化工进展 ›› 2020, Vol. 39 ›› Issue (2): 567-576.DOI: 10.16085/j.issn.1000-6613.2019-0544
CuZnTiO2/SAPO-34双功能催化剂的设计、制备及其用于CO2加氢制烯烃性能
陈静宇( ),张建红,盛浩,吴大凯,高新华(
),张建红,盛浩,吴大凯,高新华( ),马清祥,张建利(
),马清祥,张建利( ),范素兵,赵天生
),范素兵,赵天生
- 省部共建煤炭高效利用与绿色化工国家重点实验室(宁夏大学),宁夏 银川 750021
-
收稿日期:2019-04-09出版日期:2020-02-05发布日期:2020-03-12 -
通讯作者:高新华,张建利 -
作者简介:陈静宇(1993—),女,硕士研究生,研究方向为煤基应用催化。E-mail:852858917@qq.com 。 -
基金资助:国家自然科学基金(21666030);宁夏重点研发计划(2018BEE03010);宁夏自然科学基金(2018AAC02002);宁夏大学研究生创新项目(GIP2018035)
Design and preparation of CuZnTiO2/SAPO-34 bifunctional catalyst and its catalytic performance in CO2 hydrogenation to light olefins
Jingyu CHEN( ),Jianhong ZHANG,Hao SHENG,Dakai WU,Xinhua GAO(
),Jianhong ZHANG,Hao SHENG,Dakai WU,Xinhua GAO( ),Qingxiang MA,Jianli ZHANG(
),Qingxiang MA,Jianli ZHANG( ),Subing FAN,Tiansheng ZHAO
),Subing FAN,Tiansheng ZHAO
- State Key Laboratory of High-efficiency Utilization of Coal and Green Chemical Engineering, Ningxia University, Yinchuan 750021, Ningxia, China
-
Received:2019-04-09Online:2020-02-05Published:2020-03-12 -
Contact:Xinhua GAO,Jianli ZHANG
摘要:
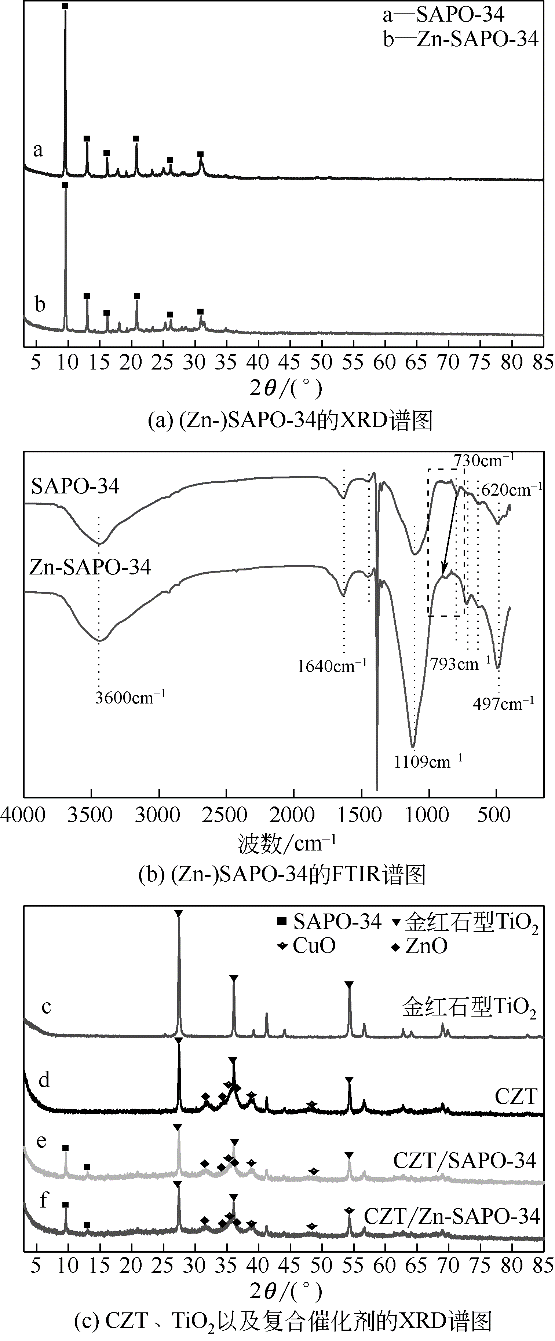
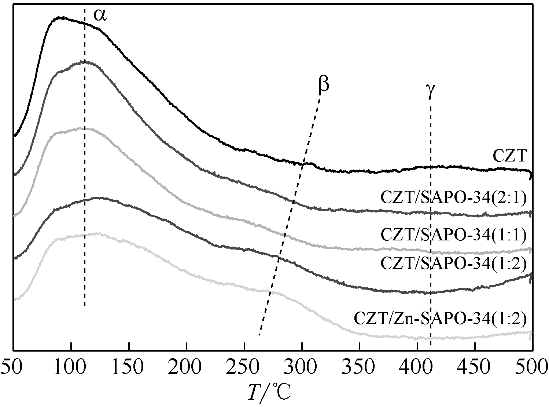
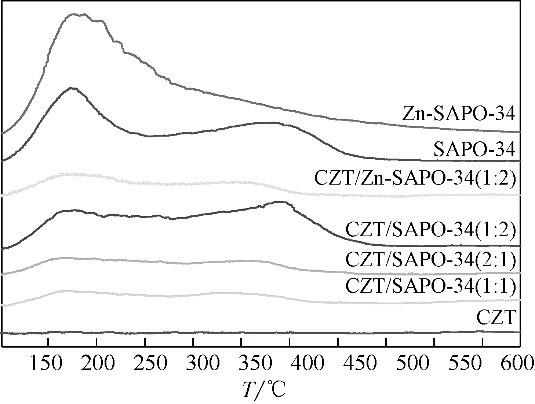
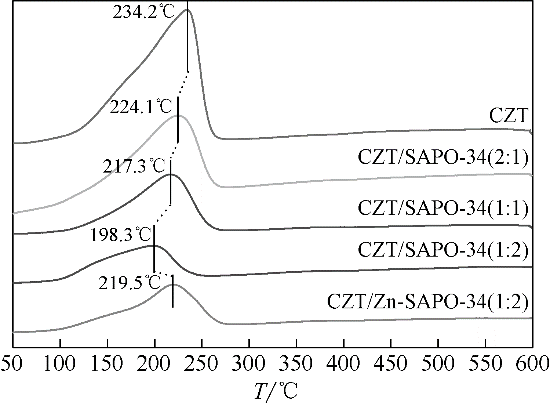
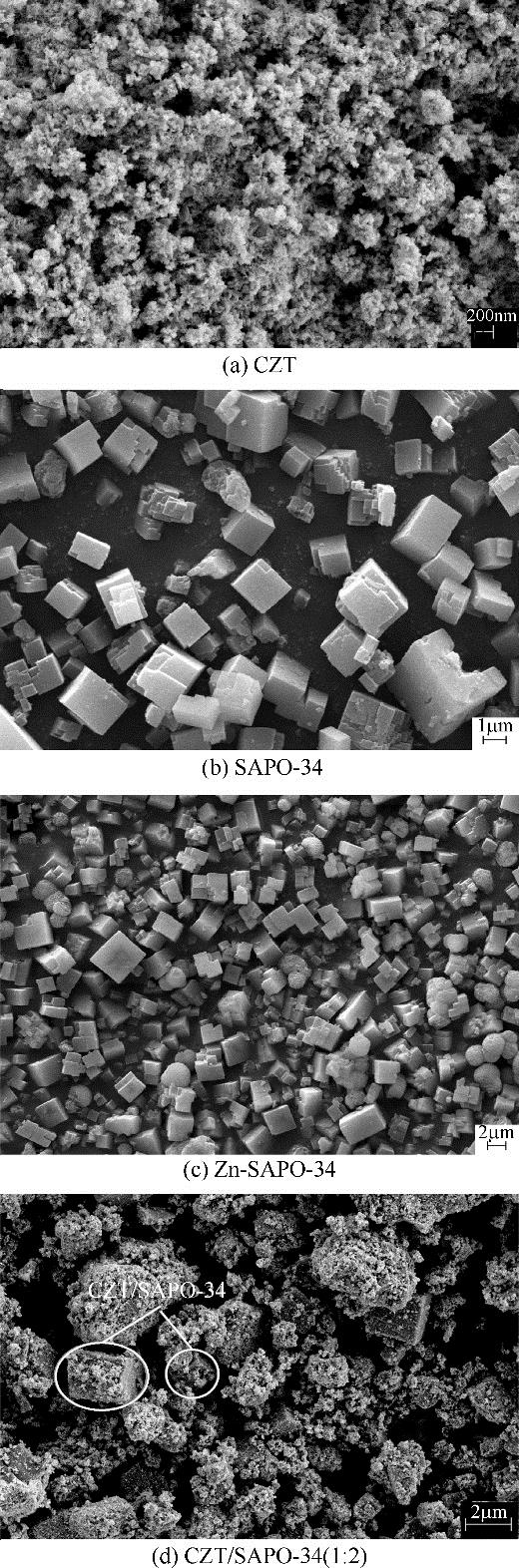
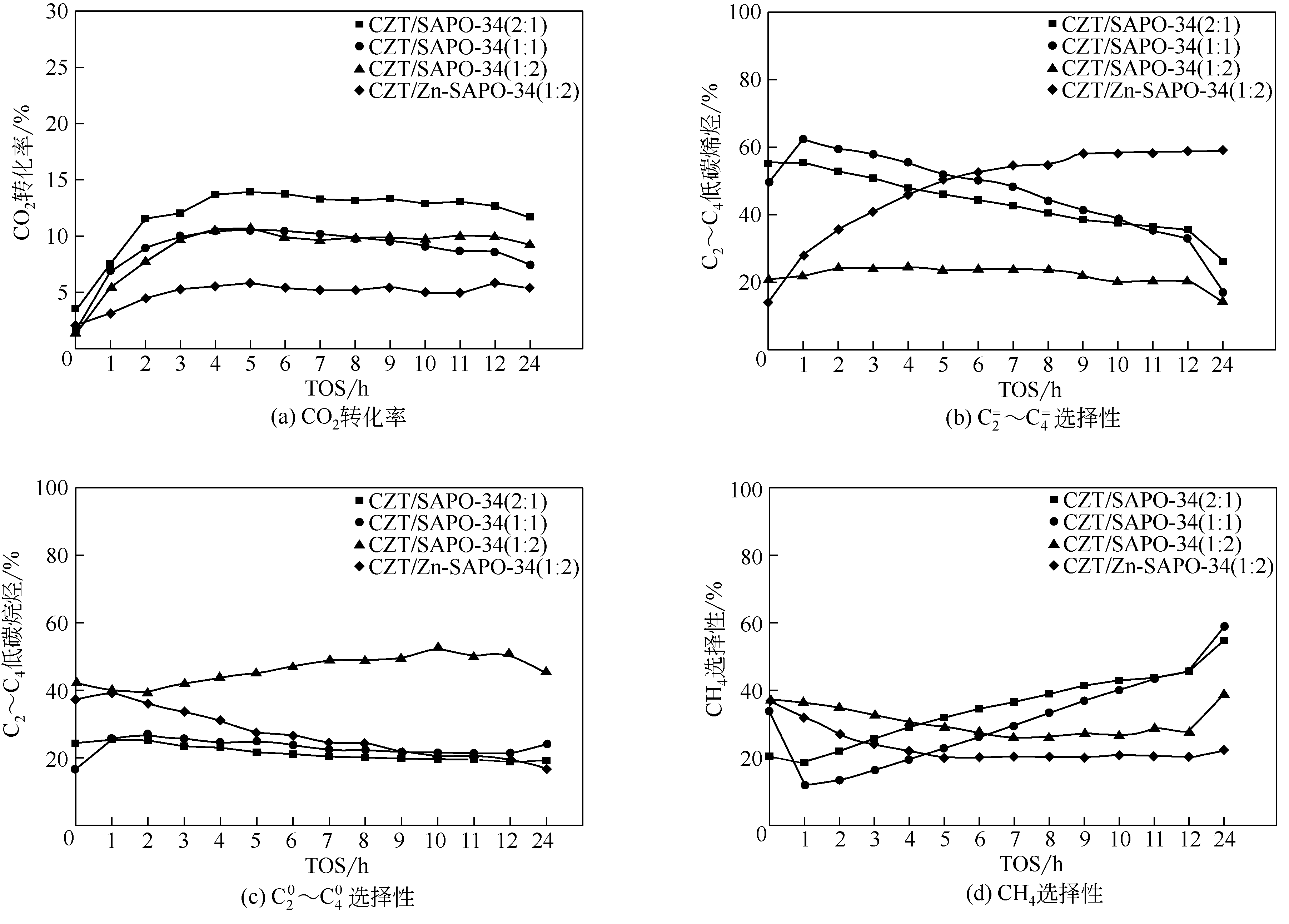
CO2加氢经甲醇(含氧中间体)制低碳烯烃工艺路线,可实现成醇、脱水两步反应串联协同进行,打破费托合成产物Anderson-Schulz-Flory(ASF)分布限制,高选择性地制取低碳烯烃。传统甲醇合成Cu基催化剂加氢能力较强,在两步反应中产物以CH4、低碳烷烃为主。实验设计、制备了CuZnTiO2/(Zn-)SAPO-34复合催化剂,实现了CO2加氢在Cu基复合催化剂上高选择性合成C2~C4烯烃(约60%)。研究表明,两步反应过程中甲醇体积分数较低(<6%),且高温下逆水煤气变换反应严重,导致催化剂酸性变化对产物分布的影响较大。调变两类活性位点比例发现,CH4的产生与串联反应存在竞争关系,SAPO-34酸量的增加抑制了CH4的生成,促进串联反应正向进行;合适的酸性有助于生成C2~C4烯烃。控制成醇、脱水两类活性位点接触距离可调变烯烃的二次反应,降低加氢能力,改善产物分布。
中图分类号:
引用本文
陈静宇,张建红,盛浩,吴大凯,高新华,马清祥,张建利,范素兵,赵天生. CuZnTiO2/SAPO-34双功能催化剂的设计、制备及其用于CO2加氢制烯烃性能[J]. 化工进展, 2020, 39(2): 567-576.
Jingyu CHEN,Jianhong ZHANG,Hao SHENG,Dakai WU,Xinhua GAO,Qingxiang MA,Jianli ZHANG,Subing FAN,Tiansheng ZHAO. Design and preparation of CuZnTiO2/SAPO-34 bifunctional catalyst and its catalytic performance in CO2 hydrogenation to light olefins[J]. Chemical Industry and Engineering Progress, 2020, 39(2): 567-576.
| 催化剂 | 弱酸 | 强酸 | 总酸量/mmol·g-1 | ||
|---|---|---|---|---|---|
| 温度/℃ | 酸量/mmol·g-1 | 温度/℃ | 酸量/mmol·g-1 | ||
| CZT | — | — | — | — | — |
| CZT/SAPO-34(2∶1) | 168 | 0.02 | 341 | 0.02 | 0.04 |
| CZT/SAPO-34(1∶1) | 163 | 0.03 | 375 | 0.02 | 0.04 |
| CZT/SAPO-34(1∶2) | 166 | 0.05 | 396 | 0.09 | 0.14 |
| SAPO-34 | 172 | 0.13 | 389 | 0.09 | 0.22 |
| Zn-SAPO-34 | 174 | 0.27 | 442 | 0.08 | 0.35 |
| CZT/Zn-SAPO-34(1∶2) | 169 | 0.04 | — | 0.02 | 0.06 |
表1 不同样品的NH3-TPD酸量
| 催化剂 | 弱酸 | 强酸 | 总酸量/mmol·g-1 | ||
|---|---|---|---|---|---|
| 温度/℃ | 酸量/mmol·g-1 | 温度/℃ | 酸量/mmol·g-1 | ||
| CZT | — | — | — | — | — |
| CZT/SAPO-34(2∶1) | 168 | 0.02 | 341 | 0.02 | 0.04 |
| CZT/SAPO-34(1∶1) | 163 | 0.03 | 375 | 0.02 | 0.04 |
| CZT/SAPO-34(1∶2) | 166 | 0.05 | 396 | 0.09 | 0.14 |
| SAPO-34 | 172 | 0.13 | 389 | 0.09 | 0.22 |
| Zn-SAPO-34 | 174 | 0.27 | 442 | 0.08 | 0.35 |
| CZT/Zn-SAPO-34(1∶2) | 169 | 0.04 | — | 0.02 | 0.06 |
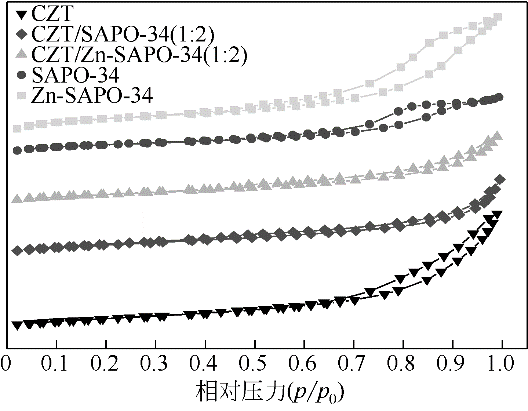
| 催化剂 | 比表面积/m2·g-1 | 总孔体积/cm3·g-1 | 孔径/nm |
|---|---|---|---|
| CZT | 36.4 | 0.12 | 2.86 |
| CZT/SAPO-34(1∶2) | 64.6 | 0.10 | 2.60 |
| CZT/Zn-SAPO-34(1∶2) | 60.7 | 0.09 | 2.11 |
| SAPO-34 | 492.6 | 0.31 | 0.45 |
| Zn-SAPO-34 | 416.3 | 0.32 | 0.45 |
表2 催化剂结构性质
| 催化剂 | 比表面积/m2·g-1 | 总孔体积/cm3·g-1 | 孔径/nm |
|---|---|---|---|
| CZT | 36.4 | 0.12 | 2.86 |
| CZT/SAPO-34(1∶2) | 64.6 | 0.10 | 2.60 |
| CZT/Zn-SAPO-34(1∶2) | 60.7 | 0.09 | 2.11 |
| SAPO-34 | 492.6 | 0.31 | 0.45 |
| Zn-SAPO-34 | 416.3 | 0.32 | 0.45 |
| 催化剂 | CO2转化率/% | 产物选择性/% | 烃类选择性/% | O/P | |||||
|---|---|---|---|---|---|---|---|---|---|
| CO | CH | CH3OH | CH4 | ||||||
| CZT | 17.93 | 93.34 | 2.55 | 4.11 | 94.62 | 2.56 | 2.82 | 0 | 0.91 |
| CZT/SAPO-34(2∶1) | 11.7 | 83.94 | 16.06 | — | 54.77 | 26.08 | 19.14 | 0 | 1.36 |
| CZT/SAPO-34(1∶1) | 7.38 | 82.96 | 17.04 | — | 58.87 | 16.96 | 24.17 | 0 | 0.7 |
| CZT/SAPO-34(1∶2) | 9.23 | 86.12 | 13.88 | — | 38.98 | 14.28 | 45.51 | 1.23 | 0.31 |
| CZT/Zn-SAPO-34(1∶2) | 5.34 | 76.18 | 23.82 | — | 22.37 | 59.2 | 16.81 | 1.63 | 3.52 |
表3 催化剂催化活性数据
| 催化剂 | CO2转化率/% | 产物选择性/% | 烃类选择性/% | O/P | |||||
|---|---|---|---|---|---|---|---|---|---|
| CO | CH | CH3OH | CH4 | ||||||
| CZT | 17.93 | 93.34 | 2.55 | 4.11 | 94.62 | 2.56 | 2.82 | 0 | 0.91 |
| CZT/SAPO-34(2∶1) | 11.7 | 83.94 | 16.06 | — | 54.77 | 26.08 | 19.14 | 0 | 1.36 |
| CZT/SAPO-34(1∶1) | 7.38 | 82.96 | 17.04 | — | 58.87 | 16.96 | 24.17 | 0 | 0.7 |
| CZT/SAPO-34(1∶2) | 9.23 | 86.12 | 13.88 | — | 38.98 | 14.28 | 45.51 | 1.23 | 0.31 |
| CZT/Zn-SAPO-34(1∶2) | 5.34 | 76.18 | 23.82 | — | 22.37 | 59.2 | 16.81 | 1.63 | 3.52 |
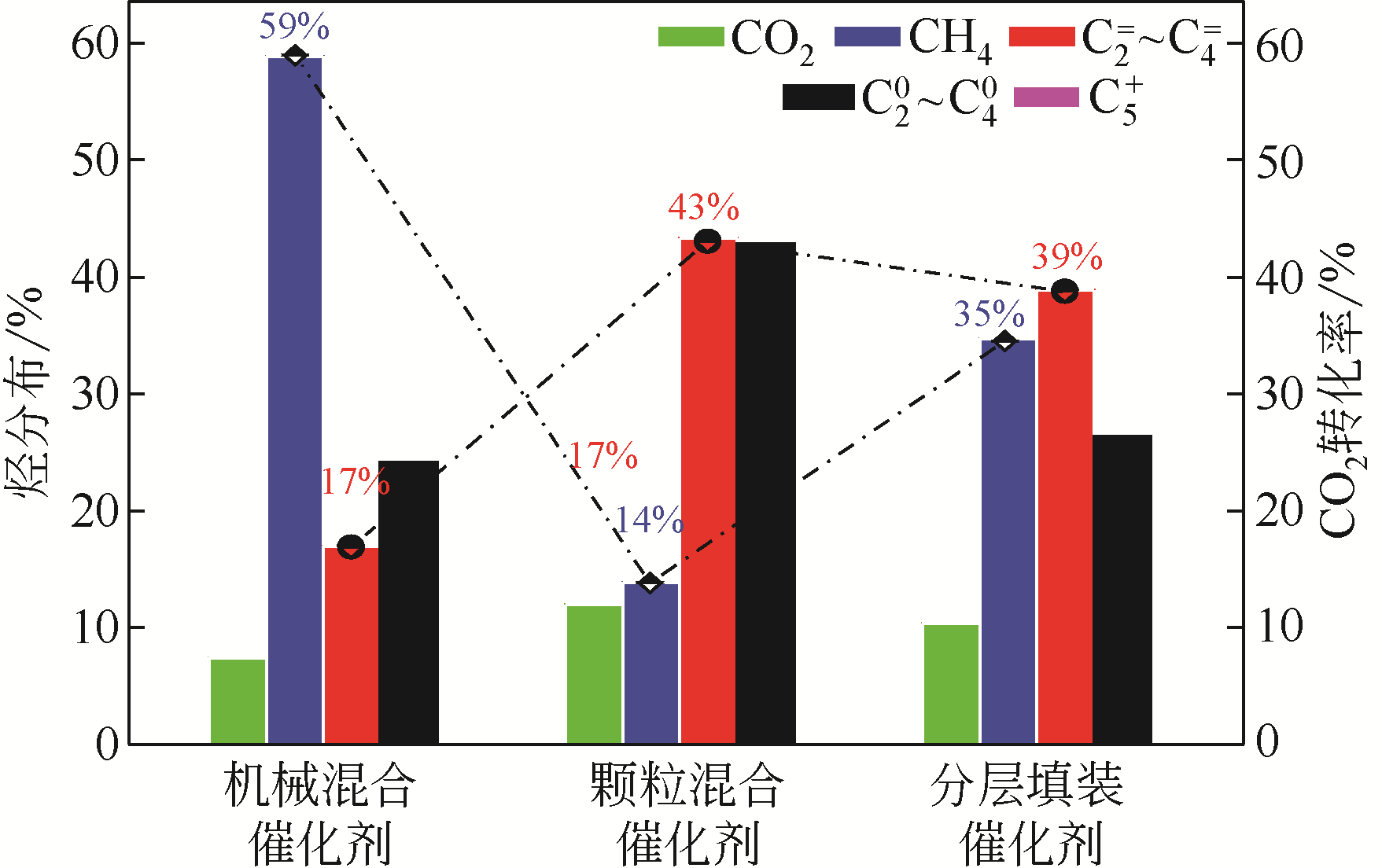
图9 活性组分的复合方式对催化性能的影响[还原条件为30%H2+70%N2,常压,350℃/6h;反应条件为H2/CO2=3、380℃、2MPa、3000h-1;反应时间24h;(a)CZT与SAPO-34粉末以质量比1∶1研磨在一起,再压片至20~40目;(b)CZT与SAPO-34压至20~40目,然后以质量比为1∶1均匀混合;(c)CZT与SAPO-34用石英棉隔开,石英棉上层为CZT 20~40目颗粒,下层为SAPO-34 20~40目颗粒,质量比为1∶1装入反应管中]
| 1 | CORMA A, MELO F V, SAUVANAUD L, et al. Light cracked naphtha processing: controlling chemistry for maximum propylene production[J]. Catalysis Today, 2005, 107: 699-706. |
| 2 | DIERCKS R, ARNDT J D, FREYER S, et al. Raw material changes in the chemical industry[J]. Chem. Eng. Technol., 2008, 31: 631-637. |
| 3 | CENTI G, QUADRELLI E A, PERATHONER S. Catalysis for CO2 conversion: a key technology for rapid introduction of renewable energy in the value chain of chemical industries[J]. Energy & Environmental Science, 2013, 6(6): 1711-1731. |
| 4 | 丁云杰. 用于合成气高选择性直接制备低碳烯烃的碳化钴纳米棱柱结构[J]. 催化学报, 2017, 38(1): 1-4. |
| DING Y J. Co2C nanoprisms for syngas conversion to lower olefins with highselectivity[J]. Chinese Journal of Catalysis, 2017, 38(1): 1-4. | |
| 5 | 张跃, 李静, 严生虎, 等. Ce助剂对CuO-ZnO-Al2O3/HZSM-5在CO2加氢合成二甲醚中的性能影响[J]. 化工进展, 2011, 30(3): 542-546. |
| ZHANG Y, LI J, YAN S H, et al. Effect of Ce loading in CuO-ZnO-Al2O3/HZSM-5 on catalytic performance of dimethyl ether synthesis from carbon dioxide[J]. Chemical Industry and Engineering Progress, 2011, 30(3): 542-546. | |
| 6 | PACHECO M A, MARSHALL C L. Review of dimethyl carbonate (DMC) manufacture and its characteristics as a fuel additive[J]. Energy Fuels, 1997, 11: 2-9. |
| 7 | 马光远, 徐艳飞, 王捷, 等. 合成气直接法制取低碳烯烃铁基催化体系研究进展[J]. 化工进展, 2018, 37(3): 992-1000. |
| MA G Y, XU Y F, WANG J, et al. Research progress of iron-based catalyst for converting syngas directly to light olefins[J]. Chemical Industry and Engineering Progress, 2018, 37(3): 992-1000. | |
| 8 | ZHANG J L, LU S P, SU X J, et al. Selective formation of light olefins from CO2 hydrogenation over Fe-Zn-K catalysts[J]. Journal of CO2 Utilization, 2015, 12: 95-100. |
| 9 | JIAO F, LI J, PAN X, et al. Selective conversion of syngas to light olefins[J]. Science, 2016, 351(6277): 1065-1068. |
| 10 | CHENG K, GU B, LIU X L, et al. Direct and highly selective conversion of synthesis gas into lower olefns: design of a bifunctional catalyst combining methanol synthesis and carbon-carbon coupling[J]. Angew. Chem. Int. Ed., 2016, 55: 4725-4728. |
| 11 | GAO P, DANG S S, LI S G, et al. Direct production of lower olefns from CO2 conversion via bifunctional catalysis[J]. ACS Catal., 2017, 8: 571-578. |
| 12 | FUJIWARA M, SOUMA Y. Hydrocarbon synthesis from carbon dioxide and hydrogen over Cu-Zn-Cr oxide/zeolite hybrid catalysts[J]. Journal of the Chemical Society, Chemical Communications, 1992 (10): 767-768. |
| 13 | PARK Y K, PARK K C, IHM S K. Hydrocarbon synthesis through CO2 hydrogenation over CuZnOZrO2/zeolite hybrid catalysts[J]. Catalysis Today, 1998, 44(1/2/3/4): 165-173. |
| 14 | 张鲁湘, 张永春, 陈绍云. 助剂TiO2对CO2催化加氢制甲醇催化剂CuO-ZnO-Al2O3性能的影响[J]. 燃料化学学报, 2011, 39(12):912-917. |
| ZHANG L X, ZHANG Y C, CHEN S Y. Effect of promoter TiO2 on the performance of CuO-ZnO-Al2O3 catalyst for CO2 catalytic hydrogenation to methanol[J]. Journal of Fuel Chemistry and Technology, 2011, 39(12): 912-917. | |
| 15 | BAO Y F, HUANG C L, CHEN L M, et al. Highly efficient Cu/anatase TiO2 {001}-nanosheets catalysts for methanol synthesis from CO2[J]. Journal of Energy Chemistry, 2018, 27(2): 381-388. |
| 16 | TAGAWA T, NOMURA N, SHIMAKAGE M, et al. Effect of supports on copper catalysts for methanol synthesis from CO2+ H2[J]. Research on Chemical Intermediates, 1995, 21(2): 193-202. |
| 17 | NOMURA N, TAGAWA T, GOTO S. Effect of acid-base properties on copper catalysts for hydrogenation of carbon dioxide[J]. Reaction Kinetics and Catalysis Letters, 1998, 63(1): 21-25. |
| 18 | NOMURA N, TAGAWA T, GOTO S. Titania supported copper catalysts for methanol synthesis from carbon dioxide[J]. Reaction Kinetics and Catalysis Letters, 1998, 63(1): 9-13. |
| 19 | ÁLVAREZ A, BANSODE A, URAKAWA A, et al. Challenges in the greener production of formates/formic acid, methanol, and DME by heterogeneously catalyzed CO2 hydrogenation processes[J]. Chemical Reviews, 2017, 117(14): 9804-9838. |
| 20 | CHEN J Y, WANG X, WU D K, et al. Hydrogenation of CO2 to light olefins on CuZnZr@(Zn-) SAPO-34 catalysts: strategy for product distribution[J]. Fuel, 2019, 239: 44-52. |
| 21 | SŁOCZYŃSKI J, GRABOWSKI R, OLSZEWSKI P, et al. Effect of metal oxide additives on the activity and stability of Cu/ZnO/ZrO2 catalysts in the synthesis of methanol from CO2 and H2[J]. Applied Catalysis A: General, 2006, 310: 127-137. |
| 22 | 郭晓明, 毛东森, 卢冠忠, 等. CO2加氢合成甲醇催化剂的研究进展[J]. 化工进展, 2012, 31(3): 477-488. |
| GUO X M, MAO D S, LU G Z, et al. Progress in catalysts for methanol synthesis from CO2 hydrogenation[J]. Chemical Industry and Engineering Progress, 2012, 31(3): 477-488. | |
| 23 | AHMADI S M A, ASKARI S, HALLADJ R. A review on kinetic modeling of deactivation of SAPO-34 catalyst during methanol to olefins (MTO) process[J]. Afinidad, 2013, 70(562): 130-138. |
| 24 | 赵云鹏. 温室气体CO2加氢合成甲醇CuO-ZnO/TiO2催化剂的制备与性能研究[D]. 哈尔滨:哈尔滨工业大学, 2013. |
| ZHAO Y P. Preparation and preformance study of CuO-ZnO/TiO2 catalyst for methanol synthesis from greenhouse gas carbon dioxide hydrogenation[D]. Harbin:Harbin Institute of Technology, 2013. | |
| 25 | NAJAFI N, ASKARI S, HALLADJ R. Hydrothermal synthesis of nanosized SAPO-34 molecular sieves by different combinations of multi templates[J]. Powder technology, 2014, 254: 324-330. |
| 26 | RAVEENDRA G, LI C, CHENG Y, et al. Direct transformation of syngas to lower olefins synthesis over hybrid Zn-Al2O3/SAPO-34 catalysts[J]. New Journal of Chemistry, 2018, 42(6): 4419-4431. |
| 27 | LI Z, MARTÍNEZ-TRIGUERO J, CONCEPCIÓN P, et al. Methanol to olefins: activity and stability of nanosized SAPO-34 molecular sieves and control of selectivity by silicon distribution[J]. Physical Chemistry Chemical Physics, 2013, 15(35): 14670-14680. |
| 28 | 袁奋云. 过渡金属改性SAPO-34催化甲醇制烯烃[D]. 太原:太原理工大学, 2012. |
| YUAN F Y. Transition metals-modified SAPO-34 for methanol conversion to light olefins[D]. Taiyuan: Taiyuan University of Technology, 2012. | |
| 29 | AGHAEI E, HAGHIGHI M. High temperature synthesis of nanostructured Ce-SAPO-34 catalyst used in conversion of methanol to light olefins: effect of temperature on physicochemical properties and catalytic performance[J]. Journal of Porous Materials, 2015, 22(1): 187-200. |
| 30 | XU L, LIU Z M, DU A P, et al. Synthesis, characterization, and MTO performance of MeAPSO-34 molecular sieves[J]. Studies in Surface Science and Catalysis, 2004, 147: 445-450. |
| 31 | 季先进, 王继元, 曾崇余, 等. 焙烧温度对 TiO2 成型载体性能的影响[J]. 工业催化, 2008, 16(12): 30-33. |
| JI X J, WANG J Y, ZENG C Y, et al. Effects of calcinations temperature on properties of formed TiO2 support[J]. Industrial Catalysis, 2008, 16(12): 30-33. | |
| 32 | 张同旺, 武雪峰, 侯拴弟. 催化剂积炭对甲醇制低碳烯烃效果的影响[J]. 石油学报(石油加工), 2011, 27(6):891-896. |
| ZHANG T W, WU X F, HOU S D. The effect of coke deposition of catalyst on the reaction of methanol to olefins[J]. Acta Petrolei Sinica(Petroleum Processing Section), 2011, 27(6):891-896. | |
| 33 | NIE R F, LEI H, PAN S Y, et al. Core-shell structured CuO-ZnO@H-ZSM-5 catalysts for CO hydrogenation to dimethyl ether[J]. Fuel, 2012, 96: 419-425. |
| 34 | SUN J, YANG G H, MA Q X, et al. Fabrication of active Cu-Zn nanoalloys on H-ZSM5 zeolite for enhanced dimethyl ether synthesis via syngas[J]. Journal of Materials Chemistry A, 2014, 2(23): 8637-8643. |
| 35 | YANG R Q, YU X C, ZHANG Y I, et al. A new method of low-temperature methanol synthesis on Cu/ZnO/Al2O3 catalysts from CO/CO2/H2[J]. Fuel, 2008, 87(4/5): 443-450. |
| 36 | 辛勤, 罗孟飞. 现代催化研究方法[M].北京: 科学出版社, 2009: 21, 277. |
| XIN Q, LUO M F. Modern catalytic research method[M]. Beijing: Science Press, 2009: 21, 277. | |
| 37 | JARONIEC M, SOLOVYOV L A. Improvement of the Kruk-Jaroniec-Sayar method for pore size analysis of ordered silicas with cylindrical mesopores[J]. Langmuir, 2006, 22: 6757-6760. |
| 38 | 魏民, 于跃, 许跃, 等. 水热合成法与机械混合法制备SAPO-34/ZSM-5复合分子筛的比较研究[J]. 应用化工, 2015(9):1694-1697. |
| WEI M, YU Y, XU Y, et al. Comparison of composite molecular sieve SAPO-34/ZSM-5 synthesized by hydrothermal synthesis and physical mixing[J]. Applied Chemical Industry, 2015(9):1694-1697. | |
| 39 | LU P, SUN J, SHEN D M, et al. Direct syngas conversion to liquefed petroleum gas: importance of a multifunctional metal-zeolite interface[J]. Appl. Energy, 2018, 209: 1-7. |
| 40 | LI Z L, WANG J J, QU Y Z, et al. Highly selective conversion of carbon dioxide to lower olefns[J]. ACS Catal., 2017, 7: 8544-8548. |
| 41 | KIRILIN A V, DEWILDE J F, SANTOS V, et al. Conversion of synthesis gas to light olefins: impact of hydrogenation activity of methanol synthesis catalyst on the hybrid process selectivity over Cr-Zn and Cu-Zn with SAPO-34[J]. Industrial & Engineering Chemistry Research, 2017, 56(45): 13392-13401. |
| 42 | NIESKENS D L S, CIFTCI A, GROENENDIJK P E, et al. Production of light hydrocarbons from syngas using a hybrid catalyst[J]. Industrial & Engineering Chemistry Research, 2017, 56(10): 2722-2732. |
| 43 | ZHOU H B, LIU S, SU J J, et al. Light olefin synthesis from syngas over sulfide-zeolite composite catalyst[J]. Industrial & Engineering Chemistry Research, 2018, 57(20): 6815-6820. |
| 44 | LIU X L, WANG M H, ZHOU C, et al. Selective transformation of carbon dioxide into lower olefins with a bifunctional catalyst composed of ZnGa2O4 and SAPO-34[J]. Chemical Communications, 2018, 54(2): 140-143. |
| 45 | LIU X L, ZHOU W, YANG Y D, et al. Design of efficient bifunctional catalysts for direct conversion of syngas into lower olefins via methanol/dimethyl ether intermediates[J]. Chemical Science, 2018, 9(20): 4708-4718. |
| [1] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [2] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [3] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [4] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [5] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [6] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [7] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [8] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [9] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [10] | 毛善俊, 王哲, 王勇. 基团辨识加氢:从概念到应用[J]. 化工进展, 2023, 42(8): 3917-3922. |
| [11] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [12] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| [13] | 王兰江, 梁瑜, 汤琼, 唐明兴, 李学宽, 刘雷, 董晋湘. 快速热解铂前体合成高分散的Pt/HY催化剂及其萘深度加氢性能[J]. 化工进展, 2023, 42(8): 4159-4166. |
| [14] | 黄玉飞, 李子怡, 黄杨强, 金波, 罗潇, 梁志武. 光催化CO2和CH4重整催化剂研究进展[J]. 化工进展, 2023, 42(8): 4247-4263. |
| [15] | 王晓晗, 周亚松, 于志庆, 魏强, 孙劲晓, 姜鹏. 不同晶粒尺寸Y分子筛的合成及其加氢裂化反应性能[J]. 化工进展, 2023, 42(8): 4283-4295. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||