化工进展 ›› 2020, Vol. 39 ›› Issue (4): 1485-1492.DOI: 10.16085/j.issn.1000-6613.2019-0671
二 恶英生成抑制剂及其阻滞机理研究现状与展望
- 1.同济大学环境科学与工程学院,上海 200092
2.上海污染控制与生态安全研究院,上海 200092
-
收稿日期:2019-04-28出版日期:2020-04-05发布日期:2020-04-28 -
通讯作者:何品晶 -
作者简介:章骅(1978—),女,博士,教授,博士生导师,研究方向为固体废物处理处置与资源化利用。E-mail:zhanghua_tj@tongji.edu.cn 。 -
基金资助:国家自然科学基金面上项目(21577102)
Progresses in the inhibitors for suppressing de novo formation ofPCDD/Fs: a review
Hua ZHANG1,2( ),Rui YANG1,2,Liming SHAO1,2,Pinjing HE1,2(
),Rui YANG1,2,Liming SHAO1,2,Pinjing HE1,2( )
)
- 1.College of Environmental Science and Engineering, Tongji University, Shanghai 200092, China
2.Shanghai Institute of Pollution Control and Ecological Security, Shanghai 200092, China
-
Received:2019-04-28Online:2020-04-05Published:2020-04-28 -
Contact:Pinjing HE
摘要:
固体废物焚烧过程产生的二 英类物质具剧毒性且可在环境中造成持久性污染,国内外学者一直致力于探究高效的二
英类物质具剧毒性且可在环境中造成持久性污染,国内外学者一直致力于探究高效的二 英类物质源头减量化技术以保护公众健康。本文综述了近年来二
英类物质源头减量化技术以保护公众健康。本文综述了近年来二 英类物质从头合成的生成阻滞研究现状,对比分析了实验研究中常采用的含硫类抑制剂、含氮类抑制剂、含OH基抑制剂及复合抑制剂对二
英类物质从头合成的生成阻滞研究现状,对比分析了实验研究中常采用的含硫类抑制剂、含氮类抑制剂、含OH基抑制剂及复合抑制剂对二 英类物质的抑制效果及其阻滞机理,进而剖析二
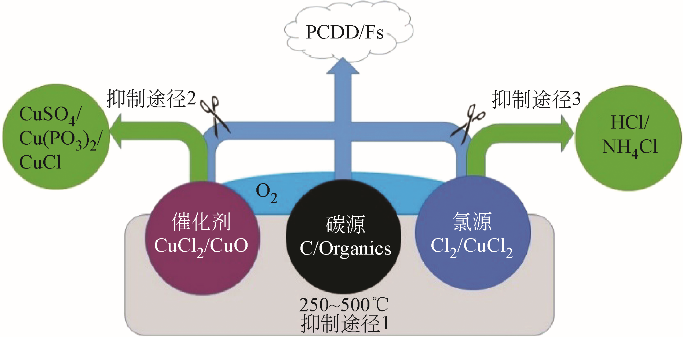
英类物质的抑制效果及其阻滞机理,进而剖析二 英生成抑制剂的研发思路进展。在当前以抑制氯源和催化剂活性为主的抑制途径基础上,依据二
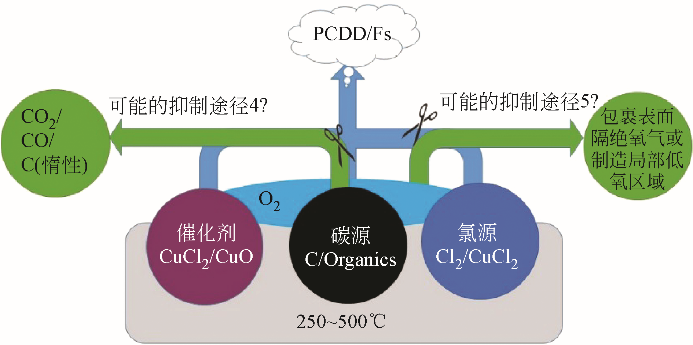
英生成抑制剂的研发思路进展。在当前以抑制氯源和催化剂活性为主的抑制途径基础上,依据二 英类物质的从头合成机理,探讨了通过阻滞碳源或氧气与反应物接触进而阻滞从头合成反应的抑制新途径,并展望了未来新型阻滞剂研发的方向,以期为二
英类物质的从头合成机理,探讨了通过阻滞碳源或氧气与反应物接触进而阻滞从头合成反应的抑制新途径,并展望了未来新型阻滞剂研发的方向,以期为二 英类物质的源头减量和污染控制提供理论支持。抑制剂工业化应用和推广仍处于尝试阶段,在实际规模的焚烧厂应用时抑制效果不稳定,未来仍需进一步开展中试和现场试验以验证抑制作用实效及其影响因素。
英类物质的源头减量和污染控制提供理论支持。抑制剂工业化应用和推广仍处于尝试阶段,在实际规模的焚烧厂应用时抑制效果不稳定,未来仍需进一步开展中试和现场试验以验证抑制作用实效及其影响因素。
中图分类号:
引用本文
章骅,杨瑞,邵立明,何品晶. 二 恶英生成抑制剂及其阻滞机理研究现状与展望[J]. 化工进展, 2020, 39(4): 1485-1492.
Hua ZHANG,Rui YANG,Liming SHAO,Pinjing HE. Progresses in the inhibitors for suppressing de novo formation ofPCDD/Fs: a review[J]. Chemical Industry and Engineering Progress, 2020, 39(4): 1485-1492.
| 抑制剂种类 | 抑制剂用量 | 飞灰⑥ | 反应条件⑦ | 抑制率 | 抑制作用机理 | 参考文献 |
|---|---|---|---|---|---|---|
| S① | S/Cl=0.5 S/Cl=1 S/Cl=2 S/Cl=3 | 实际静电除尘飞灰经甲苯索提两次去除PCDD/Fs,干燥后用于实验,3g | 10% O2+15% H2O+N2,1L/min,300℃,60min | 32.1% 44.3% 54.2% 30.1% | S与CuO/CuCl2反应生成 Cu2SO4/CuSO4,钝化铜的催化能力,阻滞PCDD/Fs从头合成,部分S单质在300℃可被氧化生成SO2/SO3,进而阻滞PCDD/Fs生成 | [ |
| SO2① | 50% SO2+ 5% O2+ 45% N2 | 0.44g实际飞灰(甲苯索提24h)+0.428mmol NaCl+1.998 mmol C+0.064mmol CuCl2·2H2O | 50% SO2+5% O2+45% N2,20mL/min,340℃,1h | 21.1% | CuCl2被SO2转化为硫酸盐,硫酸盐在热力学上更加稳定,催化能力弱 | [ |
| SO2① | 150μL/L (S/Cl=0.25) | 100g模拟飞灰:3.2% C+0.09% Cu(加入CuO,以Cu计)+石英基质,600μL/L Cl2 | 10% O2+10% H2O+N2,400mL/min,350℃,30min | 90.3% | CuO被转化为CuSO4,催化能力减弱 | [ |
| SO3① | 135μL/L (S/Cl=0.225) | 90.5% | CuO被转化为CuSO4,催化能力减弱 | [ | ||
| (NH4)2SO4①,② | 0.1g | 2g模拟飞灰:3.1% C+96.7%石英砂+0.2% CuCl2·2H2O | 10% O2+90% N2,350℃,60min | 93.0% | (NH4)2SO4可减少气相中氯气的生成进而阻断氯与有机物反应,减少PCDD/Fs的生成 | [ |
| CH4N2S①,② | 0.06g | 2g模拟飞灰:3.0% C+91.8% SiO2+5% NaCl+0.2% CuCl2·2H2O | 1.12% O2+N2,300mL/min,350℃,50min | 99.8% | 胺既可与金属结合降低金属的催化性能,亦可胺化有机物,从而抑制氯化反应生成PCDD/Fs | [ |
| (NH4)2S2O3①,② | 0.06g | 85.4% | [ | |||
| CO(NH2)2② | 0.1g | 2g模拟飞灰:3.1% C+96.7%石英砂+0.2% CuCl2·2H2O | 10% O2+90% N2,350℃,60min | 55.0% | 分解形成氨气,氨气的强还原性可将Cl2转化为氯化能力较差的物质 | [ |
| 氟化氢铵② | 5g模拟飞灰:10% CuCl2·2H2O+20% 活性炭+石英基质 | 20% O2+80% N2,1L/min,400℃,60min | 61.1%⑤ | [ | ||
| 溴化铵② | 35.8%⑤ | [ | ||||
| NH4H2PO4② | 98.1%⑤ | [ | ||||
| (NH4)2SO4①,② | 88.8%⑤ | [ | ||||
| NH3② | 350μL/L 1000μL/L | 10g模拟飞灰:石墨粉+CuCl2·2H2O(Cl为1%) | 2.5% O2+97.5% Ar,2L/min,15min升温至300℃,再恒温120min | 25.9% 55.5% | 供碳能力对PCDD/Fs形成极为重要,同时NH3对HCl也有很好的结合作用,通过凝结成NH4Cl的途径能够去除97%的HCl | [ |
| NaOH③ | 2% 5% | 5g实际飞灰,收集于静电除尘器,未添加活性炭及石灰 | 10% O2+90% N2,50mL/min,300℃,30min | 19.0% 96.0% | NaOH与金属氯化物快速反应降低其催化能力和供氯能力 | [ |
| NaHCO3③ | 2% 5% | 5g实际飞灰,收集于静电除尘器,未添加活性炭及石灰 | 10% O2+90% N2,50mL/min,300℃,30min | 25.0% 50.0% | NaHCO3与金属氯化物快速反应降低其催化能力和供氯能力 | [ |
| Ca(OH)2③ | 2% 5% | 5g实际飞灰,收集于静电除尘器,未添加活性炭及石灰 | 10% O2+90% N2,50mL/min,300℃,30min | 27.0% 67.0% | Ca(OH)2与金属氯化物快速反应降低其催化能力和供氯能力 | [ |
| H2O③ | 10% | 2g模拟飞灰:3.2% C+0.09% Cu (加入CuCl2,以Cu计)+石英基质 | 10% O2+10% H2O+N2,360mL/min,300℃,30min | 10%~40% | [ | |
| H2O2+SO2④ | 6% H2O2,0.1mmol/min | 0.44g实际飞灰(甲苯索提24h)+0.428mmol NaCl+1.998 mmol C+0.064mmol CuCl2·2H2O | 50% SO2+5% O2+45% N2,20mL/min,340℃,60 min | 77.2% | [ | |
| H2O2+H2SO4④ | 9.5% H2O2,0.1mmol/min | 85.3% | H2SO4的强氧化性催生了·OH,加快反应 | [ | ||
| (NH2)2CO+S④ | 5%(NH2)2CO+5% S | 10g模拟垃圾:72%褐煤+12.0%垃圾+6.0%聚氯乙烯 | 空气10% O2+90% N2,2L/min,400℃,30min | 93.0% | 同时发挥S及尿素的抑制作用,阻滞二 英类物质的生成 英类物质的生成 | [ |
表1 二英类物质从头合成抑制的研究进展
| 抑制剂种类 | 抑制剂用量 | 飞灰⑥ | 反应条件⑦ | 抑制率 | 抑制作用机理 | 参考文献 |
|---|---|---|---|---|---|---|
| S① | S/Cl=0.5 S/Cl=1 S/Cl=2 S/Cl=3 | 实际静电除尘飞灰经甲苯索提两次去除PCDD/Fs,干燥后用于实验,3g | 10% O2+15% H2O+N2,1L/min,300℃,60min | 32.1% 44.3% 54.2% 30.1% | S与CuO/CuCl2反应生成 Cu2SO4/CuSO4,钝化铜的催化能力,阻滞PCDD/Fs从头合成,部分S单质在300℃可被氧化生成SO2/SO3,进而阻滞PCDD/Fs生成 | [ |
| SO2① | 50% SO2+ 5% O2+ 45% N2 | 0.44g实际飞灰(甲苯索提24h)+0.428mmol NaCl+1.998 mmol C+0.064mmol CuCl2·2H2O | 50% SO2+5% O2+45% N2,20mL/min,340℃,1h | 21.1% | CuCl2被SO2转化为硫酸盐,硫酸盐在热力学上更加稳定,催化能力弱 | [ |
| SO2① | 150μL/L (S/Cl=0.25) | 100g模拟飞灰:3.2% C+0.09% Cu(加入CuO,以Cu计)+石英基质,600μL/L Cl2 | 10% O2+10% H2O+N2,400mL/min,350℃,30min | 90.3% | CuO被转化为CuSO4,催化能力减弱 | [ |
| SO3① | 135μL/L (S/Cl=0.225) | 90.5% | CuO被转化为CuSO4,催化能力减弱 | [ | ||
| (NH4)2SO4①,② | 0.1g | 2g模拟飞灰:3.1% C+96.7%石英砂+0.2% CuCl2·2H2O | 10% O2+90% N2,350℃,60min | 93.0% | (NH4)2SO4可减少气相中氯气的生成进而阻断氯与有机物反应,减少PCDD/Fs的生成 | [ |
| CH4N2S①,② | 0.06g | 2g模拟飞灰:3.0% C+91.8% SiO2+5% NaCl+0.2% CuCl2·2H2O | 1.12% O2+N2,300mL/min,350℃,50min | 99.8% | 胺既可与金属结合降低金属的催化性能,亦可胺化有机物,从而抑制氯化反应生成PCDD/Fs | [ |
| (NH4)2S2O3①,② | 0.06g | 85.4% | [ | |||
| CO(NH2)2② | 0.1g | 2g模拟飞灰:3.1% C+96.7%石英砂+0.2% CuCl2·2H2O | 10% O2+90% N2,350℃,60min | 55.0% | 分解形成氨气,氨气的强还原性可将Cl2转化为氯化能力较差的物质 | [ |
| 氟化氢铵② | 5g模拟飞灰:10% CuCl2·2H2O+20% 活性炭+石英基质 | 20% O2+80% N2,1L/min,400℃,60min | 61.1%⑤ | [ | ||
| 溴化铵② | 35.8%⑤ | [ | ||||
| NH4H2PO4② | 98.1%⑤ | [ | ||||
| (NH4)2SO4①,② | 88.8%⑤ | [ | ||||
| NH3② | 350μL/L 1000μL/L | 10g模拟飞灰:石墨粉+CuCl2·2H2O(Cl为1%) | 2.5% O2+97.5% Ar,2L/min,15min升温至300℃,再恒温120min | 25.9% 55.5% | 供碳能力对PCDD/Fs形成极为重要,同时NH3对HCl也有很好的结合作用,通过凝结成NH4Cl的途径能够去除97%的HCl | [ |
| NaOH③ | 2% 5% | 5g实际飞灰,收集于静电除尘器,未添加活性炭及石灰 | 10% O2+90% N2,50mL/min,300℃,30min | 19.0% 96.0% | NaOH与金属氯化物快速反应降低其催化能力和供氯能力 | [ |
| NaHCO3③ | 2% 5% | 5g实际飞灰,收集于静电除尘器,未添加活性炭及石灰 | 10% O2+90% N2,50mL/min,300℃,30min | 25.0% 50.0% | NaHCO3与金属氯化物快速反应降低其催化能力和供氯能力 | [ |
| Ca(OH)2③ | 2% 5% | 5g实际飞灰,收集于静电除尘器,未添加活性炭及石灰 | 10% O2+90% N2,50mL/min,300℃,30min | 27.0% 67.0% | Ca(OH)2与金属氯化物快速反应降低其催化能力和供氯能力 | [ |
| H2O③ | 10% | 2g模拟飞灰:3.2% C+0.09% Cu (加入CuCl2,以Cu计)+石英基质 | 10% O2+10% H2O+N2,360mL/min,300℃,30min | 10%~40% | [ | |
| H2O2+SO2④ | 6% H2O2,0.1mmol/min | 0.44g实际飞灰(甲苯索提24h)+0.428mmol NaCl+1.998 mmol C+0.064mmol CuCl2·2H2O | 50% SO2+5% O2+45% N2,20mL/min,340℃,60 min | 77.2% | [ | |
| H2O2+H2SO4④ | 9.5% H2O2,0.1mmol/min | 85.3% | H2SO4的强氧化性催生了·OH,加快反应 | [ | ||
| (NH2)2CO+S④ | 5%(NH2)2CO+5% S | 10g模拟垃圾:72%褐煤+12.0%垃圾+6.0%聚氯乙烯 | 空气10% O2+90% N2,2L/min,400℃,30min | 93.0% | 同时发挥S及尿素的抑制作用,阻滞二 英类物质的生成 英类物质的生成 | [ |
| 63 | CHEN Z, LIN X, LU S, et al. Suppressing formation pathway of PCDD/Fs by S-N-containing compound in full-scale municipal solid waste incinerators[J]. Chemical Engineering Journal, 2019, 359: 1391-1399. |
| 64 | KOROBEINICHEV O P, SHMAKOV A G, SHVARTSBERG V M, et al. Fire suppression by low-volatile chemically active fire suppressants using aerosol technology[J]. Fire Safety Journal, 2012, 51: 102-109. |
| 65 | LAPSHIN D, KUNIN A, SEMENOV A. Influence of chemical impurities in ammonium phosphate and ammonium sulfate on the properties of ABCE fire extinguishing dry powders[J]. Russian Journal of General Chemistry, 2016, 86(2): 439-449. |
| 66 | SU C H, CHEN C C, LIAW H J, et al. The assessment of fire suppression capability for the ammonium dihydrogen phosphate dry powder of commercial fire extinguishers[J]. Procedia Engineering, 2014, 84: 485-490. |
| 67 | JOSEPH G. A review of phosphorus-containing flame retardants[J]. Journal of Fire Sciences, 1992, 10(6): 470-487. |
| 68 | REJEESH C R, SAJU K K. Methods and materials for reducing flammability behaviour of coir fibre based composite boards: a review[J]. Materials Today: Proceedings, 2017, 4(9): 9399-9407. |
| 69 | LU S Y, HAMERTON I. Recent developments in the chemistry of halogen-free flame retardant polymers[J]. Progress in Polymer Science, 2002, 27(8): 1661-1712. |
| 70 | LEVCHIK S V, WEIL E D. A review of recent progress in phosphorus-based flame retardants[J]. Journal of Fire Sciences, 2006, 24(5): 345-364. |
| 71 | 杨瑞. 温度和抑制剂对焚烧过程氯苯类物质从头生成抑制的影响及其阻滞机理[D]. 上海: 同济大学, 2019. |
| YANG R. Effect of temperature and inhibitors on chlorobenzenes’ de novo formation during incineration process[D]. Shanghai:Tongji University, 2019. | |
| 1 | WANG Y Q, ZHANG X H, LIAO W J, et al. Investigating impact of waste reuse on the sustainability of municipal solid waste (MSW) incineration industry using emergy approach: a case study from Sichuan province, China[J]. Waste Management, 2018, 77: 252-267. |
| 2 | 何品晶. 固体废物处理与资源化技术[M]. 北京: 高等教育出版社, 2011. |
| HE P J. Solid waste treatment and beneficial reuse technology[M]. Beijing: Higher Education Press, 2011. | |
| 3 |
章骅, 何品晶, 李国建, 等. 生活垃圾焚烧飞灰中的二 英检测[J]. 同济大学学报(自然科学版), 2004, 32(12): 1655-1659. 英检测[J]. 同济大学学报(自然科学版), 2004, 32(12): 1655-1659.
|
| ZHANG H, HE P J, LI G J, et al. Dioxins measurement of municipal solid waste incineration ash[J]. Journal of Tongji University (Natural Science), 2004, 32(12): 1655-1659. | |
| 4 | BALLSCHMITER K, ZOLLER W, SCHOLZ C, et al. Occurrence and absence of polychlorodibenzofurans and polychlorodibenzodioxins in fly ash from municipal incinerators[J]. Chemosphere, 1983, 12(4): 585-594. |
| 5 | ALTARAWNEH M, DLUGOGORSKI B Z, KENNEDY E M, et al. Mechanisms for formation, chlorination, dechlorination and destruction of polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs)[J]. Progress in Energy and Combustion Science, 2009, 35(3): 245-274. |
| 6 | HITES R A. Dioxins: an overview and history[J]. Environmental Science & Technology, 2011, 45(1):16-20. |
| 7 |
齐丽, 任玥, 刘爱民, 等. 北京市某垃圾焚烧厂周边大气二 英污染特征及暴露风险[J]. 环境科学, 2017, 38(4): 1317-1326. 英污染特征及暴露风险[J]. 环境科学, 2017, 38(4): 1317-1326.
|
| QI L, REN Y, LIU A M, et al. Pollution characteristics of PCDD/Fs in ambient air and exposure risk assessment around a municipal solid waste incinerator in Beijing[J]. Environmental Science, 2017, 38(4): 1317-1326. | |
| 8 | 中华人民共和国环保部. 危险废物焚烧污染控制标准: GB 18484—2001[S]. 北京: 中国环境科学出版社, 2001. |
| Ministry of Environmental Protection of the People’s Republic of China. Pollution control standard for hazardous wastes incineration: GB 18484—2001 [S]. Beijing: China Environmental Science Press, 2001. | |
| 9 | 中华人民共和国环保部. 生活垃圾焚烧污染控制标准: GB 18485—2014[S]. 北京: 中国环境科学出版社, 2014. |
| Ministry of Environmental Protection of the People’s Republic of China. Standard for pollution control on municipal solid waste incineration: GB 18485—2014[S]. Beijing: China Environmental Science Press, 2014. | |
| 10 | 中华人民共和国环保部. 生活垃圾焚烧污染控制标准: GB 18485—2011[S]. 北京: 中国环境科学出版社, 2001. |
| Ministry of Environmental Protection of the People’s Republic of China. Standard for pollution control on municipal solid waste incineration: GB 18485—2001[S]. Beijing: China Environmental Science Press, 2001. | |
| 11 | European Parliament and Council of the European Union. Directive 2010/75/EU of the European Parliament and of the Council of 24 November 2010 on industrial emissions (integrated pollution prevention and control)[J]. Official Journal of the European Union, 2010, 334: 17-119. |
| 12 | SELDEN T M, FORREST A S, LOCKHART J E. Analyzing the reductions in US air pollution emissions: 1970 to 1990[J]. Land Economics, 1999, 75: 1-21. |
| 13 |
罗阿群, 刘少光, 林文松, 等. 二 英生成机理及减排方法研究进展[J]. 化工进展, 2016,35 (3): 910-916. 英生成机理及减排方法研究进展[J]. 化工进展, 2016,35 (3): 910-916.
|
| LUO A Q, LIU S G, LIN W S, et al. Progress of formation mechanisms and emission reduction methods of PCDD/Fs[J]. Chemical Industry and Engineering Progress, 2016, 35(3): 910-916. | |
| 14 | CHANG Y M, HUNG C Y, CHEN J H, et al. Minimum feeding rate of activated carbon to control dioxin emissions from a large-scale municipal solid waste incinerator[J]. Journal of Hazardous Materials, 2009, 161(2): 1436-1443. |
| 15 | NI Y W, ZHANG H J, SU F, et al. Emissions of PCDD/Fs from municipal solid waste incinerators in China[J]. Chemosphere, 2009, 75(9): 1153-1158. |
| 16 | 赵树青, 宋薇, 刘晶昊, 等. 我国生活垃圾焚烧二恶英污染现状及减排建议[J]. 环境工程, 2011, 29(1): 86-88. |
| ZHAO S Q, SONG W, LIU J H, et al. Pollution status and suggestions for emission reduction of dioxin from incineration of municipal solid waste in China[J]. Environmental Engineering, 2011, 29(1): 86-88. | |
| 17 | TANG Z W, HUANG Q F, YANG Y F. PCDD/Fs in fly ash from waste incineration in China: a need for effective risk management[J]. Environmental Science & Technology, 2013, 47(11):5520-5521. |
| 18 | HUANG T Y, CHIUEH P T, LO S L. Life-cycle environmental and cost impacts of reusing fly ash[J]. Resources, Conservation and Recycling, 2017, 123: 255-260. |
| 19 | 章骅, 于思源, 邵立明, 等. 烟气净化工艺和焚烧炉类型对生活垃圾焚烧飞灰性质的影响[J]. 环境科学, 2018, 39(1): 467-476. |
| ZHANG H, YU S Y, SHAO L M, et al. Influence of air pollution control (APC) systems and furnace type on the characteristics of APC residues from municipal solid waste incinerators[J]. Environmental Science, 2018, 39(1): 467-476. | |
| 20 | RATHNA R, VARJANI S, NAKKEERAN E. Recent developments and prospects of dioxins and furans remediation[J]. Journal of Environmental Management, 2018, 223: 797-806. |
| 21 | DENG D Y, QIAO J Q, LIU M Q, et al. Detoxification of municipal solid waste incinerator (MSWI) fly ash by single-mode microwave (MW) irradiation: addition of urea on the degradation of dioxin and mechanism[J]. Journal of Hazardous Materials, 2019, 369: 279-289. |
| 22 | 石德智. 基于新型分类收集系统的生活垃圾焚烧过程污染物控制及其机理研究[D]. 杭州:浙江大学, 2009. |
| SHI D Z. Mechanism of pollution control during the municipal solid waste incineration based on newly established waste source-classified collection system[D]. Hangzhou:Zhejiang University, 2009. | |
| 23 | SHI D Z, WU W X, LU S Y, et al. Effect of MSW source-classified collection on the emission of PCDDs/Fs and heavy metals from incineration in China[J]. Journal of Hazardous Materials, 2008, 153(1): 685-694. |
| 24 | 吴冰思. 生活垃圾分类收集减量效果探讨[J]. 环境卫生工程, 2013, 21(4): 24~26. |
| WU B S. Reduction effect of domestic waste sorting[J]. Environmental Sanitation Engineering, 2013, 21(4): 24~26. | |
| 25 | CHENG H F, HU Y A. Curbing dioxin emissions from municipal solid waste incineration in China: re-thinking about management policies and practices[J]. Environmental Pollution, 2010, 158(9): 2809-2814. |
| 26 | LI X M, ZHANG C M, LI Y Z, et al. The status of municipal solid waste incineration (MSWI) in China and its clean development[J]. Energy Procedia, 2016, 104: 498-503. |
| 27 | MCKAY G. Dioxin characterisation, formation and minimisation during municipal solid waste (MSW) incineration: review[J]. Chemical Engineering Journal, 2002, 86(3): 343-368. |
| 28 | ZHOU H, MENG A H, LONG Y Q, et al. A review of dioxin-related substances during municipal solid waste incineration[J]. Waste Management, 2015, 36: 106-118. |
| 29 | WIKSTRÖM E, RYAN S, TOUATI A, et al. Key parameters for de novo formation of polychlorinated dibenzo-p-dioxins and dibenzofurans[J]. Environmental Science & Technology, 2003, 37(9): 1962-1970. |
| 30 | ZHANG M M, BUEKENS A. De novo synthesis of dioxins: a review[J]. International Journal of Environment and Pollution, 2016, 60: 63-110. |
| 31 | WIKSTRÖM E, MARKLUND S. Secondary formation of chlorinated dibenzo-p-dioxins, dibenzofurans, biphenyls, benzenes, and phenols during MSW combustion[J]. Environmental Science & Technology, 2000, 34(4): 604-609. |
| 32 | FUJIMORI T, TAKAOKA M. Direct chlorination of carbon by copper chloride in a thermal process[J]. Environmental Science & Technology, 2009, 43(7): 2241-2246. |
| 33 | SHAO K, YAN J H, LI X D, et al. Inhibition of de novo synthesis of PCDD/Fs by SO2 in a model system[J]. Chemosphere, 2010, 78(10): 1230-1235. |
| 34 | CHEN T, ZHAN M X, LIN X Q, et al. Inhibition of the de novo synthesis of PCDD/Fs on model fly ash by sludge drying gases[J]. Chemosphere, 2014, 114: 226-232. |
| 35 | JUNK G A, RICHARD J J. Dioxins not detected in effluents from coal/refuse combustion[J]. Chemosphere, 1981, 10(11): 1237-1241. |
| 36 | GULLETT B K, BRUCE K R, BEACH L O. Effect of sulfur dioxide on the formation mechanism of polychlorinated dibenzodioxin and dibenzofuran in municipal waste combustors[J]. Environmental Science & Technology, 1992, 26(10): 1938-1943. |
| 37 | PEKÁREK V, PUNČOCHÁŘ M, BUREŠ M, et al. Effects of sulfur dioxide, hydrogen peroxide and sulfuric acid on the de novo synthesis of PCDD/F and PCB under model laboratory conditions[J]. Chemosphere, 2007, 66(10): 1947-1954. |
| 38 | OH J E, GULLETT B, RYAN S, et al. Mechanistic relationships among PCDDs/Fs, PCNs, PAHs, ClPhs, and ClBzs in municipal waste incineration[J]. Environmental Science & Technology, 2007, 41(13): 4705-4710. |
| 39 | CHANG M B, YAO C C, KAI H C. Reducing PCDD/F formation by adding sulfur as inhibitor in waste incineration processes[J]. Science of the Total Environment, 2006, 366(2): 456-465. |
| 40 | YAN M, QI Z F, YANG J, et al. Effect of ammonium sulfate and urea on PCDD/F formation from active carbon and possible mechanism of inhibition[J]. Journal of Environmental Sciences, 2014, 26(11): 2277-2282. |
| 41 | FU J Y, LI X D, CHEN T, et al. PCDD/Fs’ suppression by sulfur-amine/ammonium compounds[J]. Chemosphere. 2015, 123: 9-16. |
| 42 | WEBER P, DINJUS E, STIEGLITZ L. The role of copper(Ⅱ) chloride in the formation of organic chlorine in fly ash[J]. Chemosphere, 2001, 42: 579-582. |
| 43 | HAJIZADEH Y, ONWUDILI J A, WILLIAMS P T. Effects of gaseous NH3 and SO2 on the concentration profiles of PCDD/F in flyash under post-combustion zone conditions[J]. Waste Management, 2012, 32(7): 1378-1386. |
| 44 | VOGG H, METZGER M, STIEGLITZ L. Recent findings on the formation and decomposition of PCDD/PCDF in municipal solid waste incineration[J]. Waste Management & Research, 1987, 5(3): 285-294. |
| 45 | HUANG Q X, CAI X, ALHADJ MALLAH M M, et al. Effect of HCl/SO2/NH3/O2 and mineral sorbents on the partitioning behaviour of heavy metals during the thermal treatment of solid wastes[J]. Environmental Technology, 2015, 36(23): 3043-3049. |
| 46 | WANG S J, HE P J, LU W T, et al. Amino compounds as inhibitors of de novo synthesis of chlorobenzenes[J]. Scientific Reports, 2016, 6: 23197. |
| 47 | 詹明秀, 陈彤, 林晓青, 等. 氮基阻滞剂抑制二恶英生成研究综述[J]. 能源工程, 2013 (6): 43-49. |
| ZHAN M X, CHEN T, LIN X Q, et al. An overview of the suppressing effect of nitrogen-containing inhibitors on the formation of dioxins[J]. Energy Engineering, 2013 (6): 43-49. | |
| 48 | FUJIMORI T, TAKAOKA M, MORISAWA S. Chlorinated aromatic compounds in a thermal process promoted by oxychlorination of ferric chloride[J]. Environmental Science & Technology, 2010, 44(6): 1974-1979. |
| 49 | PIGNATELLO J J, OLIVEROS E, MACKAY A. Advanced oxidation processes for organic contaminant destruction based on the fenton reaction and related chemistry[J]. Critical Reviews in Environmental Science and Technology, 2006, 36(1): 1-84. |
| 50 | FUJIMORI T, FUJINAGA Y, TAKAOKA M. Deactivation of metal chlorides by alkaline compounds inhibits formation of chlorinated aromatics[J]. Environmental Science & Technology, 2010, 44(19): 7678-7684. |
| 51 | DICKSON L C, LENOIR D, HUTZINGER O, et al. Inhibition of chlorinated dibenzo-p-dioxin formation on municipal incinerator fly ash by using catalyst inhibitors[J]. Chemosphere, 1989, 19(8): 1435-1445. |
| 52 | RAGHUNATHAN K, GULLETT B K. Role of sulfur in reducing PCDD and PCDF formation[J]. Environmental Science & Technology, 1996, 30(6): 1827-1834. |
| 53 | SHAO K, YAN J H, LI X D, et al. Effects of SO2 and SO3 on the formation of polychlorinated dibenzo-p-dioxins and dibenzofurans by de novo synthesis[J]. Journal of Zhejiang University-Science A, 2010, 11(5): 363-369. |
| 54 | KUZUHARA S, SATO H, TSUBOUCHI N, et al. Effect of nitrogen-containing compounds on polychlorinated dibenzo-p-dioxin/dibenzofuran formation through de novo synthesis[J]. Environmental Science & Technology, 2005, 39(3): 795-799. |
| 55 | SHAO K, YAN J H, LI X D, et al. Experimental study on the effects of H2O on PCDD/Fs formation by de novo synthesis in carbon/CuCl2 model system[J]. Chemosphere, 2010, 78(6): 672-679. |
| 56 | PANDELOVA M, LENOIR D, SCHRAMM K W. Correlation between PCDD/F, PCB and PCBz in coal/waste combustion. Influence of various inhibitors[J]. Chemosphere, 2006, 62(7): 1196-1205. |
| 57 | LIN X, ZHAN M, YAN M, et al. Suppression of dioxins in waste incinerator emissions by recirculating SO2[J]. Chemosphere, 2015, 133: 75-81. |
| 58 | LIN X, YAN M, DAI A, et al. Simultaneous suppression of PCDD/F and NOx during municipal solid waste incineration[J]. Chemosphere, 2015, 126: 60-66. |
| 59 | WU H L, LU S Y, LI X D, et al. Inhibition of PCDD/F by adding sulphur compounds to the feed of a hazardous waste incinerator[J]. Chemosphere, 2012, 86(4): 361-367. |
| 60 | LUNDIN L, JANSSON S. The effects of fuel composition and ammonium sulfate addition on PCDD, PCDF, PCN and PCB concentrations during the combustion of biomass and paper production residuals[J]. Chemosphere, 2014, 94(Supplement C): 20-26. |
| 61 | LONG H M, LI J X, WANG P, et al. Emission reduction of dioxin in iron ore sintering by adding urea as inhibitor[J]. Ironmaking & Steelmaking, 2011, 38(4): 258-262. |
| 62 | ZHAN M, CHEN T, LIN X, et al. Suppression of dioxins after the post-combustion zone of MSWIs[J]. Waste Management, 2016, 54: 153-161. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [6] | 王正坤, 黎四芳. 双子表面活性剂癸炔二醇的绿色合成[J]. 化工进展, 2023, 42(S1): 400-410. |
| [7] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [8] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [9] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [10] | 邵博识, 谭宏博. 锯齿波纹板对挥发性有机物低温脱除过程强化模拟分析[J]. 化工进展, 2023, 42(S1): 84-93. |
| [11] | 许友好, 王维, 鲁波娜, 徐惠, 何鸣元. 中国炼油创新技术MIP的开发策略及启示[J]. 化工进展, 2023, 42(9): 4465-4470. |
| [12] | 耿源泽, 周俊虎, 张天佑, 朱晓宇, 杨卫娟. 部分填充床燃烧器中庚烷均相/异相耦合燃烧[J]. 化工进展, 2023, 42(9): 4514-4521. |
| [13] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [14] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [15] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||