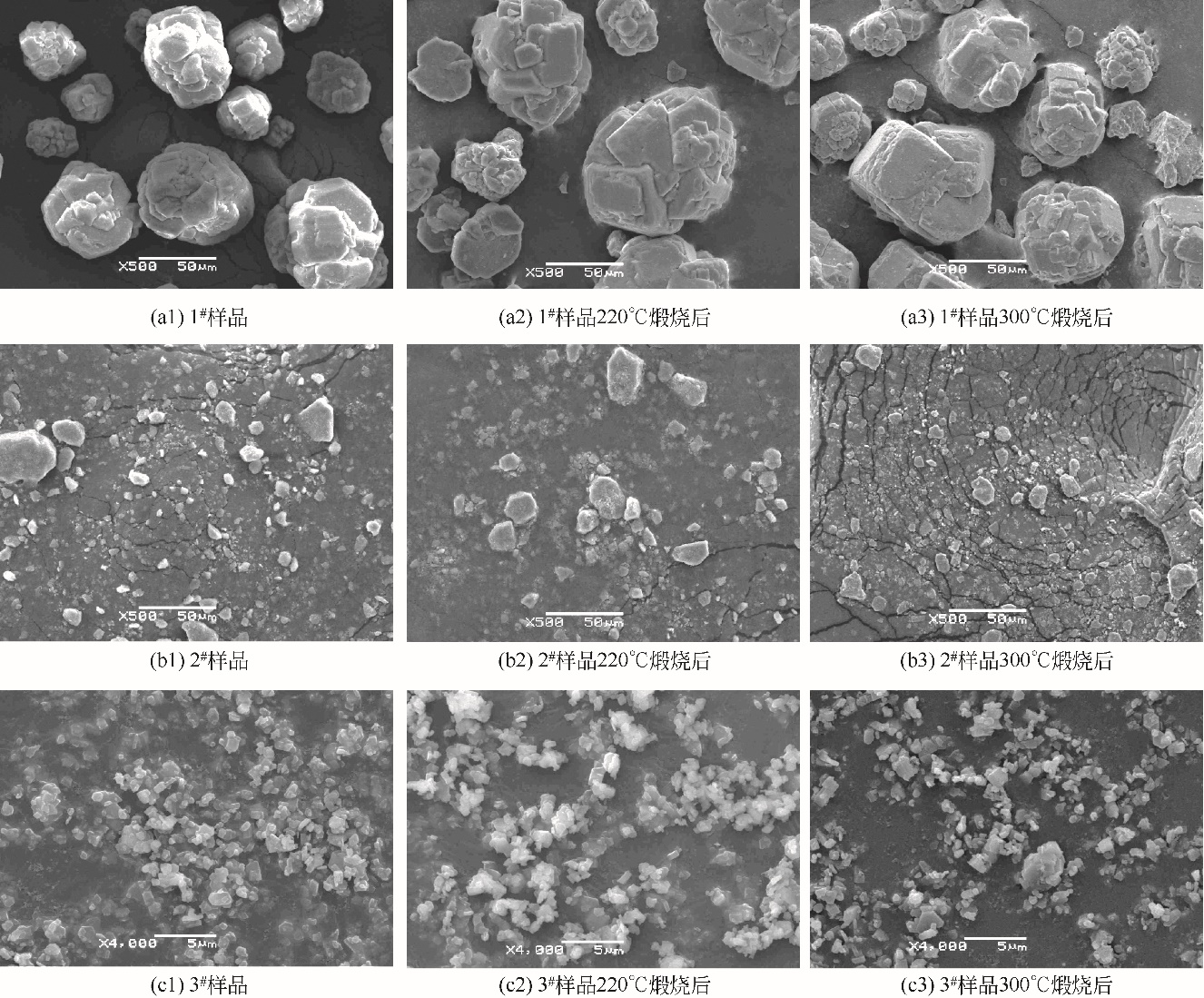
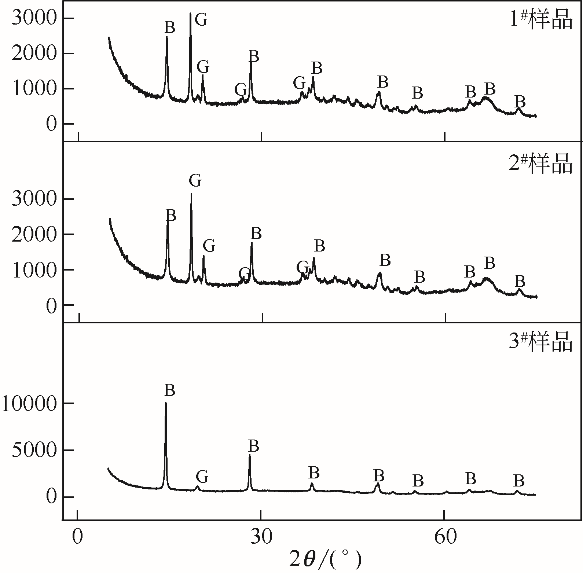
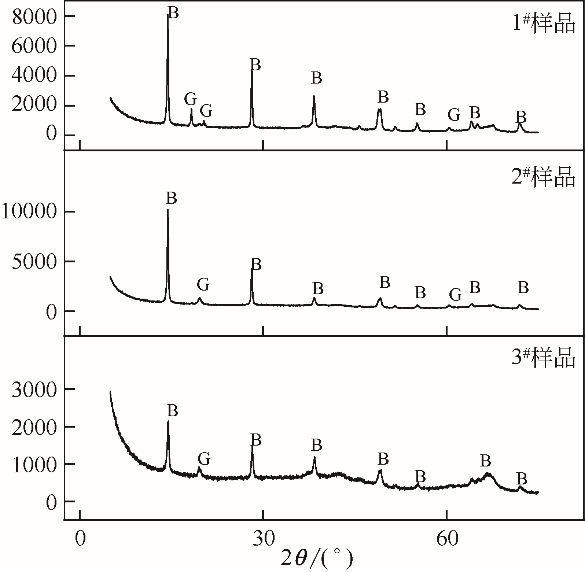
| 1 |
李旺兴.氧化铝生产理论与工艺[M].长沙:中南大学出版社,2010:176-180.
|
|
LI Wangxing.Theory and technics of alumina production [M].Changsha:Centrol South University Press,2010:176-180
|
| 2 |
李广慈,柳云骐,刘迪,等.不同形貌纳米薄水铝石的水/溶剂热合成及其催化应用[J].化工进展,2010,29(7):1215-1222.
|
|
LI Guangci,LIU Yunqi,LIU Di,et al.Hydrothermal/solvothermal synthesis and potential catalytic application of nanoscale boehmite with different morphologies[J].Chemical Industry and Engineering Progress,2010,29(7):1215-1222.
|
| 3 |
吴彩虹,郑国源,王吉林,等.高分散纳米薄水铝石和纳米氧化铝的制备及其对甲基橙的吸附性能[J].无机化学学报,2019,36(3):449-458.
|
|
WU Caihong,ZHENG Guoyuan,WANG Jilin,et al.Preparation and adsorption properties for melthyl orange of high dispersed boehmite and alumina nanostrctures[J].Chinese Journal of Inorganic Chemistry,2019,36(3):449-458.
|
| 4 |
李学礼,蔡进军,谭争国,等.活性氧化铝及其前驱体的性质及应用[J].石化技术与应用,2015,33(3):276-281.
|
|
LI Xueli,CAI Jinjun,TAN Zhengguo,et al.Property and application of activated alumina and its precursor[J].Petrochemical Technology & Application,2015,33(3):276-281.
|
| 5 |
LIU Q,WANG A Q,WANG X H,et al.Characterization and catalytic applications of mesoporous γ-alumina from boehmite sol[J].Microporous Meterials,2008,111(4):323-333.
|
| 6 |
权婷婷,郭小蕊,谢天翼,等.捆束状分级结构γ-AlOOH的无模板水热制备及吸附性能研究[J].陶瓷学报,2017,38(3):398-403.
|
|
QUAN Tingting,GUO Xiaorui,XIE Tianyi,et al.Synthesis of bundle-like hierarchical γ-AlOOHvia template-flee hydrothermal method and its adsorption[J].Journal of Ceramics,2017,38(3):398-403.
|
| 7 |
纪惟惟,王智杰,马敬红,等.勃姆石改性氧化铝膜对刚果红染料吸附的研究[J].膜科学与技术,2015,35(6):57-62.
|
|
JI Weiwei,WANG Zhijie,MA Jinghong,et al.Investigation of alumina membrane modified with boehmite for Congo red dye adsorption[J].Membrane Science and Technology,2015,35(6):57-62.
|
| 8 |
HYUCK H,RICHARAD J R.Formation of CoAl layered double hydroxide on the boehmite surface and its role in tungstete sorption[J].Journal of Environmental Sciences,2018,65(3):103-115.
|
| 9 |
STELLA G,FLORIAN H,ANJA M S,et al.Impact of crystalline and amorphous iron and alumina hydroxide on mechanisms of phosphate adsorption and desorption[J].Journal of Environmental Sciences,2018,70(8):178-192.
|
| 10 |
安亚强,张汉鸿,吴春丹,等.低水分勃姆石涂层隔膜研究进展[J].广东化工,2019,46(2):105-107.
|
|
AN Yaqiang,ZHANG Hanhong,WU Chundan,et al.Research progress of low moisture bomsite coating separators[J].Guangdong Chemical Industry,2019,46(2):105-107.
|
| 11 |
柳彦梅,张存良,海士坤,等.勃姆石阻燃和增强聚碳酸亚丙酯复合材料的制备和表征[J].塑料科技,2018,46(9):73-77.
|
|
LIU Yanmei,ZHANG Cunliang,Shikun HAI,et al.Preparation and characterization of flame retardant and reinforced PPC composites with boehmite[J].Plastics Science and Technology,2018,46(9):73-77.
|
| 12 |
王娜,赵莹,朱明,等.勃姆石阻燃改性PP复合材料的制备及其性能研究[J].塑料科技,2017,45(11):36-40.
|
|
WANG Na,ZHAO Ying,ZHU Ming,et al.Study on properties of boehmite flame retardant modified PP composites and its preparation[J].Plastics Science and Technology,2017,45(11):36-40.
|
| 13 |
KANGHEE C,JUNGSU K,HEE T B,et al.Synthesis of CuCl/boehmite adsorbents that exhibit CO selectivity in CO/CO2 separation[J].Journal of Hazardous Materials,2018,344(15):857-864.
|
| 14 |
ARASH G,ZEINAB S,BAHMAN T.Tribromide ion supported on boehmite nanoparticles as a reusable catalyst for organic reactions[J].Comptes Rendus Chimie,2018,21(11):1011-1022.
|
| 15 |
王瑞,郭彦鑫.勃姆石催化浆态床甲醇脱水制二甲醚[J].工业催化,2015,23(1):54-58.
|
|
WANG Rui,Guo Yanxin.Catalytic performance of boehmite catalysts for methanol dehydration to dim ethyl ether in slurry reactor[J].Industrial Catalysis,2015,23(1):54-58.
|
| 16 |
CHIVAS C,LONGUETB C,POURCHEZC J.Physical, morphological and chemical modification of Al-based nanofillers in by-products of incinerated nanocomposites and related biological outcome[J].Journal of Hazardous Materials,2019,365(3):405-412.
|
| 17 |
MALYALA P,LAERA D,CIANETTI S.The preparation and physico-chemical characterization of aluminum hydroxide/TLR7a, a noval vaccine adjuvant comprising a small molecule adsorbed to aluminum hydroxide[J].Journal of Pharmaceutical Sciences,2018,107(6):1577-1585.
|
| 18 |
刘超,王晶,杨雨佳.水热处理异丙醇铝及其煅烧产物的显微结构[J].硅酸盐学报,2014,42(12):1554-1559.
|
|
LIU Chao,WANG Jing,YANG Yujia.Microstructure of productsvia hydrothermol treatment and calcination of aluminum isopropoxide[J].Journal of the Chinese Ceramic Society,2014,42(12):1554-1559.
|
| 19 |
熊敏,肖汉宇.制备工艺对Al2O3纳滤膜的结构及结构性能的影响[J].硅酸盐学报,2018,46(12):1733-1741.
|
|
XIONG Min,XIAO Hanyu.Effect of preparation process on structure and perpeability of Al2O3 nanofiltration membrane[J].Journal of the Chinese Ceramic Society,2018,46(12):1733-1741.
|
| 20 |
黄静,夏举佩,罗中秋.碳酸钠沉淀法制备氢氧化铝及其形成机理讨论[J].化工进展,2017,36(3):1120-1125.
|
|
HUANG Jing,XIA Jupei,LUO Zhongqiu.Preparation and growth mechanism of aluminum hydroxide by precipitation method with sodium carbonate[J].Chemical Industry and Engineer Progress,2017,36(3):1120-1125.
|
| 21 |
李长刚,赵萍,王维勋,等.微乳/水热法制备勃姆石[J].齐鲁工业大学学报(自然科学版),2017,31(5):13-16.
|
|
LI Changgang,ZHAO Ping,WANG Weixun,et al.Preparation of boehmite by microemulsion/hydrothermal method[J].Journal of Shandong Polytechnic University,2017,31(5):13-16.
|
| 22 |
彭志宏,李琼芳,周秋生.氢氧化铝脱水过程的动力学研究[J].轻金属,2010(5):16-18.
|
|
PENG Zhihong,LI Qiongfang,ZHOU Qiusheng.Studies on dehydration kinetics of aluminum hydroxide[J].Light Metals,2010(5):16-18.
|
| 23 |
龚念.特殊形貌氢氧化铝煅烧过程的微观形貌演变及动力学分析[D].大连:大连交通大学,2013.
|
|
GONG Nian.Microsopic morphology evolution and dynamics analysis of the special morphology of aluminum hydroxide during calcination process[D].Dalian:Dalian Jiaotong University,2013.
|
| 24 |
关昕,王晶,史忠祥.水热法制备薄水铝石粉体及其脱水动力学分析[J].大连交通大学学报,2018,39(4):77-82.
|
|
GUAN Xin,WANG Jing,SHI Zhongxiang.Preparation and kinetics of dehydration of boehmite by hydrothermal treatment[J].Journal of Dalian Jiaotong University,2018,39(4):77-82.
|
| 25 |
KISSINGER H E.Reaction kinetics in differential thermal analysis[J].Analytical Chemistry,1957,29(11):1702-1706.
|
| 26 |
OZAWA T.Kinetics of non-isothermal crystallization[J].Polymer,1971,12(3):150-158.
|
| 27 |
CRANE L W.Analysis of curing kinetics in polymer conposite[J].Journal of Polymer Science,1973,11(8):533-540.
|
 ),Wangxing LI1(
),Wangxing LI1( ),Dongzhan HAN1,2,Chunhui ZHENG2
),Dongzhan HAN1,2,Chunhui ZHENG2