化工进展 ›› 2019, Vol. 38 ›› Issue (08): 3701-3710.DOI: 10.16085/j.issn.1000-6613.2018-2121
碳球表面缺陷密度对其负载铜催化剂甲醇氧化羰基化反应性能的影响
贾东森( ),张国强(
),张国强( ),尹娇,张亮亮,赵丹,郑华艳,李忠(
),尹娇,张亮亮,赵丹,郑华艳,李忠( )
)
- 太原理工大学煤科学与技术教育部和山西省重点实验室,山西 太原 030024
-
收稿日期:2018-10-29出版日期:2019-08-05发布日期:2019-08-05 -
通讯作者:张国强,李忠 -
作者简介:贾东森(1993—),男,硕士研究生,研究方向为甲醇高效转化合成碳酸二甲酯。E-mail:458603793@qq.com 。 -
基金资助:国家自然科学基金(U1510203);山西省青年科技研究基金(201701D221043)
Effect of surface defects density of carbon spheres on the catalytic performance of the supported Cu catalyst for oxidativecarbonylation of methanol
Dongsen JIA( ),Guoqiang ZHANG(
),Guoqiang ZHANG( ),Jiao YIN,Liangliang ZHANG,Dan ZHAO,Huayan ZHENG,Zhong LI(
),Jiao YIN,Liangliang ZHANG,Dan ZHAO,Huayan ZHENG,Zhong LI( )
)
- Key Laboratory of Coal Science and Technology of Ministry of Education and Shanxi Province, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
-
Received:2018-10-29Online:2019-08-05Published:2019-08-05 -
Contact:Guoqiang ZHANG,Zhong LI
摘要:
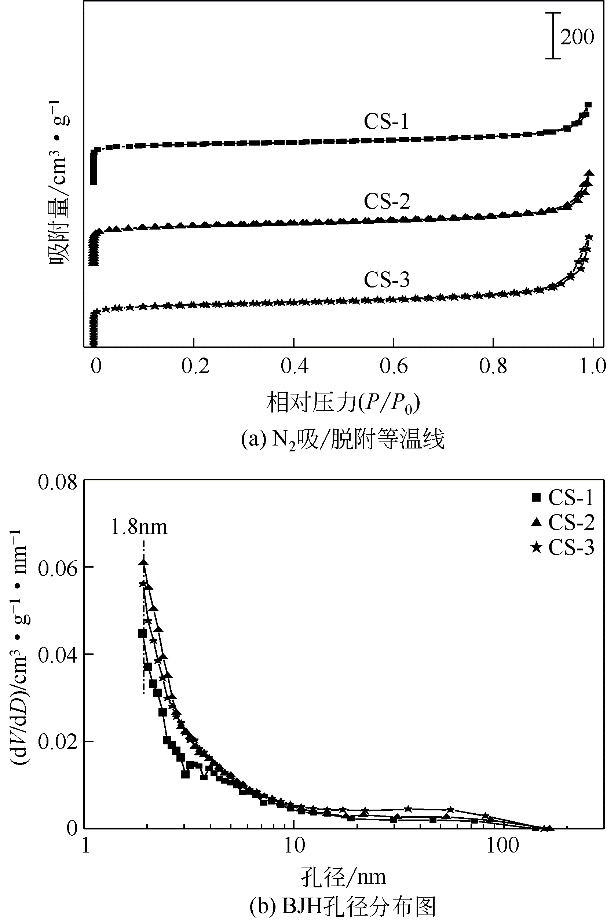
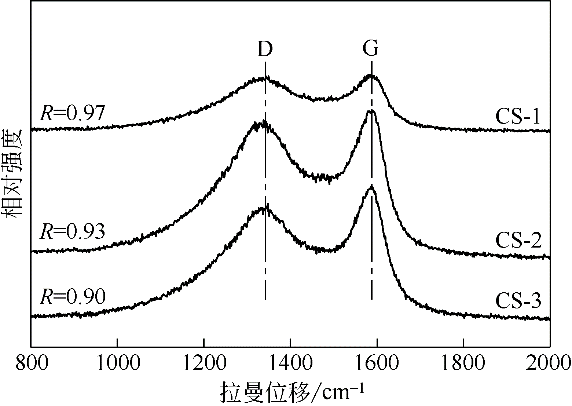
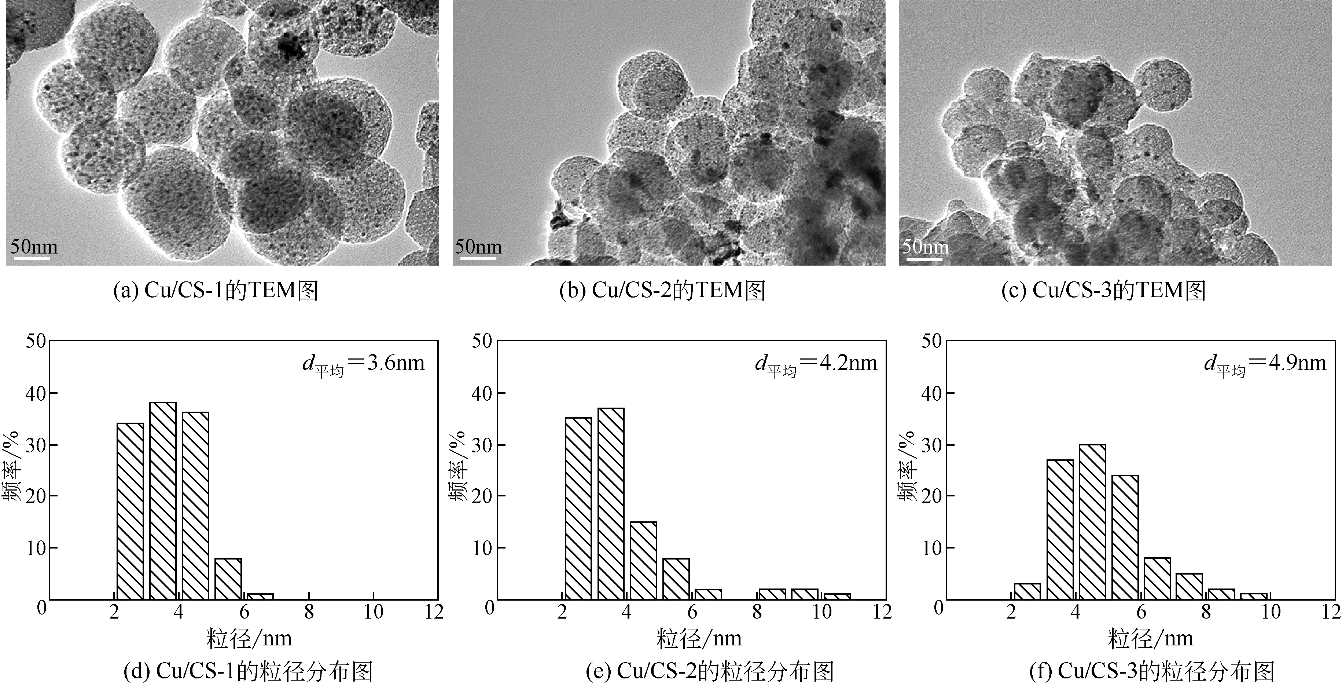
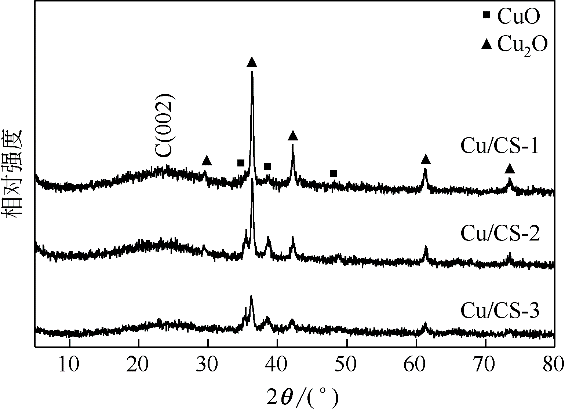
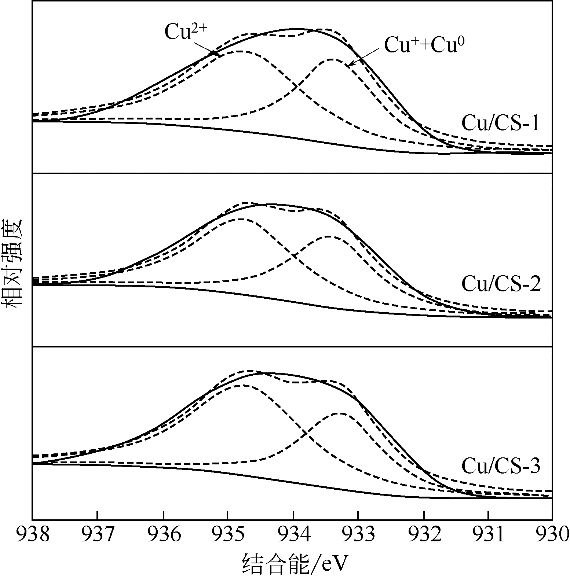
采用水热聚合法合成了一系列表面缺陷密度不同的有序微孔碳球(CS),并以其为载体制备了表面负载铜催化剂(Cu/CS),用于催化气相甲醇氧化羰基化合成碳酸二甲酯。结合表征结果,研究了载体表面缺陷密度对Cu/CS催化剂结构及催化性能的影响。结果表明,CS的表面缺陷密度随其粒径增大而增大,且其缺陷密度越大,催化剂中Cu物种分散度越高;同时,较大的表面缺陷密度有利于增强载体与Cu物种间的相互作用力,促进CuO自还原为活性物种Cu2O和Cu,从而提高催化活性。长期评价结果表明,Cu物种的氧化和团聚是造成Cu/CS催化剂失活的原因。CS的表面缺陷抑制了反应过程中活性Cu物种的氧化,且缺陷密度越大,Cu物种抗氧化能力越强;但表面缺陷密度大的催化剂中Cu物种颗粒尺寸小,表面能高,因而更容易发生团聚。
中图分类号:
引用本文
贾东森,张国强,尹娇,张亮亮,赵丹,郑华艳,李忠. 碳球表面缺陷密度对其负载铜催化剂甲醇氧化羰基化反应性能的影响[J]. 化工进展, 2019, 38(08): 3701-3710.
Dongsen JIA,Guoqiang ZHANG,Jiao YIN,Liangliang ZHANG,Dan ZHAO,Huayan ZHENG,Zhong LI. Effect of surface defects density of carbon spheres on the catalytic performance of the supported Cu catalyst for oxidativecarbonylation of methanol[J]. Chemical Industry and Engineering Progress, 2019, 38(08): 3701-3710.
| 样品 | 比表面积/m2·g-1 | 孔体积/m3·g-1 | 最可几 孔径③/nm | ||
|---|---|---|---|---|---|
| 总比表面积 | 微孔比表面积① | 总孔体积 | 微孔体积② | ||
| CS-1 | 613 | 525 | 0.61 | 0.50 | 1.84 |
| CS-2 | 600 | 505 | 0.62 | 0.51 | 1.86 |
| CS-3 | 626 | 523 | 0.64 | 0.53 | 1.85 |
| Cu/CS-1 | 588 | 495 | 0.59 | 0.49 | 1.84 |
| Cu/CS-2 | 590 | 491 | 0.61 | 0.50 | 1.86 |
| Cu/CS-3 | 617 | 509 | 0.62 | 0.51 | 1.85 |
表1 CS和Cu/CS催化剂的织构参数
| 样品 | 比表面积/m2·g-1 | 孔体积/m3·g-1 | 最可几 孔径③/nm | ||
|---|---|---|---|---|---|
| 总比表面积 | 微孔比表面积① | 总孔体积 | 微孔体积② | ||
| CS-1 | 613 | 525 | 0.61 | 0.50 | 1.84 |
| CS-2 | 600 | 505 | 0.62 | 0.51 | 1.86 |
| CS-3 | 626 | 523 | 0.64 | 0.53 | 1.85 |
| Cu/CS-1 | 588 | 495 | 0.59 | 0.49 | 1.84 |
| Cu/CS-2 | 590 | 491 | 0.61 | 0.50 | 1.86 |
| Cu/CS-3 | 617 | 509 | 0.62 | 0.51 | 1.85 |
| 催化剂 | W Cu/% | d Cu ①/nm | S Cu ②/m2·g-1 | D Cu ③/% | C CH3OH/% | S DMC/% | STYDMC /mg·g-1·h-1 |
|---|---|---|---|---|---|---|---|
| Cu/CS-1 | 10 | 3.6 | 42.9 | 63.2 | 3.4 | 70.0 | 276.6 |
| Cu/CS-2 | 10 | 4.2 | 37.0 | 54.6 | 2.4 | 71.3 | 199.2 |
| Cu/CS-3 | 10 | 4.9 | 27.3 | 40.3 | 2.0 | 69.5 | 161.1 |
| Cu/AC[ | 12 | 32.0 | — | — | 6.0 | 62.1 | 156.0 |
| Cu/SCS[ | 10 | 9.9 | — | — | 0.7 | 52.7 | 47.4 |
表2 Cu催化剂在甲醇氧化羰基化合成DMC中的催化性能
| 催化剂 | W Cu/% | d Cu ①/nm | S Cu ②/m2·g-1 | D Cu ③/% | C CH3OH/% | S DMC/% | STYDMC /mg·g-1·h-1 |
|---|---|---|---|---|---|---|---|
| Cu/CS-1 | 10 | 3.6 | 42.9 | 63.2 | 3.4 | 70.0 | 276.6 |
| Cu/CS-2 | 10 | 4.2 | 37.0 | 54.6 | 2.4 | 71.3 | 199.2 |
| Cu/CS-3 | 10 | 4.9 | 27.3 | 40.3 | 2.0 | 69.5 | 161.1 |
| Cu/AC[ | 12 | 32.0 | — | — | 6.0 | 62.1 | 156.0 |
| Cu/SCS[ | 10 | 9.9 | — | — | 0.7 | 52.7 | 47.4 |
| 催化剂 | Cu2p3/2结合能/eV | 表面Cu物种的摩尔分数/% | ||
|---|---|---|---|---|
| Cu2+ | Cu++Cu0 | Cu2+ | Cu++Cu0 | |
| Cu/CS-1 | 934.7 | 933.3 | 54.3 | 45.7 |
| Cu/CS-2 | 934.8 | 933.4 | 56.3 | 43.7 |
| Cu/CS-3 | 934.7 | 933.2 | 62.4 | 37.6 |
| 反应后Cu/CS-1 | 934.9 | 933.1 | 57.0 | 43.0 |
| 反应后Cu/CS-2 | 934.8 | 933.1 | 60.8 | 39.2 |
| 反应后Cu/CS-3 | 935.7 | 933.5 | 80.3 | 19.7 |
表3 基于Cu2p3/2 XPS拟合结果的反应前后催化剂表面Cu物种组成
| 催化剂 | Cu2p3/2结合能/eV | 表面Cu物种的摩尔分数/% | ||
|---|---|---|---|---|
| Cu2+ | Cu++Cu0 | Cu2+ | Cu++Cu0 | |
| Cu/CS-1 | 934.7 | 933.3 | 54.3 | 45.7 |
| Cu/CS-2 | 934.8 | 933.4 | 56.3 | 43.7 |
| Cu/CS-3 | 934.7 | 933.2 | 62.4 | 37.6 |
| 反应后Cu/CS-1 | 934.9 | 933.1 | 57.0 | 43.0 |
| 反应后Cu/CS-2 | 934.8 | 933.1 | 60.8 | 39.2 |
| 反应后Cu/CS-3 | 935.7 | 933.5 | 80.3 | 19.7 |
| 1 | FU Z H , ONO Y . Two-step synthesis of diphenyl carbonate from dimethyl carbonate and phenol using MoO3/SiO2 catalysts[J]. Journal of Molecular Catalysis A Chemical, 1997, 118(3): 293-299. |
| 2 | ONO Y . Catalysis in the production and reactions of dimethyl carbonate, an environmentally benign building block[J]. Applied Catalysis A: General, 1997, 155(2): 133-166. |
| 3 | WANG Y J , ZHAO X Q , YUAN B G , et al . Synthesis of dimethyl carbonate by gas-phase oxidative carbonylation of methanol on the supported solid catalyst Ⅰ. Catalyst preparation and catalytic properties[J]. Applied Catalysis A: General, 1998, 171(2): 255-260. |
| 4 | FIORANI G , PEROSA A , SELVA M . Dimethyl carbonate: a versatile reagent for a sustainable valorization of renewables[J]. Green Chemistry, 2017, 20(2): 288-322. |
| 5 | TUNDO P , SELVA M . The chemistry of dimethyl carbonate[J]. ACC Chem. Res., 2002, 35(9): 706-716. |
| 6 | 宋一兵, 罗爱国, 杜玉海, 等 . 甲醇直接气相氧化羰基化合成碳酸二甲酯[J]. 化学进展, 2008, 20(2): 221-226. |
| SONG Y B , LUO A G , DU Y H , et al . Synthesis of dimethyl carbonate by direct vapor-phase oxycarbonylation of methanol[J]. Progress of Chemistry, 2008, 20(2): 221-226. | |
| 7 | WANG R Y , LI Z , ZHENG H Y . Preparation of chlorine-free Cu/AC catalyst and its catalytic properties for vapor phase oxidative carbonylation of methanol[J]. Chinese Journal of Catalysis, 2010, 31(7): 851-856. |
| 8 | LI Z , WEN C M , WANG R Y , et al . Chloride-free Cu2O/AC catalyst prepared by pyrolysis of copper acetate and catalytic oxycarbonylation[J]. Chemical Journal of Chinese Universities, 2009, 30(10): 2024-2031. |
| 9 | REN J , WANG W , WANG D L , et al . A theoretical investigation on the mechanism of dimethyl carbonate formation on Cu/AC catalyst[J]. Applied Catalysis A: General, 2014, 472(472): 47-52. |
| 10 | ZHANG R G , SONG L Z , WANG B J , et al . A density functional theory investigation on the mechanism and kinetics of dimethyl carbonate formation on Cu2O catalyst.[J]. Journal of Computational Chemistry, 2012, 33(11): 1101-1110. |
| 11 | FU T J , WANG X , ZHENG H Y , et al . Effect of Cu location and dispersion on carbon sphere supported Cu catalysts for oxidative carbonylation of methanol to dimethyl carbonate[J]. Carbon, 2017, 115: 363-374. |
| 12 | ZHANG G Q , YAN J F , WANG J J , et al . Effect of carbon support on the catalytic performance of Cu-based nanoparticles for oxidative carbonylation of methanol[J]. Applied Surface Science, 2018, 455: 696-704. |
| 13 | DESHMUKH A A , MHLANGA S D , COVILLE N J . Carbon spheres[J]. Materials Science & Engineering R, 2010, 70(1/2):1-28. |
| 14 | MORENO C C . Colloidal and micro-carbon spheres derived from low-temperature polymerization reactions[J]. Advances in Colloid & Interface Science, 2016, 236: 113-141. |
| 15 | WANG J , HAO P P , SHI R N , et al . Fabrication of yolk-shell Cu@C nanocomposites as high-performance catalysts in oxidative carbonylation of methanol to dimethyl carbonate[J]. Nanoscale Research Letters, 2017, 12(1): 481. |
| 16 | HAO P P , REN J , YANG L L , et al . Direct and generalized synthesis of carbon-based yolk-shell nanocomposites from metal-oleate precursor[J]. Chemical Engineering Journal, 2016, 283: 113-141. |
| 17 | LI H X , ZHAO J X , SHI R N , et al . Remarkable activity of nitrogen-doped hollow carbon spheres encapsulated Cu on synthesis of dimethyl carbonate: role of effective nitrogen[J]. Applied Surface Science, 2018, 436: 803-813. |
| 18 | FANG Y , GU D , ZOU Y , et al . A low-concentration hydrothermal synthesis of biocompatible ordered mesoporous carbon nanospheres with tunable and uniform size[J]. Angewandte Chemie, 2010, 49(43): 7987-7991. |
| 19 | SING K S W , WILLIAMS R T . Physisorption hysteresis loops and the characterization of nanoporous materials[J]. Adsorption Science & Technology, 2004, 22(10): 773-782. |
| 20 | SONG S , JIANG S . Selective catalytic oxidation of ammonia to nitrogen over CuO/CNTs: the promoting effect of the defects of CNTs on the catalytic activity and selectivity[J]. Applied Catalysis B: Environmental, 2012, 117/118(1): 346-350. |
| 21 | SUZUKI S , HIBINO H . Characterization of doped single-wall carbon nanotubes by Raman spectroscopy[J]. Carbon, 2011, 49(7): 2264-2272. |
| 22 | LIN C R , SU C H , CHANG C Y , et al . Synthesis of nanosized flake carbons by RF-chemical vapor method[J]. Surface & Coatings Technology, 2006, 200(10): 3190-3193. |
| 23 | SONG S , YANG H , RAO R , et al . Defects of multi-walled carbon nanotubes as active sites for benzene hydroxylation to phenol in the presence of HO[J]. Catalysis Communications, 2010, 11(8): 783-787. |
| 24 | RODRÍGUEZMANZO J A , CRETU O , BANHART F . Trapping of metal atoms in vacancies of carbon nanotubes and graphene[J]. ACS Nano, 2010, 4(6): 3422-3428. |
| 25 | SONG S , JIANG S , RAO R , et al . Bicomponent VO2-defects/MWCNT catalyst for hydroxylation of benzene to phenol: promoter effect of defects on catalytic performance[J]. Applied Catalysis A: General, 2011, 401(1): 215-219. |
| 26 | GROBMANN D , DREIER A , LEHMANN C W , et al . Encapsulation of copper and zinc oxide nanoparticles inside small diameter carbon nanotubes[J]. Microporous & Mesoporous Materials, 2015, 202(4): 189-197. |
| 27 | YAN B , HUANG S S , WANG S P , et al . Catalytic oxidative carbonylation over Cu2O nanoclusters supported on carbon materials: the role of the carbon support[J]. Chemcatchem, 2014, 6(9): 2671-2679. |
| 28 | SONG S Q , RAO R C , YANG H X , et al . Cu2O/MWCNTs prepared by spontaneous redox: growth mechanism and superior catalytic activity[J]. Journal of Physical Chemistry C, 2010, 114(33): 13998-14003. |
| 29 | ZHANG Z L , CHE H W , WANG Y L , et al . Template-free synthesis of Cu@Cu2O core-shell microspheres and their application as copper-based catalysts for dimethyldichlorosilane synthesis[J]. Chemical Engineering Journal, 2012, 211(47): 421-431. |
| 30 | ZHANG G Q , LI Z , ZHENG H Y , et al . Influence of the surface oxygenated groups of activated carbon on preparation of a nano Cu/AC catalyst and heterogeneous catalysis in the oxidative carbonylation of methanol[J]. Applied Catalysis B: Environmental, 2015, 179: 95-105. |
| 31 | HU Q , FAN G L , ZHANG S Y , et al . Gas phase hydrogenation of dimethyl-1,4-cyclohexane dicarboxylate over highly dispersed and stable supported copper-based catalysts[J]. Journal of Molecular Catalysis A: Chemical, 2015, 397: 134-141. |
| 32 | CHAKRABORTY A K , WOOLLEY R A J , BUTENKO Y V , et al . A photoelectron spectroscopy study of ion-irradiation induced defects in single-wall carbon nanotubes[J]. Carbon, 2007, 45(14): 2744-2750. |
| 33 | ESPINÓS J P , MORALES J , BARRANCO A , et al . Interface effects for Cu, CuO, and Cu2O deposited on SiO2 and ZrO2. XPS determination of the valence state of copper in Cu/SiO2 and Cu/ZrO2 catalysts[J]. Journal of Physical Chemistry B, 2002, 106(27): 6921-6929. |
| 34 | ZHANG G Q , LI Z , ZHENG H Y , et al . Influence of surface oxygenated groups on the formation of active Cu species and the catalytic activity of Cu/AC catalyst for the synthesis of dimethyl carbonate[J]. Applied Surface Science, 2016, 390: 68-77. |
| 35 | HANSEN T W , DELARIVA A T , CHALLA S R , et al . Sintering of catalytic nanoparticles: particle migration or Ostwald ripening[J]. Accounts of Chemical Research, 2013, 46(8): 1720-1730. |
| 36 | YE R P , LIN L , LI Q H , et al . Recent progress in improving the stability of copper-based catalysts for hydrogenation of carbon-oxygen bonds[J]. Catalysis Science & Technology, 2018, 8(14): 3428-3449. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [6] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [7] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [8] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [9] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [10] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [11] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [12] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [13] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| [14] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [15] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||