化工进展 ›› 2019, Vol. 38 ›› Issue (01): 315-323.DOI: 10.16085/j.issn.1000-6613.2018-1106
高比表面积二氧化锆的合成及其催化应用
- 大连理工大学化工学院,宾州-大连联合能源研究中心,精细化工国家重点实验室,辽宁 大连 116024
-
收稿日期:2018-05-29修回日期:2018-10-12出版日期:2019-01-05发布日期:2019-01-05 -
通讯作者:郭新闻 -
作者简介:朱杰(1994—),男,博士研究生,研究方向为多孔材料。E-mail:<email>dut_zj@163.com</email>。|郭新闻,教授,博士生导师,研究方向为多孔材料、新催化反应及工艺。E-mail:<email>guoxw@dlut.edu.cn</email>。 -
基金资助:国家重点研发计划(2016YFB0600902-5);国家重点研发计划(2016YFB0600902-5)。
Preparation of high-surface-area ZrO2 and its application in catalysis
Jie ZHU( ),Wenhui LI,Bangjian LIU,Minchen MU,Xinwen GUO(
),Wenhui LI,Bangjian LIU,Minchen MU,Xinwen GUO( )
)
- State Key Laboratory of Fine Chemicals,PSU-DUT Joint Center for Energy Research,School of Chemical Engineering,Dalian University of Technology,Dalian 116024,Liaoning,China
-
Received:2018-05-29Revised:2018-10-12Online:2019-01-05Published:2019-01-05 -
Contact:Xinwen GUO
摘要:
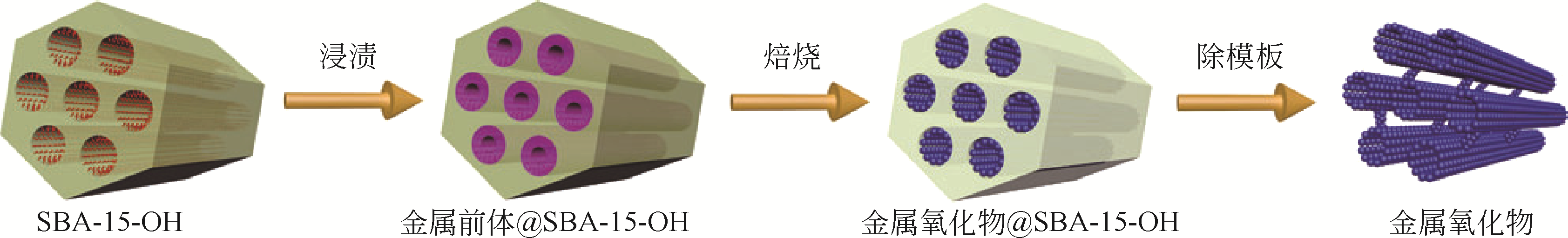
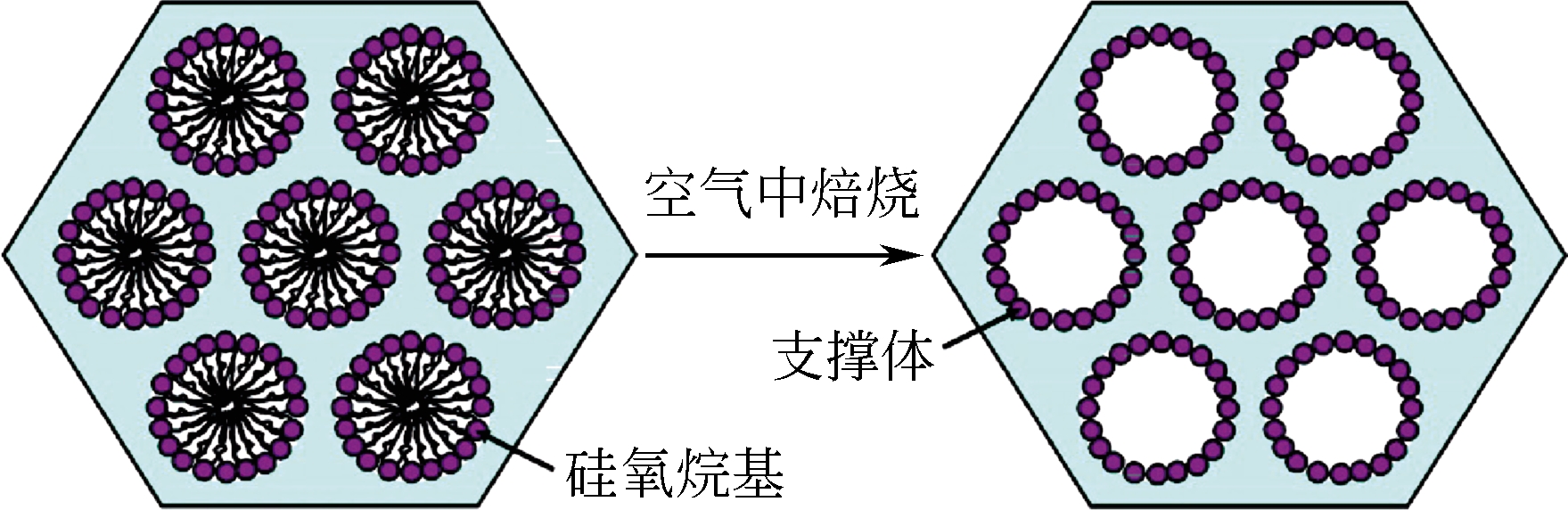
二氧化锆(ZrO2)是一种优异的催化材料,同时具有表面酸碱性,易产生氧空位,耐高温、抗腐蚀,机械强度高。然而传统方法合成的二氧化锆比表面积和孔容较小,限制了其应用。本文介绍了近年来高比表面积多孔二氧化锆的合成方法和技术,包括模板法、MOF热解法、静电纺丝等以及提高其热稳定性的措施,同时简述了其在催化领域的应用。研究表明,此类高比表面积的二氧化锆可以提高负载金属分散度,加强金属-载体相互作用,进而提高催化剂活性和稳定性,同时其粒径大小、形貌、孔结构均会影响其催化性能。高效低成本合成热稳定的高比表面积二氧化锆并对对其形貌和结构进行精准调控,将使其未来具有更广的催化应用前景。
中图分类号:
引用本文
朱杰, 李文慧, 刘邦荐, 慕旻辰, 郭新闻. 高比表面积二氧化锆的合成及其催化应用[J]. 化工进展, 2019, 38(01): 315-323.
Jie ZHU, Wenhui LI, Bangjian LIU, Minchen MU, Xinwen GUO. Preparation of high-surface-area ZrO2 and its application in catalysis[J]. Chemical Industry and Engineering Progress, 2019, 38(01): 315-323.
| 锆源 | 模板 | 比表面积 /m2·g-1 | 孔容 /cm3·g-1 | 孔径 /nm | 晶型 | 文献 |
|---|---|---|---|---|---|---|
| 氧氯化锆 | F127 | 97 | 0.14 | 4.1 | 四方相 | [11] |
| 四氯化锆 | CTAB | 573 | 0.76 | 5.2 | 单斜相 | [12] |
| 氧氯化锆 | SDS | 113 | — | 5.0 | 单斜相 | [13] |
| 正丁醇锆 | F127 | 124 | 0.16 | 4.8 | 四方相 | [14] |
| 氧氯化锆 | SBA-15 | 248 | 0.30 | 3.0~4.0 | 四方相 | [15] |
| 氧氯化锆 | KIT-6 | 391 | 0.54 | 3.4 | 四方相 | [16] |
| 四氯化锆 | 多孔SiO2 | 293 | 0.60 | 7.3 | 四方相 | [17] |
| UiO-66 | UiO-66 | 174 | 0.21 | 5.0~8.0 | 四方相 | [18] |
表1 模板法合成的高比表面积ZrO2
| 锆源 | 模板 | 比表面积 /m2·g-1 | 孔容 /cm3·g-1 | 孔径 /nm | 晶型 | 文献 |
|---|---|---|---|---|---|---|
| 氧氯化锆 | F127 | 97 | 0.14 | 4.1 | 四方相 | [11] |
| 四氯化锆 | CTAB | 573 | 0.76 | 5.2 | 单斜相 | [12] |
| 氧氯化锆 | SDS | 113 | — | 5.0 | 单斜相 | [13] |
| 正丁醇锆 | F127 | 124 | 0.16 | 4.8 | 四方相 | [14] |
| 氧氯化锆 | SBA-15 | 248 | 0.30 | 3.0~4.0 | 四方相 | [15] |
| 氧氯化锆 | KIT-6 | 391 | 0.54 | 3.4 | 四方相 | [16] |
| 四氯化锆 | 多孔SiO2 | 293 | 0.60 | 7.3 | 四方相 | [17] |
| UiO-66 | UiO-66 | 174 | 0.21 | 5.0~8.0 | 四方相 | [18] |
| 1 | KARWACKI C J , GANESH P , KENT P R C , et al . Structure-activity relationship of Au/ZrO2 catalyst on formation of hydroxyl groups and its influence on CO oxidation[J]. Journal of Materials Chemistry A, 2013, 1(19): 6051-6062. |
| 2 | LIU Y C , FANG K G , CHEN J G , et al . Effect of pore size on the performance of mesoporous zirconia-supported cobalt Fischer-Tropsch catalysts[J]. Green Chem., 2007, 9(6): 611-615. |
| 3 | LI W H , NIE X W , JIANG X , et al . ZrO2 support imparts superior activity and stability of Co catalysts for CO2 methanation[J]. Applied Catalysis B: Environmental, 2018, 220: 397-408. |
| 4 | ZHANG X P , ZHANG Q D , TSUBAKI N , et al . Carbon dioxide reforming of methane over Ni nanoparticles incorporated into mesoporous amorphous ZrO2 matrix[J]. Fuel, 2015, 147: 243-252. |
| 5 | OTROSHCHENKO T , SOKOLOV S , STOYANOVA M , et al . ZrO2-based alternatives to conventional propane dehydrogenation catalysts: active sites, design, and performance[J]. Angew. Chem. Int. Ed., 2015, 54(52): 15880-15883. |
| 6 | SHUKLA S , SEAL S . Mechanisms of room temperature metastable tetragonal phase stabilisation in zirconia[J]. International Materials Reviews, 2013, 50(1): 45-64. |
| 7 | GARVLE R C . Stabilization of the tetragonal structure in zirconia microcrystals[J]. The Journal of Physical Chemistry, 1978, 82: 218-224. |
| 8 | PARK J N , NOH J , CHANG J S , et al . Ethylbenzene to styrene in the presence of carbon dioxide over zirconia [J]. Catalysis Letters, 2000, 65: 75-78. |
| 9 | MA Z Y, YANG C , WEI W , et al . Catalytic performance of copper supported on zirconia polymorphs for CO hydrogenation[J]. Journal of Molecular Catalysis A: Chemical, 2005, 231(1/2): 75-81. |
| 10 | LIU Y D , GOEBL J , YIN Y D . Templated synthesis of nanostructured materials[J]. Chem. Soc. Rev., 2013, 42(7): 2610-2653. |
| 11 | YUAN Q , LI L L , LU S L , et al . Facile synthesis of Zr-based functional materials with highly ordered mesoporous structures[J]. The Journal of Physical Chemistry C, 2009, 113: 4117-4124. |
| 12 | TSONCHEVA T , IVANOVA L , PANEVA D , et al . Cobalt and iron oxide modified mesoporous zirconia: preparation, characterization and catalytic behaviour in methanol conversion[J]. Microporous and Mesoporous Materials, 2009, 120(3): 389-396. |
| 13 | CHANG Y L , WANG C , LIANG T X , et al . Sol-gel synthesis of mesoporous spherical zirconia[J]. RSC Advances, 2015, 5(127): 104629-104634. |
| 14 | DAS S K , BHUNIA M K , SINHA A K , et al . Self-assembled mesoporous zirconia and sulfated zirconia nanoparticles synthesized by triblock copolymer as template[J]. The Journal of Physical Chemistry C, 2009, 113: 8918-8923. |
| 15 | GONG L , SUN L B , SUN Y H , et al . Exploring in situ functionalization strategy in a hard template process: preparation of sodium-modified mesoporous tetragonal zirconia with superbasicity[J]. The Journal of Physical Chemistry C, 2011, 115(23): 11633-11640. |
| 16 | GU D , SCHMIDT W , PICHLER C M , et al . Surface-casting synthesis of mesoporous zirconia with a CMK-5-like structure and high surface area[J]. Angew. Chem. Int. Ed., 2017, 56(37): 11222-11225. |
| 17 | XIAO W M , YANG S Z , ZHANG P F , et al . Facile synthesis of highly porous metal oxides by mechanochemical nanocasting[J]. Chemistry of Materials, 2018, 30(9): 2924-2929. |
| 18 | YAN X L , LU N Y , FAN B B , et al . Synthesis of mesoporous and tetragonal zirconia with inherited morphology from metal-organic frameworks[J]. CrystEngComm, 2015, 17(33): 6426-6433. |
| 19 | YU Z C , LIU B X , ZHOU H F , et al . Mesoporous ZrO2 fibers with enhanced surface area and the application as recyclable absorbent[J]. Applied Surface Science, 2017, 399: 288-297. |
| 20 | YU G , ZHU L Y , ZHANG G L , et al . Preparation and characterization of the continuous titanium-doped ZrO2 mesoporous fibers with large surface area[J]. Journal of Porous Materials, 2013, 21(1): 105-112. |
| 21 | SONG Y H , LI X , SUN L L , et al . Metal/metal oxide nanostructures derived from metal-organic frameworks[J]. RSC Advances, 2015, 5(10): 7267-7279. |
| 22 | PING D , DONG X F , ZANG Y H , et al . Highly efficient MOF-templated Ni catalyst towards CO selective methanation in hydrogen-rich reformate gases[J]. International Journal of Hydrogen Energy, 2017, 42(23): 15551-15556. |
| 23 | LIPPI R , HOWARD S C , BARRON H , et al . Highly active catalyst for CO2 methanation derived from a metal organic framework template[J]. Journal of Materials Chemistry A, 2017, 5(25): 12990-12997. |
| 24 | D’SOUZA L , SUCHOPAR A , ZHU K , et al . Preparation of thermally stable high surface area mesoporous tetragonal ZrO2 and Pt/ZrO2: an active hydrogenation catalyst[J]. Microporous and Mesoporous Materials, 2006, 88(1/2/3): 22-30. |
| 25 | DESHMANE V G , ADEWUYI Y G . Synthesis of thermally stable, high surface area, nanocrystalline mesoporous tetragonal zirconium dioxide (ZrO2): effects of different process parameters[J]. Microporous and Mesoporous Materials, 2012, 148(1): 88-100. |
| 26 | 尹双凤,徐柏庆 . 碱液回流老化制备高比表面积二氧化锆[J]. 催化学报,2002,23(3): 214-218. |
| YIN S F , XU B Q . Preparation of high surface area zirconia by reflux digestion in basic solutions[J]. Chinese Journal of Catalysis, 2002, 23(3): 214-218. | |
| 27 | WANG P Y , UENO K , TAKIGAWA H , et al . Versatility of one-pot, single-step synthetic approach for spherical porous (metal) oxide nanoparticles using supercritical alcohols[J]. The Journal of Supercritical Fluids, 2013, 78: 124-131. |
| 28 | 石国亮,于峰,王琰, 等 . 溶剂辅助超细二氧化锆纳米晶体的可控合成[J]. 化工进展,2016,35(8): 2518-2522. |
| SHI G L , YU F , WANG Y , et al . Solvent-assisted controllable synthesis of ultrafine zirconia nanocrystals[J]. Chemical Industry and Engineering Progress, 2016, 35(8): 2518-2522. | |
| 29 | ZINK N , EMMERLING F , HÄGER T , et al . Low temperature synthesis of monodisperse nanoscaled ZrO2 with a large specific surface area[J]. Dalton Trans., 2013, 42(2): 432-440. |
| 30 | GU D , SCHUTH F . Synthesis of non-siliceous mesoporous oxides[J]. Chem. Soc. Rev., 2014, 43(1): 313-344. |
| 31 | CHEN H R , SHI J L , YU J , et al . Synthesis of titanium-doped ordered porous zirconium oxide with high-surface-area[J]. Microporous and Mesoporous Materials, 2000, 39: 171-176. |
| 32 | LIU S G , MA J, GUAN L X , et al . Mesoporous CaO-ZrO2 nano-oxides: a novel solid base with high activity and stability[J]. Microporous and Mesoporous Materials, 2009, 117: 466-471. |
| 33 | CIESLA U , FRÖBA M , STUCKY G , et al . Highly ordered porous zirconias from surfactant-controlled synthesis: zirconium oxide-sulfate and zirconium oxo phosphate[J]. Chem. Mater.,1999,11: 227-234. |
| 34 | CHEN S Y , JANG L Y , CHENG S . Synthesis of thermally stable zirconia-based mesoporous materials via a facile post-treatment[J].Phys J. Chem. B, 2660,110: 11761-11771. |
| 35 | LIU B , BAKER R T . Factors affecting the preparation of ordered mesoporous ZrO2 using the replica method[J]. Journal of Materials Chemistry, 2008, 18(43): 5200-5207. |
| 36 | LYU Y Y , YI S H , SHON J K , et al . Highly stable mesoporous metal oxides using nano-propping hybrid gemini surfactant[J]. J.Am. Chem. Soc., 2004, 126: 2310-2311. |
| 37 | CIESLA U , SCHACHT S , STUCKY G , et al . Formation of a porous zirconium oxo phosphate with a high surface area by a surfactant-assisted synthesis[J]. Angew. Chem. Int. Ed.,1996,35: 541-543. |
| 38 | AKUNE T , MORITA Y , SHIRAKAWA S , et al . ZrO2 nanocrystals as catalyst for synthesis of dimethylcarbonate from methanol and carbon dioxide: catalytic activity and elucidation of active sites[J]. Langmuir, 2018, 34(1): 23-29. |
| 39 | LAOSIRIPOJANA N , KIATKITTIPONG W , ASSABUMRUNGRAT S . Partial oxidation of palm fatty acids over Ce-ZrO2: roles of catalyst surface area, lattice oxygen capacity and mobility[J]. AIChE Journal, 2011, 57(10): 2861-2869. |
| 40 | SAKITANI K , NAKAMURA K I , IKENAGA N O , et al . Oxidative dehydrogenation of ethane over NiO-loaded high surface area ZrO2 catalysts[J]. Journal of the Japan Petroleum Institute, 2010,53(6): 327-335. |
| 41 | WANG Z Q , MA Y C, LIN J X . Ruthenium catalyst supported on high-surface-area basic ZrO2 for ammonia synthesis[J]. Journal of Molecular Catalysis A: Chemical, 2013, 378: 307-313. |
| 42 | CHEN H , WU Y L , QI S T , et al . Deoxygenation of octanoic acid catalyzed by hollow spherical Ni/ZrO2 [J]. Applied Catalysis A: General, 2017, 529: 79-90. |
| 43 | WANG H Q , CHEN H , NI B , et al . Mesoporous ZrO2 nanoframes for biomass upgrading[J]. ACS Appl. Mater. Interfaces., 2017, 9(32): 26897-26906. |
| 44 | AN K , ALAYOGLU S , MUSSELWHITE N , et al . Designed catalysts from Pt nanoparticles supported on macroporous oxides for selective isomerization of n-hexane[J].Am J. Chem. Soc., 2014, 136(19): 6830-6833. |
| 45 | YANG L P , LIN X J , ZHANG X , et al . General synthetic strategy for hollow hybrid microspheres through a progressive inward crystallization process[J].Am J. Chem. Soc., 2016, 138(18): 5916-5922. |
| 46 | JIN Z , WANG F , WANG F , et al . Metal nanocrystal-embedded hollow mesoporous TiO2 and ZrO2 microspheres prepared with polystyrene nanospheres as carriers and templates[J]. Advanced Functional Materials, 2013, 23(17): 2137-2144. |
| 47 | JOO J B , VU A , ZHANG Q , et al . A sulfated ZrO2 hollow nanostructure as an acid catalyst in the dehydration of fructose to 5-hydroxymethylfurfural[J]. ChemSusChem, 2013, 6(10): 2001-2008. |
| 48 | HUANG X Q , GUO C Y , ZUO J Q , et al . An assembly route to inorganic catalytic nanoreactors containing sub-10-nm gold nanoparticles with anti-aggregation properties[J]. Small, 2009, 5(3): 361-365. |
| 49 | SHUKLA A , SINGHA R K , SENGUPTA M , et al . Surfactant-induced preparation of highly dispersed Ni-nanoparticles supported on nanocrystalline ZrO2 for chemoselective reduction of nitroarenes[J]. Chemistry Select, 2018, 3(4): 1129-1141. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [6] | 王正坤, 黎四芳. 双子表面活性剂癸炔二醇的绿色合成[J]. 化工进展, 2023, 42(S1): 400-410. |
| [7] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [8] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [9] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [10] | 许友好, 王维, 鲁波娜, 徐惠, 何鸣元. 中国炼油创新技术MIP的开发策略及启示[J]. 化工进展, 2023, 42(9): 4465-4470. |
| [11] | 耿源泽, 周俊虎, 张天佑, 朱晓宇, 杨卫娟. 部分填充床燃烧器中庚烷均相/异相耦合燃烧[J]. 化工进展, 2023, 42(9): 4514-4521. |
| [12] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [13] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [14] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [15] | 高彦静. 单原子催化技术国际研究态势分析[J]. 化工进展, 2023, 42(9): 4667-4676. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||