Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (7): 3803-3812.DOI: 10.16085/j.issn.1000-6613.2020-1535
• Materials science and technology • Previous Articles Next Articles
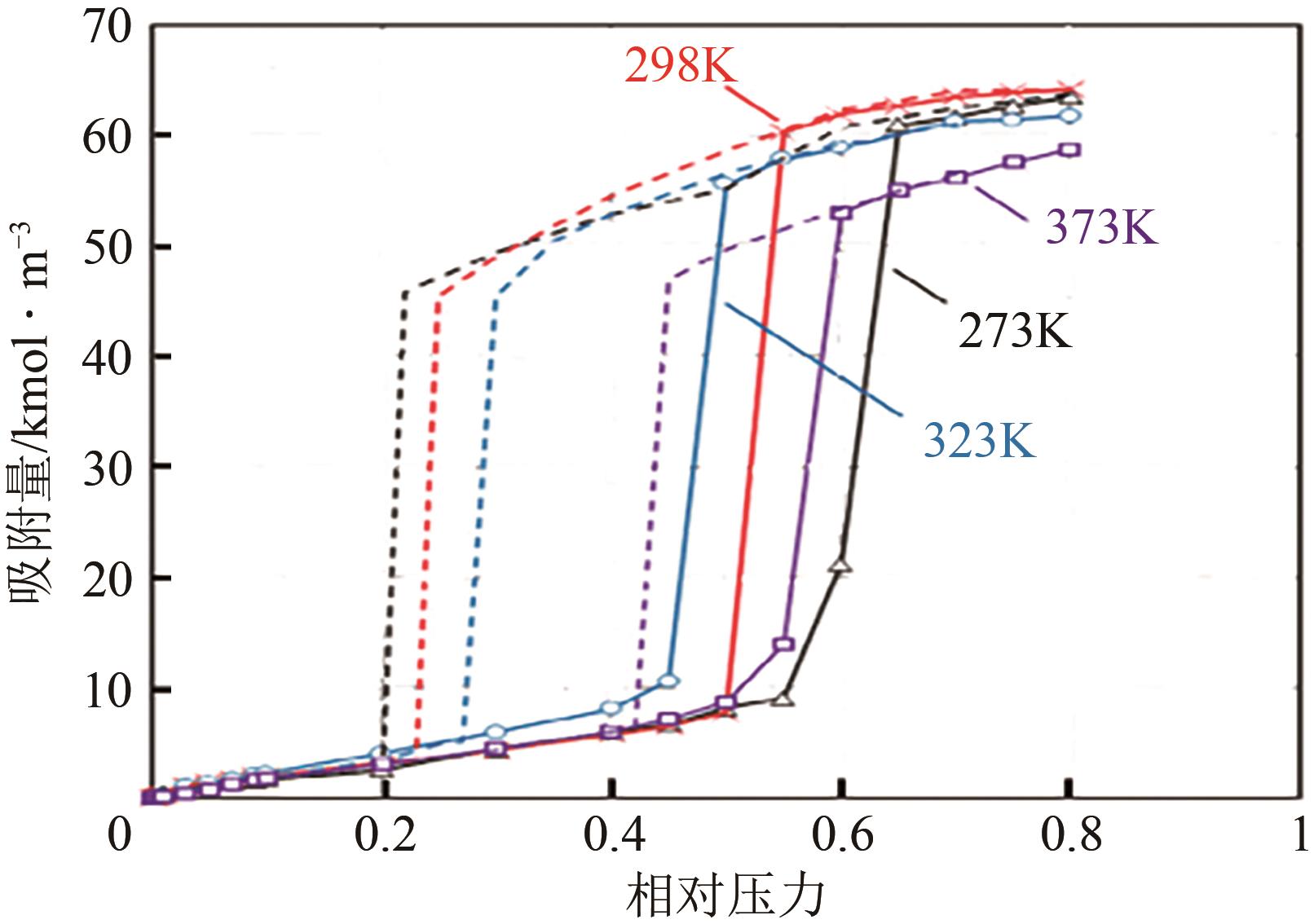
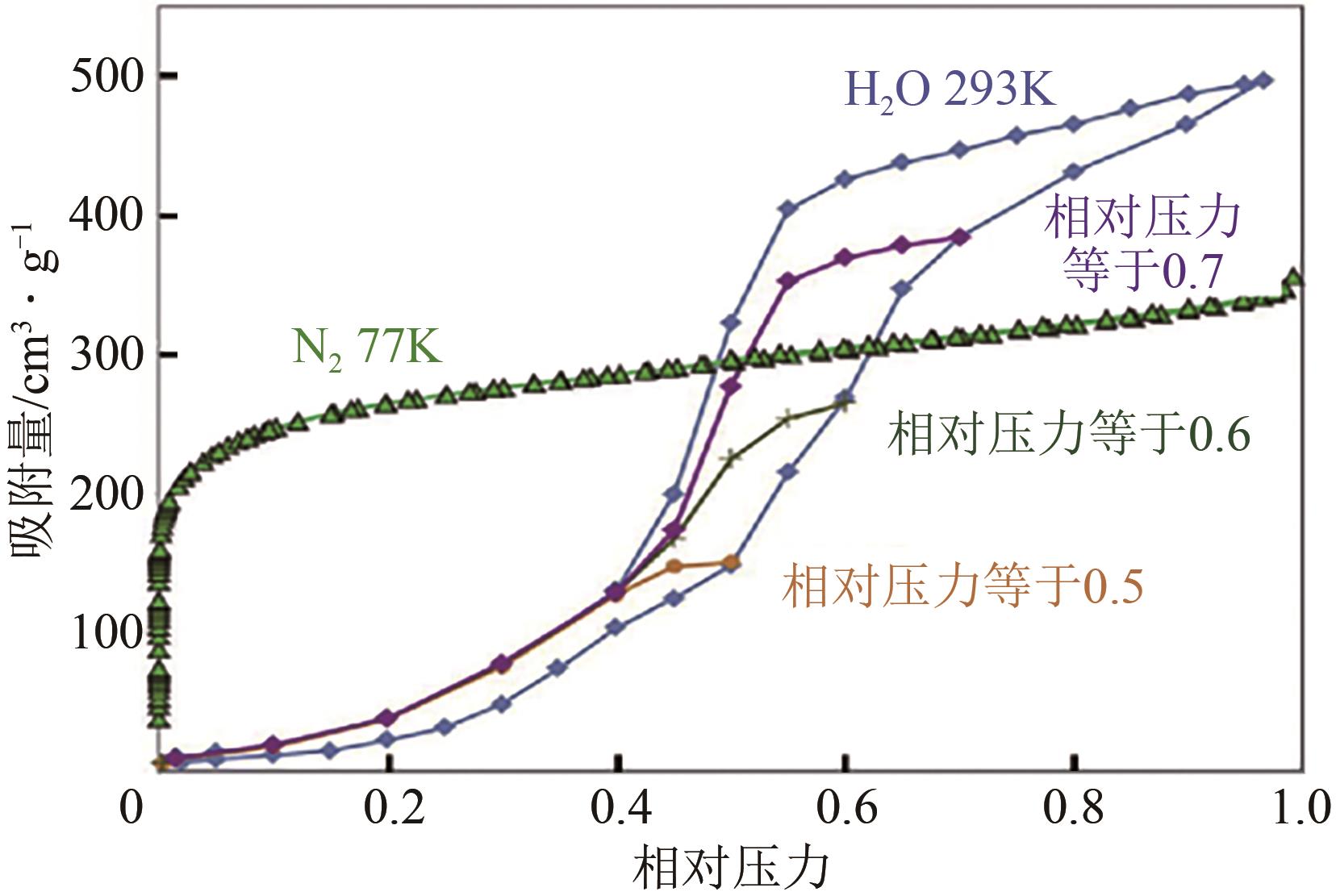
Adsorption behavior of water molecules on porous carbon materials
ZHENG Chao( ), KANG Kai, ZHOU Shuyuan, SONG Hua(
), KANG Kai, ZHOU Shuyuan, SONG Hua( ), BAI Shupei(
), BAI Shupei( )
)
- State Key Laboratory of National Biological Chemical Protection, Chemical Defense Research Institute, Beijing 100089, China
-
Received:2020-08-03Revised:2020-10-10Online:2021-07-19Published:2021-07-06 -
Contact:SONG Hua,BAI Shupei
水分子在多孔炭材料上的吸附行为研究进展
- 军事科学院防化研究院,国民核生化灾害防护国家重点实验室,北京 100089
-
通讯作者:宋华,白书培 -
作者简介:郑超(1994—),男,硕士研究生,研究方向为环境工程。E-mail:zchao94@163.com 。 -
基金资助:国家自然科学基金(21701186)
CLC Number:
Cite this article
ZHENG Chao, KANG Kai, ZHOU Shuyuan, SONG Hua, BAI Shupei. Adsorption behavior of water molecules on porous carbon materials[J]. Chemical Industry and Engineering Progress, 2021, 40(7): 3803-3812.
郑超, 康凯, 周术元, 宋华, 白书培. 水分子在多孔炭材料上的吸附行为研究进展[J]. 化工进展, 2021, 40(7): 3803-3812.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2020-1535
| 1 | 郑经堂, 黄振兴. 多孔炭材料[M]. 北京: 化学工业出版社, 2015. |
| ZHENG Jingtang, HUANG Zhenxing. Porous carbon materials[M]. Beijing: Chemical Industry Press, 2015. | |
| 2 | 林舒媛, 张儒静, 姜欣, 等. 碳质材料的气体吸附性能及其在空气净化中的应用[J]. 新型炭材料, 2015, 30(6): 502-510. |
| LIN Shuyuan, ZHANG Rujing, JIANG Xin, et al. Gas adsorption properties of carbon materials and their applications in air purification [J]. New Carbon Materials, 2015, 30(6): 502-510. | |
| 3 | CHELLA S, VENUGOPAL V, GEORGE J, et al. Role of nanomaterials in water treatment applications: a review[J]. Chemical Engineering Journal, 2016, 306: 1116-1137. |
| 4 | ZHANG Xueyang, GAO Bin, ANNE E C, et al. Adsorption of VOCs onto engineered carbon materials: a review[J]. Journal of Hazardous Materials, 2017, 338: 102-123. |
| 5 | 许伟, 刘军利, 孙康, 等. 活性炭吸附法在挥发性有机物治理中的应用研究进展[J]. 化工进展, 2016, 35(4): 1223-1229. |
| XU Wei, LIU Junli, SUN Kang, et al. Application progresses in the treatment of volatile organic compounds by adsorption on activated carbon[J]. Chemical Industry and Engineering Progress, 2016, 35(4): 1223-1229. | |
| 6 | 程代云, 史喜成. 军用吸附技术[M]. 北京: 国防工业出版社, 2012. |
| CHENG Daiyun, SHI Xicheng. Principle of military adsorption technology[M]. Beijing: National Defense Industry Press, 2012. | |
| 7 | JIA Lijuan, SHI Jialu, LONG Chao, et al. VOCs adsorption on activated carbon with initial water vapor contents: adsorption mechanism and modified characteristic curves[J]. Science of the Total Environment, 2020, 731: 139184. |
| 8 | 王稚真, 卢晗锋, 张波, 等. 水蒸气对改性椰壳活性炭吸附VOCs的影响[J]. 环境工程学报, 2010, 4(11): 2566-2570. |
| WANG Zhizhen, LU Hanfeng, ZHANG Bo, et al. Effect of water vapor on adsorption of VOCs by modified cocoanut activated carbon[J]. Chinese Journal of Environmental Engineering, 2010, 4(11): 2566-2570. | |
| 9 | 罗兰多. 纳米多孔材料内的吸附与扩散[M]. 史喜成, 白书培, 译. 北京: 国防工业出版社, 2018. |
| ROLANDO M A. Adsorption and diffusion in nanoporous materials[M]. SHI Xicheng, BAI Shupei, trans. Beijing: National Defense Industry Press, 2018. | |
| 10 | DEWEY C S, LEFFORGE P K, CABOT G L. Moisture sorption by carbon black[J]. Industrial and Engineering Chemistry, 1932, 24(9): 1045-1050. |
| 11 | MCBAIN J W, PORTER J L, SESSIONS R F. The nature of the sorption of water by charcoal[J]. Journal of the American Chemical Society, 1933, 55(6): 2294-2304. |
| 12 | EMMETT P H. Adsorption and pore-size measurements on charcoals and whetlerites[J]. Chemical Reviews, 1948, 43(1): 69-148. |
| 13 | DUBININ M M, ZAVERINA E D, SERPINSKY V V. The sorption of water vapor by active carbon[J]. Journal of the Chemical Society (Resumed), 1955: 1760. |
| 14 | DUBININ M M, SERPINSKY V V. Isotherm equation for water vapor adsorption by microporous carbonaceous adsorbents[J]. Carbon, 1981, 19(5): 402-403. |
| 15 | BARTON S S, EVANS M J B, MACDONALD J A F. The adsorption of water vapor by porous carbon[J]. Carbon, 1991, 29(8): 1099-1105. |
| 16 | BARTON S S, EVANS M J B, MACDONALD J A F. Adsorption of water vapor on nonporous carbon[J]. Langmuir, 1994, 10(11): 4250-4252. |
| 17 | BARTON S S, EVANS M J B, MACDONALD J A F. Adsorption and immersion enthalpies on BPL carbon[J]. Carbon, 1998, 36(7/8): 969-972. |
| 18 | STOECKLI F, JAKUBOV T, LAVANCHY A. Water adsorption in active carbons described by the Dubinin-Astakhov equation[J]. Journal of the Chemical Society, Faraday Transactions, 1994, 90(5): 783-786. |
| 19 | LODEWYCKX P,RAYMUNDO-PIÑERO E, VACLAVIKOVA M, et al. Suggested improvements in the parameters used for describing the low relative pressure region of the water vapour isotherms of activated carbons[J]. Carbon, 2013, 60: 556-558. |
| 20 | DE HORIKAWA T, SEKIDA T, HAYASHI J, et al. A new adsorption-desorption model for water adsorption in porous carbons[J]. Carbon, 2011, 49(2): 416-424. |
| 21 | LAGORSSE S, CAMPO M C, MAGALHÃES F D, et al. Water adsorption on carbon molecular sieve membranes: experimental data and isotherm model[J]. Carbon, 2005, 43(13): 2769-2779. |
| 22 | RUTHERFORD S W, COONS J E. Equilibrium and kinetics of water adsorption in carbon molecular sieve: theory and experiment[J]. Langumir, 2004, 20(20): 8681-8687. |
| 23 | RUTHERFORD S W. Modeling water adsorption in carbon micropores: study of water in carbon molecular sieves[J]. Langmuir, 2006, 22(2): 702-708. |
| 24 | OHTA N, NISHI Y, MORISHITA T, et al. Preparation of microporous carbon foams for adsorption/desorption of water vapor in ambient air [J]. New Carbon Materials, 2008, 23(3): 216-220. |
| 25 | OHTA N, NISHI Y, MORISHITA T, et al. Water vapor adsorption of microporous carbon films prepared from fluorinated aromatic polyimides[J]. Adsorption Science & Technology, 2008, 26(5): 373-382. |
| 26 | KANEKO K, HANZAWA Y, IIYAMA T, et al. Cluster-mediated water adsorption on carbon nanopores[J]. Adsorption, 1999, 5(1): 7-13. |
| 27 | KIM Pyoungchung, AGNIHOTRI S. Application of water-activated carbon isotherm models to water adsorption isotherms of single-walled carbon nanotubes[J]. Journal of Colloid and Interface Science, 2008, 325(1): 64-73. |
| 28 | KIM Pyoungchung, MEYER H M, AGNIHOTRI S. Effect of surface oxygen and temperature on external and micropore adsorption of water in single-walled carbon nanotubes by gravimetric and spectroscopic experiments[J]. The Journal of Physical Chemistry C, 2009, 113(28): 12109-12117. |
| 29 | MAO Shenghua, KLEINHAMMES A, WU Yue. NMR study of water adsorption in single-walled carbon nanotubes[J]. Chemical Physics Letters, 2006, 421(4/5/6): 513-517. |
| 30 | NAKAMURA M, OHBA T, BRANTON P, et al. Equilibration-time and pore-width dependent hysteresis of water adsorption isotherm on hydrophobic microporous carbons[J]. Carbon, 2010, 48(1): 305-308. |
| 31 | KUMAR K V, PREUSS K, GUO Zhengxiao, et al. Understanding the hydrophilicity and water adsorption behavior of nanoporous nitrogen-doped carbons[J]. The Journal of Physical Chemistry C, 2016, 120(32): 18167-18179. |
| 32 | YAN Jian, YU Ying, MA Chen, et al. Adsorption isotherms and kinetics of water vapor on novel adsorbents MIL-101(Cr)@GO with super-high capacity[J]. Applied Thermal Engineering, 2015, 84: 118-125. |
| 33 | ROUQUEROL F, ROUQUEROL J, SING K S W. Adsorption by powder and porous solids[M]. San Diego: Academic Press, 1999. |
| 34 | DO D D, NICHOLSON D, DO H D. On the anatomy of the adsorption heat versus loading as a function of temperature and adsorbate for a graphitic surface[J]. Journal of Colloid and Interface Science, 2008, 325(1): 7-22. |
| 35 | LIU Lumeng, TAN Shiliang J, HORIKAWA T, et al. Water adsorption on carbon—A review[J]. Advances in Colloid and Interface Science, 2017, 250: 64-78. |
| 36 | SCHEINER S. Forty years of progress in the study of the hydrogen bond[J]. Structural Chemistry, 2019, 30: 1119-1128. |
| 37 | 王林双, 王榕树. 水分子簇研究进展[J]. 化学进展, 2001, 13(2): 81-86. |
| WANG Linshuang, WANG Rongshu. Progress in studies of water cluster[J]. Progress in Chemistry, 2001, 13(2): 81-86. | |
| 38 | IIYAMA T, KOBAYASHI Y, KANEKO K, et al. In situ small-angle X-ray scattering study of cluster formation in carbon micropores[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2004, 241(1/2/3): 207-213. |
| 39 | OHBA T, KANOH H, KANEKO K. Cluster-growth-induced water adsorption in hydrophobic carbon nanopores[J]. The Journal of Physical Chemistry B, 2004, 108(39): 14964-14969. |
| 40 | DO D D, DO H D. A model for water adsorption in activated carbon[J]. Carbon, 2000, 38(5): 767-773. |
| 41 | DO D D, JUNPIROM S, DO H D. A new adsorption-desorption model for water adsorption in activated carbon[J]. Carbon, 2009, 47(6): 1466-1473. |
| 42 | SONG Yan, CHONG Yuan, RAGHAVAN A,et al. Nucleation and growth process of water adsorption in micropores of activated carbon revealed by NMR[J]. The Journal of Physical Chemistry C, 2017, 121(15): 8504-8509. |
| 43 | OHBA T, KANOH H, KANEKO K. Affinity transformation from hydrophilicity to hydrophobicity of water molecules on the basis of adsorption of water in graphitic nanopores[J]. Journal of the American Chemical Society, 2004, 126(5): 1560-1562. |
| 44 | OHBA T, KANEKO K. Surface oxygen-dependent water cluster growth in carbon nanospaces with GCMC simulation-aided in situ SAXS[J]. The Journal of Physical Chemistry C, 2007, 111(17): 6207-6214. |
| 45 | VAITHEESWARAN S, YIN Hao, RASAIAH J C, et al. Water clusters in nonpolar cavities[J]. Proceedings of the National Academy of Sciences, 2004, 101(49): 17002-17005. |
| 46 | OHBA T, KANOH H, KANEKO K. Structures and stability of water nanoclusters in hydrophobic nanospaces[J]. Nano Letters, 2005, 5(2): 227-230. |
| 47 | JORGE M, SCHUMACHER C, SEATON N A. Simulation study of the effect of the chemical heterogeneity of activated carbon on water adsorption[J]. Langmuir, 2002, 18(24): 9296-9306. |
| 48 | NGUYEN T X, BHATIA S K. How water adsorbs in hydrophobic nanospaces[J]. The Journal of Physical Chemistry C, 2011, 115(33): 16606-16612. |
| 49 | SUNG Baekman, KIM Jongwoo, STAMBAUGH C, et al. Direct measurement of activation time and nucleation rate in capillary-condensed water nanomeniscus[J]. Applied Physics Letters, 2013, 103(21): 213107. |
| 50 | MULLER E A, RULL L F, VEGA L F, et al. Adsorption of water on activated carbons: a molecular simulation study[J]. The Journal of Physical Chemistry, 1996, 100(4): 1189-1196. |
| 51 | SARKISOV L, CENTINEO A, BRANDANI S. Molecular simulation and experiments of water adsorption in a high surface area activated carbon: hysteresis, scanning curves and spatial organization of water clusters[J]. Carbon, 2017, 118: 127-138. |
| 52 | LODEWYCKX P. The effect of water uptake in ultramicropores on the adsorption water vapour in activated carbon[J]. Carbon, 2010, 48(9): 2549-2553. |
| 53 | LIU Lumeng, ZENG Yonghong, DO D D, et al. On the mechanism of water adsorption in carbon micropores: a molecular simulation study [J]. Chemical Engineering Journal, 2019, 357: 358-366. |
| 54 | HORIKAWA T, ZENG Yonghong, DO D D, et al. On the isosteric heat of adsorption of non-polar and polar fluids on highly graphitized carbon black[J]. Journal of Colloid and Interface Science, 2015, 439: 1-6. |
| 55 | KLOMKLIANG N, KAEWMANEE R, SAIMOEY S, et al. Adsorption of water and methanol on highly graphitized thermal carbon black: the effects of functional group and temperature on the isosteric heat at low loadings[J]. Carbon, 2016, 99: 361-369. |
| 56 | VELASCO L F, SNOECK D, MIGNON A, et al. Role of the surface chemistry of the adsorbent on the initialization step of the water sorption process[J]. Carbon, 2016, 106: 284-288. |
| 57 | PURI B R. Chemisorbed oxygen evolved as carbon dioxide and its influence on surface reactivity of carbons[J]. Carbon, 1966, 4(3): 391-400. |
| 58 | NGUYEN V T, DE HORIKAWA T, DO D D, et al. Water as a potential molecular probe for functional groups on carbon surfaces[J]. Carbon, 2014, 67: 72-78. |
| 59 | FLETCHER A J, UYGUR Y, THOMAS K M. Role of surface functional groups in the adsorption kinetics of water vapor on microporous activated carbons[J]. The Journal of Physical Chemistry C, 2007, 111(23): 8349-8359. |
| 60 | MCCALLUM C L, BANDOSZ T J, GUBBINS K E, et al. A molecular model for adsorption of water on activated carbon: comparison of simulation and experiment[J]. Langmuir, 1999, 15(2): 533-544. |
| 61 | STRIOLO A, CHIALVO A A, CUMMING P T, et al. Water adsorption in carbon-slit nanopores[J]. Langmuir, 2003, 19(20): 8583-8591. |
| 62 | HORIKAWA T, SAKAON, DO D D. Effects of temperature on water adsorption on controlled microporous and mesoporous carbonaceous solids[J]. Carbon, 2013, 56: 183-192. |
| 63 | FURMANIAK S, HATA K, GAUDEN P A, et al. Water at curved carbon surface: mechanisms of adsorption revealed by first calorimetric study[J]. The Journal of Physical Chemistry C, 2015, 119: 2703-2715. |
| 64 | WANG Haijing, XI Xuekui, KLEINHAMMES A, et al. Temperature-induced hydrophobic-hydrophilic transition observed by water adsorption[J]. Science, 2008, 322(5898): 80-83. |
| 65 | TACHIKAWA M, SHIGA M. Geometrical H/D isotope effect on hydrogen bonds in charged water clusters[J]. Journal of the American Chemical Society, 2005, 127(34): 11908-11909. |
| 66 | VIDELA P E, ROSSKY P J, LARIA D. Communication: isotopic effects on tunneling motions in the water trimer[J]. The Journal of Chemical Physics, 2016, 144(6): 061101. |
| 67 | LANIN S N, KOVALEVA N V, LITVINCHEVA L A. The adsorption of water isotopomers on carbon adsorbents[J]. Russian Journal of Physical Chemistry A, 2009, 83(2): 281-284. |
| 68 | FLETCHER A J, THOMAS K M. Kinetic isotope quantum effects in the adsorption of H2O and D2O on porous carbons[J]. The Journal of Physical Chemistry C, 2007, 111(5): 2107-2115. |
| 69 | ONO Y, FUTAMURA R, HATTORI Y, et al. Isotope effect on water adsorption on hydrophobic carbons of different nanoporosities[J]. Carbon, 2017, 119: 251-256. |
| 70 | FAN Chunyan, NGUYEN Van Thuong, ZENG Yonghong, et al. Novel approach to the characterization of the pore structure and surface chemistry of porous carbon with Ar, N2, H2O and CH3OH adsorption [J]. Microporous and Mesoporous Materials, 2015, 209: 79-89. |
| 71 | ZENG Yonghong, PRASWTYO L, NGUYEN Van Thuong, et al. Characterization of oxygen functional groups on carbon surfaces with water and methanol adsorption[J]. Carbon, 2015, 81: 447-457. |
| 72 | DO D D, NICHOLSON D, DO H D. On the Henry constant and isosteric heat at zero loading in gas phase adsorption[J]. Journal of Colloid and Interface Science, 2008, 324(1/2): 15-24. |
| 73 | VELASCO L F, GUILLET-NICOLAS R, DOBOSG, et al. Towards a better understanding of water adsorption hysteresis in activated carbons by scanning isotherms[J]. Carbon, 2016, 96: 753-758. |
| 74 | THOMMES M, MORLAY C, AHMAD R, et al. Assessing surface chemistry and pore structure of active carbons by a combination of physisorption (H2O, Ar, N2, CO2), XPS and TPD-MS[J]. Adsorption, 2011, 17(3): 653-661. |
| 75 | THOMMES M, MORELL J, CYCHOSZ K, et al. Combining nitrogen, argon, and water adsorption for advanced characterization of ordered mesoporous carbons (CMKs) and periodic mesoporous organosilicas (PMOs) [J]. Langmuir, 2013, 29(48): 14893-14902. |
| 76 | HORIKAWA T, MUGURUMAT, DO D D, et al. Scanning curves of water adsorption on graphitized thermal carbon black and ordered mesoporous carbon[J]. Carbon, 2015, 95: 137-143. |
| [1] | CUI Shoucheng, XU Hongbo, PENG Nan. Simulation analysis of two MOFs materials for O2/He adsorption separation [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 382-390. |
| [2] | CHEN Chongming, CHEN Qiu, GONG Yunqian, CHE Kai, YU Jinxing, SUN Nannan. Research progresses on zeolite-based CO2 adsorbents [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 411-419. |
| [3] | XU Chunshu, YAO Qingda, LIANG Yongxian, ZHOU Hualong. Research progress on functionalization strategies of covalent organic frame materials and its adsorption properties for Hg(Ⅱ) and Cr(Ⅵ) [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 461-478. |
| [4] | GU Yongzheng, ZHANG Yongsheng. Dynamic behavior and kinetic model of Hg0 adsorption by HBr-modified fly ash [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 498-509. |
| [5] | GUO Qiang, ZHAO Wenkai, XIAO Yonghou. Numerical simulation of enhancing fluid perturbation to improve separation of dimethyl sulfide/nitrogen via pressure swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 64-72. |
| [6] | WANG Shengyan, DENG Shuai, ZHAO Ruikai. Research progress on carbon dioxide capture technology based on electric swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 233-245. |
| [7] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [8] | YANG Ying, HOU Haojie, HUANG Rui, CUI Yu, WANG Bing, LIU Jian, BAO Weiren, CHANG Liping, WANG Jiancheng, HAN Lina. Coal tar phenol-based carbon nanosphere prepared by Stöber method for adsorption of CO2 [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5011-5018. |
| [9] | ZHANG Zhen, LI Dan, CHEN Chen, WU Jinglan, YING Hanjie, QIAO Hao. Separation and purification of salivary acids with adsorption resin [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4153-4158. |
| [10] | JIANG Jing, CHEN Xiaoyu, ZHANG Ruiyan, SHENG Guangyao. Research progress of manganese-loaded biochar preparation and its application in environmental remediation [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4385-4397. |
| [11] | YU Jingwen, SONG Luna, LIU Yanchao, LYU Ruidong, WU Mengmeng, FENG Yu, LI Zhong, MI Jie. An indole-bearing hypercrosslinked polymer In-HCP for iodine adsorption from water [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3674-3683. |
| [12] | LI Yanling, ZHUO Zhen, CHI Liang, CHEN Xi, SUN Tanglei, LIU Peng, LEI Tingzhou. Research progress on preparation and application of nitrogen-doped biochar [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3720-3735. |
| [13] | BAI Yadi, DENG Shuai, ZHAO Ruikai, ZHAO Li, YANG Yingxia. Exploration on standardized test scheme and experimental performance of temperature swing adsorption carbon capture unit [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3834-3846. |
| [14] | ZHANG Xuewei, HUANG Yaji, XU Yueyang, CHENG Haoqiang, ZHU Zhicheng, LI Jinlei, DING Xueyu, WANG Sheng, ZHANG Rongchu. Adsorption characteristics of SO3 from coal flue gas by alkaline adsorbent [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3855-3864. |
| [15] | WANG Zhicai, LIU Weiwei, ZHOU Cong, PAN Chunxiu, YAN Honglei, LI Zhanku, YAN Jingchong, REN Shibiao, LEI Zhiping, SHUI Hengfu. Synthesis and performance of a superplasticizer based on coal-based humic acid [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3634-3642. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||