化工进展 ›› 2022, Vol. 41 ›› Issue (4): 2054-2059.DOI: 10.16085/j.issn.1000-6613.2021-0750
可视化高通量检测天冬氨酸转氨甲酰酶活性的方法
- 1.北京理工大学化学与化工学院,北京 100081
2.清华大学化学工程系,北京 100084
Visual and high-throughput method for detecting the activity of aspartate transcarbamylase
GAO Bo1( ), FENG Xudong1, LI Chun1,2(
), FENG Xudong1, LI Chun1,2( )
)
- 1.School of Chemistry and Chemical Engineering, Beijing Institute of Technology, Beijing 100081, China
2.Department of Chemical Engineering, Tsinghua University, Beijing 100084, China
摘要:
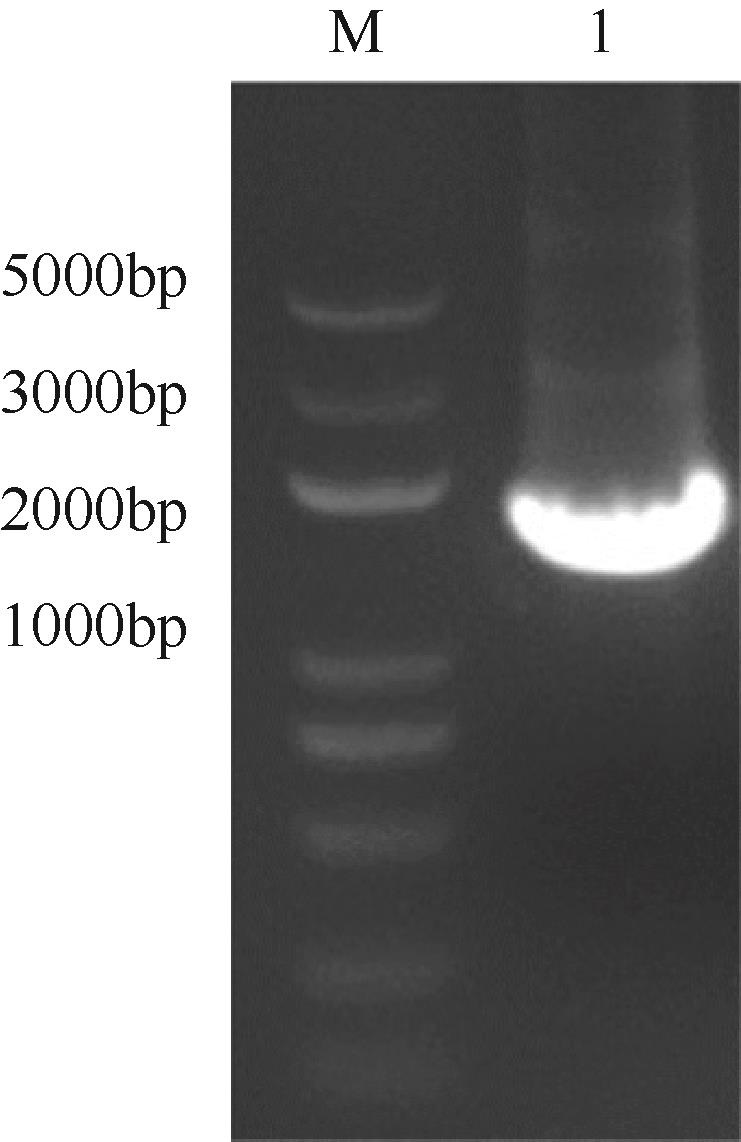
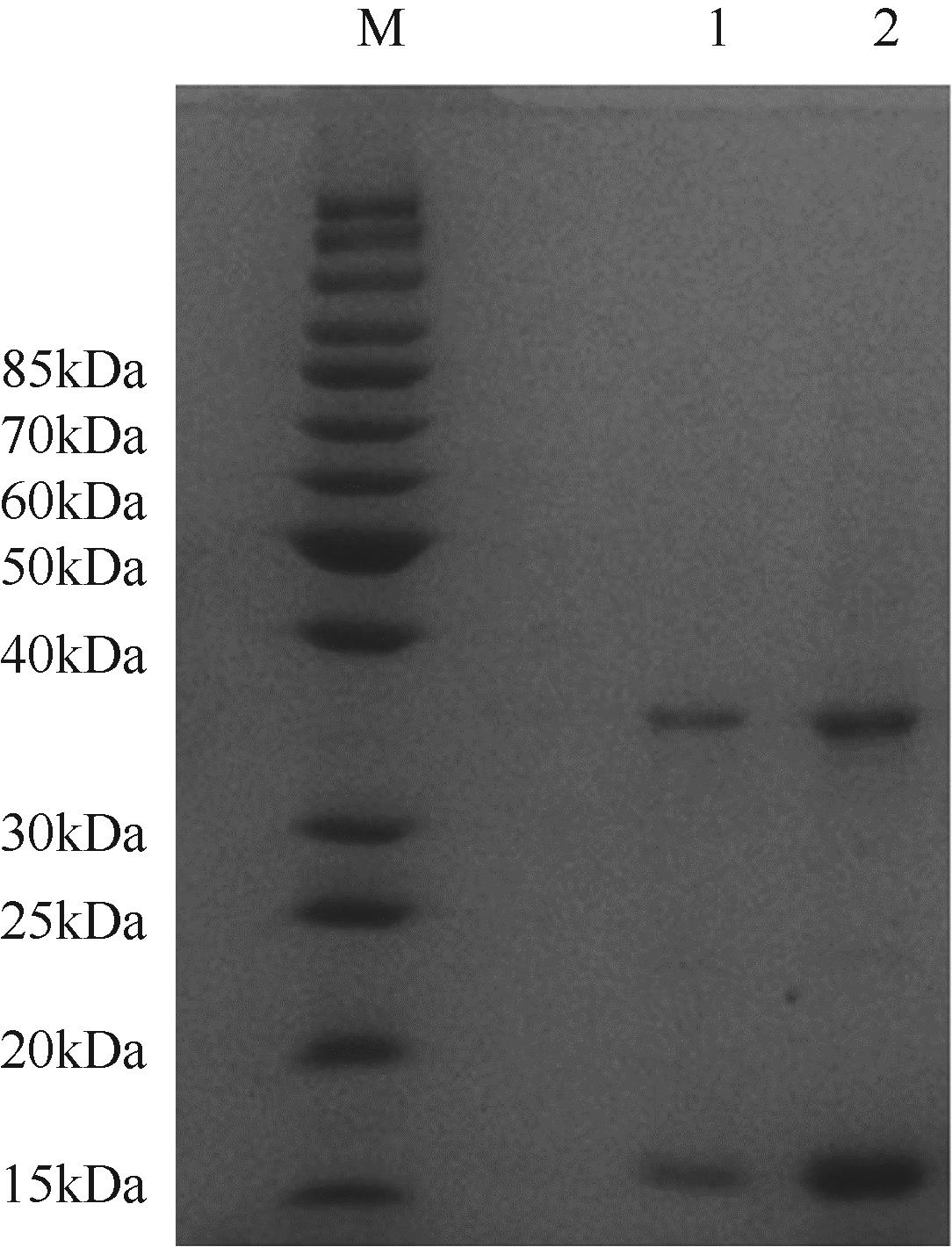
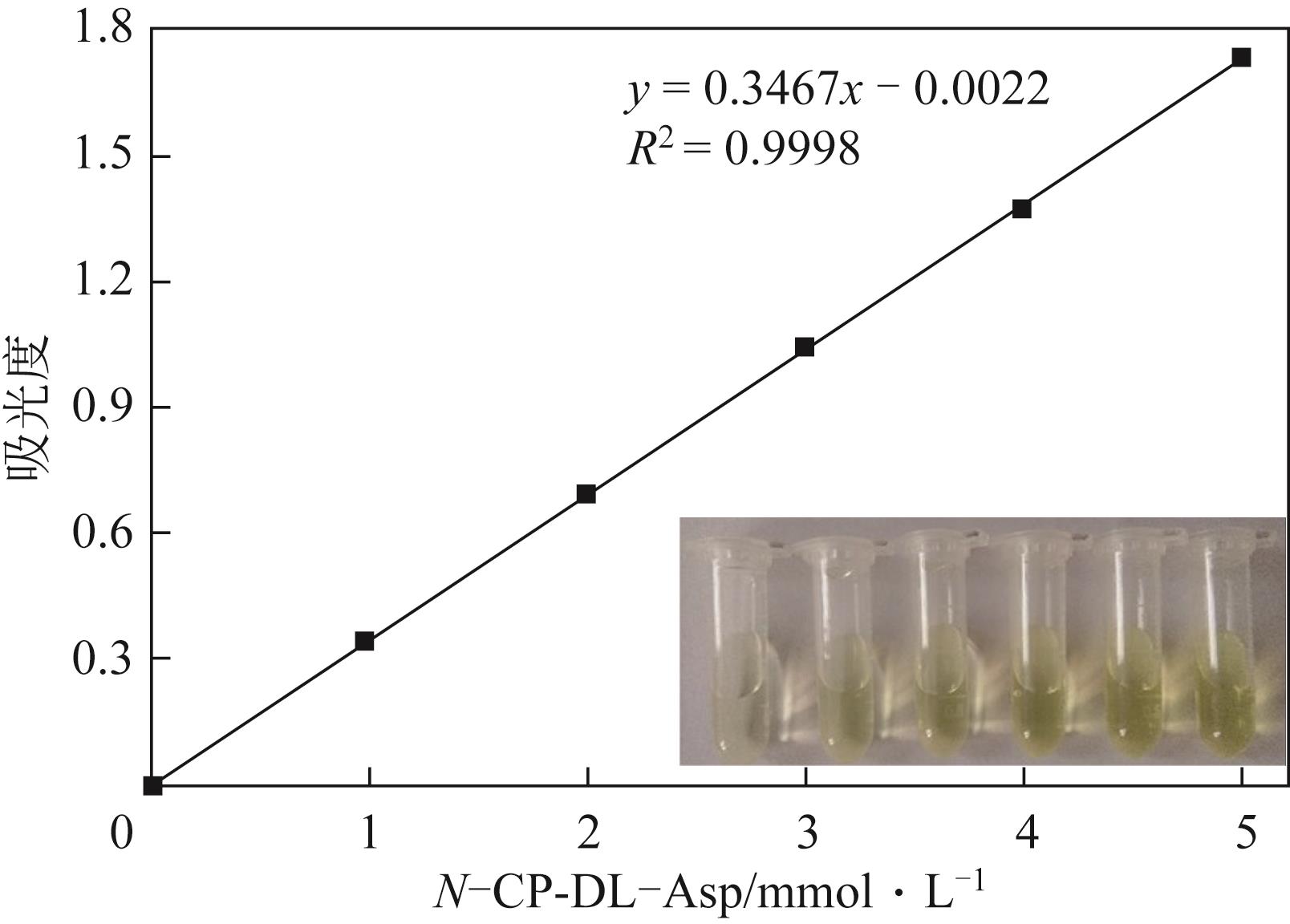
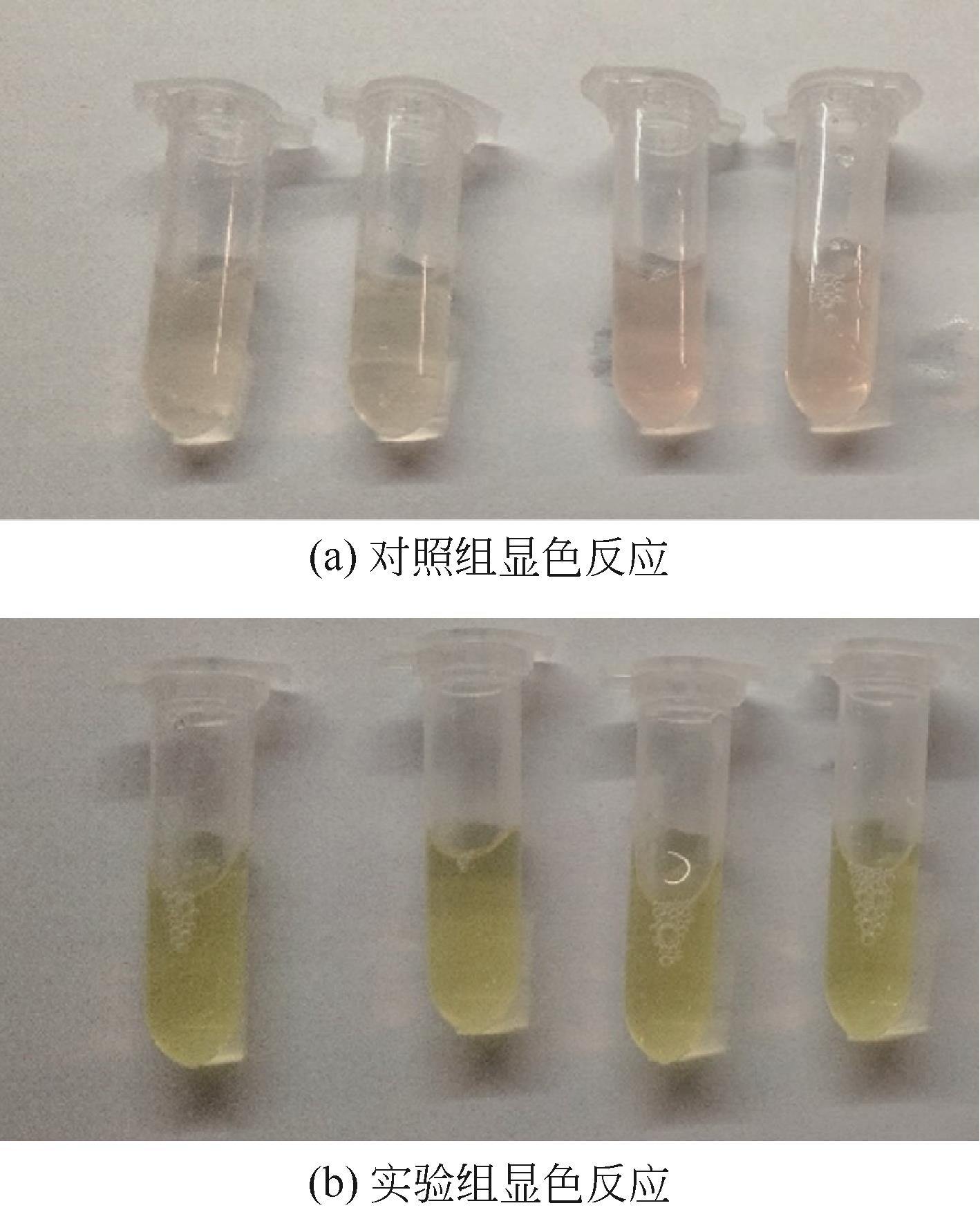
天冬氨酸转氨甲酰酶(ATCase)是嘧啶生物合成途径的第一个酶,其活性的反馈调节机制在控制嘌呤和嘧啶合成途径的平衡中起重要作用。目前,检测该酶活性的方法是基于安替比林和2,3-丁二酮肟的显色法。然而,该方法需先避光反应16h,再在45℃水浴30min并均匀光照,操作比较繁琐。本研究以对二甲氨基苯甲醛(PDAB)盐酸溶液为显色试剂,建立了一种检测ATCase活性的方法。该方法的原理是ATCase催化产生的N-氨甲酰基-L-天冬氨酸(N-CP-L-Asp)与PDAB在室温下反应15min,可生成黄色物质并能通过比色法定量检测。研究表明,在0.1~5mmol/L N-CP-DL-Asp的浓度范围内,黄色随化合物浓度的增加而加深,且在438nm的吸光度具有良好的线性关系,精密度RSD为0.87%~1.52%,加标回收率为96.6%~101.9%。该方法成功用于重组表达ATCase的活性测试,测得比酶活为56.83U/mg。该方法可以高效、快速和可视化地检测ATCase的活性,并且可以通过酶标仪实现高通量检测。
中图分类号: