化工进展 ›› 2021, Vol. 40 ›› Issue (1): 463-476.DOI: 10.16085/j.issn.1000-6613.2020-0474
金属有机框架材料吸附VOCs影响因素研究进展
杨建成1,2( ), 王诗宁1, 杨硕1, 杨明涛1, 沈伯雄1,2(
), 王诗宁1, 杨硕1, 杨明涛1, 沈伯雄1,2( ), 张笑1
), 张笑1
- 1.河北工业大学能源与环境工程学院,天津市清洁能源利用与污染物控制重点实验室,天津 300401
2.河北省动力系统污染物控制技术创新中心,天津 300401
-
收稿日期:2020-03-27出版日期:2021-01-05发布日期:2021-01-12 -
通讯作者:沈伯雄 -
作者简介:杨建成(1981—),男,博士,硕士生导师,研究方向为烟气污染控制理论及技术。E-mail:yangjch1023@hebut.edu.cn 。 -
基金资助:河北省高等学校科学技术研究重点项目(ZD2017019);科技部国家重点研发计划(2016YFC0209202);国家自然科学基金(5180081987)
Influence factors of VOCs adsorption on metal-organic frameworks: the reviews
Jiancheng YANG1,2( ), Shining WANG1, Shuo YANG1, Mingtao YANG1, Boxiong SHEN1,2(
), Shining WANG1, Shuo YANG1, Mingtao YANG1, Boxiong SHEN1,2( ), Xiao ZHANG1
), Xiao ZHANG1
- 1.Tianjin Key Laboratory of Clean Energy and Pollutant Control, School of Energy and Environmental Engineering, Hebei University of Technology, Tianjin 300401, China
2.Hebei Engineering Research Center of Pollution Control in Power System, Tianjin 300401
-
Received:2020-03-27Online:2021-01-05Published:2021-01-12 -
Contact:Boxiong SHEN
摘要:
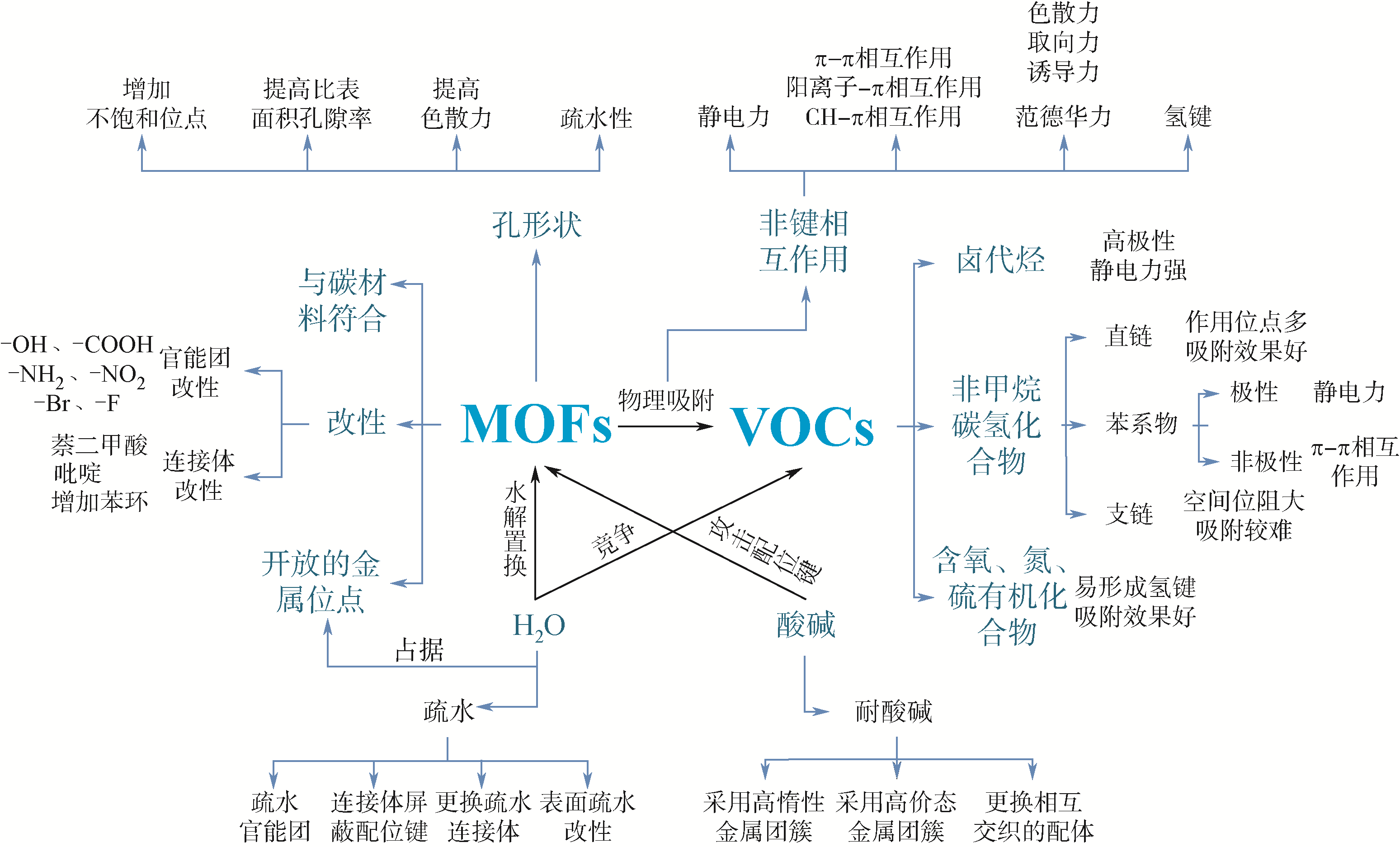
挥发性有机化合物(VOCs)对环境和人体健康均具有严重危害,而吸附法作为有效的VOCs脱除技术已得到广泛应用。在众多吸附剂中,金属有机框架材料(MOFs)以其极大的比表面积、可调节的孔径和可修饰性等优势,在VOCs脱除领域展示出良好的应用前景。本文首先介绍了在吸附过程中涉及到的吸附机理,从影响因素角度回顾了近年MOFs在VOCs吸附方面的研究进展。按照吸附质与吸附剂的几何结构、改性官能团、MOFs的金属位点、酸碱、水和碳材料复合等多个方面剖析了吸附过程中的影响因素,并将其分为内部影响因素和外部影响因素两大部分。针对影响因素归纳了提高吸附量的主要方法,并对MOFs吸附VOCs的吸附量进行了汇总。最后总结并展望了未来应用MOFs吸附VOCs的研究发展方向,期望为深入研究VOCs脱除技术提供有价值的参考。
中图分类号:
引用本文
杨建成, 王诗宁, 杨硕, 杨明涛, 沈伯雄, 张笑. 金属有机框架材料吸附VOCs影响因素研究进展[J]. 化工进展, 2021, 40(1): 463-476.
Jiancheng YANG, Shining WANG, Shuo YANG, Mingtao YANG, Boxiong SHEN, Xiao ZHANG. Influence factors of VOCs adsorption on metal-organic frameworks: the reviews[J]. Chemical Industry and Engineering Progress, 2021, 40(1): 463-476.
| 吸附剂 | 吸附质 | 相互作用力 | 参考文献 |
|---|---|---|---|
| MIL-101 | 苯 | π-π | [ |
| 甲苯 | π-π、阳离子-π | [ | |
| 丙酮 | 静电力、范德华力 | [ | |
| HKUST-1 | 丙酮 | 静电力、范德华力 | [ |
| 苯 | π-π | [ | |
| HKUST-1/ZSM-5复合材料 | 苯 | 阳离子-π | [ |
| UiO-66(NH2) | 甲苯 | 弱氢键 | [ |
| ZIF-67 | 甲苯 | 弱氢键 | [ |
| CMP-200-In/Mg | CH4、C2H4 | 范德华力 | [ |
| C2H3Cl、C2H2Cl2、CH2Cl2、CHCl3 | 卤素键、静电力 | [ | |
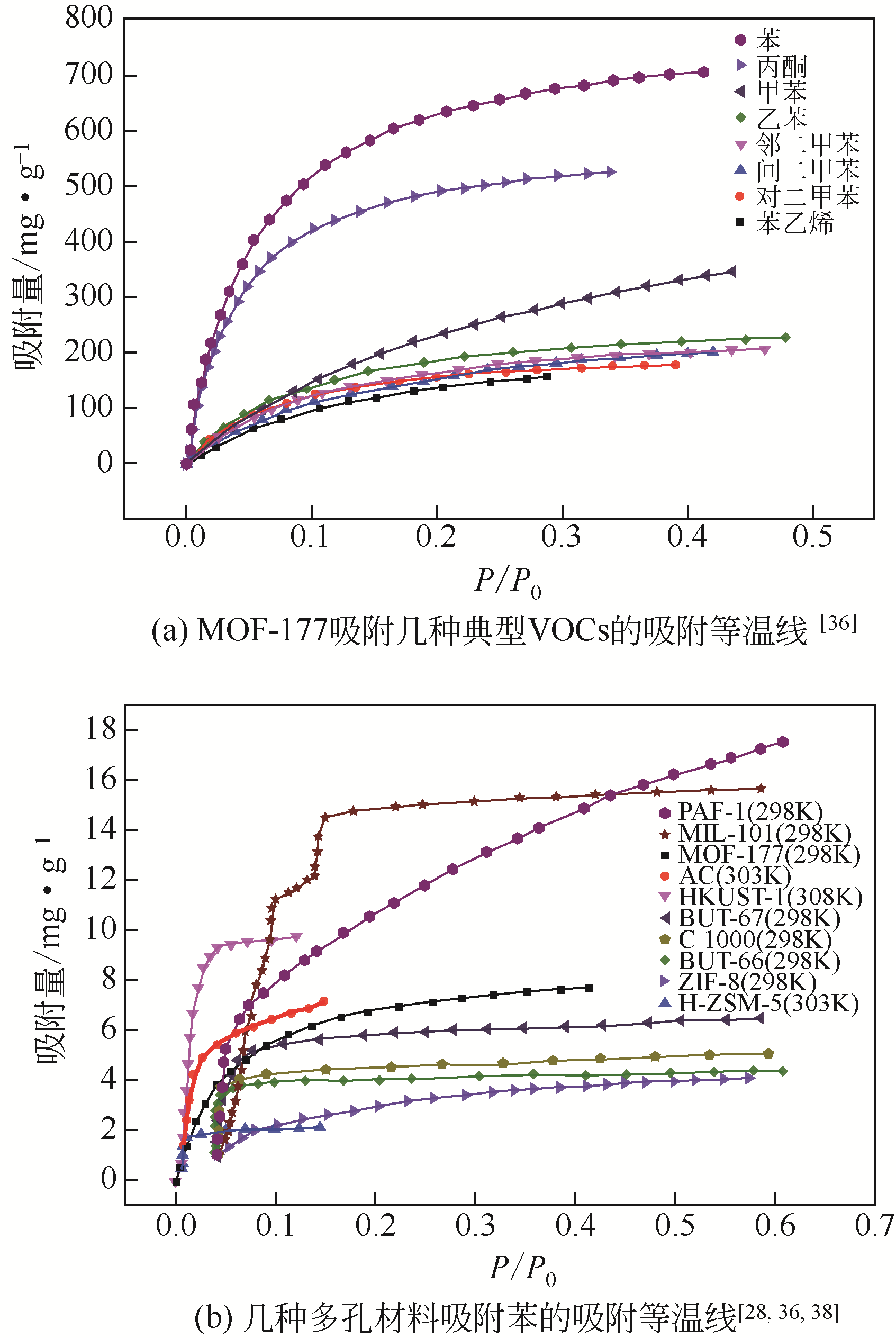
| MOF-177 | 苯 | π-π | [ |
| 丙酮 | 静电力、范德华力 | [ |
表1 不同MOFs材料与吸附剂间主要的相互作用力
| 吸附剂 | 吸附质 | 相互作用力 | 参考文献 |
|---|---|---|---|
| MIL-101 | 苯 | π-π | [ |
| 甲苯 | π-π、阳离子-π | [ | |
| 丙酮 | 静电力、范德华力 | [ | |
| HKUST-1 | 丙酮 | 静电力、范德华力 | [ |
| 苯 | π-π | [ | |
| HKUST-1/ZSM-5复合材料 | 苯 | 阳离子-π | [ |
| UiO-66(NH2) | 甲苯 | 弱氢键 | [ |
| ZIF-67 | 甲苯 | 弱氢键 | [ |
| CMP-200-In/Mg | CH4、C2H4 | 范德华力 | [ |
| C2H3Cl、C2H2Cl2、CH2Cl2、CHCl3 | 卤素键、静电力 | [ | |
| MOF-177 | 苯 | π-π | [ |
| 丙酮 | 静电力、范德华力 | [ |
| 吸附剂 | BET比表面积/m2·g-1 | Langmuir比表面积/m2·g-1 | 孔容积/cm3·g-1 | 孔尺寸/? |
|---|---|---|---|---|
| HKUST-1 | 1568.5 | 2081.4 | 0.75 | 9~10 |
| MOF-177 | 2970 | 4170 | 1.11 | 9.4 |
| MIL-101 | 2925 | 4977 | 1.56 | 笼子30~40,窗口12~16 |
| BUT-66 | 1096 | 1291 | 0.46 | 孔道6,窗口4 |
| BUT-67 | 984 | 1141 | 0.41 | 孔道7,窗口5.5 |
| AC | 1600 | — | 2 | 4~4.6 |
| PAF-1 | 2380 | 3209 | 1.99 | 7~12(三维孔) |
| ZIF-8 | 1510 | 2008 | 0.85 | 笼子12.5,窗口3.3 |
| HZSM-5 | 550 | — | 0.2 | — |
| Carboxen 1000 | 1017 | 1213 | 0.61 | 10~12 |
表2 MOFs和几种其他典型吸附剂的几何性质
| 吸附剂 | BET比表面积/m2·g-1 | Langmuir比表面积/m2·g-1 | 孔容积/cm3·g-1 | 孔尺寸/? |
|---|---|---|---|---|
| HKUST-1 | 1568.5 | 2081.4 | 0.75 | 9~10 |
| MOF-177 | 2970 | 4170 | 1.11 | 9.4 |
| MIL-101 | 2925 | 4977 | 1.56 | 笼子30~40,窗口12~16 |
| BUT-66 | 1096 | 1291 | 0.46 | 孔道6,窗口4 |
| BUT-67 | 984 | 1141 | 0.41 | 孔道7,窗口5.5 |
| AC | 1600 | — | 2 | 4~4.6 |
| PAF-1 | 2380 | 3209 | 1.99 | 7~12(三维孔) |
| ZIF-8 | 1510 | 2008 | 0.85 | 笼子12.5,窗口3.3 |
| HZSM-5 | 550 | — | 0.2 | — |
| Carboxen 1000 | 1017 | 1213 | 0.61 | 10~12 |
| 吸附剂 | 吸附质 | 吸附量/mmol·g-1 | 温度/K | 压力/kPa | 参考文献 |
|---|---|---|---|---|---|
| MIL-Z1 | 苯① | 3.35 | 298 | 1 | [ |
| 苯② | 2.94 | 298 | 1 | [ | |
| 苯③ | 2.63 | 298 | 1 | [ | |
| HKUST-1 | 苯 | 9.1 | 298 | 0.05 | [ |
| 苯④ | 6.55 | 308 | 0.15 | [ | |
| 苯 | 9.97 | 298 | 0.0521 | [ | |
| 水 | 26~31 | 298 | 0.25-0.3 | [ | |
| 甲苯 | 1.72 | 293 | 101.3 | [ | |
| 甲苯 | 6.6 | 298 | P/P0>0.1 | [ | |
| BUT-66 | 苯 | 2.54 | 298 | 0.012 | [ |
| Carboxen 1000 | 苯 | 2.27 | 298 | 0.012 | [ |
| PDVB | 甲苯 | 5.215 | 298 | 101.3 | [ |
| 乙酸乙酯 | 1.31 | 298 | 101.3 | [ | |
| ZIF-8/PDVB | 甲苯 | 5.9707 | 298 | 101.3 | [ |
| 乙酸乙酯 | 1.668 | 298 | 101.3 | [ | |
| ZIF-8 | 甲苯 | 0.84 | 298 | 101.3 | [ |
| 乙酸乙酯 | 1.1 | 298 | 101.3 | [ | |
| 苯 | 0.03 | 298 | 0.012 | [ | |
| MCM-41 | 苯 | 10.49 | 298 | 10 | [ |
| MIL-101 | 苯 | 15.84 | 298 | 10 | [ |
| 对二甲苯 | 10.9 | 288 | 0.6 | [ | |
| 甲苯 | 0.626 | 298 | 101.3 | [ | |
| 甲苯 | 22.96 | 298 | 101.3 | [ | |
| 甲苯 | 15.1 | 298 | P/P0>0.1 | [ | |
| PAF-1 | 苯 | 20.59 | 298 | 10 | [ |
| CPM-200-In/Mg | 甲醛 | 13 | 293 | 100 | [ |
| CPM-200-In/Mg-NH2(site2) | 甲醛 | 13.67 | 298 | 100 | [ |
| CPM-5 | 甲苯 | 4.22 | 298 | 101.3 | [ |
| 438-MOF | 甲醛 | 7.333 | 293 | 100 | [ |
| 活性炭 | 甲醛 | 0.3 | 293 | 100 | [ |
| MOF-177 | 丙酮 | 10.155 | 298 | 100 | [ |
| 苯 | 10.256 | 298 | 100 | [ | |
| 甲苯 | 6.359 | 298 | 100 | [ | |
| 乙苯 | 2.557 | 298 | 100 | [ | |
| 二甲苯 | 2.01~2.557 | 298 | 100 | [ | |
| 苯乙烯 | 2.25 | 298 | 100 | [ | |
| MIL-100 | 甲烷 | 0.36 | 298 | 100 | [ |
| 乙烷 | 2.22 | 298 | 100 | [ | |
| 丙烷 | 6.78 | 298 | 100 | [ | |
| UiO-66 | 甲苯 | 1.8 | 293 | 101.3 | [ |
| 二氯甲烷 | 6.0 | 298 | 44 | [ | |
| UiO-66(NH2) | 甲苯 | 2.739 | 293 | 101.3 | [ |
| ZIF-67 | 甲苯 | 2.43 | 293 | 101.3 | [ |
| 4A Zeolite | 甲苯 | 0.334 | 293 | 101.3 | [ |
| 颗粒活性炭 | 二甲苯 | 1.88 | 298 | 101.3 | [ |
| UL-ZSM5 | 二甲苯 | 3.5 | 303 | 0.6 | [ |
| MIL-53 | 甲苯 | 7.93 | 298 | 101.3 | [ |
表3 MOFs与其他多孔材料吸附VOCs吸附量
| 吸附剂 | 吸附质 | 吸附量/mmol·g-1 | 温度/K | 压力/kPa | 参考文献 |
|---|---|---|---|---|---|
| MIL-Z1 | 苯① | 3.35 | 298 | 1 | [ |
| 苯② | 2.94 | 298 | 1 | [ | |
| 苯③ | 2.63 | 298 | 1 | [ | |
| HKUST-1 | 苯 | 9.1 | 298 | 0.05 | [ |
| 苯④ | 6.55 | 308 | 0.15 | [ | |
| 苯 | 9.97 | 298 | 0.0521 | [ | |
| 水 | 26~31 | 298 | 0.25-0.3 | [ | |
| 甲苯 | 1.72 | 293 | 101.3 | [ | |
| 甲苯 | 6.6 | 298 | P/P0>0.1 | [ | |
| BUT-66 | 苯 | 2.54 | 298 | 0.012 | [ |
| Carboxen 1000 | 苯 | 2.27 | 298 | 0.012 | [ |
| PDVB | 甲苯 | 5.215 | 298 | 101.3 | [ |
| 乙酸乙酯 | 1.31 | 298 | 101.3 | [ | |
| ZIF-8/PDVB | 甲苯 | 5.9707 | 298 | 101.3 | [ |
| 乙酸乙酯 | 1.668 | 298 | 101.3 | [ | |
| ZIF-8 | 甲苯 | 0.84 | 298 | 101.3 | [ |
| 乙酸乙酯 | 1.1 | 298 | 101.3 | [ | |
| 苯 | 0.03 | 298 | 0.012 | [ | |
| MCM-41 | 苯 | 10.49 | 298 | 10 | [ |
| MIL-101 | 苯 | 15.84 | 298 | 10 | [ |
| 对二甲苯 | 10.9 | 288 | 0.6 | [ | |
| 甲苯 | 0.626 | 298 | 101.3 | [ | |
| 甲苯 | 22.96 | 298 | 101.3 | [ | |
| 甲苯 | 15.1 | 298 | P/P0>0.1 | [ | |
| PAF-1 | 苯 | 20.59 | 298 | 10 | [ |
| CPM-200-In/Mg | 甲醛 | 13 | 293 | 100 | [ |
| CPM-200-In/Mg-NH2(site2) | 甲醛 | 13.67 | 298 | 100 | [ |
| CPM-5 | 甲苯 | 4.22 | 298 | 101.3 | [ |
| 438-MOF | 甲醛 | 7.333 | 293 | 100 | [ |
| 活性炭 | 甲醛 | 0.3 | 293 | 100 | [ |
| MOF-177 | 丙酮 | 10.155 | 298 | 100 | [ |
| 苯 | 10.256 | 298 | 100 | [ | |
| 甲苯 | 6.359 | 298 | 100 | [ | |
| 乙苯 | 2.557 | 298 | 100 | [ | |
| 二甲苯 | 2.01~2.557 | 298 | 100 | [ | |
| 苯乙烯 | 2.25 | 298 | 100 | [ | |
| MIL-100 | 甲烷 | 0.36 | 298 | 100 | [ |
| 乙烷 | 2.22 | 298 | 100 | [ | |
| 丙烷 | 6.78 | 298 | 100 | [ | |
| UiO-66 | 甲苯 | 1.8 | 293 | 101.3 | [ |
| 二氯甲烷 | 6.0 | 298 | 44 | [ | |
| UiO-66(NH2) | 甲苯 | 2.739 | 293 | 101.3 | [ |
| ZIF-67 | 甲苯 | 2.43 | 293 | 101.3 | [ |
| 4A Zeolite | 甲苯 | 0.334 | 293 | 101.3 | [ |
| 颗粒活性炭 | 二甲苯 | 1.88 | 298 | 101.3 | [ |
| UL-ZSM5 | 二甲苯 | 3.5 | 303 | 0.6 | [ |
| MIL-53 | 甲苯 | 7.93 | 298 | 101.3 | [ |
| 复合材料 | 组合 | 吸附质 | 吸附量/mmol·g-1 | 温度/K | 参考文献 |
|---|---|---|---|---|---|
| MIL-101@GO | MIL-101与氧化石墨 | 正戊烷 | 13.4 | 298 | [ |
| 正己烷 | 11.9 | 298 | [ | ||
| 正庚烷 | 10.7 | 298 | [ | ||
| 正辛烷 | 9.3 | 298 | [ | ||
| 丙酮 | 20.1 | 288 | [ | ||
| MIL-101/TC-40 | MIL-101与烟草茎 | 丙酮 | 19.58 | 288 | [ |
| MIL-101/TC-30 | MIL-101与烟草茎 | 丙酮 | 19.33 | 288 | [ |
| MC-500-6 | HKUST-1与葡萄糖 | 苯 | 12.8 | 298 | [ |
| KC-SB | MOF-5与葡萄糖、蔗糖 | 正己烷 | 10 | 298 | [ |
表4 复合材料吸附VOCs吸附量
| 复合材料 | 组合 | 吸附质 | 吸附量/mmol·g-1 | 温度/K | 参考文献 |
|---|---|---|---|---|---|
| MIL-101@GO | MIL-101与氧化石墨 | 正戊烷 | 13.4 | 298 | [ |
| 正己烷 | 11.9 | 298 | [ | ||
| 正庚烷 | 10.7 | 298 | [ | ||
| 正辛烷 | 9.3 | 298 | [ | ||
| 丙酮 | 20.1 | 288 | [ | ||
| MIL-101/TC-40 | MIL-101与烟草茎 | 丙酮 | 19.58 | 288 | [ |
| MIL-101/TC-30 | MIL-101与烟草茎 | 丙酮 | 19.33 | 288 | [ |
| MC-500-6 | HKUST-1与葡萄糖 | 苯 | 12.8 | 298 | [ |
| KC-SB | MOF-5与葡萄糖、蔗糖 | 正己烷 | 10 | 298 | [ |
| 1 | WORLD HEALTH O. Indoor air quality: organic pollutants[J]. Environmental Technology Letters, 1989, 10(9): 855-858. |
| 2 | ZHANG X, XUE Z, LI H, et al. Ambient volatile organic compounds pollution in China[J]. Journal of Environmental Sciences, 2017, 55: 69-75. |
| 3 | ZHANG X, GAO B, CREAMER A E, et al. Adsorption of VOCs onto engineered carbon materials: a review[J]. Journal of Hazardous Materials, 2017, 338: 102-123. |
| 4 | ZHENG C H, SHEN J L, ZHANG Y X, et al. Quantitative assessment of industrial VOC emissions in China: historical trend, spatial distribution, uncertainties, and projection[J]. Atmospheric Environment, 2017, 150: 116-125. |
| 5 | WEI W, WANG S X, HAO J M, et al. Trends of chemical speciation profiles of anthropogenic volatile organic compounds emissions in China, 2005—2020 [J]. Frontiers of Environmental Science & Engineering, 2012, 8(1): 27-41. |
| 6 | 杨新兴, 李世莲, 尉鹏, 等. 环境中的VOCs及其危害[J]. 前沿科学, 2013, 7(28): 21-35. |
| YANG X X, LI S L, WEI P, et al. Volatile organic compounds in the environment and their harms[J]. Frontier Science, 2013, 7(28): 21-35. | |
| 7 | BERNSTEIN J A, ALEXIS N, BACCHUS H, et al. The health effects of non-industrial indoor air pollution[J]. The Journal of Allergy and Clinical Immunology, 2008, 121(3): 585-591. |
| 8 | KAMAL M S, RAZZAK S A, HOSSAIN M M. Catalytic oxidation of volatile organic compounds (VOCs)—A review[J]. Atmospheric Environment, 2016, 140:117-134. |
| 9 | XU Z N, HUANG X, NIE W, et al. Influence of synoptic condition and holiday effects on VOCs and ozone production in the Yangtze River delta region, China[J]. Atmospheric Environment, 2017, 168: 112-124. |
| 10 | EHN M, THORNTON J A, KLEIST E, et al. A large source of low-volatility secondary organic aerosol[J]. Nature, 2014, 506(7489): 476-479. |
| 11 | ZAITAN H, MANERO M H, VALDES H. Application of high silica zeolite ZSM-5 in a hybrid treatment process based on sequential adsorption and ozonation for VOCs elimination[J]. Journal of Environmental Sciences, 2016, 41: 59-68. |
| 12 | BLÄKER C, PASEL C, LUCKAS M, et al. Investigation of load-dependent heat of adsorption of alkanes and alkenes on zeolites and activated carbon[J]. Microporous and Mesoporous Materials, 2017, 241: 1-10. |
| 13 | 吕双春, 葛云丽, 赵倩, 等. 高硅分子筛的合成及其在VOCs吸附去除领域的应用[J]. 环境化学, 2017, 36(7): 1492-1505. |
| LYU S C, GE Y L, ZHAO Q, et al. Synthesis of high silica molecular sieves and their application in VOCs adsorption removal[J]. Environmental Chemistry, 2017, 36(7): 1492-1505. | |
| 14 | BAUR G B, BESWICK O, SPRING J, et al. Activated carbon fibers for efficient VOC removal from diluted streams: the role of surface functionalities[J]. Adsorption, 2015, 21(4): 255-264. |
| 15 | BAUR G B, YURANOV I, RENKEN A, et al. Activated carbon fibers for efficient VOC removal from diluted streams: the role of surface morphology[J]. Adsorption, 2015, 21(6/7): 479-488. |
| 16 | LI X Q, ZHANG L, YANG Z Q, et al. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: a review[J]. Separation and Purification Technology, 2020, 235: 116213. |
| 17 | TWUMASI E, FORSLUND M, NORBERG P, et al. Carbon-silica composites prepared by the precipitation method. Effect of the synthesis parameters on textural characteristics and toluene dynamic adsorption[J]. Journal of Porous Materials, 2011, 19(3): 333-343. |
| 18 | 胡莹. 活性炭再生技术研究与发展[J]. 煤炭与化工, 2018, 41(4): 136-139. |
| HU Y. Research and development on activated carbon regeneration technologies[J]. Coal and Chemical Industry, 2018, 41(4): 136-139. | |
| 19 | 李莹, 张红星, 闫柯乐, 等. MOFs材料对挥发性有机物VOCs的吸附研究[J]. 广州化工, 2016, 44(8): 27-29. |
| LI Y, ZHANG H X, YAN K L, et al. Research progress on VOCs adsorption of metal-organic frameworks (MOFs)[J]. Guangzhou Chemical Industry, 2016, 44(8): 27-29. | |
| 20 | FARHA O K, ERYAZICI I, JEONG N C, et al. Metal-organic framework materials with ultrahigh surface areas: is the sky the limit?[J]. Journal of the American Chemical Society, 2012, 134(36): 15016-15021. |
| 21 | HK C, DY S P, JAHEON K, et al. A route to high surface area, porosity and inclusion of large molecules in crystals[J]. Nature, 2004, 427(6974): 523-527. |
| 22 | STEPHEN R C, ANTEK G F, ADMA J M. Dramatic tuning of carbon dioxide uptake via metal substitution in a coordination polymer with cylindrical pores[J]. Journal of the American Chemical Society, 2008, 130(33): 10870-10871. |
| 23 | FRANCISCO D L, ANA M C, SOFIA C. Selective separation of BTEX mixtures using metal-organic frameworks[J]. The Journal of Physical Chemistry C, 2014, 118(24): 13126-13136. |
| 24 | EDDAOUDII M, LI H, YAGHI O M. Highly porous and stable metal organic frameworks structure[J]. Journal of the American Chemical Society, 2000, 122(7): 1391-1397. |
| 25 | YANG K, SUN Q, XUE F, et al. Adsorption of volatile organic compounds by metal-organic frameworks MIL-101: influence of molecular size and shape[J]. Journal of Hazardous Materials, 2011, 195: 124-131. |
| 26 | VELLINGIRI K, KUMAR P, DEEP A, et al. Metal-organic frameworks for the adsorption of gaseous toluene under ambient temperature and pressure[J]. Chemical Engineering Journal, 2017, 307: 1116-1126. |
| 27 | BRITT D, TRANCHEMONTAGNE D, YAGHI O M. Metal-organic frameworks with high capacity and selectivity for harmful gases[J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(33): 11623-11627. |
| 28 | XIE L H, LIU X, HE L, et al. Metal-organic frameworks for the capture of trace aromatic volatile organic compounds[J]. Chem., 2018, 4(8): 1911-1927. |
| 29 | MA S, ZHOU H C. Gas storage in porous metal-organic frameworks for clean energy applications[J]. Chem. Commun., 2010, 46(1): 44-53. |
| 30 | 张景梅, 高歌. 金属有机框架多孔材料(MOFs)的制备及其应用研究[J]. 现代化工, 2018, 38(11): 53-57. |
| ZHANG J M, GAO G. Preparation and application of metal-organic frameworks(MOFs) porous materials[J]. Modern Chemical Industry, 2018, 38(11): 53-57. | |
| 31 | WANG B, XIE L H, WANG X, et al. Applications of metal-organic frameworks for green energy and environment: new advances in adsorptive gas separation, storage and removal[J]. Green Energy & Environment, 2018, 3(3): 191-228. |
| 32 | HUANG C, SONG M, GU Z, et al. Probing the adsorption characteristic of metal-organic framework MIL-101 for volatile organic compounds by quartz crystal microbalance[J]. Environmental Science & Technology, 2011, 45(10): 4490-4496. |
| 33 | LIU X L, CHEN G H, WANG X J, et al. Theoretical study on the gas adsorption capacity and selectivity of CPM-200-In/Mg and CPM-200-In/Mg-X (-X=-NH2, -OH, -N, -F)[J]. Physical Chemistry Chemical Physics, 2017, 19(44): 29963-29974. |
| 34 | 王曦, 麦裕良, 张俊杰, 等. MOFs材料对气态硫化合物的吸附研究进展[J]. 现代化工, 2018, 38(7): 62-66. |
| WANG X, MAI Y L, ZHANG J J, et al. Research progress on adsorption of gaseous sulfur compounds by metal-organic frameworks[J]. Modern Chemical Industry, 2018, 38(7): 62-66. | |
| 35 | JIANG J, SANDLER S I. Monte carlo simulation for the adsorption and separation of linear and branched alkanes in IRMOF-1[J]. Langmuir, 2006, 22:5702-5707. |
| 36 | YANG K, XUE F, SUN Q, et al. Adsorption of volatile organic compounds by metal-organic frameworks MOF-177[J]. Journal of Environmental Chemical Engineering, 2013, 1(4): 713-718. |
| 37 | LOW J J, BENIN A I, JAKUBCZAK P, et al. Virtual high throughput screening confirmed experimentally, porous coordination polymer hydration[J]. American Chemical Society, 2009, 131: 15834-15842. |
| 38 | ZHAO Z, WANG S, YANG Y, et al. Competitive adsorption and selectivity of benzene and water vapor on the microporous metal organic frameworks (HKUST-1)[J]. Chemical Engineering Journal, 2015, 259: 79-89. |
| 39 | BAHRI M, HAGHIGHAT F, KAZEMIAN H, et al. A comparative study on metal organic frameworks for indoor environment application: adsorption evaluation[J]. Chemical Engineering Journal, 2017, 313: 711-723. |
| 40 | TRENS P, BELARBI H, SHEPHERD C, et al. Coadsorption of n-hexane and benzene vapors onto the chromium terephthalate-based porous material MIL-101(Cr) an experimental and computational study[J]. The Journal of Physical Chemistry C, 2012, 116(49): 25824-25831. |
| 41 | ZHAO Z, LI X, LI Z. Adsorption equilibrium and kinetics of p-xylene on chromium-based metal organic framework MIL-101[J]. Chemical Engineering Journal, 2011, 173(1): 150-157. |
| 42 | SAINI V K, PIRES J. Development of metal organic fromwork-199 immobilized zeolite foam for adsorption of common indoor VOCs[J]. Journal of Environmental Sciences, 2017, 55: 321-330. |
| 43 | YUAN B, WANG X, ZHOU X, et al. Novel room-temperature synthesis of MIL-100(Fe) and its excellent adsorption performances for separation of light hydrocarbons[J]. Chemical Engineering Journal, 2019, 355: 679-686. |
| 44 | SHAFIEI M, ALIVAND M S, RASHIDI A, et al. Synthesis and adsorption performance of a modified micro-mesoporous MIL-101(Cr) for VOCs removal at ambient conditions[J]. Chemical Engineering Journal, 2018, 341: 164-174. |
| 45 | XU F, XIAN S, XIA Q, et al. Effect of textural properties on the adsorption and desorption of toluene on the metal-organic frameworks HKUST-1 and MIL-101[J]. Adsorption Science & Technology, 2013, 31(4): 325-339. |
| 46 | 陈建东, 许伟城, 吴军良, 等. 金属有机框架ZIF-8/聚二乙烯基苯纳米复合材料的合成及其吸附VOCs的性能[J]. 环境科学学报, 2017, 37(5): 1877-1883. |
| CHEN J D, XU W C, WU J L, et al. Synthesis of metal-organic framework ZIF-8/polydivinylbenzene nanohybrid composite and its adsorption property of VOCs[J]. Acta Scientiae Circumstantiae, 2017, 37(5): 1877-1883. | |
| 47 | GREATHOUSE J A, OCKWIG N W, CRISCENTI L J, et al. Computational screening of metal-organic frameworks for large-molecule chemical sensing[J]. Physical Chemistry Chemical Physics, 2010, 12(39): 12621-12629. |
| 48 | HORCAJADA P, SERRE C, MAURIN G, et al. Flexible porous metal-organic frameworks for a controlled drug delivery[J]. Journal of the American Chemical Society, 2008, 130: 6774-6780. |
| 49 | ZHU Meiping HU P, TONG Z, et al. Enhanced hydrophobic MIL(Cr) metal-organic framework with high capacity and selectivity for benzene VOCs capture from high humid air[J]. Chemical Engineering Journal, 2017, 313: 1122-1131. |
| 50 | ZHOU L, ZHANG X, CHEN Y. Modulated synthesis of zirconium metal–organic framework UiO-66 with enhanced dichloromethane adsorption capacity[J]. Materials Letters, 2017, 197: 167-170. |
| 51 | LI L, WANG S B, FENG Q C, et al. Removal of o-xylene from off-gas by a combination of bioreactor and adsorption[J]. Asia-Pacific Journal of Chemical Engineering, 2008, 3(5): 489-496. |
| 52 | HUANG Q, VINH-THANG H, MALEKIAN A, et al. Adsorption of n-heptane, toluene and o-xylene on mesoporous UL-ZSM5 materials[J]. Microporous and Mesoporous Materials, 2006, 87(3): 224-234. |
| 53 | 吴永标, 刘德飞, 吴颖, 等. MOF-5上甲醇、乙醛和丙酮吸附机理的密度泛函理论研究[J]. 化工学报, 2013, 64(8): 2891-2897. |
| WU Y B, LIU D F, WU Y, et al. Adsorpation mechanism of methanol, acetaldehyde and acetone on MOF-5 with density functional theory[J]. CIESC Journal, 2013, 64(8): 2891-2897. | |
| 54 | MA F J, LIU S X, LIANG D D, et al. Adsorption of volatile organic compounds in porous metal-organic frameworks functionalized by polyoxometalates[J]. Journal of Solid State Chemistry, 2011, 184(11): 3034-3039. |
| 55 | 李竞草, 吴冬霞, 常丽萍, 等. 疏水性金属-有机骨架材料的研究进展[J]. 化工进展, 2020, 39(1): 224-232. |
| LI J C, WU D X, CHANG L P, et al. Research progress of hydrophobic metal-organic framework materials[J]. Chemical Industry and Engineering Progress, 2020, 39(1): 224-232. | |
| 56 | XIAN S K, YU Y, XIAO J, et al. Competitive adsorption of water vapor with VOCs dichloroethane, ethyl acetate and benzene on MIL-101(Cr) in humid atmosphere[J]. Royal Socirty of Chemistry Advances, 2015, 5(3): 1827-1834. |
| 57 | KALMUTZKI M J, DIERCKS C S, YAGHI O M. Metal-organic frameworks for water harvesting from air[J]. Advanced Materials, 2018, 30(37): 1704304. |
| 58 | CHEN T H, POPOV I, ZENASNI O, et al. Superhydrophobic perfluorinated metal-organic frameworks[J]. Chemical Communications, 2013, 49(61): 6846-6848. |
| 59 | BELLAROSA L, GUTIERREZ-SEVILLANO J J, CALERO S, et al. How ligands improve the hydrothermal stability and affect the adsorption in the IRMOF family[J]. Physical Chemistry Chemical Physics, 2013, 15(40): 17696-17704. |
| 60 | NGUYEN J G, COHEN S M. Moisture-resistant and superhydrophobic metal-organic frameworks obtained via postsynthetic modification[J]. Journal of the American Chemical Society, 2010, 132: 4560-4561. |
| 61 | GAO M L, ZHAO S Y, CHEN Z Y, et al. Superhydrophobic/superoleophilic MOF composites for oil-water separation[J]. Inorganic Chemistry, 2019, 58(4): 2261-2264. |
| 62 | ZHANG L, HU H Y. A systematic investigation of decomposition of nano Zn4O(C8H4O4)3 metal-organic framework[J]. The Journal of Physical Chemistry C, 2010, 114(6): 2566-2572. |
| 63 | JIAO L, SEOW J Y R, SKINNER W S, et al. Metal-organic frameworks: structures and functional applications[J]. Materials Today, 2019, 27: 43-68. |
| 64 | CAVKA J H, JAKOBSEN S, OLSBYE U, et al. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability[J]. Journal of the American Chemical Society, 2008, 130(42): 13850-13851. |
| 65 | FENG D, GU Z Y, LI J R, et al. Zirconium-metalloporphyrin PCN-222: mesoporous metal-organic frameworks with ultrahigh stability as biomimetic catalysts[J]. Angewandte Chemie: International Ed., 2012, 51(41): 10307-10310. |
| 66 | LYU X L, WANG K, WANG B, et al. A base-resistant metalloporphyrin metal-organic framework for C-H bond halogenation[J]. Journal of the American Chemical Society, 2017, 139(1): 211-217. |
| 67 | KANG I J, KHAN N A, HAQUE E, et al. Chemical and thermal stability of isotypic metal-organic frameworks: effect of metal ions[J]. Chemistry, 2011, 17(23): 6437-6442. |
| 68 | WANG W J, LI Z, ZHANG S H, et al. From porous aromatic frameworks to nanoporous carbons: a novel solid-phase microextraction coating[J]. Talanta, 2018, 190: 327-334. |
| 69 | SUN X J, LI Y J, XI H X, et al. Adsorption performance of a MIL-101(Cr)/graphite oxide composite for a series of n-alkanes[J]. Royal Socirty of Chemistry Advances, 2014, 4(99): 56216-56223. |
| 70 | ZHOU X, HUANG W Y, SHI J, et al. A novel MOF/graphene oxide composite GrO@MIL-101 with high adsorption capacity for acetone[J]. Journal of Materials Chemistry A, 2014, 2(13): 4722-4730. |
| 71 | SUN X J, XIA Q B, ZHAO Z X, et al. Synthesis and adsorption performance of MIL-101(Cr)/graphite oxide composites with high capacities of n-hexane[J]. Chemical Engineering Journal, 2014, 239: 226-232. |
| 72 | LI D H, LI L Q, CHEN R F, et al. A MIL-101 composite doped with porous carbon from tobacco stem for enhanced acetone uptake at normal temperature[J]. Industrial & Engineering Chemistry Research, 2018, 57(18): 6226-6235. |
| 73 | WANG C P, YIN H, TIAN P J, et al. Remarkable adsorption performance of MOF-199 derived porous carbons for benzene vapor[J]. Environmental Research, 2020, 184: 109323. |
| 74 | SUN X J, WU T T, YAN Z M, et al. Novel MOF-5 derived porous carbons as excellent adsorption materials for n-hexane[J]. Journal of Solid State Chemistry, 2019, 271: 354-360. |
| [1] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [2] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [3] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [4] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [5] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [6] | 张婷婷, 左旭乾, 田玲娣, 王世猛. 化工园区挥发性有机物排放清单及因子库构建方法[J]. 化工进展, 2023, 42(S1): 549-557. |
| [7] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [8] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [9] | 葛全倩, 徐迈, 梁铣, 王凤武. MOFs材料在光电催化领域应用的研究进展[J]. 化工进展, 2023, 42(9): 4692-4705. |
| [10] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [11] | 许中硕, 周盼盼, 王宇晖, 黄威, 宋新山. 硫铁矿介导的自养反硝化研究进展[J]. 化工进展, 2023, 42(9): 4863-4871. |
| [12] | 陈翔宇, 卞春林, 肖本益. 温度分级厌氧消化工艺的研究进展[J]. 化工进展, 2023, 42(9): 4872-4881. |
| [13] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [14] | 姜晶, 陈霄宇, 张瑞妍, 盛光遥. 载锰生物炭制备及其在环境修复中应用研究进展[J]. 化工进展, 2023, 42(8): 4385-4397. |
| [15] | 张振, 李丹, 陈辰, 吴菁岚, 应汉杰, 乔浩. 吸附树脂对唾液酸的分离纯化[J]. 化工进展, 2023, 42(8): 4153-4158. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||