| 1 |
CHEN P , XIONG Z , LUO J , et al . Interaction of hydrogen with metal nitrides and imides[J]. Nature, 2002, 420(6913): 302-304.
|
| 2 |
CIPRIANI G , DIO V D, GENDUSO F , et al . Perspective on hydrogen energy carrier and its automotive applications[J]. International Journal of Hydrogen Energy, 2014, 39(16): 8482-8494.
|
| 3 |
FIHRI A , ARTERO V , RAZAVET M , et al . Cobaloxime-based photocatalytic devices for hydrogen production[J]. Angew. Chem. Int. Ed. Engl., 2010, 47(3): 564-567.
|
| 4 |
SCHMID T , E W . Hydrazine and its derivatives: preparation, properties, applications[M]. Hoboken: Wiley, 2001.
|
| 5 |
HE L , HUANG Y Q , WANG A Q , et al . Surface modification of Ni/Al2O3 with Pt: highly efficient catalysts for H2 generation via selective decomposition of hydrous hydrazine[J]. Journal of Catalysis, 20132, 98(1): 1-9.
|
| 6 |
DU Y , SU J , LUO W , et al . Graphene-supported nickel–platinum nanoparticles as efficient catalyst for hydrogen generation from hydrous hydrazine at room temperature[J]. ACS Applied Materials & Interfaces, 2015, 7(2): 1301-1034.
|
| 7 |
WANG H L , YAN J M , LI S J , et al . Noble-metal-free NiFeMo nanocatalyst for hydrogen generation from the decomposition of hydrous hydrazine[J]. Journal of Materials Chemistry A, 2014, 3(1): 121-124.
|
| 8 |
DU X , CAI P , LUO W , et al . Facile synthesis of P-doped Rh nanoparticles with superior catalytic activity toward dehydrogenation of hydrous hydrazine[J]. International Journal of Hydrogen Energy, 2017, 42(9): 6137-6143.
|
| 9 |
SINGH S K , XU Q . Bimetallic Ni-Pt nanocatalysts for selective decomposition of hydrazine in aqueous solution to hydrogen at room temperature for chemical hydrogen storage[J]. Inorganic Chemistry, 2010, 49(13): 6148-6152.
|
| 10 |
SINGH S K , IIZUKA Y , XU Q . Nickel-palladium nanoparticle catalyzed hydrogen generation from hydrous hydrazine for chemical hydrogen storage[J]. International Journal of Hydrogen Energy, 2011, 36(18): 11794-11801.
|
| 11 |
SINGH S K , XU Q . Complete conversion of hydrous hydrazine to hydrogen at room temperature for chemical hydrogen storage[J]. American Chemical Society, 2009, 131(50): 18032-18033.
|
| 12 |
WU D , WEN M , GU C , et al . 2D NiFe/CeO2 basic-site-enhanced catalyst via in-situ topotactic reduction for selectively catalyzing the H2 generation from N2H4·H2O[J]. ACS Applied Materials & Interfaces, 2017, 9(19): 16103-16108.
|
| 13 |
DAI H , QIU Y P , DAI H B , et al . A study of degradation phenomenon of Ni–Pt/CeO2 catalyst towards hydrogen generation from hydrous hydrazine[J]. International Journal of Hydrogen Energy, 2017, 42(26):16355-16361.
|
| 14 |
KANG W , VARMA A . Hydrogen generation from hydrous hydrazine over Ni/CeO2 catalysts prepared by solution combustion synthesis[J]. Applied Catalysis B:Environmental, 2017, 220: 409-416.
|
| 15 |
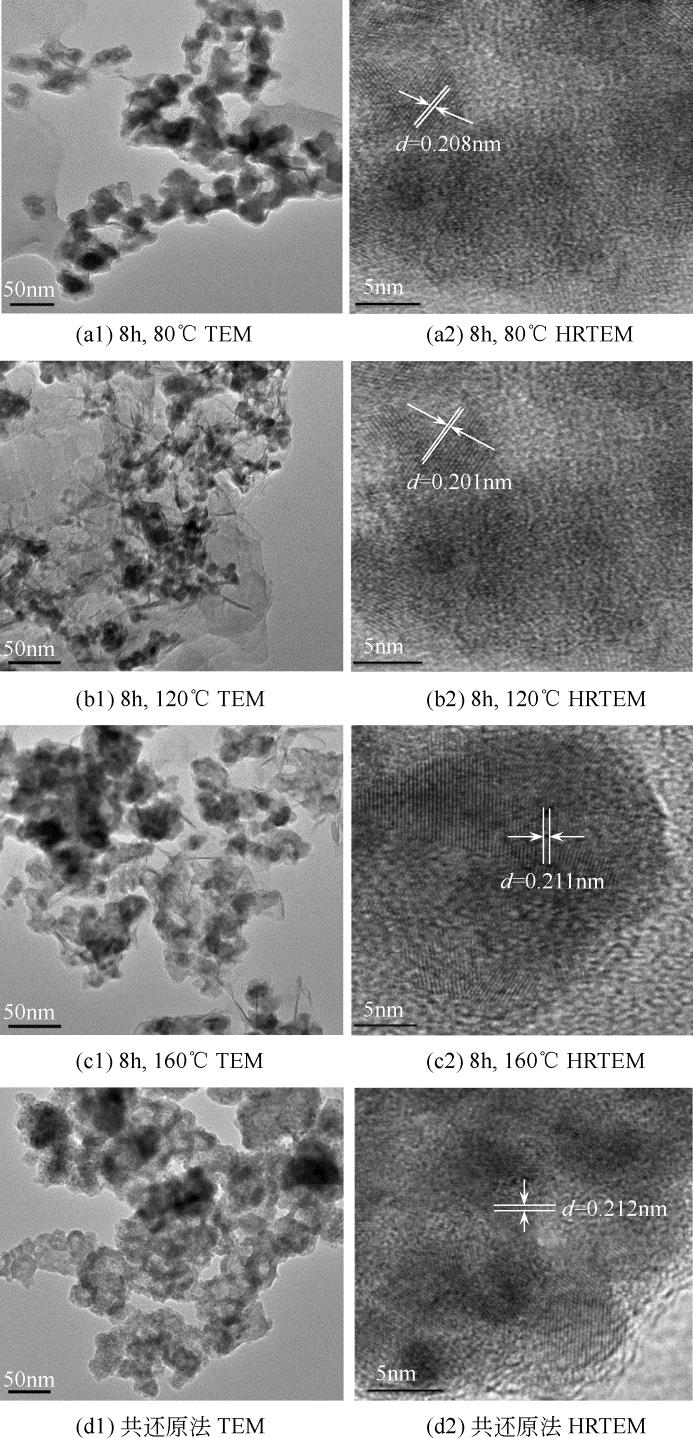
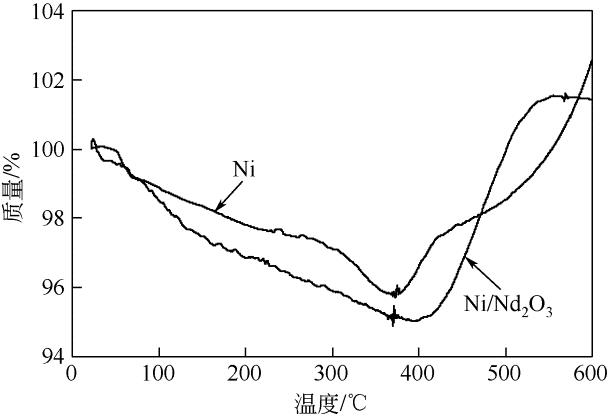
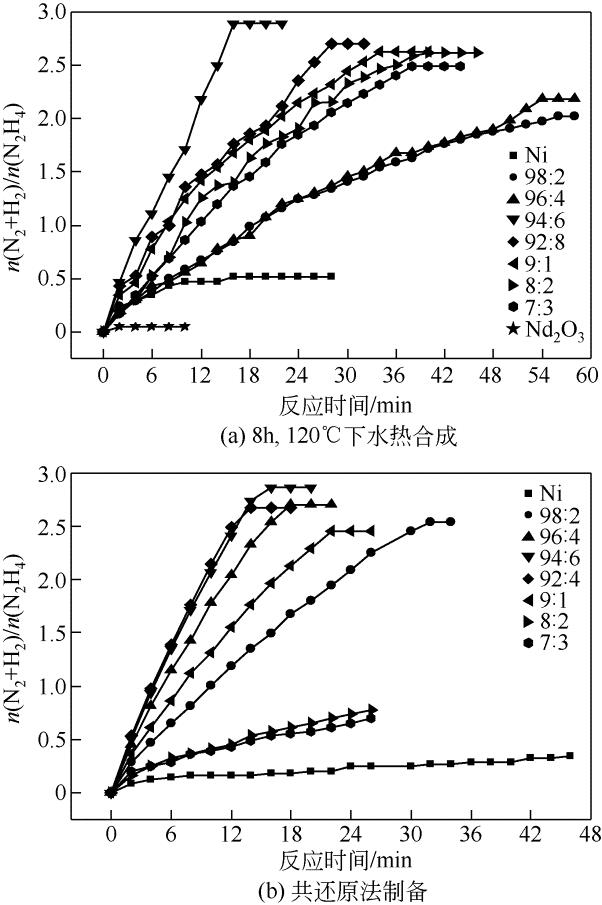
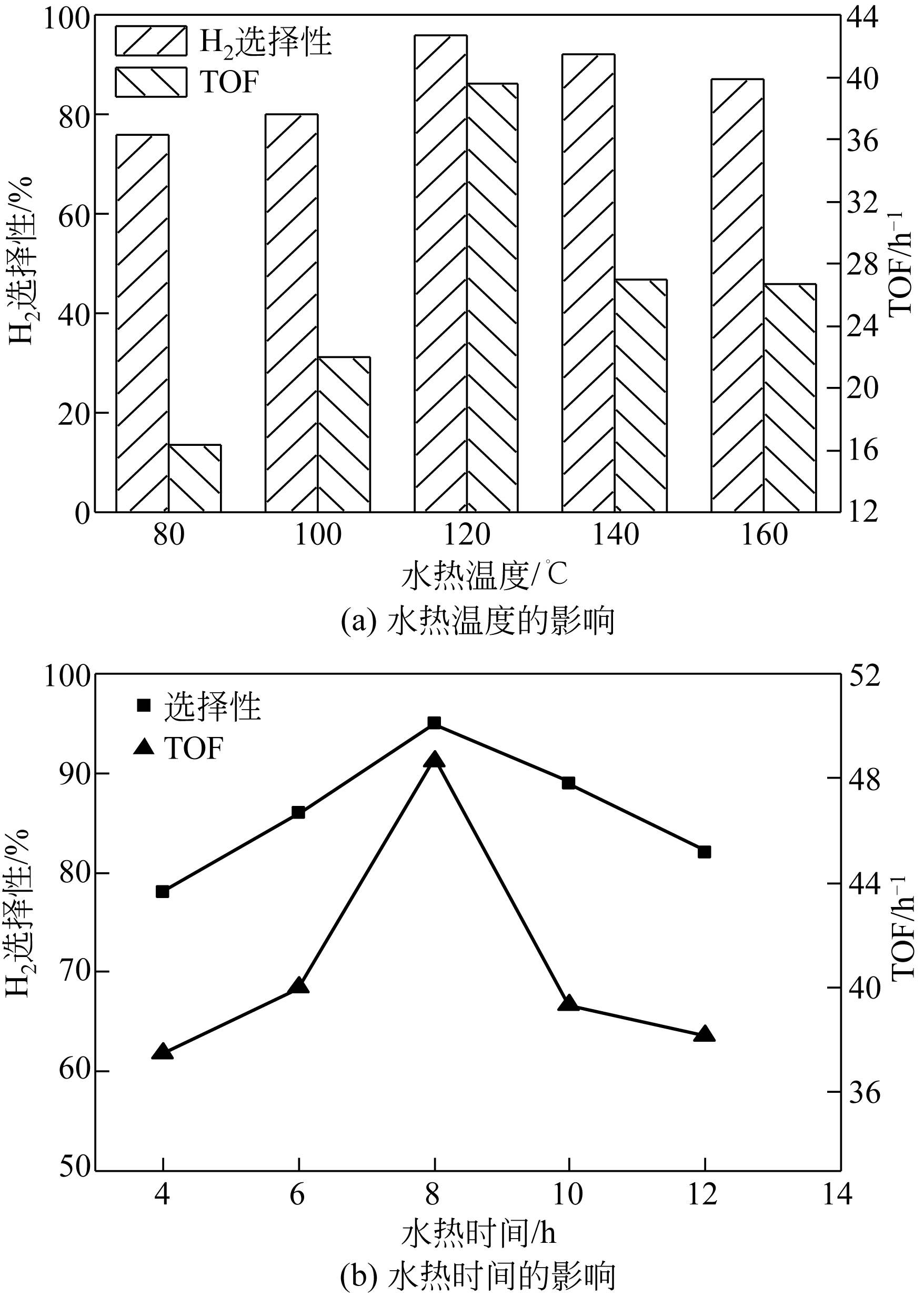
康雪婧, 马敬环, 刘莹 . Ni-Nd2O3纳米颗粒的制备及其催化性能研究[J]. 化工新型材料, 2018, 46(2): 193-196.
|
|
KANG X J , MA J H, LIU Y . Synthesis and characterization of Ni-Nd2O3 nanoparticle and the catalytic property of hydrazine hydrate[J]. New Chemical Materials, 2018, 46(2): 193-196.
|
| 16 |
高宾, 张晓军 . 水热法合成MoO3单晶纳米带及其形成机理[J]. 材料导报, 2017, 31(2):112-116.
|
|
GAO B , ZHANG X J . Hydrothermal synthesis of single crystal α-MoO3 nanobelts and their formation mechanism[J]. Material Review, 2017, 31(2): 112-116.
|
| 17 |
马利海, 张建利, 范素兵, 等 . 水热法Fe-Mn催化剂制备及其合成气制低碳烯烃催化活性[J]. 燃料化学学报, 2013, 41(11): 1356-1360.
|
|
MA L H, ZHANG J L , FAN S B , et al . Preparation of Fe-Mn catalyst by hydrothermal method and its catalytic activity for the synthesis of light olefins from CO hydrogenation[J]. Journal of Fuel Chemistry and Technology, 2013, 41(11): 1356-1360.
|
| 18 |
李寅瑞, 王鲜, 刘卫沪, 等 . 水热法一步合成VO2粉末及其微观形貌调控[J]. 材料导报:纳米与新材料专辑, 2017(s1): 263-268.
|
|
LI Y R , WANG X , LIU W H , et al . One-step hydrothermal synthesis of VO2 powders with micro-morphology control[J]. Materials Review, 2017(s1): 263-268.
|
| 19 |
LI F , LI Y , SONG Z , et al . Grain growth characteristics of hydrothermally prepared yttria stabilized zirconia nanocrystals during calcination[J]. Rare Metal Materials & Engineering, 2017, 46(4): 899-905.
|
| 20 |
MILLANGE F , WALTON R I , O'HARE D . Time-resolved in situ X-ray diffraction study of the liquid-phase reconstruction of Mg–Al–carbonate hydrotalcite-like compounds[J]. Journal of Materials Chemistry, 2000, 10(7): 1713-1720.
|
| 21 |
汪志新, 黄少斌, 费志宾, 等 . 水热法制备Eu复合BiVO4纳米片及其光催化降解性能[J]. 化工进展, 2016, 35(4): 1121-1125.
|
|
WANG Z X , HONG S B , FEI Z B , et al . Synthesis and photocatalytic activity of Eu-doped BiVO4 nanosheets by hydrothermal method[J]. Chemical Industry and Engineeing Progress, 2016, 35(4): 1121-1125.
|
| 22 |
王芳芳, 郝露, 徐山青, 等 . 镧系元素改性TiO2催化剂的研究进展[J]. 化工新型材料, 2015(12): 21-23.
|
|
WANG F F , HAO L , XU S Q , et al . Research progress of Lanthanide modified TiO2 catalysts[J]. New Chemical Materials, 2015(12): 21-23.
|
| 23 |
LIU P , GU X , WU Y , et al . Construction of bimetallic nanoparticles immobilized by porous functionalized metal-organic frameworks toward remarkably enhanced catalytic activity for the room-temperature complete conversion of hydrous hydrazine into hydrogen[J]. International Journal of Hydrogen Energy, 2017, 42(30): 19096-19105.
|
| 24 |
ZHANG L , ZHOU L , YANG K , et al . Pd-Ni nanoparticles supported on MIL-101 as high-performance catalysts for hydrogen generation from ammonia borane[J]. Journal of Alloys & Compounds, 2016, 677: 87-95.
|
| 25 |
WANG J , LI Y , ZHANG Y . Precious-metal-free nanocatalysts for highly efficient hydrogen production from hydrous hydrazine[J]. Advanced Functional Materials, 2015, 24(45): 7073-7077.
|
| 26 |
ÖZDEMIR H , ÖKSUZOMER M A F , GÜRKAYNAK M A . Preparation and characterization of Ni based catalysts for the catalytic partial oxidation of methane: effect of support basicity on H2/CO ratio and carbon deposition[J]. International Journal of Hydrogen Energy, 2010, 35(22): 12147-12160.
|
| 27 |
WU P P , ZHANG Z K , SONG G P . Preparation of Nd2O3 nanorods in SDBS micelle system[J]. Journal of Rare Earths, 2014, 32(11): 1027-1031.
|
| 28 |
MEN Y, DU X , CHENG G , et al . CeO x -modified NiFe nanodendrits grown on rGO for efficient catalytic hydrogen generation from alkaline solution of hydrazine[J]. International Journal of Hydrogen Energy, 2017, 42(44): 27165-27173.
|
| 29 |
ZHONG Y J , DAI H B , ZHU M , et al . Catalytic decomposition of hydrous hydrazine over Ni-Pt/La2O3 catalyst: a high-performance hydrogen storage system[J]. International Journal of Hydrogen Energy, 2016, 41(26): 11042-11049.
|
| 30 |
DAI H , DAI H B , ZHONG Y J , et al . Kinetics of catalytic decomposition of hydrous hydrazine over CeO2-supported bimetallic Ni-Pt nanocatalysts[J]. International Journal of Hydrogen Energy, 2016, 42(9): 5684-5693.
|
 ),马敬环1,马云超2,刘莹1(
),马敬环1,马云超2,刘莹1( ),王嘉豪1,苏彦铭1,刘晓涯1,戴莉3
),王嘉豪1,苏彦铭1,刘晓涯1,戴莉3
 ),Jinghuan MA1,Yunchao MA2,Ying LIU1(
),Jinghuan MA1,Yunchao MA2,Ying LIU1( ),Jiahao WANG1,Yanming SU1,Xiaoya LIU1,Li DAI3
),Jiahao WANG1,Yanming SU1,Xiaoya LIU1,Li DAI3