| 1 |
FU B, LIU G, MIAN M M, et al. Co-combustion of industrial coal slurry and sewage sludge: thermochemical and emission behavior of heavy metals[J]. Chemosphere, 2019, 233: 440-451.
|
| 2 |
RONDA A, GOMEZ-BAREA A, HARO P, et al. Elements partitioning during thermal conversion of sewage sludge[J]. Fuel Processing Technology, 2019, 186: 156-166.
|
| 3 |
ZHANG S, JIANG X, LÜ G, et al. Effect of chlorine, sulfur, moisture and ash content on the partitioning of As, Cr, Cu, Mn, Ni and Pb during bituminous coal and pickling sludge co-combustion[J]. Fuel, 2019, 239: 601-610.
|
| 4 |
严玉朋, 黄亚继, 王昕晔, 等. 高岭土对焚烧烟气中Pb、Cd排放的控制特性研究[J]. 燃料化学学报, 2014, 42(10): 1273-1280.
|
|
YAN Yupeng, HUANG Yaji, WANG Xinye, et al. Control of Pb and Cd emission by kaolin during waste incineration[J]. Journal of Fuel Chemistry and Technology, 2014, 42(10): 1273-1280.
|
| 5 |
李园, 陈娟, 张平安, 等. 高岭土同时吸附Na、Pb化合物的机理研究[J]. 工程热物理学报, 2013, 34(1): 168-172.
|
|
LI Yuan, CHEN Juan, ZHANG Ping’an, et al. Simultaneous adsorption of Na and Pb compounds of kaolinite[J]. Journal of Engineering Thermophysics, 2013, 34(1): 168-172.
|
| 6 |
WU L, ZHONG D, DU Y, et al. Emission and control characteristics for incineration of sedum plumbizincicola biomass in a laboratory-scale entrained flow tube furnace[J]. International Journal of Phytoremediation, 2013, 15(3): 13.
|
| 7 |
YAO H, NARUSE I. Control of trace metal emissions by sorbents during sewage sludge combustion[J]. Proceedings of the Combustion Institute, 2005, 30(2): 3009-3016.
|
| 8 |
YAO H, NARUSE I. Using sorbents to control heavy metals and particulate matter emission during solid fuel combustion[J]. Particuology, 2009, 7(6): 477-482.
|
| 9 |
SCOTTO M V, UBEROI M, PETERSON T W, et al. Metal capture by sorbents in combustion processes[J]. Fuel Processing Technology, 1994, 39(1/2/3): 357-372.
|
| 10 |
DAVIS S B, WENDT J O L. Mechanism and kinetics of lead capture by kaolinite in a downflow combustor[J]. Proceedings of the Combustion Institute, 2000, 28(a): 2743-2749.
|
| 11 |
MWABE P O, WENDT J O L. Mechanisms governing trace sodium capture by kaolinite in a downflow combustor[C]//Twenty-sixth Symposium (International) on Combustion, 1996: 2447-2453.
|
| 12 |
YAO H, MKILAHA I S N, NARUSE I. Screening of sorbents and capture of lead and cadmium compounds during sewage sludge combustion[J]. Fuel, 2004, 83(7/8): 1001-1007.
|
| 13 |
WANG X, HUANG Y, PAN Z, et al. Theoretical investigation of lead vapor adsorption on kaolin surfaces with DFT calculations[J]. Journal of Hazardous Materials, 2015, 295: 43-54.
|
| 14 |
DING Shuli, ZHANG Leilei, XU Bohui. Review and prospect of surface modification of kaolin[J]. Advanced Materials Research, 2012, 432: 1382-1385.
|
| 15 |
PTCEK P, SOUKAL F, OPRAVIL T, et al. The kinetic analysis of the thermal decomposition of kaolin by DTG technique[J]. Powder Technology, 2011, 208(1): 20-25.
|
| 16 |
FROST R L, VASSALLO A M. The dehydroxylation of the kaolinite clay minerals using infrared emission spectroscopy[J]. Clay Clay Miner, 1996, 44(5): 635-651.
|
| 17 |
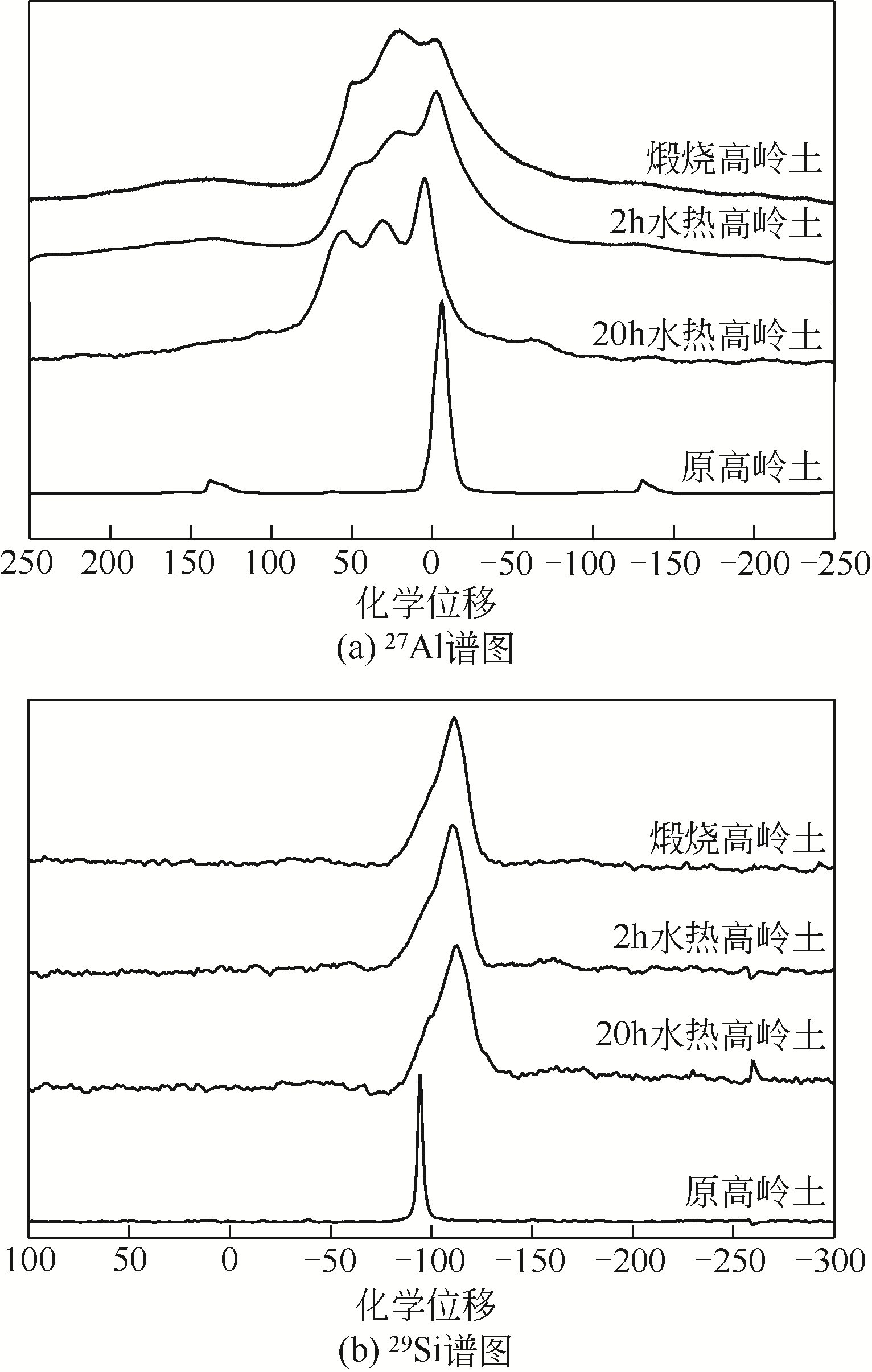
何宏平, 胡澄, 郭九皋, 等. 高岭石及其热处理产物的29Si, 27Al魔角旋转核磁共振研究[J]. 科学通报, 1993(6): 570-572.
|
|
HE Hongping, HU Cheng, GUO Jiugao, et al. Study on 29Si, 27Al magic Angle rotating NMR of kaolinite and its heat treatment products[J]. Chinese Science Bulletin, 1993(6): 570-572.
|
| 18 |
GÜVEN N. The coordination of aluminum ions in the palygorskite structure[J]. Clay Clay Miner, 1992, 40(4): 457-461.
|
| 19 |
郭九皋, 何宏平, 王辅亚, 等. 高岭石-莫来石反应系列: 27Al和29Si MAS NMR研究[J]. 矿物学报, 1997(3): 250-259.
|
|
GUO Jiugao, HE Hongping, WANG Fuya, et al. Kaolinite-mullite reaction series: A 27Al and 29Si MAS NMR study[J]. Acta Mineralogica Sinica, 1997(3): 250-259.
|
| 20 |
ROCHA J, KLINOWAKI J. Solid-state NMR studies of the structure and reactivity of metakaolinite[J]. Angew. Chem. Int. Ed. Engl., 1990, 29(5): 553-554
|
| 21 |
谢晶晶, 陈天虎, 陈冬, 等. 27Al魔角旋转核磁共振研究热处理凹凸棒石结构中Al配位变化[J]. 硅酸盐学报, 2013, 41(2): 235-239.
|
|
XIE Jingjing, CHEN Tianhu, CHEN Dong, et al. Study of Al coordination structure change in palygorskite via27Al MAS NMR analysis[J]. Journal of the Chinese Ceramic Society, 2013, 41(2): 235-239.
|
| 22 |
SI J, LIU X, XU M, et al. Effect of kaolin additive on PM2.5 reduction during pulverized coal combustion: importance of sodium and its occurrence in coal[J]. Applied Energy, 2014, 114: 434-444.
|
| 23 |
XING H, LIU H, ZHANG X, et al. Enhanced sodium adsorption capacity of kaolinite using a combined method of thermal pre-activation and intercalation-exfoliation: Alleviating the problems of slagging and fouling during the combustion of Zhundong coal[J]. Fuel, 2019, 239: 312-319.
|
| 24 |
HALL C, HAMILTON A, WILSON M. The influence of temperature on rehydroxylation [RHX] kinetics in archaeological pottery[J]. J. Archaeol. Sci., 2013, 40: 305-312.
|
| 25 |
ZEMENOVA P, KLOUŽKOVÁ A, KOHOUTKOWA M, et al. Investigation of the first and second dehydroxylation of kaolinite[J]. Journal of Thermal Analysis and Calorimetry, 2014, 116(2): 633-639.
|
| 26 |
程运, 王昕晔, 吕文婷, 等. 高岭土高温吸附重金属和碱金属的研究进展[J]. 化工进展, 2019, 38(8): 3852-3865.
|
|
CHENG Yun, WANG Xinye, Wenting LÜ, et al. A review of heavy metals and alkali metals adsorption by kaolin at high temperature[J]. Chemical Industry and Engineering Progress, 2019, 38(8): 3852-3865.
|
| 27 |
LEE S G, CHOI J I, KOH W, et al. Adsorption of β-d-glucose and cellobiose on kaolinite surfaces: density functional theory (DFT) approach[J]. Applied Clay Science, 2013, 71: 73-81.
|
| 28 |
AULLON G, BELLAMY D, BRAMMER L, et al. Metal-bound chlorine accepts hydrogen bonds[J]. Chemical Communications, 1998, 6: 653-654.
|
 ),黄亚继(
),黄亚继( ),夏志鹏,查键锐,于梦竹,丁守一,胡华军,戚二兵
),夏志鹏,查键锐,于梦竹,丁守一,胡华军,戚二兵
 ),Yaji HUANG(
),Yaji HUANG( ),Zhipeng XIA,Jianrui ZHA,Mengzhu YU,Shouyi DING,Huajun HU,Erbing QI
),Zhipeng XIA,Jianrui ZHA,Mengzhu YU,Shouyi DING,Huajun HU,Erbing QI