化工进展 ›› 2019, Vol. 38 ›› Issue (06): 2697-2706.DOI: 10.16085/j.issn.1000-6613.2018-1742
丙烷氧化脱氢制丙烯研究进展
- 浙江大学化学系,浙江 杭州 310027
-
收稿日期:2018-08-30出版日期:2019-06-05发布日期:2019-06-05 -
通讯作者:范杰 -
作者简介:杜凯敏(1993—),男,硕士研究生,研究方向为多相催化。E-mail:<email>dkmzju@163.com</email>。 -
基金资助:国家自然科学基金(91545113)
Research progress on oxidative dehydrogenation of propane to propene
- Department of Chemistry,Zhejiang University,Hangzhou 310027,Zhejiang,China
-
Received:2018-08-30Online:2019-06-05Published:2019-06-05 -
Contact:Jie FAN
摘要:
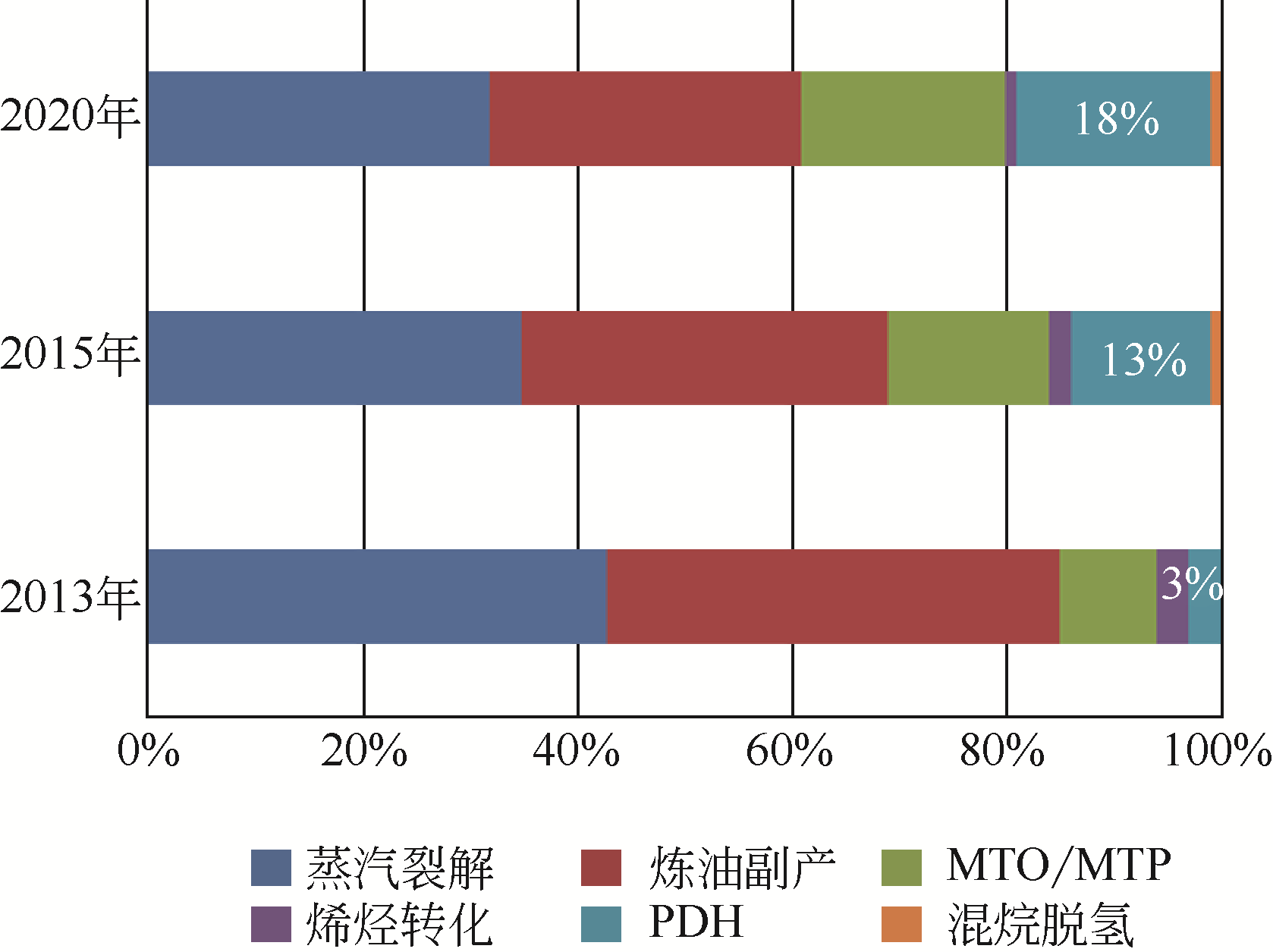
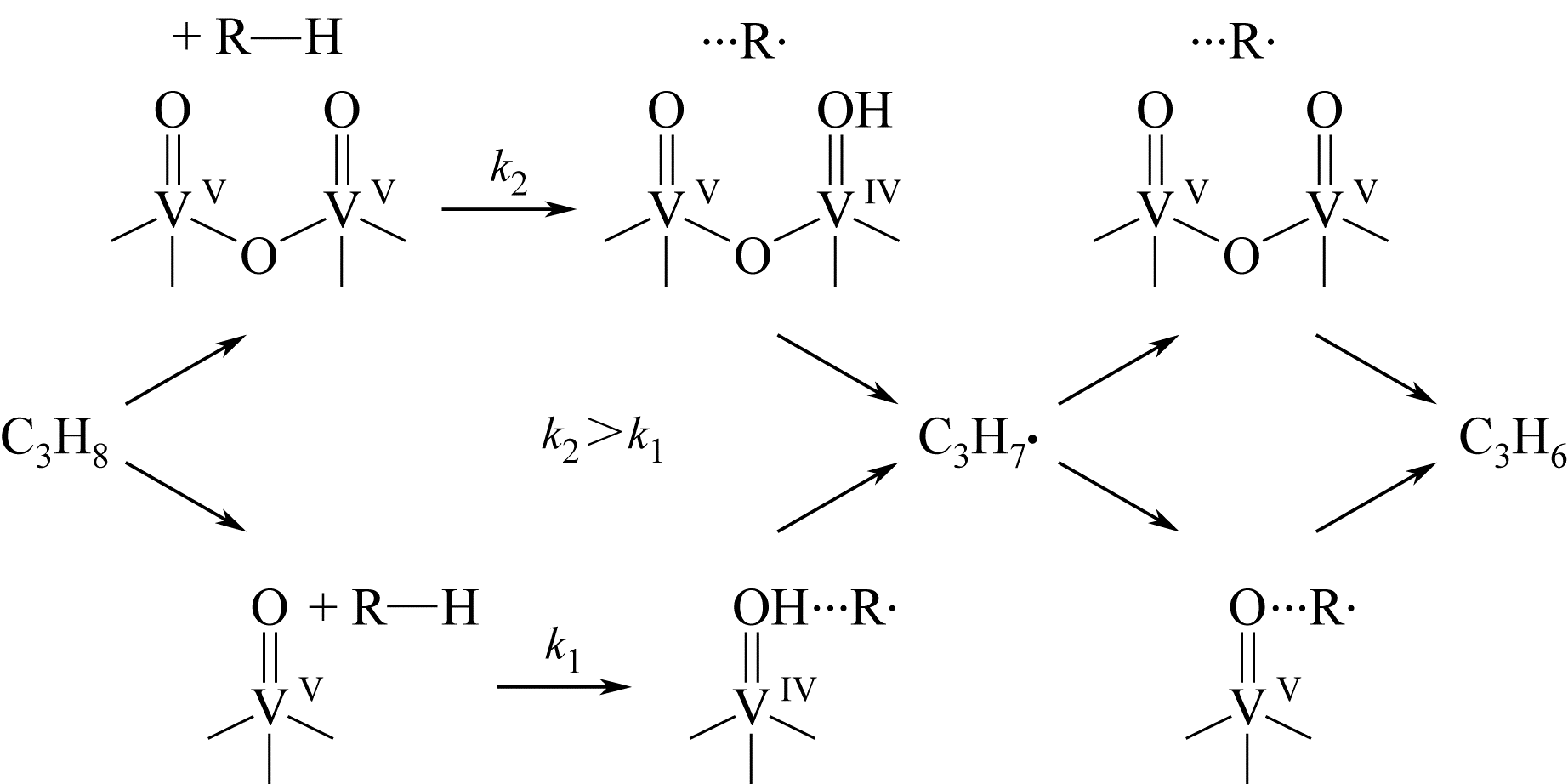
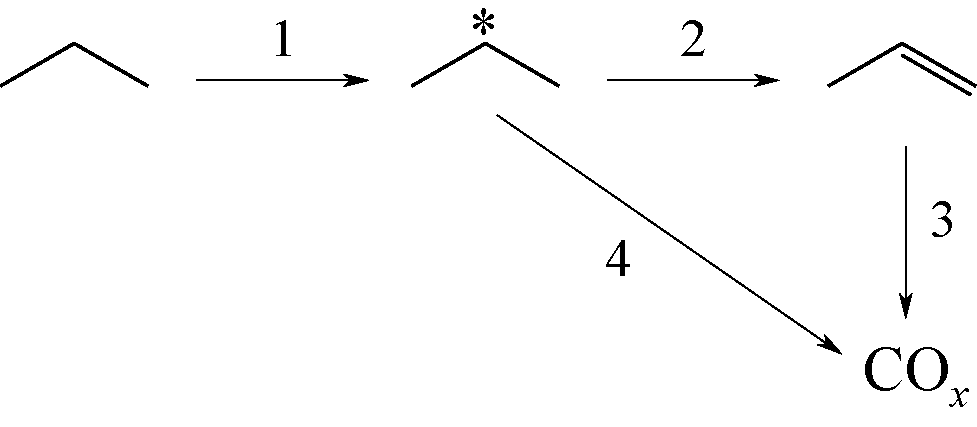
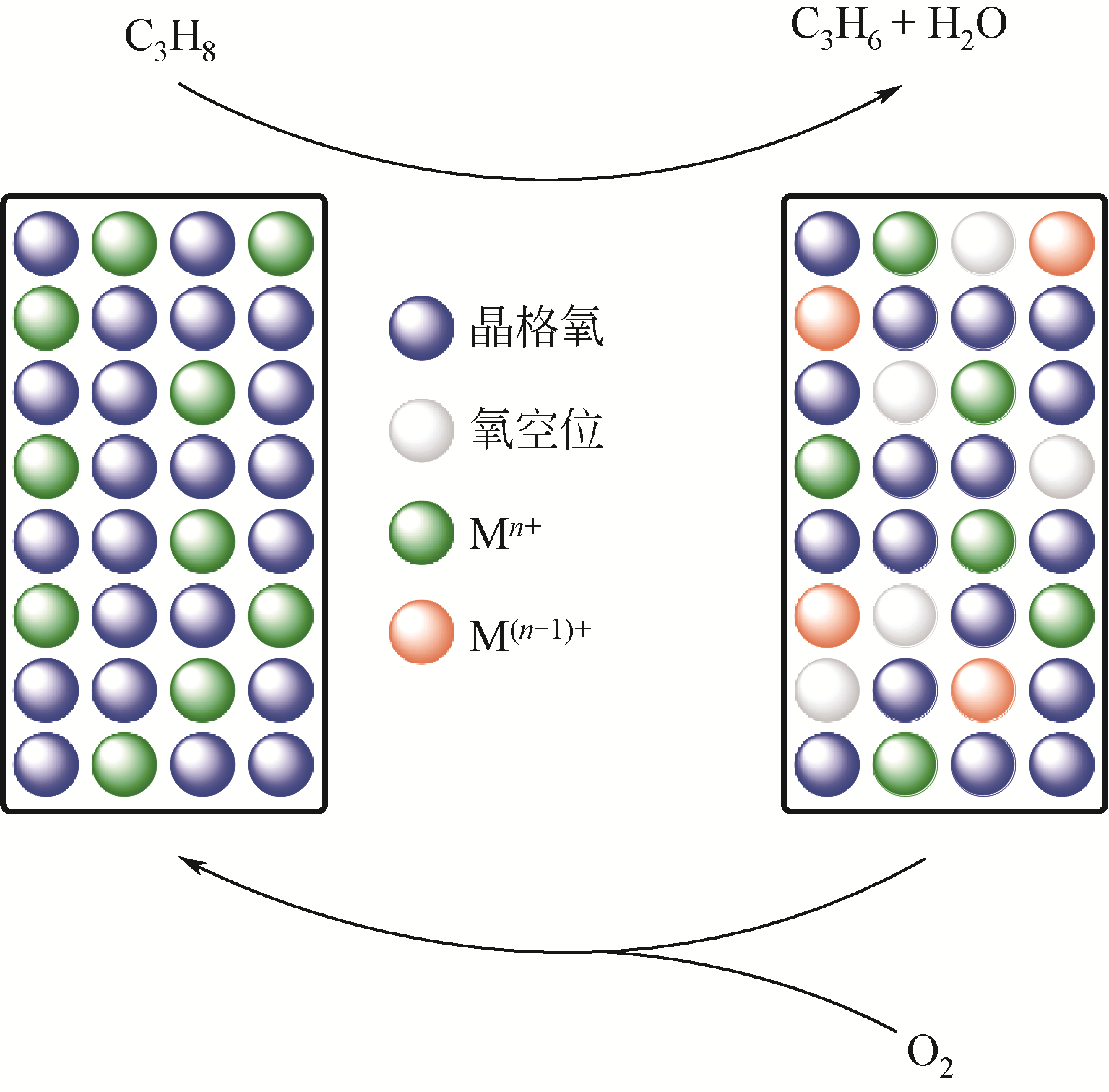
开发新型丙烯制备工艺对于满足人们日益增长的丙烯需求具有重要意义。由于商业化无氧脱氢技术存在热力学平衡限制、反应温度高、催化剂易积炭等不足,近年来,人们将研究重心转向了丙烷氧化脱氢技术。本文简述了丙烷氧化脱氢制丙烯的发展现状,综述了近年来文献报道的丙烷氧化脱氢催化剂体系(V基、Cr基、Co基、Ni基、Mo基、Pt基、Ce基和非金属基催化剂)、机理研究和不同氧化剂选择,并对各自的优势和不足进行了简单分析。分析发现,虽然目前丙烷氧化脱氢催化剂的种类非常广泛,但产物丙烯的收率仍有待提高,机理研究也需要更加系统和深入。最后指出,系统研究丙烷氧化脱氢机理,并在此基础上开发先进催化剂,进一步提高丙烯的选择性和收率是未来丙烷氧化脱氢研究的重要方向。
中图分类号:
引用本文
杜凯敏, 范杰. 丙烷氧化脱氢制丙烯研究进展[J]. 化工进展, 2019, 38(06): 2697-2706.
Kaimin DU, Jie FAN. Research progress on oxidative dehydrogenation of propane to propene[J]. Chemical Industry and Engineering Progress, 2019, 38(06): 2697-2706.
| 工艺 | 反应器 | 催化剂 | 再生方式 | 寿命 /年 | 温度 /℃ | 压力 /bar | 转化率 /% | 选择性 /% |
|---|---|---|---|---|---|---|---|---|
| Catofin | 固定床 | Cr2O3/Al2O3 | 空气燃烧 | 2 | 550~620 | 0.5 | 55~60 | 90 |
| Oleflex | 移动床 | Pt-Sn/Al2O3 | 连续移出 | 4~5 | 550~600 | >1 | 35~40 | 84 |
| STAR | 固定床 | Pt-Sn/ ZnAl2O4 | 切换,空气燃烧 | 1~2 | 480~620 | 3~7 | 30~40 | 80~90 |
| LINDE | 固定床 | Pt/沸石 | 切换,空气燃烧 | >2 | 550~620 | 1~2 | 32~50 | 91~93 |
| FBD | 流化床 | Cr2O3/Al2O3 | 连续移出再生流化床,空气燃烧 | — | 540~590 | 1~2 | 40 | 84 |
表1 DHP工艺对比
| 工艺 | 反应器 | 催化剂 | 再生方式 | 寿命 /年 | 温度 /℃ | 压力 /bar | 转化率 /% | 选择性 /% |
|---|---|---|---|---|---|---|---|---|
| Catofin | 固定床 | Cr2O3/Al2O3 | 空气燃烧 | 2 | 550~620 | 0.5 | 55~60 | 90 |
| Oleflex | 移动床 | Pt-Sn/Al2O3 | 连续移出 | 4~5 | 550~600 | >1 | 35~40 | 84 |
| STAR | 固定床 | Pt-Sn/ ZnAl2O4 | 切换,空气燃烧 | 1~2 | 480~620 | 3~7 | 30~40 | 80~90 |
| LINDE | 固定床 | Pt/沸石 | 切换,空气燃烧 | >2 | 550~620 | 1~2 | 32~50 | 91~93 |
| FBD | 流化床 | Cr2O3/Al2O3 | 连续移出再生流化床,空气燃烧 | — | 540~590 | 1~2 | 40 | 84 |
| 催化剂 体系 | 代表 | 特点 | 研究 |
|---|---|---|---|
| 钒基 | V-Mg-O[ | 目前效果较好的催化体系,性能与活性相分散度及助剂酸碱性 有极大关系, 也是研究机理的主要体系,但起活温度较高(>500℃), 且长时间高温易挥发 | 多研究负载型催化剂,钒的分散性及 负载量,载体酸碱性效应及反应活性中心研究 |
| 铬基 | Cr/Al2O3 [ | 低温活性很高,多为负载型,极容易深度氧化 | 负载量及载体研究 |
| 钴基 | Co-Al-O[ | 低温活性很高,丙烯选择性较低,整体效果不佳,极容易深度氧化, 且稳定性差 | 研究较少,强调低温高收率,选择性 提高是关键 |
| 镍基 | Ni-M-O[ | 一般都有较好的低温性能,有一定的尺寸效应,收率一般都比较好, 且裂解产物较少 | 掺杂助剂,尺寸效应研究 |
| 钼基 | Mo/Al2O3 [ | 选择性较高,但低温活性差,一般低温活性低,需较高温度下才有活性(>500℃),且高温下易挥发 | 多研究非负载型催化剂 |
| 铂基 | Pt8-10/SnO/AAO[ | 一般要求Pt原子簇 | 制备方法研究 |
| 稀土铈基 | 3%Cs2O/ CeO2-2CeF3 [ | 丰富的氧空位结构,促进表面活性氧的迁移,经氟化物修饰的 稀土氧化物表现出极好的催化性能 | 助剂及氧化脱氢机理研究 |
| 非金属 | B-CNTs[ | 多以碳基为活性相,经P、B助剂的修饰可大幅提高丙烯选择性, 但稳定性不佳 | 助剂及氧化脱氢机理研究 |
表2 丙烷氧化脱氢催化剂体系汇总
| 催化剂 体系 | 代表 | 特点 | 研究 |
|---|---|---|---|
| 钒基 | V-Mg-O[ | 目前效果较好的催化体系,性能与活性相分散度及助剂酸碱性 有极大关系, 也是研究机理的主要体系,但起活温度较高(>500℃), 且长时间高温易挥发 | 多研究负载型催化剂,钒的分散性及 负载量,载体酸碱性效应及反应活性中心研究 |
| 铬基 | Cr/Al2O3 [ | 低温活性很高,多为负载型,极容易深度氧化 | 负载量及载体研究 |
| 钴基 | Co-Al-O[ | 低温活性很高,丙烯选择性较低,整体效果不佳,极容易深度氧化, 且稳定性差 | 研究较少,强调低温高收率,选择性 提高是关键 |
| 镍基 | Ni-M-O[ | 一般都有较好的低温性能,有一定的尺寸效应,收率一般都比较好, 且裂解产物较少 | 掺杂助剂,尺寸效应研究 |
| 钼基 | Mo/Al2O3 [ | 选择性较高,但低温活性差,一般低温活性低,需较高温度下才有活性(>500℃),且高温下易挥发 | 多研究非负载型催化剂 |
| 铂基 | Pt8-10/SnO/AAO[ | 一般要求Pt原子簇 | 制备方法研究 |
| 稀土铈基 | 3%Cs2O/ CeO2-2CeF3 [ | 丰富的氧空位结构,促进表面活性氧的迁移,经氟化物修饰的 稀土氧化物表现出极好的催化性能 | 助剂及氧化脱氢机理研究 |
| 非金属 | B-CNTs[ | 多以碳基为活性相,经P、B助剂的修饰可大幅提高丙烯选择性, 但稳定性不佳 | 助剂及氧化脱氢机理研究 |
| 1 | 王卅 .我国丙烯下游产业产品市场情况[J].化工进展,2014,33(9):2517-2520. |
| WANG Sa . The market of propene downstream industrial link in China[J]. Chemical Industry and Engineering Progress,2014,33(9):2517-2520. | |
| 2 | 王中银 .丙烯下游产品市场现状和产业链选择[J].煤炭加工和综合利用,2016(4):19-20. |
| WANG Zhongyin . The market and choice of propene downstream industrial link[J]. Coal Processing & Comprehensive Utilization,2016(4):19-20. | |
| 3 | 杨学萍 .国内外丙烯生产技术进展及市场分析[J].石油化工技术与经济,2017,33(6):11-15. |
| YANG Xueping . Production technology progress and market analysis of propene at home and abroad[J]. Technology & Economics in Petrochemicals,2017,33(6):11-15. | |
| 4 | 丙烷脱氢对丙烯市场的影响[J].石油化工,2016,45(6):724. |
| The effect of propane dehydrogenation on propene market[J]. Petrochemical Technology,2016,45(6):724. | |
| 5 | SATTLER J J , RUIZ-MARTINEZ J , SANTILLAN-JIMENEZ E ,et al .Catalytic dehydrogenation of light alkanes on metals and metal oxides[J]. Chemical Reviews,2014,114(20):10613-10653. |
| 6 | KUNG H H .Oxidative dehydrogenation of light (C2 to C4) alkanes[J].Advances in Catalysis,1994,40:1-38. |
| 7 | 韩香莲 .丙烯生产工艺研究进展[J].山东化工,2013,42(5):60-62. |
| HAN Xianglian . Production technology progress of propene[J].Shandong Chemical Industry,2013,42(5):60-62. | |
| 8 | 盖希坤,田原宇,夏道宏 .丙烷催化脱氢制丙烯工艺分析[J].炼油技术与工程,2010,40(12):27-32. |
| GAI Xikun , TIAN Yuanyu , XIA Daohong . Technology progress analysis of the catalytic dehydrogenation of propane[J].Petroleum Refinery Engineering,2010,40(12):27-32. | |
| 9 | CAVANI F , BALLARINI N , CERICOLA A .Oxidative dehydrogenation of ethane and propane:how far from commercial implementation?[J]. Catalysis Today,2007,127(1/2/3/4):113-131. |
| 10 | GAO Xingtao , RUIZ P , GUO Xiexian , et al .Effect of coexistence of magnesium vanadate phases in the selectivity oxidation of propane to propene[J]. Journal of Catalysis, 1994,148:56-67. |
| 11 | BLASCO T , GALLI A , LOPEZ-NIETO J M ,et al .Oxidative dehydrogenation of ethane and n-butane on VO x /Al2O3 catalysts[J]. Journal of Catalysis,1997,169:203-211. |
| 12 | FRANK B , DINSE A , OVSITSER O ,et al .Mass and heat transfer effects on the oxidative dehydrogenation of propane (ODP) over a low loaded VO x /Al2O3 catalyst[J]. Applied Catalysis A:General,2007,323:66-76. |
| 13 | LUO Qunxing , ZHANG Xiaokang , HOU Bouli ,et al . Catalytic function of VO x /Al2O3 for oxidative dehydrogenation of propane: support microstructure-dependent mass transfer and diffusion[J]. Catalysis Science & Technology,2018,8:4864-4876. |
| 14 | LIU Yongmei , CAO Yong , YI Nan ,et al .Vanadium oxide supported on mesoporous SBA-15 as highly selective catalysts in the oxidative dehydrogenation of propane[J].Journal of Catalysis,2004,224(2):417-428. |
| 15 | CHAAR M A , PATEL D , KUNG H H .Selective oxidative dehydrogenation of propane over V-Mg-O catalysts[J].Journal of Catalysis,1988,109:463-467. |
| 16 | LIU Lulu , HAN Xinyou , ZHOU Juan ,et al . Oxidative dehydrogenation of propane over three-dimensionally ordered macroporous VMgO catalysts with different vanadium doping[J]. Journal of Porous Materials,2018,25(4):955-963. |
| 17 | AYANDIRAN A A , BAKARE I A , BINOUS H ,et al .Oxidative dehydrogenation of propane to propylene over VO x /CaO-γ-Al2O3 using lattice oxygen[J]. Catalysis Science & Technology,2016,6(13):5154-5167. |
| 18 | SOLSONA B , BLASCO T , LOPEZ-NIETO J M ,et al .Vanadium oxide supported on mesoporous MCM-41 as selective catalysts in the oxidative dehydrogenation of alkanes[J]. Journal of Catalysis,2001,203(2):443-452. |
| 19 | DINSE A , FRANK B , HESS C ,et al .Oxidative dehydrogenation of propane over low-loaded vanadia catalysts: impact of the support material on kinetics and selectivity[J].Journal of Molecular Catalysis A:Chemical,2008,289(1/2):28-37. |
| 20 | LIU Yongmei , FENG Weiliang , LI Tingcheng ,et al .Structure and catalytic properties of vanadium oxide supported on mesocellulous silica foams (MCF) for the oxidative dehydrogenation of propane to propylene[J]. Journal of Catalysis,2006,239(1):125-136. |
| 21 | ROZANSKA X , FORTRIE R , SAUER J .Size-dependent catalytic activity of supported vanadium oxide species: oxidative dehydrogenation of propane[J].Journal of the American Chemical Society,2014,136(21):7751-7761. |
| 22 | BARMAN S , MAITY N , BHATTE K ,et al .Single-site VO x moieties generated on silica by surface organometallic chemistry: a way to enhance the catalytic activity in the oxidative dehydrogenation of propane[J]. ACS Catalysis,2016,6(9):5908:5921. |
| 23 | JIBRIL B Y . Propane oxidative dehydrogenation over chromium oxide-based catalysts[J].Applied Catalysis A:General, 2004,264(2):193-202. |
| 24 | JIBRIL B Y , AL-ZAHRANI S M , ABASAEED A E ,et al .Effects of reducibility on propane oxidative dehydrogenation over γ-Al2O3-supported chromium oxide-based catalysts[J]. Catalysis Letters,2003,87(3/4):121-132. |
| 25 | DAVIES T E , GARCIA T , SOLSONA B ,et al .Nanocrystalline cobalt oxide: a catalyst for selective alkane oxidation under ambient conditions[J]. Chemical Communications,2006(32):3417-3419. |
| 26 | HUANG Mingxiang , WU Xin , YI Xiaodong ,et al .Highly dispersed CoO x in layered double oxides for oxidative dehydrogenation of propane: guest-host interactions[J].RSC Advance,2017,7(24):14846-14856. |
| 27 | LI Zhanyong , PETERS A W , BERNALES V ,et al .Metal-organic framework supported cobalt catalysts for the oxidative dehydrogenation of propane at low temperature[J].ACS Central Science,2017,3(1):31-38. |
| 28 | BOIZUMAULT-MORICEAU P , PENNEAUIN A , GRZYBOWSKA B ,et al .Oxidative dehydrogenation of propane on Ni-Ce-O oxide: effect of the preparation method, effect of potassium addition and physical characterization[J].Applied Catalysis A:General, 2003,245(1):55-67. |
| 29 | WU Ying , HE Yiming , CHEN Tong ,et al .Low temperature catalytic performance of nanosized Ti-Ni-O for oxidative dehydrogenation of propane to propene[J]. Applied Surface Science,2006,252(14):5220-5226. |
| 30 | HE Yiming , WU Ying , CHEN Tong ,et al .Low-temperature catalytic performance for oxidative dehydrogenation of propane on nanosized Ti(Zr)-Ni-O prepared by modified sol-gel method[J].Catalysis Communications,2006,7(5):268-271. |
| 31 | HERACLEOUS E , LEMONIDOU A A .Ni-Me-O mixed metal oxides for the effective oxidative dehydrogenation of ethane to ethylene—Effect of promoting metal Me[J]. Journal of Catalysis,2010,270:67-75. |
| 32 | ZHU Haibo , DEVON C R , MOUSSAB H ,et al .Ni-M-O(M = Sn, Ti, W) catalysts prepared by a dry mixing method for oxidative dehydrogenation of ethane[J]. ACS Catalysis,2016,6:2852-2866. |
| 33 | LI Jianhui , WANG Caicai , Chuanjing HUNG ,et al . Mesoporous nickel oxides as effective catalysts for oxidative dehydrogenation of propane to propene[J]. Applied Catalysis A:General,2010,382(1),99-105. |
| 34 | ABELLO M C , GOMEZ M F , FERRETTI O . Mo/γ-Al2O3 catalysts for the oxidative dehydrogenation of propane: effect of Mo loading[J].Applied Catalysis A:General,2001,207(1):421-431. |
| 35 | STERN D L , GRASSELLI R K .Propane oxydehydrogenation over molybdate-based catalysts[J]. Journal of Catalysis,1997,167:550-559. |
| 36 | MAZZOCCHIA C , ABOUMRAD C , DIAGNE C ,et al .On the NiMoO4 oxidative dehydrogenation of propane to propene: some physical correlations with the catalytic activity[J].Catalysis Letters,1991,10:181-192. |
| 37 | ZAVOIANU R , DIAS C R , PORTELA M F . Stabilisation of β-NiMoO4 in TiO2-supported catalysts[J].Catalysis Communications,2001,2:37-42. |
| 38 | DIAS C R , ZAVOIANU R , PORTELA M F .Isobutane oxydehydrogenation on SiO2-supported nickel molybdate catalysts: effect of the active phase loading[J].Catalysis Communications,2002,3:85-90. |
| 39 | FARIN B , DEVILLERS M , GAIGNEAUX E M . Nanostructured hybrid materials as precursors of mesoporous NiMo-based catalysts for the propane oxidative dehydrogenation[J].Microporous and Mesoporous Materials,2017,242:200-207. |
| 40 | MDERIA L M , PORTELA M F , MAZZOCCHIA C .Nickel molybdate catalysts and their use in the selective oxidation of hydrocarbons[J].Catalysis Reviews,2004,46(1):53-110. |
| 41 | SUN Mia , ZHANG Jizhe , PUTAJ P ,et al .Catalytic oxidation of light alkanes (C1-C4) by heteropoly compounds[J]. Chemical Reviews,2014,114(2):981-1019. |
| 42 | VAJDA S , PELLIN M J , GREELEY J P ,et al .Subnanometre platinum clusters as highly active and selective catalysts for the oxidative dehydrogenation of propane[J]. Nature Materials,2009,8(3):213-216. |
| 43 | TROVARELLI A .Catalytic properties of ceria and CeO2-containing materials[J].Catalysis Reviews,1996,38(4):439-520. |
| 44 | WAN Huilin , ZHOU Xiaoping , WENG Weizheng ,et al .Catalytic performance, structure, surface properties and activity oxygen species of the fluoride-containing rare earth(alkaine earth)-based catalysts for the oxidative coupling of methane and oxidative dehydrogenation of light alkanes[J]. Catalysis today,1999,51:161-175. |
| 45 | TROTUS L T , TEODORESCU C M , PARVULESCU V I ,et al .Enhancing oxidative dehydrogenation selectivity of ceria-based catalysts with phosphorus as additive[J].ChemCatChem,2013,5(3):757-765. |
| 46 | FRANK B , MORASSUTTO M , SCHOMACKE R ,et al .Oxidative dehydrogenation of ethane over multiwalled carbon nanotubes[J].ChemCatChem,2010,2(6):644-648. |
| 47 | FRANK B , ZHANG Jian , BLUME R ,et al .Heteroatoms increase the selectivity in oxidative dehydrogenation reactions on nanocarbons[J].Angewandte Chemie,2009,48(37):6913-6917. |
| 48 | HU Zhongpan , ZHAO Hui , CHEN Chong ,et al . Castanea mollissima shell-derived porous carbons as metal-free catalysts for highly efficient dehydrogenation of propane to propylene[J]. Catalysis Today,2018,316:214-222. |
| 49 | ZHANG Jian , LIU Xi , BLUME R ,et al .Surface-modified carbon nanotubes catalyze oxidative dehydrogenation of n-butane[J].Science,2008,322(5898):73-77. |
| 50 | ZHANG Jian , SU Dangsheng , ZHANG Aihua ,et al .Nanocarbon as robust catalyst: mechanistic insight into carbon-mediated catalysis[J].Angewandte Chemie,2007,119(38):7460-7464. |
| 51 | SUN Xiaoyan , DING Yuxiao , ZHANG Bingsen ,et al . New insights into the oxidative dehydrogenation of propane on borate-modified nanodiamond[J].Chemical Communications,2015,51(44):9145-9148. |
| 52 | GUO Xiaoling , QI Wei , LIU Wei ,et al .Oxidative dehydrogenation on nanocarbon: revealing the catalytic mechanism using model catalysts[J]. ACS Catalysis, 2017,7(2):1424-1427. |
| 53 | QI Wei , LIU Wei , ZHANG Binsen ,et al .Oxidative dehydrogenation on nanocarbon: identification and quantification of active sites by chemical titration[J].Angewandte Chemie,2013,52(52):14224:14228. |
| 54 | LI Jiaquan , YU Peng , XIE Jingxin ,et al .Improving the alkene selectivity of nanocarbon-catalyzed oxidative dehydrogenation of n-butane by refinement of oxygen species[J].ACS Catalysis,2017,7(10):7305-7311. |
| 55 | GRANT J T , CARRERO C A , GOELTL F ,et al .Selective oxidative dehydrogenation of propane to propene using boron nitride catalysts[J].Science,2016,354:1570-1573. |
| 56 | SHI Lei , WANG Dongqi , SONG Wei ,et al .Edge-hydroxylated boron nitride for oxidative dehydrogenation of propane to propylene[J].ChemCatChem,2017,9(10):1788-1793. |
| 57 | CAVANI F , TRIFIRO F .The oxidative dehydrogenation of ethane and propane as an alternative way for the production of light olefins[J].Catalysis Today,1995,24:307-313. |
| 58 | MICHALAKOS P M , BIRKELAND K , KUNG H H . Selective oxidation of pentane over Al2O3- and SiO2-supported vanadia catalysys[J].Journal of Catalysis,1996,158:349-353. |
| 59 | 陈淑 .过渡金属氧化物纳米介孔材料在丙烷选择氧化脱氢制丙烯反应中的研究[D]. 杭州:浙江大学,2015. |
| CHEN Shu . The study of transition metal oxide nano-mesoporous material in oxidative dehydrogenation of propane to propene[D]. Hangzhou:Zhejiang University,2015. | |
| 60 | GRABOWSKI R .Kinetics of oxidative dehydrogenation of C2-C3 alkanes on oxide catalysts[J].Catalysis Reviews, 2006,48(2):199-268. |
| 61 | CHRISTODOULAKIS A , MACHLI M , LEMONIDOU A A ,et al .Molecular structure and reactivity of vanadia-based catalysts for propane oxidative dehydrogenation studied by in situ Raman spectroscopy and catalytic activity measurements[J]. Journal of Catalysis,2004,222(2):293-306. |
| 62 | YOU Rui , ZHANG Xuanyu , LUO Liangfeng ,et al .NbO x /CeO2-rods catalysts for oxidative dehydrogenation of propane: Nb-CeO2 interaction and reaction mechanism[J]. Journal of Catalysis,2017,348:189-199. |
| 63 | XUE Xuliang , LANG Wanzhong , YAN Xi ,et al .Dispersed vanadium in three-dimensional dendritic mesoporous silica nanospheres: active and stable catalysts for the oxidative dehydrogenation of propane in the presence of CO2 [J]. ACS Applied Materials & Interfaces,2017,9(18):15408-15423. |
| 64 | NOWICKA E , REECE C , ALTHAHBAN S M ,et al . Elucidating the role of CO2 in the soft oxidative dehydrogenation of propane over ceria-based catalysts[J]. ACS Catalysis,2018,8(4):3454-3468. |
| 65 | 郑雯,程党国,陈丰秋,等 . N2O作为氧化脱氢剂的研究进展[J].化工进展,2006,25(12):1391-1394. |
| ZHENG Wen , CHENG Dangguo , CHEN Fengqiu ,et al . The progress of N2O as oxidant in oxidative dehydrogenation[J]. Chemical Industry and Engineering Progress,2006,25(12):1391-1394. | |
| 66 | KONDRATENKO E V , CHERIAN M , BAERNS M ,et al .Oxidative dehydrogenation of propane over V/MCM-41 catalysts: comparison of O2 and N2O as oxidants[J].Journal of Catalysis,2005,234(1):131-142. |
| 67 | GASPAR N J , PASTERNAK I S , VADEKAR M .H2S promoted oxidative dehydrogenation of hydrocarbons in molten media[J].The Canadian Journal of Chemical Engineering,1974,52:793-797. |
| 68 | PREMJI Z A ,LO J M H, CLARK P D .Experimental and ab initio investigations of H2S-assisted propane oxidative dehydrogenation reactions[J]. The Journal of Physical Chemistry A,2014,118(9):1541-1556. |
| 69 | ADAMS C T , BRANDENBERGER S G , DUBOIS J B ,et al . Dehydrogenation and coupling reactions in the presence of iodine and molten salt hydrogen iodide acceptors[J].The Journal of Organic Chemistry,1977,42(1):1-6. |
| 70 | XIE Qunhua , ZHANG Huamin , KANG Jincan ,et al . Oxidative dehydrogenation of propane to propylene in the presence of HCl catalyzed by CeO2 and NiO-modified CeO2 Nanocrystals[J]. ACS Catalysis,2018,8(6):4902-4916. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [6] | 李化全, 王明华, 邱贵宝. 硫酸酸解钙钛矿相精矿的行为[J]. 化工进展, 2023, 42(S1): 536-541. |
| [7] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [8] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [9] | 杨建平. 降低HPPO装置反应系统原料消耗的PSE[J]. 化工进展, 2023, 42(S1): 21-32. |
| [10] | 王福安. 300kt/a环氧丙烷工艺反应器降耗减排分析[J]. 化工进展, 2023, 42(S1): 213-218. |
| [11] | 王谨航, 何勇, 史伶俐, 龙臻, 梁德青. 气体水合物阻聚剂研究进展[J]. 化工进展, 2023, 42(9): 4587-4602. |
| [12] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [13] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [14] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [15] | 高彦静. 单原子催化技术国际研究态势分析[J]. 化工进展, 2023, 42(9): 4667-4676. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||