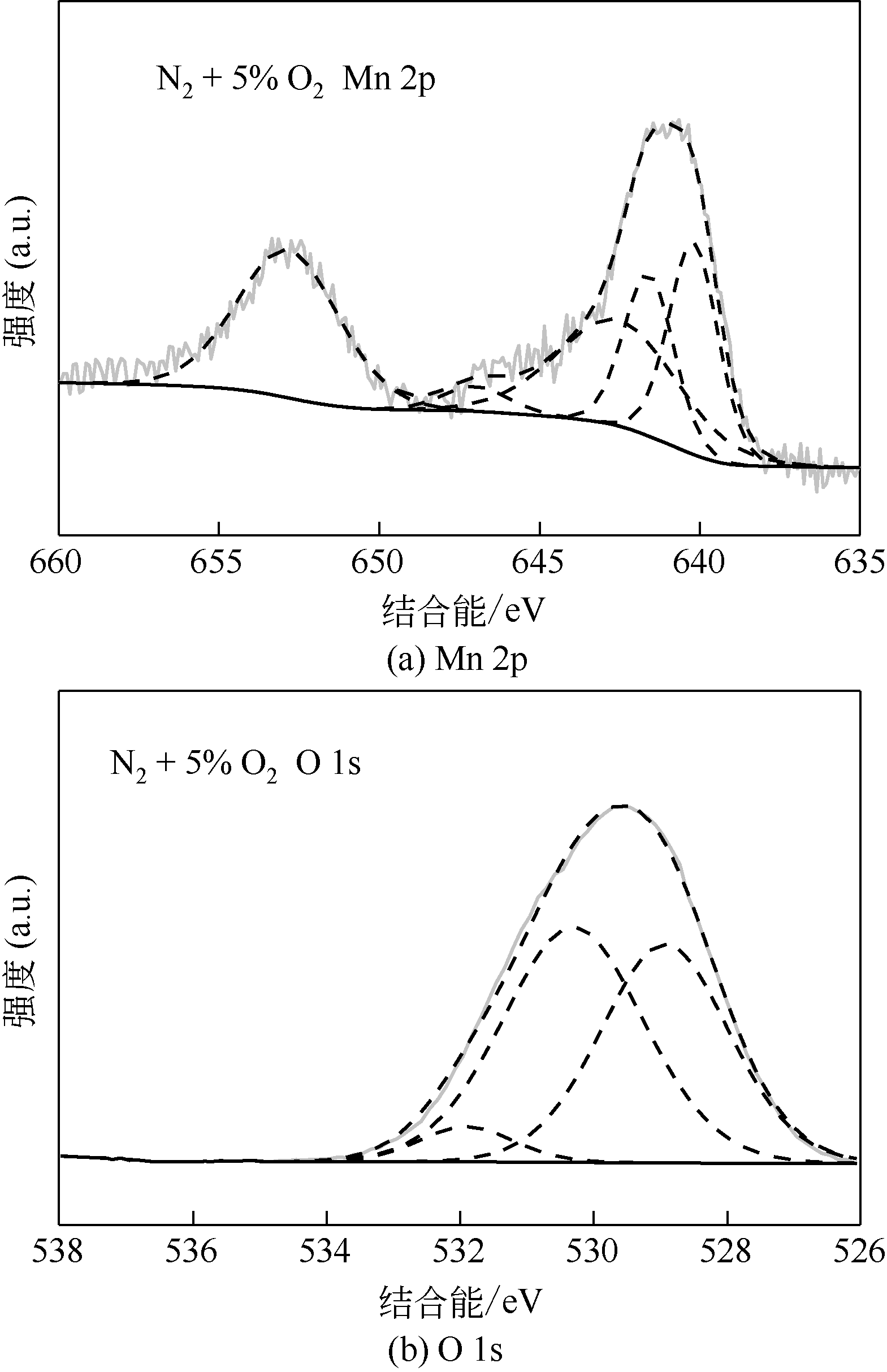
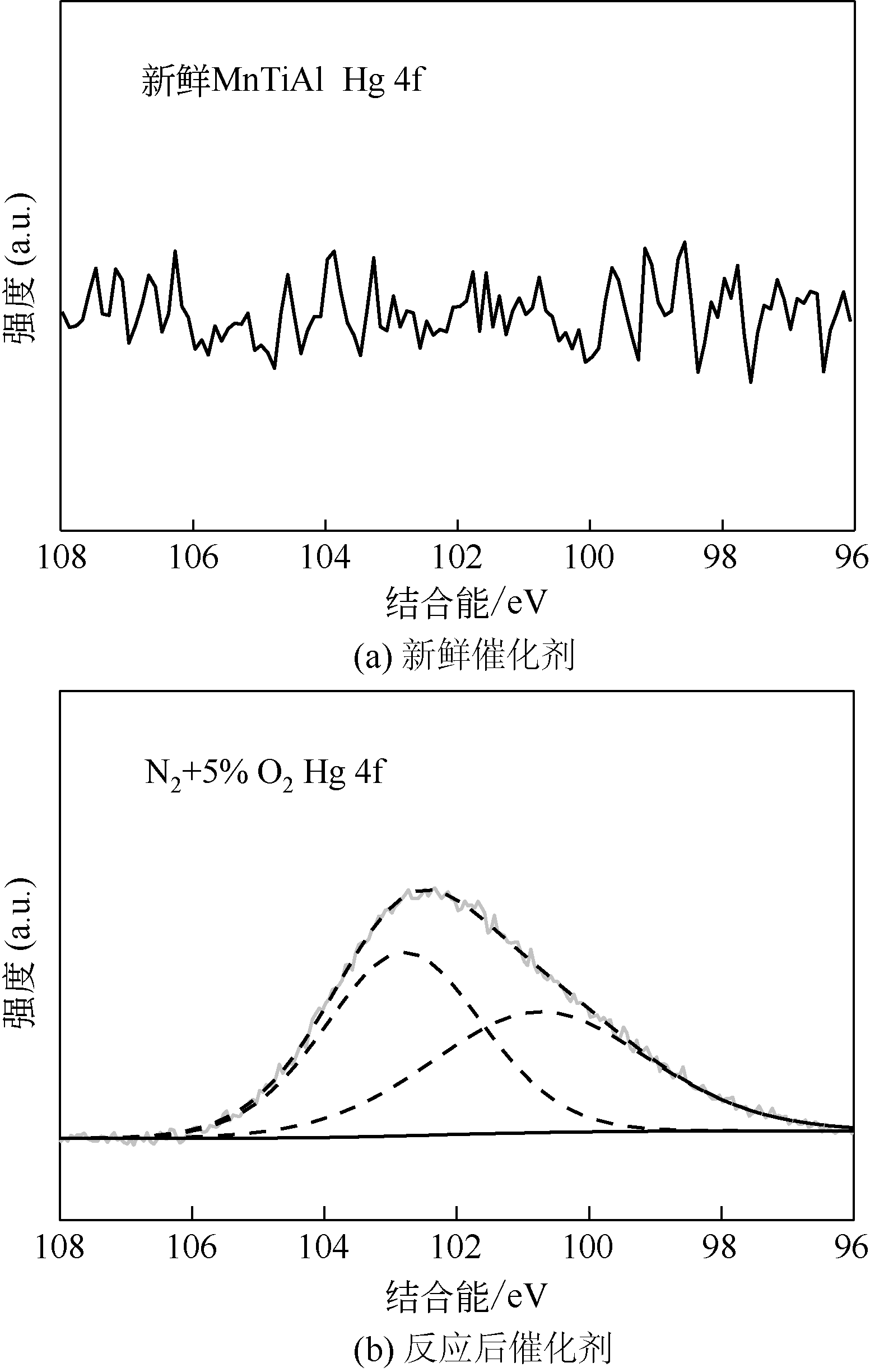
| 1 |
ZHAO S, PUDASAINEE D, DUAN Y, et al. A review on mercury in coal combustion process: content and occurrence forms in coal, transformation, sampling methods, emission and control technologies[J]. Progress in Energy and Combustion Science, 2019, 73: 26-64.
|
| 2 |
YANG Y, LIU J, WANG Z. Reaction mechanisms and chemical kinetics of mercury transformation during coal combustion[J]. Progress in Energy and Combustion Science, 2020, 79: 100844.
|
| 3 |
ZHANG S, ZHAO Y, YANG J, et al. Fe-modified MnOx/TiO2 as the SCR catalyst for simultaneous removal of NO and mercury from coal combustion flue gas[J]. Chemical Engineering Journal, 2018, 348: 618-629.
|
| 4 |
ZHAO B, YI H, TANG X, et al. Using CuO-MnOx/AC-H as catalyst for simultaneous removal of Hg0 and NO from coal-fired flue gas[J]. Journal of Hazardous Materials, 2019, 364: 700-709.
|
| 5 |
洪亚光, 段钰锋, 朱纯, 等. 载溴高硫石油焦活性炭脱汞实验研究[J]. 中国电机工程学报, 2014(11): 1762-1768.
|
|
HONG Yaguang, DUAN Yufeng, ZHU Chun, et al. Experimental study on mercury removal of high-sulfur petroleum coke activated carbon impregnated with bromine[J]. Proceedings of the CSEE, 2014(11): 1762-1768.
|
| 6 |
YANG W, CHEN H, HAN X, et al. Preparation of magnetic Co-Fe modified porous carbon from agricultural wastes by microwave and steam activation for mercury removal[J]. Journal of Hazardous Materials, 2020, 381: 120981.
|
| 7 |
LI H, WANG S, WANG X, et al. Catalytic oxidation of Hg0 in flue gas over Ce modified TiO2 supported Co-Mn catalysts: characterization, the effect of gas composition and co-benefit of NO conversion[J]. Fuel, 2017, 202: 470-482.
|
| 8 |
YANG Z, LI H, LIU X, et al. Promotional effect of CuO loading on the catalytic activity and SO2 resistance of MnOx/TiO2 catalyst for simultaneous NO reduction and Hg0 oxidation[J]. Fuel, 2018, 227: 79-88.
|
| 9 |
XIE J, YAN N, YANG S, et al. Synthesis and characterization of nano-sized Mn-TiO2 catalysts and their application to removal of gaseous elemental mercury[J]. Research on Chemical Intermediates, 2012, 38 (9): 2511-2522.
|
| 10 |
ZHANG S, ZHAO Y, WANG Z, et al. Integrated removal of NO and mercury from coal combustion flue gas using manganese oxides supported on TiO2[J]. Journal of Environmental Sciences, 2017, 53: 141-150.
|
| 11 |
赵琳琳, 黄亚继, 宋静, 等. V-W/TiO2/γ-Al2O3催化剂催化还原NO的研究[J]. 环境科学与技术, 2012, 35 (5): 85-89.
|
|
ZHAO Linlin, HUANG Yaji, SONG Jing, et al. Comparative study on V-W/TiO2/γ-Al2O3 for selective catalytic reduction of NO[J]. Environmental Science & Technology, 2012, 35 (5): 85-89.
|
| 12 |
ZHU G, YANG B, WANG S. Nanocrystallites-forming hierarchical porous Ni/Al2O3-TiO2 catalyst for dehydrogenation of organic chemical hydrides[J]. International Journal of Hydrogen Energy, 2011, 36 (21): 13603-13613.
|
| 13 |
LIU X, LIU Y, LI X, et al. Cyclopropanation on a highly active heterogeneous catalyst: CuO/TiO2-Al2O3[J]. Applied Catalysis A: General, 2003, 239 (1): 279-286.
|
| 14 |
ZHANG J, LI C, ZHAO L, et al. A sol-gel Ti-Al-Ce-nanoparticle catalyst for simultaneous removal of NO and Hg0 from simulated flue gas[J]. Chemical Engineering Journal, 2017, 313: 1535-1547.
|
| 15 |
ZHAO B, RAN R, GUO X, et al. Nb-modified Mn/Ce/Ti catalyst for the selective catalytic reduction of NO with NH3 at low temperature[J]. Applied Catalysis A: General, 2017, 545: 64-71.
|
| 16 |
CHIU C, HSI H, LIN H, et al. Effects of properties of manganese oxide-impregnated catalysts and flue gas condition on multipollutant control of Hg0 and NO[J]. Journal of Hazardous Materials, 2015, 291: 1-8.
|
| 17 |
GAO X, JIANG Y, ZHONG Y, et al. The activity and characterization of CeO2-TiO2 catalysts prepared by the sol-gel method for selective catalytic reduction of NO with NH3[J]. Journal of Hazardous Materials, 2010, 174 (1): 734-739.
|
| 18 |
JOSSEN R, HEINE M, PRATSINIS S, et al. Thermal stability and catalytic activity of flame-made silica-vanadia-tungsten oxide-titania[J]. Applied Catalysis B: Environmental, 2007, 69 (3): 181-188.
|
| 19 |
WU J, ZHAO Z, HUANG T, et al. Removal of elemental mercury by Ce-Mn co-modified activated carbon catalyst[J]. Catalysis Communications, 2017, 93: 62-66.
|
| 20 |
WU P, ZHANG Y, ZHUANG K, et al. Promoting effect and mechanism of neodymium on low-temperature selective catalytic reduction with NH3 over Mn/TiO2 catalysts[J]. Journal of Rare Earths, 2020, 38(11): 1215-1223.
|
| 21 |
ZHAO B, RAN R, WU X, et al. Comparative study of Mn/TiO2 and Mn/ZrO2 catalysts for NO oxidation[J]. Catalysis Communications, 2014, 56: 36-40.
|
| 22 |
KAPTEIJN F, SINGOREDJO L, ANDREINI A, et al. Activity and selectivity of pure manganese oxides in the selective catalytic reduction of nitric oxide with ammonia[J]. Applied Catalysis B: Environmental, 1994, 3 (2): 173-189.
|
| 23 |
ZHOU Z, LIU X, LI C, et al. Seawater-assisted synthesis of MnCe/zeolite-13X for removing elemental mercury from coal-fired flue gas[J]. Fuel, 2020, 262: 116605.
|
| 24 |
YANG J, ZHAO Y, LIANG S, et al. Magnetic iron-manganese binary oxide supported on carbon nanofiber (Fe3-xMnxO4/CNF) for efficient removal of Hg0 from coal combustion flue gas[J]. Chemical Engineering Journal, 2018, 334: 216-224.
|
| 25 |
YANG J, ZHANG M, LI H, et al. Simultaneous NO reduction and Hg0 oxidation over La0.8Ce0.2MnO3 perovskite catalysts at low temperature[J]. Industrial & Engineering Chemistry Research, 2018, 57 (29): 9374-9385.
|
 ), 黄亚继1(
), 黄亚继1( ), 丁守一1, 程好强1, 王圣2, 段钰锋1
), 丁守一1, 程好强1, 王圣2, 段钰锋1
 ), Yaji HUANG1(
), Yaji HUANG1( ), Shouyi DING1, Haoqiang CHENG1, Sheng WANG2, Yufeng DUAN1
), Shouyi DING1, Haoqiang CHENG1, Sheng WANG2, Yufeng DUAN1