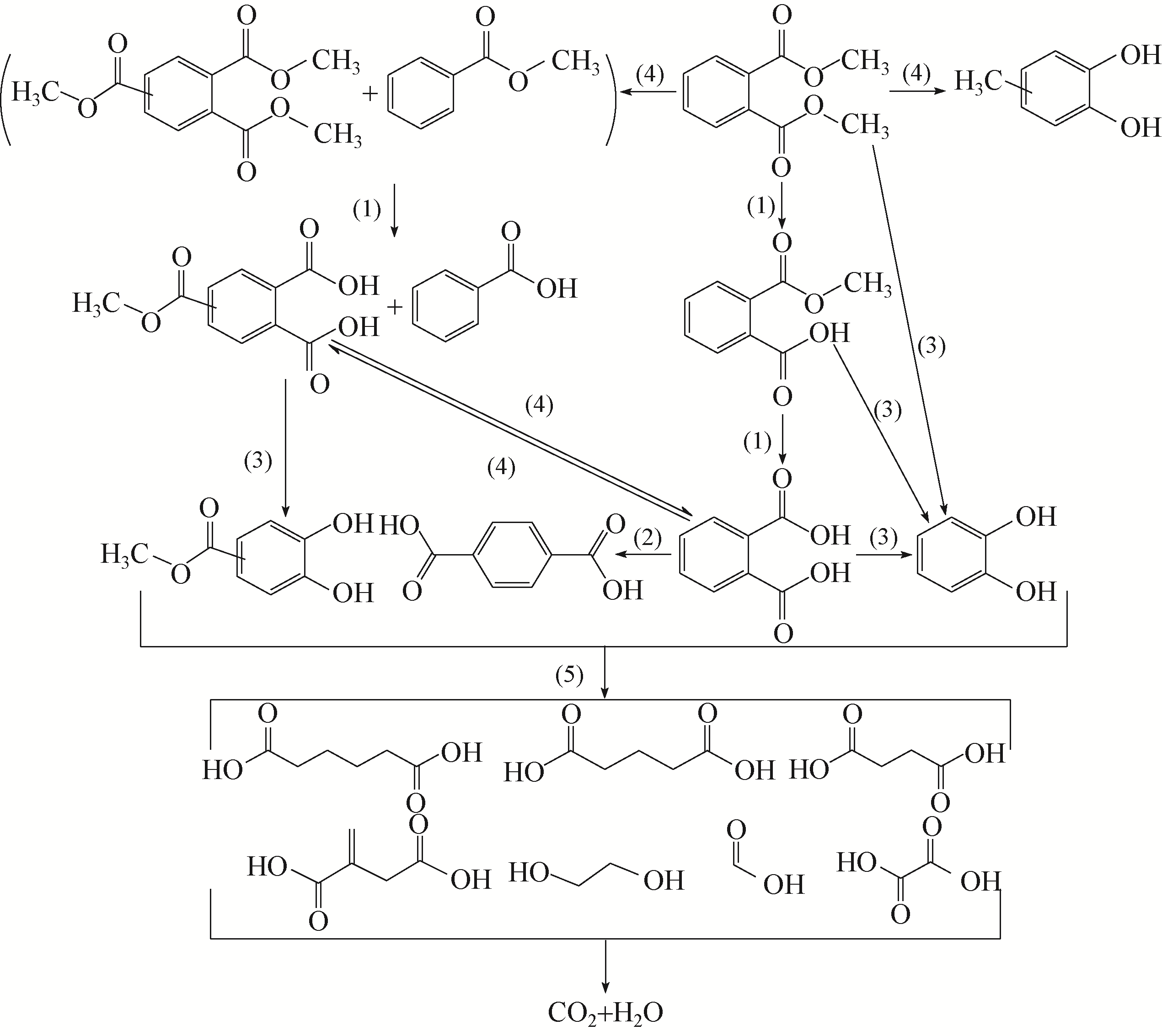
 ) in aqueous solution[J]. Applied Catalysis B: Environmental, 2001, 33(3): 239-248.
) in aqueous solution[J]. Applied Catalysis B: Environmental, 2001, 33(3): 239-248.