化工进展 ›› 2021, Vol. 40 ›› Issue (1): 354-365.DOI: 10.16085/j.issn.1000-6613.2020-0558
毕赤酵母分泌表达植物病毒蛋白制备自组装纳米催化剂提高催化性能
杨坤1( ), 卢晓雪1, 杨林松1, 赵庆欢2, 朱劼1(
), 卢晓雪1, 杨林松1, 赵庆欢2, 朱劼1( )
)
- 1.常州大学制药与生命科学学院,江苏 常州 213164
2.江苏苏州工业园区苏州百拓技术服务有限公司,江苏 苏州 215123
-
收稿日期:2020-04-09出版日期:2021-01-05发布日期:2021-01-12 -
通讯作者:朱劼 -
作者简介:杨坤(1995—),男,硕士研究生,研究方向为纳米催化剂。E-mail:1015992107@qq.com 。 -
基金资助:国家自然科学基金(21676029)
Secretion of plant viral proteins in Pichia pastoris and self-assembled nanocatalyst for enhancing catalytic performance
Kun YANG1( ), Xiaoxue LU1, Linsong YANG1, Qinghuan ZHAO2, Jie ZHU1(
), Xiaoxue LU1, Linsong YANG1, Qinghuan ZHAO2, Jie ZHU1( )
)
- 1.School of Pharmaceutical Engineering & Life Science, Changzhou University, Changzhou 213164, Jiangsu, China
2.Suzhou BioTOP Technical Service Co. , Ltd. , Suzhou Industrial Park, Suzhou 215123, Jiangsu, China
-
Received:2020-04-09Online:2021-01-05Published:2021-01-12 -
Contact:Jie ZHU
摘要:
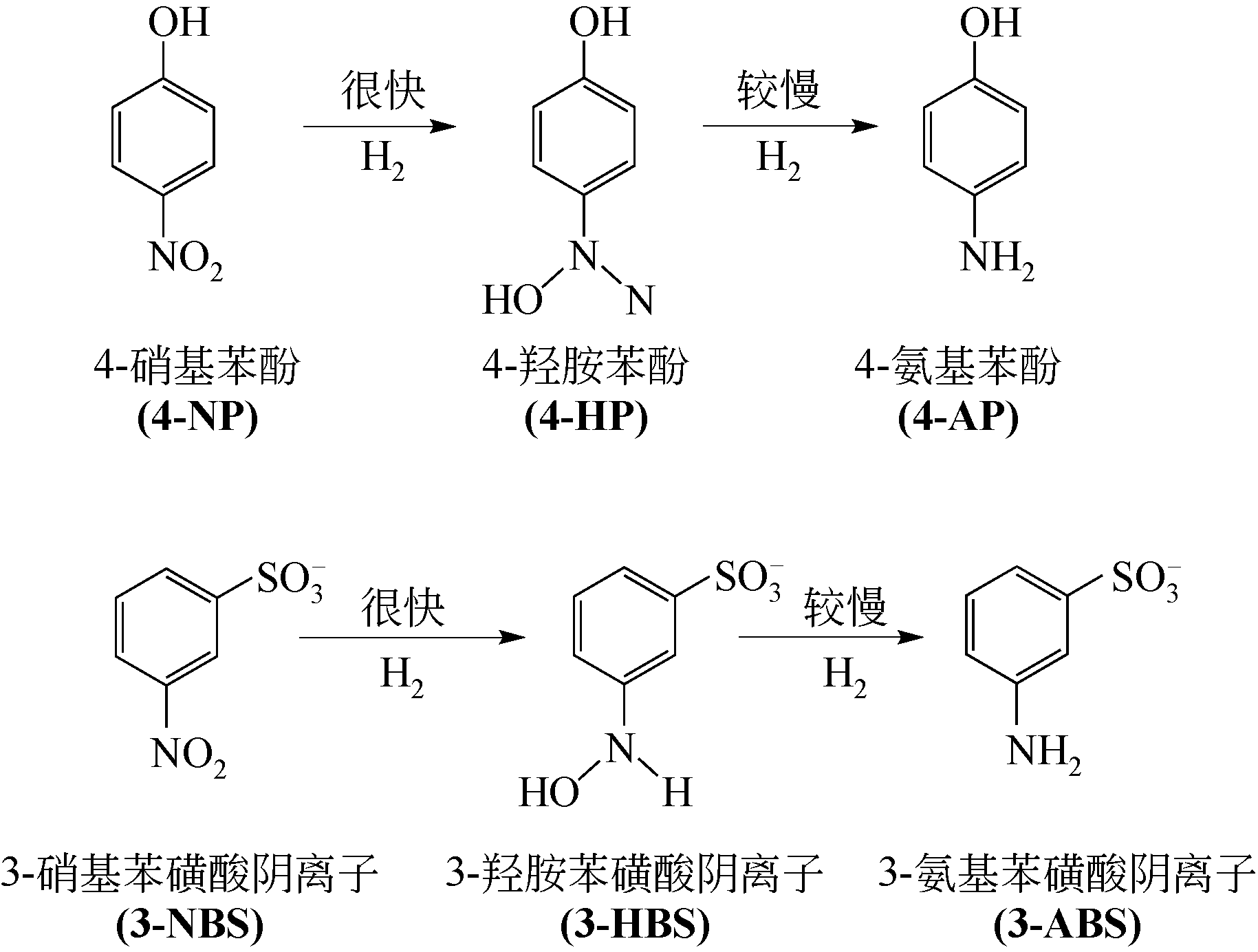
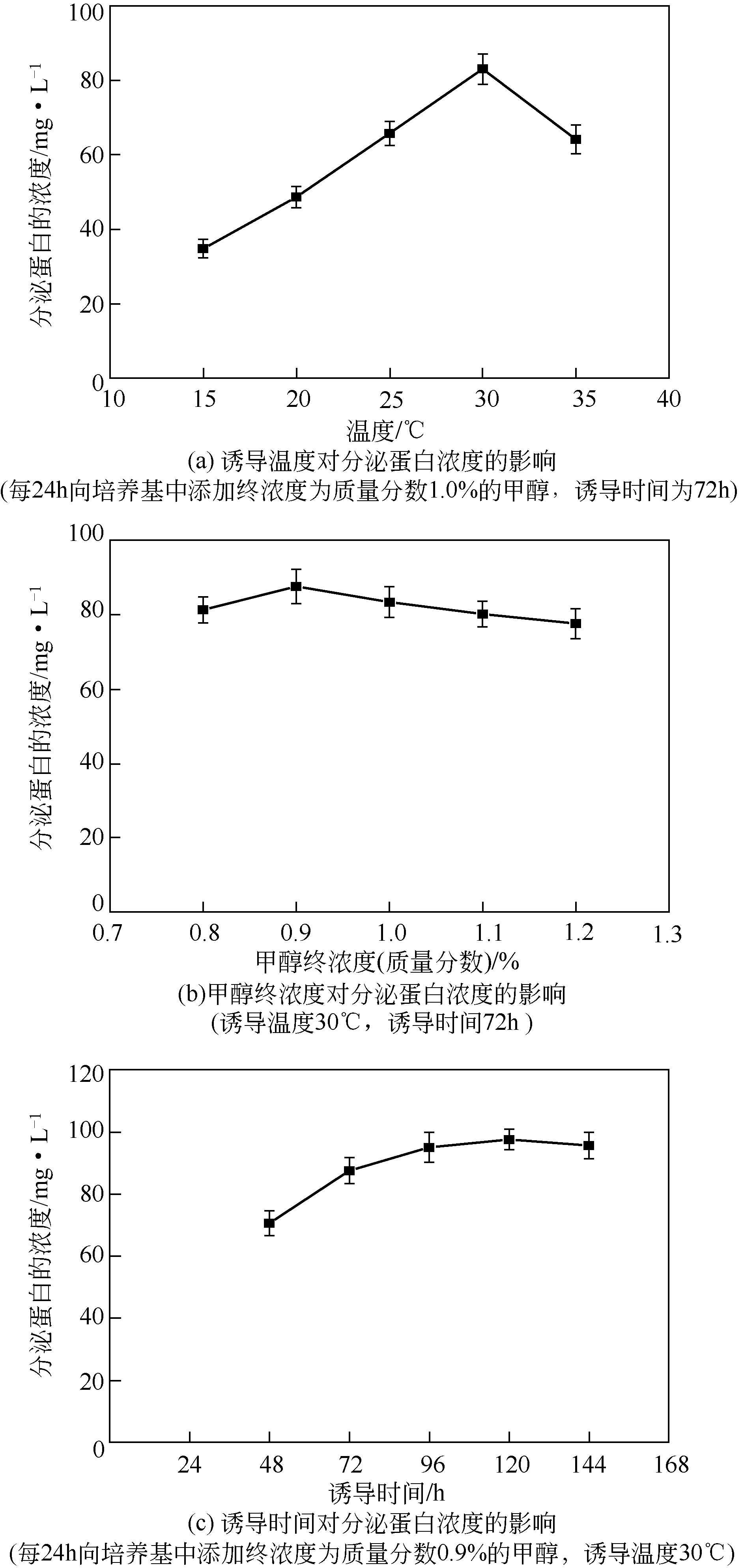
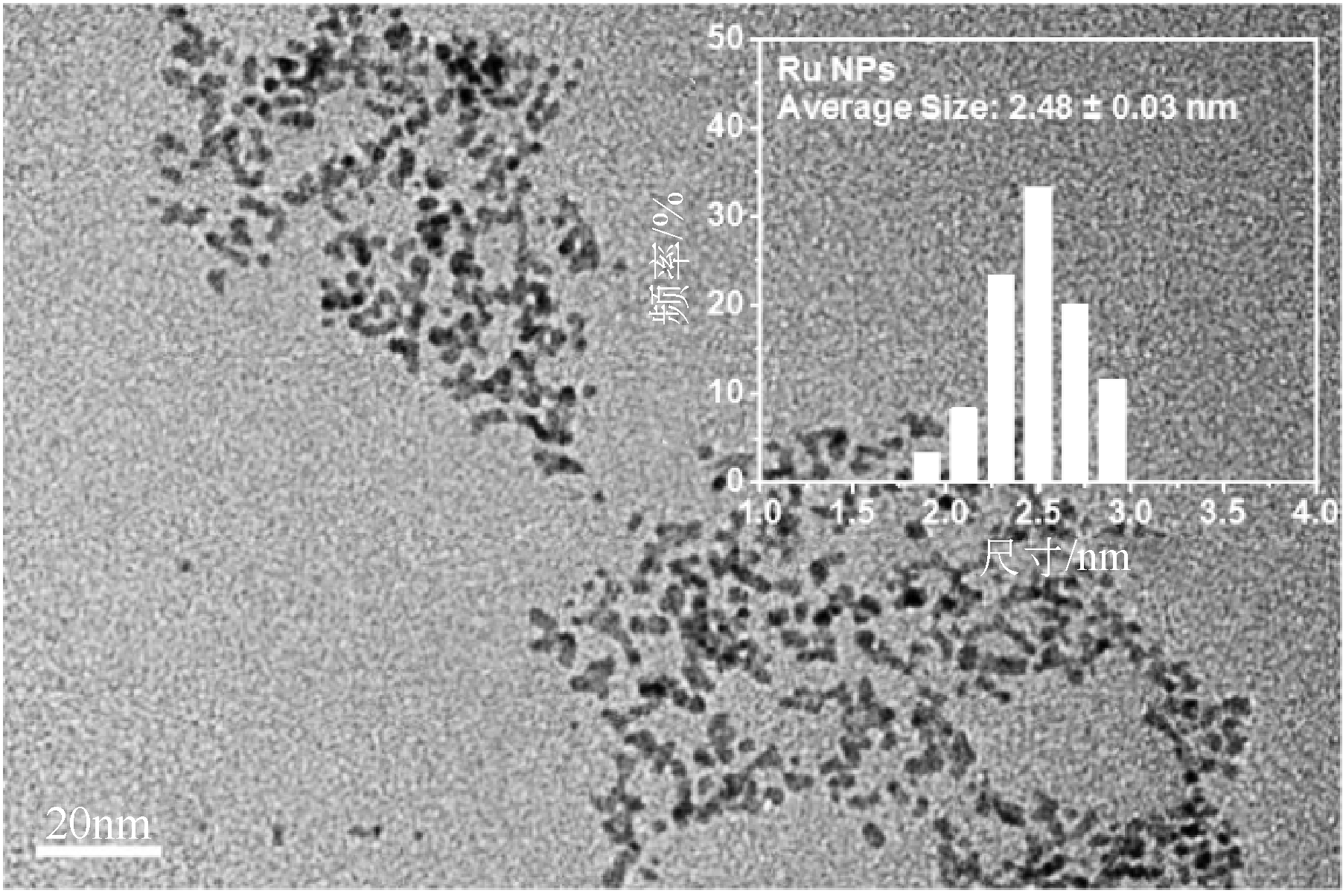
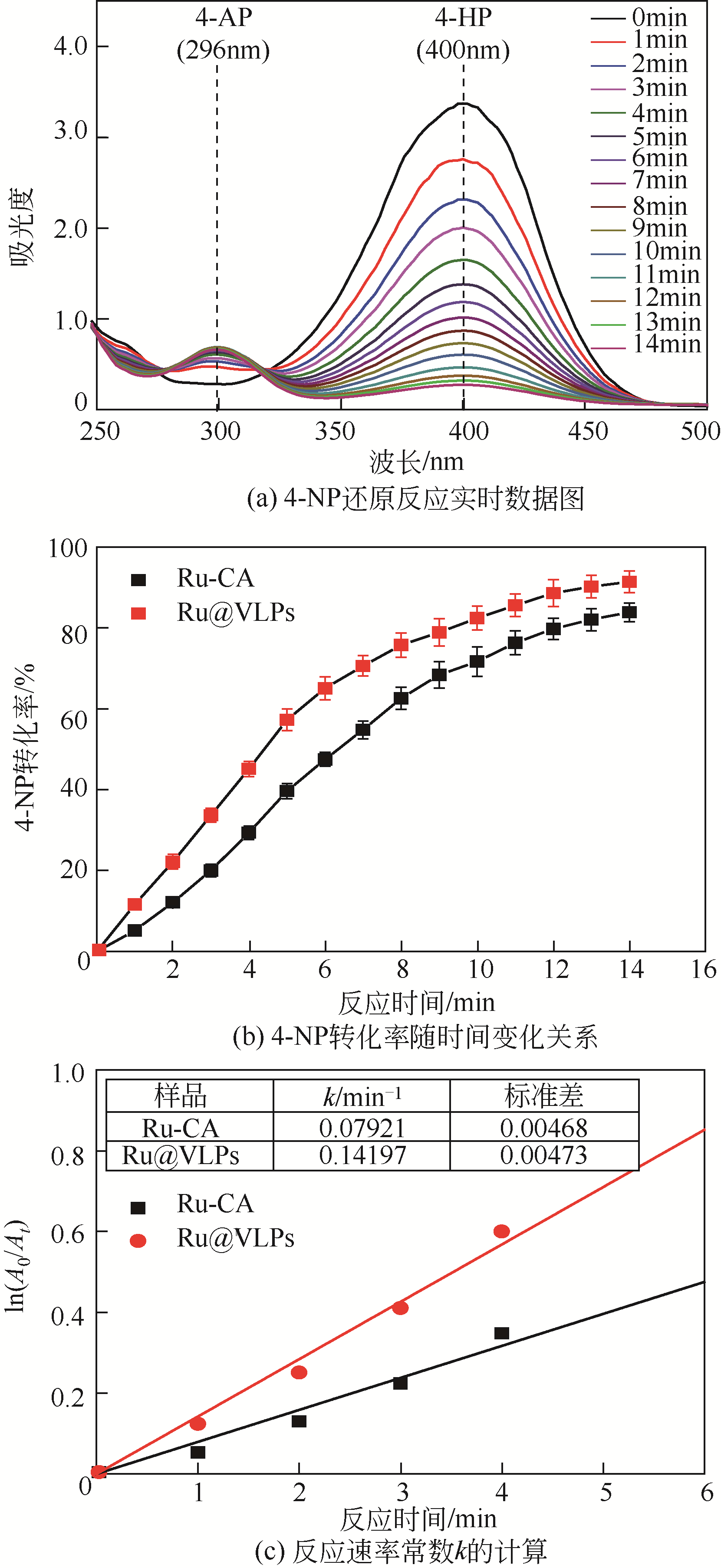
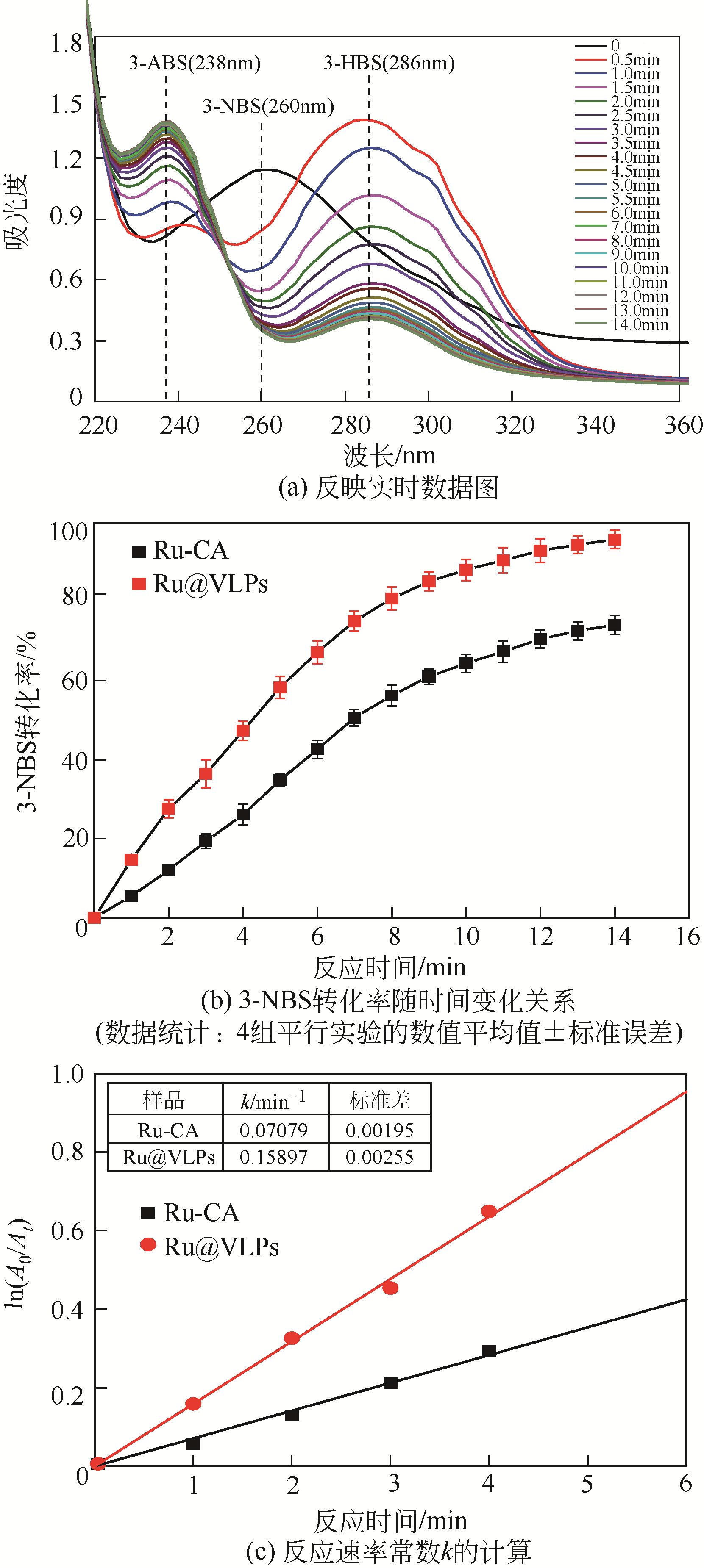
病毒样颗粒(VLPs)包裹纳米粒子的亚细胞结构仿生设计在催化领域潜力巨大。本文构建了一株基于毕赤酵母(Pichia pastoris)的基因工程菌,用于分泌表达重组豇豆褪绿斑驳病毒衣壳蛋白(CCMV CPs)。通过表达工艺的优化(诱导温度30℃、诱导时间96h、每24h添加终浓度体积分数1.0%的甲醇),可在发酵上清液中直接收获较高产量[(101.4±3.2)mg/L]和较高纯度(>90%)的衣壳蛋白。纯化后的CCMV CPs具有较强的体外自组装性能,能高效封装柠檬酸稳定的钌纳米颗粒(Ru-CA)(封装率约70%),制备具有核壳结构的复合纳米催化剂Ru@VLPs。与非负载型催化剂Ru-CA相比,该催化剂在硝基芳烃包括4-硝基苯酚(4-NP)和3-硝基苯磺酸钠(3-NBS)还原反应中显示出较高的催化活性,表观反应速率常数(k)分别达到约0.14min-1(4-NP还原反应)和0.16min-1(3-NBS还原反应)。经计算,它催化的4-NP还原反应活化能为32kJ/mol,略低于Ru-CA(39kJ/mol)。Ru@VLPs较高的催化性能可归因于CCMV CPs封装Ru-CA后其分散性和稳定性的提高,以及Ru纳米颗粒与衣壳蛋白功能基团(如氨基,—NH2)之间的协同作用。同时表明,Ru@VLPs亦具有较好的回收再利用性能。
中图分类号:
引用本文
杨坤, 卢晓雪, 杨林松, 赵庆欢, 朱劼. 毕赤酵母分泌表达植物病毒蛋白制备自组装纳米催化剂提高催化性能[J]. 化工进展, 2021, 40(1): 354-365.
Kun YANG, Xiaoxue LU, Linsong YANG, Qinghuan ZHAO, Jie ZHU. Secretion of plant viral proteins in Pichia pastoris and self-assembled nanocatalyst for enhancing catalytic performance[J]. Chemical Industry and Engineering Progress, 2021, 40(1): 354-365.
| 序号 | A/℃ | B/h | C/% | CCMV蛋白含量/mg·L-1 |
|---|---|---|---|---|
| 1 | 1(27) | 1(84) | 1(0.8) | 81.3±3.6 |
| 2 | 1(27) | 2(96) | 2(0.9) | 89.2±2.8 |
| 3 | 1(27) | 3(108) | 3(1.0) | 92.3±4.5 |
| 4 | 2(30) | 1(84) | 2(0.9) | 94.1±4.6 |
| 5 | 2(30) | 2(96) | 3(1.0) | 101.4±3.2 |
| 6 | 2(30) | 3(108) | 1(0.8) | 96.6±2.2 |
| 7 | 3(33) | 1(84h) | 3(1.0) | 83.5±4.1 |
| 8 | 3(33) | 2(96) | 1(0.8) | 89.6±3.4 |
| 9 | 3(33) | 3(108) | 2(0.9) | 87.7±3.8 |
| K1 | 262.8 | 258.9 | 267.5 | |
| K2 | 292.1 | 280.2 | 271.0 | |
| K3 | 260.8 | 276.6 | 277.2 | |
| 极差 | 31.3 | 21.3 | 9.7 | |
| 最优方案 | A2B2C3 | |||
表1 CCMV蛋白表达条件优化L9(33)正交实验结果
| 序号 | A/℃ | B/h | C/% | CCMV蛋白含量/mg·L-1 |
|---|---|---|---|---|
| 1 | 1(27) | 1(84) | 1(0.8) | 81.3±3.6 |
| 2 | 1(27) | 2(96) | 2(0.9) | 89.2±2.8 |
| 3 | 1(27) | 3(108) | 3(1.0) | 92.3±4.5 |
| 4 | 2(30) | 1(84) | 2(0.9) | 94.1±4.6 |
| 5 | 2(30) | 2(96) | 3(1.0) | 101.4±3.2 |
| 6 | 2(30) | 3(108) | 1(0.8) | 96.6±2.2 |
| 7 | 3(33) | 1(84h) | 3(1.0) | 83.5±4.1 |
| 8 | 3(33) | 2(96) | 1(0.8) | 89.6±3.4 |
| 9 | 3(33) | 3(108) | 2(0.9) | 87.7±3.8 |
| K1 | 262.8 | 258.9 | 267.5 | |
| K2 | 292.1 | 280.2 | 271.0 | |
| K3 | 260.8 | 276.6 | 277.2 | |
| 极差 | 31.3 | 21.3 | 9.7 | |
| 最优方案 | A2B2C3 | |||
| 表达宿主(菌株,载体) | CCMV CPs产量 | CCMV CPs性质 | 纯化步骤 | 参考文献 |
|---|---|---|---|---|
E. coli(BL21,pET23a ); E. coli(BL21(DE3), pET28a ) | 75~100mg/L | 细胞内表达 包涵体 | 细胞破裂,变性,复性,超速离心 | [ |
| E. coli(Rosetta 2, pET19b) | 25.92mg/L | 细胞内表达 可溶性蛋白 | 细胞破裂,亲和色谱 | [ |
| Pseudomonas fluorescens(DC487,pDOW3250) | 2.6g/L | 细胞内表达 可溶性蛋白 | 细胞破裂,PEG沉淀,蔗糖密度梯度离心 | [ |
| Pichia pastoris(pPICZA) | 0.05~0.5mg/g细胞湿重; 4.8g/L | 细胞内表达 可溶性蛋白 | 细胞破裂,PEG沉淀,CsCl梯度离心 | [ |
| Pichia pastoris(pPIC9K) | 约101mg/L | 分泌表达 可溶性蛋白 | 阴离子交换层析法 | 本文 |
表2 CCMV CPs在一些基因工程菌中的表达
| 表达宿主(菌株,载体) | CCMV CPs产量 | CCMV CPs性质 | 纯化步骤 | 参考文献 |
|---|---|---|---|---|
E. coli(BL21,pET23a ); E. coli(BL21(DE3), pET28a ) | 75~100mg/L | 细胞内表达 包涵体 | 细胞破裂,变性,复性,超速离心 | [ |
| E. coli(Rosetta 2, pET19b) | 25.92mg/L | 细胞内表达 可溶性蛋白 | 细胞破裂,亲和色谱 | [ |
| Pseudomonas fluorescens(DC487,pDOW3250) | 2.6g/L | 细胞内表达 可溶性蛋白 | 细胞破裂,PEG沉淀,蔗糖密度梯度离心 | [ |
| Pichia pastoris(pPICZA) | 0.05~0.5mg/g细胞湿重; 4.8g/L | 细胞内表达 可溶性蛋白 | 细胞破裂,PEG沉淀,CsCl梯度离心 | [ |
| Pichia pastoris(pPIC9K) | 约101mg/L | 分泌表达 可溶性蛋白 | 阴离子交换层析法 | 本文 |
| 1 | SANTISO E E,KOSTOV M K,GEORGE A M,et al. Confinement effects on chemical reactions-toward an integrated rational catalyst design[J]. Applied Surface Science, 2007, 253(13): 5570-5579. |
| 2 | ANDREAS S, STEPHEN G R, NICHOLAS D P. Perspective on the influence of interactions between hard and soft templates and precursors on morphology of hierarchically structured porous materials[J]. Procedia Engineering, 2014, 56(3): 696-701. |
| 3 | CHRISSOPOULOU K, ANASTASIADIS S H. Effects of nanoscopic-confinement on polymer dynamics[J]. Soft Matter, 2015, 11(19): 3746-3766. |
| 4 | LEENDERS S H A M, GRAMAGE-DORIA R, DEBRUIN B, et al. Transition metal catalysis in confined spaces[J]. Chemical Society Reviews, 2015, 44(2): 433-448. |
| 5 | DAI W, ZHANG S, YU Z, et al. Zeolite structural confinement effects enhance one-pot catalytic conversion of ethanol to butadiene[J]. ACS Catalysis, 2017, 7: 3703-3706. |
| 6 | FLORIAN G, MICHEL C, ANDRIKOPOULOS P, et al. Computationally exploring confinement effects in the methane-to-methanol conversion over iron-oxo centers in zeolites[J]. ACS Catalysis, 2016, 6(12): 8404-8409. |
| 7 | JANDA A, VLAISAVLJEVICH B, LIN L C, et al. Effects of zeolite structural confinement on adsorption thermodynamics and reaction kinetics for monomolecular cracking and dehydrogenation of n-butane[J]. Journal of the American Chemical Society, 2016, 138(14): 4739-4756. |
| 8 | XIAO J, PAN X, ZHANG F, et al. Size-dependence of carbon nanotube confinement in catalysis[J]. Chemical Science, 2017, 8: 278-283. |
| 9 | DENG D, CHEN X, YU L, et al. A single iron site confined in a graphene matrix for the catalytic oxidation of benzene at room temperature[J]. Science Advances, 2015, 1(11): e1500462. |
| 10 | MINERS S A, RANCE G A, KHLOBYSTOV A N. Regioselective control of aromatic halogenation reactions in carbon nanotube nanoreactors[J]. Chemical Communications, 2013(49): 5586-5588. |
| 11 | BRUNET G, SAFIN D A, ROBEYNES K, et al. Confinement effects of a crystalline sponge on ferrocene and ferrocene carboxaldehyde[J]. Chemical Communications, 2017(41): 5645-5648. |
| 12 | WANG Y, CUI H, WEI Z, et al. Engineering catalytic coordination space in a chemically stable Ir-porphyrin MOF with a confinement effect inverting conventional Si-H insertion chemoselectivity[J]. Chemical Science, 2017, 8: 775-780. |
| 13 | JIANG Y, ZHANG X, DAI X, et al. Microwave-assisted synthesis of ultrafine Au nanoparticles immobilized on MOF-199 in high loading as efficient catalysts for a three-component coupling reaction[J]. Nano Research, 2017, 10(3): 876-889. |
| 14 | LI T, LIN H, ZHANG Y, et al. Improved characteristics and protective efficacy in an animal model of E.coli-derived recombinant double-layered rotavirus virus-like particles[J]. Vaccine, 2014, 32(17): 1921-1931. |
| 15 | LIU J, DAI S, WANG M, et al. Virus like particle-based vaccines against emerging infectious disease viruses[J]. Virologica Sinica, 2016, 21(4): 279-287. |
| 16 | LUO Q, HOU C, BAI Y, et al. Protein assembly: versatile approaches to construct highly ordered nanostructures[J]. Chemical Reviews, 2016, 116(22): 13571-13632. |
| 17 | YANG L, LIU A, CAO S, et al. Self-assembly of proteins: towards supramolecular materials[J]. Chemistry: A European Journal, 2016, 22: 1-14. |
| 18 | DOUGLAS T, YOUNG M. Viruses: making friends with old foes[J]. Science, 2006, 312(5775): 873-875. |
| 19 | LEI Y, HAMADA Y, CONG L, et al. Targeted tumor delivery and controlled release of neuronal drugs with ferritin nanoparticles to regulate pancreatic cancer progression[J]. Control Release, 2016, 232: 131-142. |
| 20 | ROHOVIE M, NAGASAWA M, SWARTZ J. Virus-like particles: next-generation nanoparticles for targeted therapeutic delivery[J]. Bioeng. Transl. Med., 2017, 2: 43-57. |
| 21 | LIU A, TRAULSEN, CORNELISSEN, et al. Nitroarene reduction by a virus protein cage based nanoreactor[J]. ACS Catalysis, 2016, 6: 3084-3091. |
| 22 | BRASCH M, PUTRI M, RUITER V, et al. Assembling enzymatic cascade pathways inside virus-based nanocages using dual-tasking nucleic acid tags[J]. Journal of the American Chemical Society, 2017, 139: 1512-1519. |
| 23 | ESCOSURA A, NOLTE R, CORNELISSEN. Viruses and protein cages as nanocontainers and nanoreactors[J]. Chemical, 2009, 19(16): 2274-2278. |
| 24 | SPEIR A, MUNSHI S, WANG G, et al. Structures of the native and swollen forms of cowpea chlorotic mottle virus determined by X-ray crystallography and cryo-electron microscopy[J]. Structure, 1995, 3(1): 63-78. |
| 25 | LAVELLE L, MICHEL J, GINGERY M. The disassembly, reassembly and stability of CCMV protein capsids[J]. Journal of Virological Methods, 2007, 146(1/2): 311-316. |
| 26 | DOUGLAS T, YOUNG M. Host-guest encapsulation of materials by assembled virus protein cages[J]. Nature, 1998, 393: 152-155. |
| 27 | ZHANG Y, ARDEJANI M, ORNEN B. Design and applications of protein-cage-based nanomaterials[J]. Chemistry: An Asian Journal, 2016, 11: 2814-2828. |
| 28 | CHAUDHARY A, YADAV R. A review on virus protein self-assembly[J]. Journal of Nanoparticle Research, 2019, 21(11): 254. |
| 29 | BHASKAR S, LIM S. Engineering protein nanocages as carriers for biomedical applications[J]. NPG Asia Materials, 2017, 9(4): e371. |
| 30 | ALI A, ROOSSINCK M. Rapid and efficient purification of cowpea chlorotic mottle virus by sucrose cushion ultracentrifugation[J]. Journal of Virological Methods, 2007, 141(1): 84-86. |
| 31 | WU Y, YANG H, SHIN H J. Expression and self assembly of cowpea chlorotic mottle virus capsid proteins in Pichia pastoris and encapsulation of fluorescent myoglobin[J]. MRS Proceedings, 2011, 1317: 6-14. |
| 32 | BRUMFIELD S, WILLITS D, TANG L, et al. Heterologous expression of the modified coat protein of cowpea chlorotic mottle bromovirus results in the assembly of protein cages with altered architectures and function[J]. Journal of General Virology, 2004, 85(4): 1049-1053. |
| 33 | WU Y, KIM W, KIM S, et al. Expression and self-assembly of heterocapsa circularisquama RNA virus-like particles synthesized in Pichia pastoris[J] Chinese Sci. Bull., 2012, 57: 3288-3293. |
| 34 | TOME-AMAT J, FLEISCHER L, PARKER S, et al. Secreted production of assembled norovirus virus-like particles from Pichia pastoris[J]. Microbial Cell Factories, 2014, 13(1): 134. |
| 35 | DAMASCENO L, HUANG C, BATT C. Protein secretion in Pichia pastoris and advances in protein production[J]. Applied Microbiology & Biotechnology, 2012, 93(1): 31-39. |
| 36 | HASSANI-MEHRABAN A, CREUTZBURG S, HEEREVELD L, et al. Feasibility of cowpea chlorotic mottle virus-like particles as scaffold for epitope presentations[J]. BMC Biotechnology, 2015, 15(1): 80. |
| 37 | PHELPS J, DAO P, JIN H, et al. Expression and self-assembly of cowpea chlorotic mottle virus-like particles in pseudomonas fluorescens[J]. Biotechnology, 2007, 128(2): 290-296. |
| 38 | DOAZ-VALLE A, GARCIA-SALCEDO Y, CHAVEZ-CALVILLO G, et al. Highly efficient strategy for the heterologous expression and purification of soluble cowpea chlorotic mottle virus capsid protein and in vitro pH-dependent assembly of virus-like particles[J]. Journal of Virological Methods, 2015, 225: 23-29. |
| 39 | ARNET C, LANGE J, BOYD A, et al. Expression and secretion of active Moringa oleifera coagulant protein in Bacillus subtilis[J]. Applied Microbiology & Biotechnology, 2019, 103(23/24): 9411-9422. |
| 40 | FREUDL R. Signal peptides for recombinant protein secretion in bacterial expression systems[J]. Microbial Cell Factories, 2018, 17(1): 52. |
| 41 | SHI L, WANG D, CHAN W, et al. Efficient expression and purification of human interferon alpha2b in the methylotrophic yeast, Pichia pastoris[J]. Protein Expression and Purification, 2007, 54(2): 220-226. |
| 42 | WU Y, LI J, YANG H, et al. Targeted cowpea chlorotic mottle virus-based nanoparticles with tumor-homing peptide F3 for photothermal therapy[J]. Biotechnology & Bioprocess Engineering, 2017, 22(6): 700-708. |
| 43 | ALLISON R, JANDA M, AHLQUIST P. Sequence of cowpea chlorotic mottle virus RNAs 2 and 3 and evidence of a recombination event during bromovirus evolution[J]. Virology, 1989, 172(1): 321-330. |
| 44 | BARAN N, NASROL, LAHZADEH M, et al. Pd nanoparticles stabilized on the Schiff base-modified boehmite: catalytic role in suzuki coupling reaction and reduction of nitroarenes[J]. Journal of Organometallic Chemistry, 2019, 900: 120916-120925. |
| 45 | 蓝娜娜, 张薷月, 苏然, 等. 非甲醇诱导重组毕赤酵母表达木聚糖酶的条件优化[J]. 食品与发酵工业, 2018, 44(3): 8-14. |
| LAN Nana, ZHANG Ru, SU Ran, et al. Optimization of xylanase expression by recombinant Komagataella phaffii without methanol[J]. Food and Fermentation Industries, 2018, 44(3): 8-14. | |
| 46 | 廖锡豪, 陈明祥, 谢万勇, 等. 低温诱导对毕赤酵母表达重组外源蛋白的影响[J]. 中国酿造, 2013(2): 14-17. |
| LIAO Xihao, CHEN Mingxiang, XIE Wanyong, et al. Influence of low inducing temperature on foreign protein production in recombinant Pichia pastoris[J]. China Brewing, 2013(2): 14-17. | |
| 47 | LIM H, CHOI S, KIM K, et al. Dissolved-oxygen-stat controlling two variables for methanol induction of guamerin in Pichia pastoris and its application to repeated fed-batch[J]. Applied Microbiology & Biotechnology, 2003, 62: 342-348. |
| 48 | COS O, RAMON R, MONTESINOS J, et al. Operational strategies, monitoring and control of heterologous protein production in the methylotrophic yeast Pichia pastoris under different promoters: a review[J]. Microbial Cell Factories, 2006, 5: 17. |
| 49 | JAHIC M, GUSTAVSSON M, JANSEN A, et al. Analysis and control of proteolysis of a fusion protein in Pichia pastoris fed-batch processes[J]. Biotechnology, 2003, 102: 45-53. |
| 50 | ZHAO X, FOX J, OLSON N, et al. In vitro assembly of cowpea chlorotic mottle virus from coat protein expressed in Escherichia coli and in vitro-transcribed viral cDNA[J]. Virology, 1995, 207: 486-494. |
| 51 | KONG X, ZHU H, CHEN C, et al. Insights into the reduction of 4-nitrophenol to 4-aminophenol on catalysts[J]. Chemical Physics Letters, 2017, 684: 148-152. |
| 52 | FEDORCZYK A, RATAJCZAK J, KUZMYCH O, et al. Kinetic studies of catalytic reduction of 4-nitrophenol with NaBH4 by means of Au nanoparticles dispersed in a conducting polymer matrix[J]. Solid State Electronics, 2015, 19: 2849-2858. |
| 53 | RICCIARDI R, HUSKENS J, VERBOOM W. Influence of the Au/Ag ratio on the catalytic activity of dendrimer-encapsulated bimetallic nanoparticles in microreactors[J]. Journal of Flow Chemistry, 2015, 5: 228-233. |
| [1] | 邓少碧, 边洲峰. 核壳结构在甲烷干重整中的应用[J]. 化工进展, 2023, 42(1): 247-254. |
| [2] | 高亚, 徐丹, 王树元, 朱地. 原子层沉积构建高性能催化剂的研究进展[J]. 化工进展, 2021, 40(8): 4242-4252. |
| [3] | 徐晨, 武向南, 张庆新, 瞿雄伟. 增韧导热环氧树脂/氮化硼复合材料的制备与表征[J]. 化工进展, 2018, 37(12): 4752-4757. |
| [4] | 李素娟, 陈勐, 郑星, 郑经堂, 许倩, 胡平, 郭建波. 核壳型PS@ZnO纳米复合材料的制备及其光催化性能[J]. 化工进展, 2016, 35(08): 2513-2517. |
| [5] | 陈 丹,舒 婷,廖世军. 核壳结构低铂催化剂:设计、制备及核的组成及结构的影响[J]. 化工进展, 2013, 32(05): 1053-1059. |
| [6] | 李 磊,苏碧云,詹国雄,刘 祥. Beta沸石合成研究进展[J]. 化工进展, 2012, 31(11): 2465-2469. |
| [7] | 张 强1,Anders Thygesen2,Anne Belinda Thomsen2. 不同脱毒方法对玉米秸秆水解液酒精发酵的影响 [J]. 化工进展, 2011, 30(4): 739-. |
| [8] | 叶 晓 云. 二氧化硅/二氧化锆核壳复合材料的制备及性能 [J]. 化工进展, 2010, 29(9): 1710-. |
| [9] | 彭子青1,谌伟庆2,马洪波1,黄思富1,石秋杰1. 核壳结构纳米复合材料在催化中的应用 [J]. 化工进展, 2010, 29(8): 1461-. |
| [10] | 张 强1,戈兴炜2,Anders Thygesen3,Anne Belinda Thomsen3. 利用树干毕赤酵母发酵玉米秸秆制备燃料酒精 [J]. 化工进展, 2010, 29(12): 2270-. |
| [11] | 叶晓云1,蔡曙光1,周钰明2. Ag@SiO2核壳纳米粒子的制备与表征 [J]. 化工进展, 2010, 29(1): 100-. |
| [12] | 沈江南,裘俊红,郑幸存,吴礼光,高从堦. 微乳液聚合在分离膜制备中的应用研究进展 [J]. 化工进展, 2008, 27(4): 515-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||