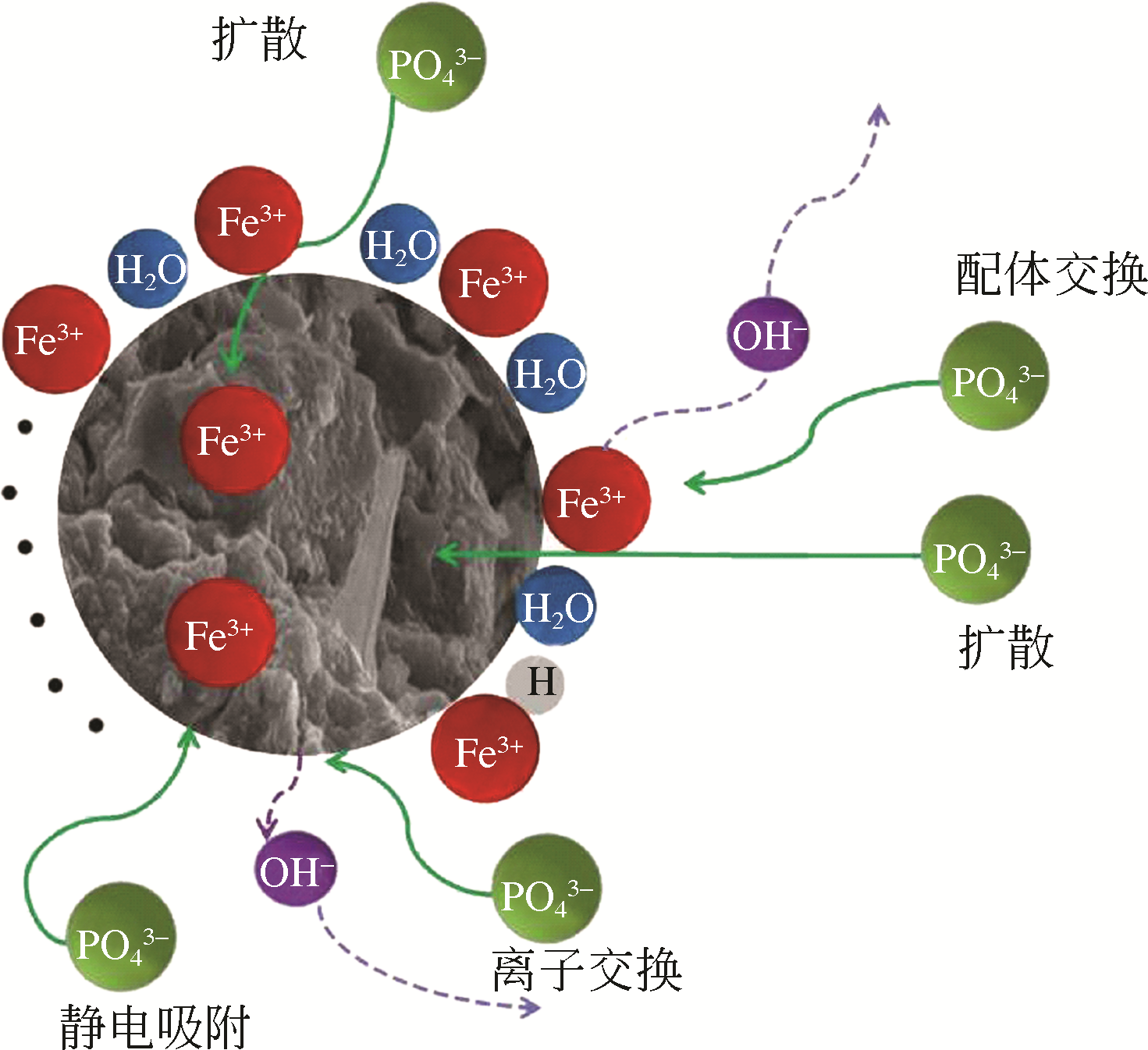
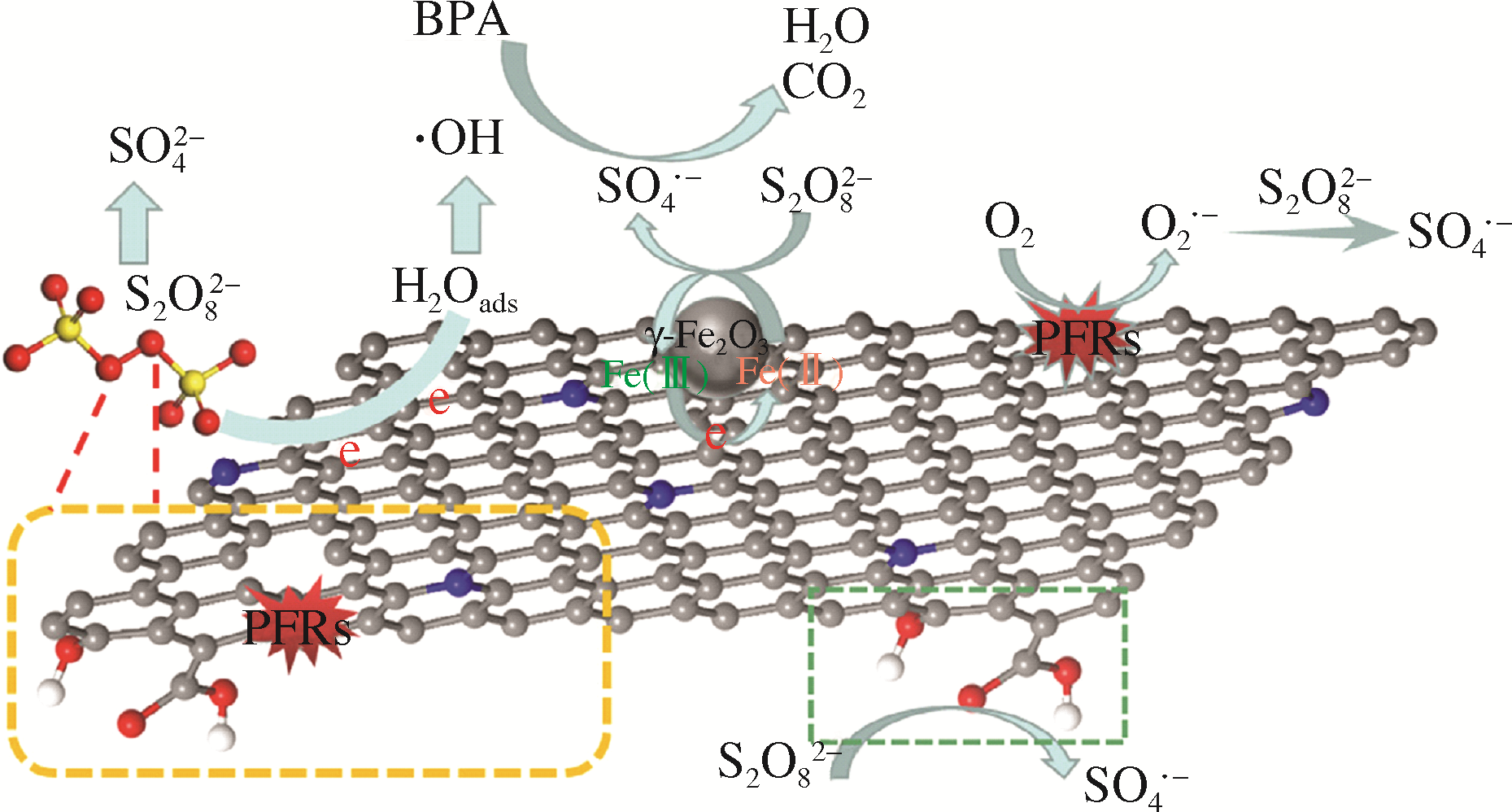
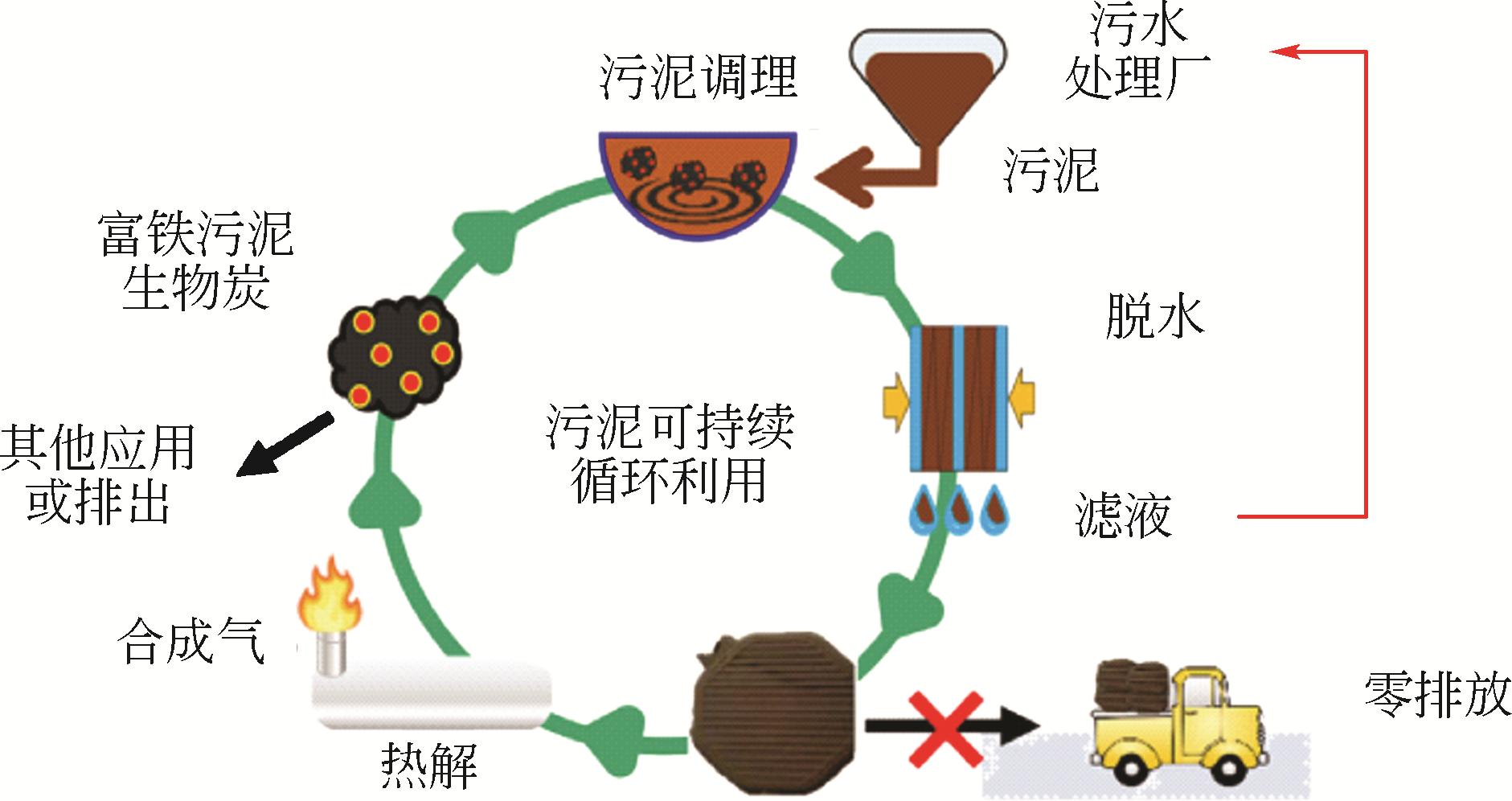
Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (2): 917-931.DOI: 10.16085/j.issn.1000-6613.2020-0736
• Materials science and technology • Previous Articles Next Articles
Preparation of biochar supported iron oxides composites and its application in water treatment
Guangzhu LI1,2( ), Shangjing ZENG1,2, Shuhai SUN1,2, Kaicheng XU3, Dejun BIAN1,2(
), Shangjing ZENG1,2, Shuhai SUN1,2, Kaicheng XU3, Dejun BIAN1,2( )
)
- 1.College of Water Conservancy and Environmental Engineering, Changchun Institute of Technology, Changchun 130012, Jilin, China
2.Jilin Provincial Key Laboratory of Municipal Wastewater Treatment, Changchun 130012, Jilin, China
3.Foshan Water Affairs and Environmental Protection Co. , Ltd. , Foshan 528000, Guangdong, China
-
Received:2020-05-06Revised:2020-07-05Online:2021-02-09Published:2021-02-05 -
Contact:Dejun BIAN
生物炭负载铁氧化物复合材料的制备及在水处理中的应用
李广柱1,2( ), 曾尚景1,2, 孙述海1,2, 许开成3, 边德军1,2(
), 曾尚景1,2, 孙述海1,2, 许开成3, 边德军1,2( )
)
- 1.长春工程学院水利与环境工程学院,吉林 长春 130012
2.吉林省城市污水处理重点实验室,吉林 长春 130012
3.佛山水务环保股份有限公司,广东 佛山 528000
-
通讯作者:边德军 -
作者简介:李广柱(1978—),男,博士,讲师,研究方向为水处理技术。E-mail:lgzzuolin@126.com 。 -
基金资助:国家自然科学基金(51878067);吉林省科技发展计划(20180101029JC);吉林省教育厅“十三五”科技项目(JJKH20180993KJ)
CLC Number:
Cite this article
Guangzhu LI, Shangjing ZENG, Shuhai SUN, Kaicheng XU, Dejun BIAN. Preparation of biochar supported iron oxides composites and its application in water treatment[J]. Chemical Industry and Engineering Progress, 2021, 40(2): 917-931.
李广柱, 曾尚景, 孙述海, 许开成, 边德军. 生物炭负载铁氧化物复合材料的制备及在水处理中的应用[J]. 化工进展, 2021, 40(2): 917-931.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2020-0736
| 铁氧化物/BC | 吸附条件 | 吸附能力 /mg·g-1 | 参考 文献 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BC | 铁氧化物 | Fe含量/% | SSA/m2·g-1 | APS③/nm | 温度/℃ | pH | 吸附剂量/g·L-1 | ||
| 橘子皮 | Fe3O4 | — | 19.4 | 6.8 | 25 | — | 6.25 | 1.24 | [ |
| 棉秆颗粒生物炭 | Fe2O3/FeOOH | — | 219.00 | 4.22 | 25 | — | 2.0 | 0.96 | [ |
| 麦秸 | 非晶态FeOOH | 47.06① | 138.56 | 2.75 | 室温 | 6.0 | 4.0 | 16.58 | [ |
| 芦苇 | Fe(OH)3 | 1.20② | — | — | 30 | 7.0 | 7. 0 | 1.185 | [ |
| 木屑 | Fe(OH)3 | 1.89② | 11.08 | — | 24 | — | 33.3 | 3.201 | [ |
| 水葫芦 | Fe3O4/Fe2O3 | 约65① | — | — | 25 | 7.0 | 5.0 | 5.07 | [ |
| ZnCl2活化生物污泥 | γ-Fe2O3/Fe3O4 | — | 156.79 | 0.888 | 22 | 7.0 | 2.0 | 61.2 | [ |
| Fe(OH)3 /α-Fe2O3 | — | 254.40 | 0.887 | 111.0 | |||||
| 竹子 | α-Fe2O3/Fe3O4 | — | 198.1 | — | 25 | 3.0 | 10.0 | 2.85 | [ |
| 含铁废物衍生真菌 | γ-Fe2O3 | 45① | 51.6 | 13.2 | 25 | — | 2.0 | 23.9 | [ |
| 铁氧化物/BC | 吸附条件 | 吸附能力 /mg·g-1 | 参考 文献 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BC | 铁氧化物 | Fe含量/% | SSA/m2·g-1 | APS③/nm | 温度/℃ | pH | 吸附剂量/g·L-1 | ||
| 橘子皮 | Fe3O4 | — | 19.4 | 6.8 | 25 | — | 6.25 | 1.24 | [ |
| 棉秆颗粒生物炭 | Fe2O3/FeOOH | — | 219.00 | 4.22 | 25 | — | 2.0 | 0.96 | [ |
| 麦秸 | 非晶态FeOOH | 47.06① | 138.56 | 2.75 | 室温 | 6.0 | 4.0 | 16.58 | [ |
| 芦苇 | Fe(OH)3 | 1.20② | — | — | 30 | 7.0 | 7. 0 | 1.185 | [ |
| 木屑 | Fe(OH)3 | 1.89② | 11.08 | — | 24 | — | 33.3 | 3.201 | [ |
| 水葫芦 | Fe3O4/Fe2O3 | 约65① | — | — | 25 | 7.0 | 5.0 | 5.07 | [ |
| ZnCl2活化生物污泥 | γ-Fe2O3/Fe3O4 | — | 156.79 | 0.888 | 22 | 7.0 | 2.0 | 61.2 | [ |
| Fe(OH)3 /α-Fe2O3 | — | 254.40 | 0.887 | 111.0 | |||||
| 竹子 | α-Fe2O3/Fe3O4 | — | 198.1 | — | 25 | 3.0 | 10.0 | 2.85 | [ |
| 含铁废物衍生真菌 | γ-Fe2O3 | 45① | 51.6 | 13.2 | 25 | — | 2.0 | 23.9 | [ |
| 铁氧化物/BC | 重金属浓度 /mg·L-1 | 吸附条件 | 吸附效果 | 参考 文献 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BC | 铁氧化物 | Fe含量/% | SSA /m2·g-1 | APS④ /nm | 温度/℃ | pH | 吸附剂量 /g·L-1 | 吸附能力 /mg·g-1 | 去除率 /% | ||
| 白杨木 | γ-Fe2O3 | 74.3① | — | — | As(Ⅴ):5~200 | 22 | — | 2.0 | 3.147 | — | [ |
| 松木 | γ-Fe2O3 | 2.95② | 193.1 | — | As(Ⅴ):1~50 | 22 | — | 2.5 | 0.429 | — | [ |
| 洋葱皮 | Fe3O4 | — | 38.58 | 21 | As(Ⅲ):10 | 25 | 7.0 | 0.1 | 57.47 | 98.9 | [ |
| 玉米秸 | γ-Fe2O3/α-Fe2O3 | 6.05② | 297.13 | 5.80 | As(Ⅴ):10 | 25 | 6.0 | 5.0 | 6.80 | 86.12 | [ |
| 废棉花 | β-FeOOH | 8.6② | 8.68 | — | As(Ⅴ):0.275 | 25 | 7.0 | 1.0 | 8.08 | >96.4 | [ |
| As(Ⅲ):0.275 | 6.04 | >96.4 | |||||||||
| 稻壳 | Fe3O4 | — | 1736.8 | 4.22 | As(Ⅴ):0.01~10 | 室温 | 7~12 | 10~50 | 5987 | >85 | [ |
| 花生壳 | γ-Fe2O3 | 20.95② | 144.01 | — | Cr(Ⅵ):10~320 | 25 | 5.13 | 2.0 | 77.54 | — | [ |
| 松香 | α-Fe2O3 | — | 5.03 | 50~150 | Cr(Ⅵ):50 | 26 | 9.0 | 0.31 | 166 | >90 | [ |
| 甘蔗渣 | Fe3O4/Fe2O3/FeO | 3.54③ | 16.18 | <100 | Cr(Ⅵ):10~300 | 30 | 4.61 | 1.0 | 71.04 | — | [ |
| 生物污泥 | Fe3O4/FeO/Fe0 | — | — | — | Cr(Ⅵ):50 | 25 | 2.0 | 4.0 | 11.56 | — | [ |
| 木屑 | Fe3O4 | — | 68 | 9.11 | Zn:0.6~19.6 | 20 | 4.4~5.5 | 1.25 | 4.55 | — | [ |
| Cu:0.6~19.2 | 7.68 | ||||||||||
| Pb:2.0~62.1 | 31.22 | ||||||||||
| 椰子壳 | Fe3O4/Fe2O3 | — | 834 | — | Pb:25~125 | 30 | 4.5 | 2.0 | 162.75 | — | [ |
| Cd:25~125 | 4.8 | 162.75 | |||||||||
| 废海带 | Fe2O3/Fe3O4 | — | 0.97 | — | Cu:1200 | 室温 | — | 10 | 47.75 | — | [ |
| Cd:1200 | 23.16 | ||||||||||
| Zn:1200 | 22.22 | ||||||||||
| 活化玉米秆 | α-FeOOH | 14.75② | 391.6 | 6.89 | Cu:50 | 25 | 7.0 | 0.25 | 144.7 | 71.9 | [ |
| 含Fe生物污泥 | Fe3O4/Fe2O3 | 14.1② | 114.57 | 32 | Cd:8 | 室温 | 5.0 | 0.8 | 14.18 | >99 | [ |
| Pb:70 | 6.0 | 1.2 | 59.50 | >99 | |||||||
| 铁氧化物/BC | 重金属浓度 /mg·L-1 | 吸附条件 | 吸附效果 | 参考 文献 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BC | 铁氧化物 | Fe含量/% | SSA /m2·g-1 | APS④ /nm | 温度/℃ | pH | 吸附剂量 /g·L-1 | 吸附能力 /mg·g-1 | 去除率 /% | ||
| 白杨木 | γ-Fe2O3 | 74.3① | — | — | As(Ⅴ):5~200 | 22 | — | 2.0 | 3.147 | — | [ |
| 松木 | γ-Fe2O3 | 2.95② | 193.1 | — | As(Ⅴ):1~50 | 22 | — | 2.5 | 0.429 | — | [ |
| 洋葱皮 | Fe3O4 | — | 38.58 | 21 | As(Ⅲ):10 | 25 | 7.0 | 0.1 | 57.47 | 98.9 | [ |
| 玉米秸 | γ-Fe2O3/α-Fe2O3 | 6.05② | 297.13 | 5.80 | As(Ⅴ):10 | 25 | 6.0 | 5.0 | 6.80 | 86.12 | [ |
| 废棉花 | β-FeOOH | 8.6② | 8.68 | — | As(Ⅴ):0.275 | 25 | 7.0 | 1.0 | 8.08 | >96.4 | [ |
| As(Ⅲ):0.275 | 6.04 | >96.4 | |||||||||
| 稻壳 | Fe3O4 | — | 1736.8 | 4.22 | As(Ⅴ):0.01~10 | 室温 | 7~12 | 10~50 | 5987 | >85 | [ |
| 花生壳 | γ-Fe2O3 | 20.95② | 144.01 | — | Cr(Ⅵ):10~320 | 25 | 5.13 | 2.0 | 77.54 | — | [ |
| 松香 | α-Fe2O3 | — | 5.03 | 50~150 | Cr(Ⅵ):50 | 26 | 9.0 | 0.31 | 166 | >90 | [ |
| 甘蔗渣 | Fe3O4/Fe2O3/FeO | 3.54③ | 16.18 | <100 | Cr(Ⅵ):10~300 | 30 | 4.61 | 1.0 | 71.04 | — | [ |
| 生物污泥 | Fe3O4/FeO/Fe0 | — | — | — | Cr(Ⅵ):50 | 25 | 2.0 | 4.0 | 11.56 | — | [ |
| 木屑 | Fe3O4 | — | 68 | 9.11 | Zn:0.6~19.6 | 20 | 4.4~5.5 | 1.25 | 4.55 | — | [ |
| Cu:0.6~19.2 | 7.68 | ||||||||||
| Pb:2.0~62.1 | 31.22 | ||||||||||
| 椰子壳 | Fe3O4/Fe2O3 | — | 834 | — | Pb:25~125 | 30 | 4.5 | 2.0 | 162.75 | — | [ |
| Cd:25~125 | 4.8 | 162.75 | |||||||||
| 废海带 | Fe2O3/Fe3O4 | — | 0.97 | — | Cu:1200 | 室温 | — | 10 | 47.75 | — | [ |
| Cd:1200 | 23.16 | ||||||||||
| Zn:1200 | 22.22 | ||||||||||
| 活化玉米秆 | α-FeOOH | 14.75② | 391.6 | 6.89 | Cu:50 | 25 | 7.0 | 0.25 | 144.7 | 71.9 | [ |
| 含Fe生物污泥 | Fe3O4/Fe2O3 | 14.1② | 114.57 | 32 | Cd:8 | 室温 | 5.0 | 0.8 | 14.18 | >99 | [ |
| Pb:70 | 6.0 | 1.2 | 59.50 | >99 | |||||||
| 铁氧化物/BC | 有机物浓度/mg·L-1 | 吸附条件 | 吸附效果 | 参考 文献 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BC | 铁氧化物 | SSA /m2·g-1 | APS① /nm | 温度 /℃ | pH | 吸附剂量 /g·L-1 | 吸附能力 /mg·g-1 | 去除率 /% | ||
| 橘子皮 | Fe3O4 | 23.4 | 7.2 | 萘:18 | 25 | — | 6.25 | 23.0 | 99.6 | [ |
| 对硝基甲苯:318 | 43.4 | 87.1 | ||||||||
| 木屑 | Fe3O4/γ-Fe2O3 | 219 | 6.69 | 菲:0.022 | 20 | — | 0.375 | — | >99.9 | [ |
| 苯酚:5-100 | 1.25 | 20.695 | — | |||||||
| 松木屑 | Fe3O4 | 125.8 | 9.6 | 磺胺甲恶唑:20.1 | 25 | 4.0 | 2.0 | 13.83 | 85.2 | [ |
| 松子和核桃壳 | Fe3O4 | 365 | — | 卡马西平:30 | 25 | 6.0 | 0.2 | 62.7 | 约40 | [ |
| 四环素:30 | 94.2 | 约50 | ||||||||
| 核桃壳 | γ-Fe2O3/Fe3O4 | 723.5 | 2.76 | 布洛芬:<10 | 25 | 3.0 | 0.5 | 75 | >95 | [ |
| 含Fe混凝污泥 | γ-Fe2O3 | 91 | — | 氧氟沙星:30 | 25 | 6.0 | 5.0 | 19.74 | 约96 | [ |
| 香蕉皮提取物 | 非晶态FexOy | — | — | 亚甲蓝:50 | 30 | 6.1 | 0.5 | 862 | >90 | [ |
| 铁氧化物/BC | 有机物浓度/mg·L-1 | 吸附条件 | 吸附效果 | 参考 文献 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BC | 铁氧化物 | SSA /m2·g-1 | APS① /nm | 温度 /℃ | pH | 吸附剂量 /g·L-1 | 吸附能力 /mg·g-1 | 去除率 /% | ||
| 橘子皮 | Fe3O4 | 23.4 | 7.2 | 萘:18 | 25 | — | 6.25 | 23.0 | 99.6 | [ |
| 对硝基甲苯:318 | 43.4 | 87.1 | ||||||||
| 木屑 | Fe3O4/γ-Fe2O3 | 219 | 6.69 | 菲:0.022 | 20 | — | 0.375 | — | >99.9 | [ |
| 苯酚:5-100 | 1.25 | 20.695 | — | |||||||
| 松木屑 | Fe3O4 | 125.8 | 9.6 | 磺胺甲恶唑:20.1 | 25 | 4.0 | 2.0 | 13.83 | 85.2 | [ |
| 松子和核桃壳 | Fe3O4 | 365 | — | 卡马西平:30 | 25 | 6.0 | 0.2 | 62.7 | 约40 | [ |
| 四环素:30 | 94.2 | 约50 | ||||||||
| 核桃壳 | γ-Fe2O3/Fe3O4 | 723.5 | 2.76 | 布洛芬:<10 | 25 | 3.0 | 0.5 | 75 | >95 | [ |
| 含Fe混凝污泥 | γ-Fe2O3 | 91 | — | 氧氟沙星:30 | 25 | 6.0 | 5.0 | 19.74 | 约96 | [ |
| 香蕉皮提取物 | 非晶态FexOy | — | — | 亚甲蓝:50 | 30 | 6.1 | 0.5 | 862 | >90 | [ |
| 铁氧化物/BC | 污染物浓度 /mg·L-1 | 反应条件 | 催化降解效果 | 参考文献 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BC | 铁氧化物 | Fe含量 /% | SSA /m2·g-1 | 催化剂量 /g·L-1 | 氧化剂浓度 /mg·L-1 | pH | 温度 /℃ | 时间 /min | 去除率 /% | 矿化率 /% | ||
| 生物污泥 | α-Fe2O3 | 19.3① | 6.30 | 罗丹明B:55.5 | 0.33④ | H2O2:200 | 4.0 | 25 | 120 | >99.8 | >59 | [ |
| 硝基苯酚:65 | H2O2:80 | 60 | 92.74 | 47.25 | ||||||||
| 厌氧硝化污泥 | α-Fe2O3 | 19.15① | 15.17 | 罗丹明B:55.5 | 0.33⑤ | H2O2:200 | 4.0 | 25 | 30 | 100 | 69 | [ |
| 甘蔗渣 | Fe3O4/FeOOH/Fe2O3 | 16.34① | 179.5 | 橙G:100 | 0.5 | H2O2:75 | 5.5 | 25 | 120 | 99.7 | 44.2 | [ |
| 生物污泥 | Fe3O4 | — | 50.10 | 亚甲蓝:100 | 1.0 | H2O2:300 | 3.0 | 室温 | 20 | 98 | 43 | [ |
| 印染废水TOC:230 | 30 | — | 49 | |||||||||
| 棕榈叶柄 | α-FeOOH | 34.92② | 221 | 硝基间苯二酚:20 | 1.0 | H2O2:850 | 4 | 25 | 40 | 100 | — | [ |
| 甘蔗渣 | Fe2O3/Fe3O4 | — | 161.8 | 甲硝唑:40 | 0.3 | H2O2:1122 | 5.61 | 30 | 120 | 100 | 32.83 | [ |
| 甘蔗渣 | Fe3O4/FeOOH/Fe2O3 | 15.61① | 185.4 | 铬黑T:100 | 0.1 | H2O2:34 | 2~3 | 25 | 120 | 56.6 | — | [ |
| K2S2O8:270 | 87.7 | 约55 | ||||||||||
| 生物污泥 | Fe(Ⅲ)/Fe(Ⅱ) | 1.37③ | — | 磺胺甲 唑:10.13 唑:10.13 | 2.0 | K2S2O8:405 | 5~9 | 25 | 180 | 94.6 | 58 | [ |
| 生物污泥 | Fe(Ⅲ)/Fe(Ⅱ) | 0.5③ | 157.4 | 三氯生:10 | 1.0 | KHSO5:122 | 7.2 | 25 | 240 | 99.2 | 32.5 | [ |
| 香蕉皮 | γ-Fe2O3 | 5.24① | 504 | 双酚A:20 | 0.3 | K2S2O8:5mmol | 6.28 | 25 | 20 | 100 | 90 | [ |
| 铁氧化物/BC | 污染物浓度 /mg·L-1 | 反应条件 | 催化降解效果 | 参考文献 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BC | 铁氧化物 | Fe含量 /% | SSA /m2·g-1 | 催化剂量 /g·L-1 | 氧化剂浓度 /mg·L-1 | pH | 温度 /℃ | 时间 /min | 去除率 /% | 矿化率 /% | ||
| 生物污泥 | α-Fe2O3 | 19.3① | 6.30 | 罗丹明B:55.5 | 0.33④ | H2O2:200 | 4.0 | 25 | 120 | >99.8 | >59 | [ |
| 硝基苯酚:65 | H2O2:80 | 60 | 92.74 | 47.25 | ||||||||
| 厌氧硝化污泥 | α-Fe2O3 | 19.15① | 15.17 | 罗丹明B:55.5 | 0.33⑤ | H2O2:200 | 4.0 | 25 | 30 | 100 | 69 | [ |
| 甘蔗渣 | Fe3O4/FeOOH/Fe2O3 | 16.34① | 179.5 | 橙G:100 | 0.5 | H2O2:75 | 5.5 | 25 | 120 | 99.7 | 44.2 | [ |
| 生物污泥 | Fe3O4 | — | 50.10 | 亚甲蓝:100 | 1.0 | H2O2:300 | 3.0 | 室温 | 20 | 98 | 43 | [ |
| 印染废水TOC:230 | 30 | — | 49 | |||||||||
| 棕榈叶柄 | α-FeOOH | 34.92② | 221 | 硝基间苯二酚:20 | 1.0 | H2O2:850 | 4 | 25 | 40 | 100 | — | [ |
| 甘蔗渣 | Fe2O3/Fe3O4 | — | 161.8 | 甲硝唑:40 | 0.3 | H2O2:1122 | 5.61 | 30 | 120 | 100 | 32.83 | [ |
| 甘蔗渣 | Fe3O4/FeOOH/Fe2O3 | 15.61① | 185.4 | 铬黑T:100 | 0.1 | H2O2:34 | 2~3 | 25 | 120 | 56.6 | — | [ |
| K2S2O8:270 | 87.7 | 约55 | ||||||||||
| 生物污泥 | Fe(Ⅲ)/Fe(Ⅱ) | 1.37③ | — | 磺胺甲 唑:10.13 唑:10.13 | 2.0 | K2S2O8:405 | 5~9 | 25 | 180 | 94.6 | 58 | [ |
| 生物污泥 | Fe(Ⅲ)/Fe(Ⅱ) | 0.5③ | 157.4 | 三氯生:10 | 1.0 | KHSO5:122 | 7.2 | 25 | 240 | 99.2 | 32.5 | [ |
| 香蕉皮 | γ-Fe2O3 | 5.24① | 504 | 双酚A:20 | 0.3 | K2S2O8:5mmol | 6.28 | 25 | 20 | 100 | 90 | [ |
| 1 | 杨金梅, 吕建波, 李莞璐, 等. 壳聚糖载纳米羟基氧化铁对水中磷的吸附[J]. 环境工程学报, 2018, 12(5): 1286-1294. |
| YANG Jinmei, Jianbo LYU, LI Wanlu, et al. Adsorption of phosphate by nano akaganeite impregnated chitosan[J]. Chinese Journal of Environmental Engineering, 2018, 12(5): 1286-1294. | |
| 2 | 段正洋, 刘树丽, 徐晓军, 等. 磁性Fe3O4纳米粒子的制备、功能化及在重金属废水中的应用[J]. 化工进展, 2017, 36(5): 1791-1801. |
| DUAN Zhengyang, LIU Shuli, XU Xiaojun, et al. Preparation and functionalization of magnetic Fe3O4 nanoparticles and its application in heavy metal wastewater[J]. Chemical Industry and Engineering Progress, 2017, 36(5): 1791-1801. | |
| 3 | 李广柱, 艾胜书, 田曦, 等. 巯基功能化磁性纳米材料去除水中 Ag(Ⅰ)和Cd(Ⅱ)[J]. 水处理技术, 2018, 44(9): 93-98. |
LI Guangzhu, AI Shengshu, TIAN Xi, et al. Efficient removal of Ag(Ⅰ) and Cd( ) from aqueous solution by sulfhydryl-functionalized magnetic nanomaterial[J]. Technology of Water Treatment, 2018, 44(9): 93-98. ) from aqueous solution by sulfhydryl-functionalized magnetic nanomaterial[J]. Technology of Water Treatment, 2018, 44(9): 93-98.
|
|
| 4 | 刘剑聪. 地下水厂铁泥制备吸附剂:矿物相变、磁性特征和吸附性能[D]. 长春: 东北师范大学, 2018. |
| LIU Jiancong. Conversion of groundwater treatment sludge into adsorbent: mineral phase transformation, magnetic property and adsorption performance[D]. Changchun: Northeast Normal University, 2018. | |
| 5 | 罗成, 李艳龙, 建纲. 四氧化三铁纳米颗粒过氧化物酶样活性的应用[J]. 科学通报, 2015, 60(35): 3478-3488. |
| LUO Cheng, LI Yanlong, JIAN Gang. Applications of iron oxide nanoparticles as peroxidase mimetics[J]. Chinese Science Bulletin, 2015, 60(35): 3478-3488. | |
| 6 | 谢之润. 基于过渡金属氧化物降解DDT性能的研究[D]. 济南: 山东大学, 2016. |
| XIE Zhirun. DDT degradation performance based on transition metal oxides[D]. Jinan: Shandong University, 2016. | |
| 7 | LI Guangzhu, ZHANG Zhuqing, GENG Chao, et al. Sulfhydryl-functionalised magnetic nanoparticles as sorbent in dispersive solid-phase extraction for the rapid enrichment of mercury species from natural water samples[J]. International Journal of Environmental Analytical Chemistry, 2017, 97(7): 657-672. |
| 8 | LI Guangzhu, LIU Miao, ZHANG Zhuqing, et al. Extraction of methylmercury and ethylmercury from aqueous solution using surface sulfhydryl-functionalized magnetic mesoporous silica nanoparticles[J]. Journal of Colloid and Interface Science, 2014, 424: 124-131. |
| 9 | 万琪, 李旭春, 潘丙才. 乙醇处理对树脂基纳米水合氧化铁结构及其除砷性能的影响[J]. 环境科学, 2013, 34(8): 3151-3155. |
| WAN Qi,LI Xuchun,PAN Bingcai. Ethanol-induced influence on the structure and arsenate adsorption of resin- based nano-hydrated ferric oxide[J]. Environmental Science, 2013, 34(8): 3151-3155. | |
| 10 | 王重庆, 王晖, 江小燕, 等. 生物炭吸附重金属离子的研究进展[J]. 化工进展, 2019, 38(1): 692-706. |
| WANG Chongqing, WANG Hui, JIANG Xiaoyan, et al. Research advances on adsorption of heavy metals by biochar[J]. Chemical Industry and Engineering Progress, 2019, 38(1): 692-706. | |
| 11 | TITIRICI Maria-Magdalena, WHITE Robin J, FALCO Camillo, et al. Black perspectives for a green future: hydrothermal carbons for environment protection and energy storage[J]. Energy & Environmental Science, 2012, 5: 6796-6822. |
| 12 | HUGGINS Tyler M, HAEGER Alexander, BIFFINGER Justin C, et al. Granular biochar compared with activated carbon for wastewater treatment and resource recovery[J]. Water Research, 2016, 94: 225-232. |
| 13 | 王哲, 骆逸飞, 郑春丽, 等. 淋溶条件下生物炭对矿区土壤中重金属迁移的影响[J]. 化工进展, 2020, 39(2): 738-746. |
| WANG Zhe, LUO Yifei, ZHENG Chunli, et al. Effect of biochar on migration of heavy metals in mining soil under leaching conditions[J]. Chemical Industry and Engineering Progress, 2020, 39(2): 738-746. | |
| 14 | QIAN Kezhen, KUMAR Ajay, ZHANG Hailin, et al. Recent advances in utilization of biochar[J]. Renewable and Sustainable Energy Reviews, 2015, 42: 1055-1064. |
| 15 | Honghong LYU, TANG Jingchun, CUI Mengke, et al. Biochar/iron (BC/Fe) composites for soil and groundwater remediation: synthesis, applications, and mechanisms[J]. Chemosphere, 2020, 246: 125609. |
| 16 | PREMARATHNAA K.S.D., RAJAPAKSHAA Anushka Upamali, SARKARB Binoy,et al. Biochar-based engineered composites for sorptive decontamination of water: a review[J]. Chemical Engineering Journal, 2019, 372: 536-550. |
| 17 | Kumar ⅥKRANT, KIMB Ki-Hyun, Yong Sik OK, et al. Engineered/designer biochar for the removal of phosphate in water and wastewater[J]. Science of the Total Environment, 2018, 616/617: 1242-1260. |
| 18 | 王靖宜, 王丽, 张文龙, 等. 生物炭基复合材料制备及其对水体特征污染物的吸附性能[J]. 化工进展, 2019, 38(8): 3838-3851. |
| WANG Jingyi, WANG Li, ZHANG Wenlong, et al. Preparation of biochar-based composites and their adsorption performances for characteristic contaminants in wastewater[J]. Chemical Industry and Engineering Progress, 2019, 38(8): 3838-3851. | |
| 19 | SHEN Yafei. Carbothermal synthesis of metal-functionalized nanostructures for energy and environmental applications[J]. Journal of Materials Chemistry A, 2015, 3: 13114-13188. |
| 20 | ZHANG Ming, GAO Bin, VARNOOSFADERANI Sima, et al. Preparation and characterization of a novel magnetic biochar for arsenic removal[J]. Bioresource Technology, 2013, 130: 457-462. |
| 21 | YUAN Shijie, DAI Xiaohu. Facile synthesis of sewage sludge-derived mesoporous material as anefficient and stable heterogeneous catalyst for photo-Fenton reaction[J]. Applied Catalysis B: Environmental, 2014, 154/155: 252-258. |
| 22 | WANG Shengsen, GAO Bin, ZIMMERMAN Andrew R, et al. Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite [J]. Bioresource Technology, 2015, 175: 391-395. |
| 23 | SINGH Vikash, SRIVASTAVA Vimal Chandra. Self-engineered iron oxide nanoparticle incorporated on mesoporous biochar derived from textile mill sludge for the removal of an emerging pharmaceutical pollutant[J]. Environmental Pollution, 2020, 259: 113822-113830. |
| 24 | LIU Yonglin, LI Yongtao, HUANG Jianfei, et al. An advanced sol-gel strategy for enhancing interfacial reactivity of iron oxide nanoparticles on rosin biochar substrate to remove Cr(Ⅵ)[J]. Science of the Total Environment, 2019, 690: 438-446. |
| 25 | LIU Wujun, JIANG Hong, YU Hanqing. Development of biochar-based functional materials: toward a sustainable platform carbon material[J]. Chemical Reviews, 2015, 115: 12251-12285. |
| 26 | CHEN Zaiming, XIAO Xin, CHEN Baoliang, et al. Quantification of chemical states, dissociation constants and contents of oxygen-containing groups on the surface of biochars produced at different temperatures[J]. Environmental Science & Technology, 2015, 49: 309-317. |
| 27 | LIAN Fei, XING Baoshan. Black carbon (biochar) in water/soil environments: molecular structure, sorption, stability, and potential risk[J]. Environmental Science & Technology, 2017, 51: 13517-13532. |
| 28 | HOCH Laurab, MACK Elizabeth J, HYDUTSKY Bianca W, et al. Carbothermal synthesis of carbon-supported nanoscale zero-valent iron particles for the remediation of hexavalent chromium[J]. Environmental Science & Technology, 2008, 42, 2600-2605. |
| 29 | 朱世殊. 改性芦苇生物炭对氧化还原反应去除水中污染物的强化机制[D]. 哈尔滨: 哈尔滨工业大学, 2019. |
| ZHU Shishu. The mechanisms of modified reed biochar-mediated redox reactions for water ecological remediation[D]. Harbin: Harbin Institute of Technology, 2019. | |
| 30 | HE Ruozhu, PENG Zhongya, Honghong LYU, et al. Synthesis and characterization of an iron-impregnated biochar for aqueous arsenic removal[J]. Science of the Total Environment, 2018, 612: 1177-1186. |
| 31 | YI Yunqiang, Guoquan TUA, TSANGC Pokeung Eric, et al. Insight into the influence of pyrolysis temperature on Fenton-like catalytic performance of magnetic biochar[J]. Chemical Engineering Journal, 2020, 380: 122518-122528. |
| 32 | CHEN Baoliang, CHEN Zaiming, Shaofang LYU. A novel magnetic biochar efficiently sorbs organic pollutants and phosphate[J]. Bioresource Technology, 2011, 102: 716-723. |
| 33 | HAN Zhantao, SANI Badruddeen, MROZIK Wojciech, et al. Magnetite impregnation effects on the sorbent properties of activated carbons and biochars[J]. Water Research, 2015, 70: 394-403. |
| 34 | REGUYALA Febelyn, SARMAHA Ajit K, GAO Wei. Synthesis of magnetic biochar from pine sawdust via oxidative hydrolysis of FeCl2 for the removal sulfamethoxazole from aqueous solution[J]. Journal of Hazardous Materials, 2017, 321: 868-878. |
| 35 | ZHANG Ping, David O’CONNOR, WANG Yinan, et al. A green biochar/iron oxide composite for methylene blue removal[J]. Journal of Hazardous Materials, 2020, 384: 121286. |
| 36 | HALE S E,ALLING V,MARTINSEN V, et al. The sorption and desorption of phosphate-P, ammonium-N and nitrate-N in cacao shell and corn cob biochars[J]. Chemosphere, 2013, 91(11): 1612-1619. |
| 37 | OLIVEIRA Luiz C A, RIOS Rachel V R A, FABRIS Jose D, et al. Activated carbon/iron oxide magnetic composites for the adsorption of contaminants in water[J]. Carbon, 2002, 40: 2177-2183. |
| 38 | WANG He, WANG Han, ZHAO Hui, et al. Adsorption and Fenton-like removal of chelated nickel from Zn-Ni alloy electroplating wastewater using activated biochar composite derived from Taihu blue algae[J]. Chemical Engineering Journal, 2020, 379: 122372-122383. |
| 39 | KUMAR Prashanth Suresh, PROT Thomas, KORVING Leon, et al. Effect of pore size distribution on iron oxide coated granular activated carbons for phosphate adsorption-Importance of mesopores[J]. Chemical Engineering Journal, 2017, 326: 231-239. |
| 40 | VENKATESWARLU Sada, Daeho LEE, YOON Minyoung. Bio-inspired 2D-carbon flakes and Fe3O4 nanoparticles composite for arsenite removal[J]. ACS Applied Materials & Interfaces, 2016, 36(8): 23876-23885. |
| 41 | WEI Yuanfeng, WEI Shudan, LIU Chengbin, et al. Effificient removal of arsenic from groundwater using iron oxide nanoneedle array-decorated biochar fibers with high Fe utilization and fast adsorption kinetics[J]. Water Research, 2019, 167: 115107-115116. |
| 42 | ZUO Xiaojun, CHEN Mindong, FU Dafang, et al. The formation of alpha-FeOOH onto hydrothermal biochar through H2O2 and its photocatalytic disinfection[J]. Chemical Engineering Journal, 2016, 294: 202-209. |
| 43 | RONG Xing, XIE Meng, KONG Lingshuai, et al. The magnetic biochar derived from banana peels as a persulfate activator for organic contaminants degradation[J]. Chemical Engineering Journal, 2019, 372: 294-303. |
| 44 | 李琪瑞, 许晨阳, 耿增超, 等. 纳米生物炭的制备方法比较及其特性研究[J].中国环境科学, 2020, 40(7): 3124-3134. |
| LI Qirui, XU Chenyang, GENG Zengchao, et al. Preparation methods and properties of nanobiochars[J]. China Environmental Science, 2020, 40(7): 3124-3134. | |
| 45 | SHAN Danna, DENG Shubo, ZHAO Tianning, et al. Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling[J]. Journal of Hazardous Materials, 2016, 305: 156-163. |
| 46 | Honghong LYU, GAO Bin, HE Feng, et al. Ball-milled carbon nanomaterials for energy and environmental applications[J]. ACS Sustainable Chemistry Engineering, 2017, 5: 9568-9585. |
| 47 | Honghong LYU, GAO Bin, HE Feng, et al. Experimental and modeling investigations of ball-milled biochar for the removal of aqueous methylene blue[J]. Chemical Engineering Journal, 2018, 335: 110-119. |
| 48 | WILFERT Philipp, KUMAR Prashanth Suresh, KORVING Leon, et al. The relevance of phosphorus and iron chemistry to the recovery of phosphorus from wastewater: a review[J]. Environmental Science & Technology, 2015, 49: 9400-9414. |
| 49 | LI Ronghua, WANG Jim J, ZHOU Baoyue, et al. Recovery of phosphate from aqueous solution by magnesium oxide decorated magnetic biochar and its potential as phosphate-based fertilizer substitute[J]. Bioresource Technology, 2016, 215: 209-214. |
| 50 | TAN Xiaofei, LIU Yunguo, ZENG Guangming, et al. Application of biochar for the removal of pollutants from aqueous solutions[J]. Chemosphere, 2015, 125: 70-85. |
| 51 | DAI Yingjie, WANG Wensi, LU Lu, et al. Utilization of biochar for the removal of nitrogen and phosphorus[J]. Journal of Cleaner Production, 2020, 257: 120573. |
| 52 | REN Jing, LI Nan, LI Lei, et al. Granulation and ferric oxides loading enable biochar derived from cotton stalk to remove phosphate from water[J]. Bioresource Technology, 2015, 178: 119-125. |
| 53 | LI Jihui, Guohua LYU, BAI Wenbo, et al. Modification and use of biochar from wheat straw (Triticum aestivum L.) for nitrate and phosphate removal from water[J]. Desalination and Water Treatment, 2014, 57(10): 1-13. |
| 54 | 唐登勇, 黄越, 胥瑞晨, 等. 改性芦苇生物炭对水中低浓度磷的吸附特征[J]. 环境科学, 2016, 37(6): 2195-2201. |
| TANG Dengyong, HUANG Yue, XU Ruichen, et al. Adsorption behavior of low concentration phosphorus from water onto modified reed biochar[J]. Environmental Science, 2016, 37(6): 2195-2201. | |
| 55 | Barbora MICHALEKOVA-RICHVEISOVA, FRISTAK Vladimír, PIPISKA Martin, et al. Iron-impregnated biochars as effective phosphate sorption materials[J]. Environmental Science and Pollution Research, 2016, 24(1): 463-475. |
| 56 | CAI Ru, WANG Xin, JI Xionghui, et al. Phosphate reclaim from simulated and real eutrophic water by magnetic biochar derived from water hyacinth[J]. Journal of Environmental Management, 2017, 187: 212-219. |
| 57 | YANG Qi, WANG Xiaolin, LUO Wei, et al. Effectiveness and mechanisms of phosphate adsorption on iron-modified biochars derived from waste activated sludge[J]. Bioresource Technology, 2018, 247: 537-544. |
| 58 | ZHU Zongqiang, HUANG C P, ZHU Yinian, et al. A hierarchical porous adsorbent of nano-α-Fe2O3/Fe3O4 on bamboo biochar (HPA-Fe/C-B) for the removal of phosphate from water[J]. Journal of Water Process Engineering, 2018, 25: 96-104. |
| 59 | JACK Joshua, HUGGINS Tyler M, HUANG Yingping, et al. Production of magnetic biochar from waste-derived fungal biomass for phosphorus removal and recovery[J]. Journal of Cleaner Production, 2019, 224: 100-106. |
| 60 | PIERCE Matthew L, MOORE Carleton B. Adsorption of arsenite and arsenate on amorphous iron hydroxide[J]. Water Research, 1982, 16: 1247-1253. |
| 61 | 胡小莲, 杨林章, 何世颖, 等. Fe3O4/BC复合材料的制备及其吸附除磷性能[J]. 环境科学研究, 2018, 31(1): 143-153. |
| HU Xiaolian, YANG Linzhang, HE Shiying, et al. Preparation of Fe3O4/BC composite and its application for phosphate adsorptive removal[J]. Research of Environmental Sciences, 2018, 31(1): 143-153. | |
| 62 | TAWFIK Dan S, VIOLA Ronald E. Arsenate replacing phosphate-alternative life chemistries and ion promiscuity[J]. Biochemistry, 2011, 50: 1128-1134. |
| 63 | YAO Ying, GAO Bin, INYANG Mandu, et al. Biochar derived from anaerobically digested sugar beet tailings: characterization and phosphate removal potential[J]. Bioresource Technology, 2011, 102: 6273-6278. |
| 64 | JUNG, Kyung-Won, AHN, Kyu-Hong. Fabrication of porosity-enhanced MgO/biochar for removal of phosphate from aqueous solution: application of a novel combined electrochemical modification method[J]. Bioresource Technology, 2016, 200: 1029-1032. |
| 65 | 蔡茹. 负载铁生物炭对富营养化水体中磷的捕集与再利用[D]. 长沙: 湖南师范大学, 2017. |
| CAI Ru. Capture and reuse of phosphorus in eutrophic water by iron—impregnated biochar[D]. Changsha: Hunan Normal University, 2017. | |
| 66 | ZHANG Hanzhi, CHEN Chengrong, GRAY Evan M, et al. Roles of biochar in improving phosphorus availability in soils: a phosphate adsorbent and a source of available phosphorus[J]. Geoderma, 2016, 276: 1-6. |
| 67 | WAN Stefan, WANG Shengsen, LI Yuncong, et al. Functionalizing biochar with Mg-Al and Mg-Fe layered double hydroxides for removal of phosphate from aqueous solutions[J]. Journal of Industrial and Engineering Chemistry, 2017, 47: 246-253. |
| 68 | NATH B K, CHALIHA C, KALITA E. Iron oxide permeated mesoporous rice-husk nanobiochar (IPMN) mediated removal of dissolved arsenic (As): chemometric modelling and adsorption dynamics[J]. Journal of Environmental Management, 2019, 246: 397-409. |
| 69 | HAN Yitong, CAO Xi, OUYANG Xin, et al. Adsorption kinetics of magnetic biochar derived from peanut hull on removal of Cr(Ⅵ) from aqueous solution: effects of production conditions and particle size[J]. Chemosphere, 2016, 145: 336-341. |
| 70 |
YI Yunqiang, TU Guoquan, ZHAO Dongye, et al. Key role of FeO in the reduction of Cr( ) by magnetic biochar synthesised using steelpickling waste liquor and sugarcane bagasse[J]. Journal of Cleaner Production, 2020, 245: 118886. ) by magnetic biochar synthesised using steelpickling waste liquor and sugarcane bagasse[J]. Journal of Cleaner Production, 2020, 245: 118886.
|
| 71 | LIU Liheng, LIU Xiu, WANG Dunqiu, et al. Removal and reduction of Cr(Ⅵ) in simulated wastewater using magnetic biochar prepared by co-pyrolysis of nano-zero-valent iron and sewage sludge[J]. Journal of Cleaner Production, 2020, 257: 120562. |
| 72 | YAPA M W, MUBARAKB N M, SAHU J N, et al. Microwave induced synthesis of magnetic biochar from agricultural biomass for removal of lead and cadmium from wastewater[J]. Journal of Industrial and Engineering Chemistry, 2017, 45: 287-295. |
| 73 | Eun-Bi SON, Kyung-Min POO, CHANG Jae-Soo, et al. Heavy metal removal from aqueous solutions using engineered magnetic biochars derived from waste marine macro-algal biomass[J]. Science of the Total Environment, 2018, 615: 161-168. |
| 74 | YANG Fan, ZHANG Shuaishuai, LI Huipeng, et al. Corn straw-derived biochar impregnated with α-FeOOH nanorods for highly effective copper removal[J]. Chemical Engineering Journal, 2018, 348: 191-201. |
| 75 | 袁健, 钱雅洁, 薛罡, 等. 活性污泥水热碳化法制备磁性炭及对水体Cd2+及Pb2+的去除[J]. 环境工程, 2020, 38(2): 55-62. |
| YUAN Jian, QIAN Yajie, XUE Gang, et al. Removal of cadmium and lead in water by magnetic carbon prepared from activated sludge with hydrothermal carbonization[J]. Environmental Engineering, 2020, 38(2): 55-62. | |
| 76 | INYANG Mandu I, GAO Bin, YAO Ying, et al. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal[J]. Critical Reviews in Environmental Science and Technology, 2016, 46: 406-433. |
| 77 | LI Hongbo, DONG Xiaoling, SILVA Evandro B DA, et al. Mechanisms of metal sorption by biochars: biochar characteristics and modifications[J]. Chemosphere, 2017, 178: 466-478. |
| 78 | YE Yuxuan, SHAN Chao, ZHANG Xiaolin, et al. Water decontamination from Cr(Ⅲ)-organic complexes based on pyrite/H2O2: performance, mechanism, and validation[J]. Environmental Science & Technology, 2018, 52: 10657-10664. |
| 79 | 赵云平. 布洛芬在铁氧化物改性生物炭上的吸附特征研究[D]. 北京: 中国地质大学(北京), 2018. |
| ZHAO Yunping. Sorption characteristics of ibuprofen to iron oxide modified biochars[D]. Beijing: China University of Geosciences (Beijing), 2018. | |
| 80 | REGUYALA Febelyn, SARMAHA Ajit K. Site energy distribution analysis and influence of Fe3O4 nanoparticles on sulfamethoxazole sorption in aqueous solution by magnetic pine sawdust biochar[J]. Environmental Pollution, 2018, 233: 510-519. |
| 81 | DONG Chengdi, CHEN Chiuwen, HUNG Changmao. Synthesis of magnetic biochar from bamboo biomass to activate persulfate for the removal of polycyclic aromatic hydrocarbons in marine sediments[J]. Bioresource Technology, 2017, 245: 188-195. |
| 82 | CHEN Yidi, BAI Shunwen, LI Ruixiang, et al. Magnetic biochar catalysts from anaerobic digested sludge: production, application and environment impact[J]. Environment International, 2019, 126: 302-308. |
| 83 | YUAN Shijie, LIAO Nianhua, DONG Bin, et al. Optimization of a digested sludge-derived mesoporous material as an efficient and stable heterogeneous catalyst for the photo-Fenton Reaction[J]. Chinese Journal of Catalysis, 2016, 37: 735-742. |
| 84 | PARK Jong-Hwan, WANG Jim J, XIAO Ran, et al. Degradation of Orange G by Fenton-like reaction with Fe-impregnated biochar catalyst[J]. Bioresource Technology, 2018, 249: 368-376. |
| 85 | ZHANG He, XUE Gang, CHEN Hong, et al. Magnetic biochar catalyst derived from biological sludge and ferric sludge using hydrothermal carbonization: preparation, characterization and its circulation in Fenton process for dyeing wastewater treatment[J]. Chemosphere, 2018, 191: 64-71. |
| 86 | Antoine TIYA-DJOWE, DOURGES Marie-Anne, BRUNEEL Jean-Luc, et al. Plasma-deposition of α-FeOOH particles on biochar using a gliding arc discharge in humid air: a green and sustainable route for producing oxidation catalysts[J]. RSC Advance, 2019, 9: 4797-4805. |
| 87 | PARK Jong-Hwan, WANG Jim J. TAFTI Negar,et al. Removal of Eriochrome Black T by sulfate radical generated from Fe-impregnated biochar/persulfate in Fenton-like reaction[J]. Journal of Industrial and Engineering Chemistry, 2019, 71: 201-209. |
| 88 | YIN Renli, GUO Wanqian, WANG Huazhe, et al. Singlet oxygen-dominated peroxydisulfate activation by sludge-derived biochar for sulfamethoxazole degradation through a nonradical oxidation pathway: performance and mechanism[J]. Chemical Engineering Journal, 2019, 357: 589-599. |
| 89 | WANG Shizong, WANG Jianlong. Activation of peroxymonosulfate by sludge-derived biochar for the degradation of triclosan in water and wastewater[J]. Chemical Engineering Journal, 2019, 356: 350-358. |
| 90 | DUAN Xiaoguang, SUN Hongqi, KANG Jian, et al. Insights into heterogeneous catalysis of persulfate activation on dimensional-structured nanocarbons[J]. ACS Catalysis, 2015, 5: 4629-4636. |
| 91 | JIANG Shunfeng, LING Lili, CHEN Wenjing, et al. High efficient removal of bisphenol A in a peroxymonosulfate/iron functionalized biochar system: mechanistic elucidation and quantification of the contributors[J]. Chemical Engineering Journal, 2019, 359: 572-583. |
| 92 | 肖鹏飞, 安璐, 韩爽. 炭质材料在活化过硫酸盐高级氧化技术中的应用进展[J]. 化工进展, 2020, 39(8): 3293-3307. |
| XIAO Pengfei, AN Lu, HAN Shuang. Novel progress on application of carbon materials in advanced oxidation technology of activated persulfate[J]. Chemical Industry and Engineering Progress, 2020, 39(8): 3293-3307. | |
| 93 |
WANG Huazhe, GUO Wanqian, YIN Renli, et al. Biochar-induced Fe( ) reduction for persulfate activation in sulfamethoxazole degradation: insight into the electron transfer, radical oxidation and degradation pathways[J]. Chemical Engineering Journal, 2019, 362: 561-569. ) reduction for persulfate activation in sulfamethoxazole degradation: insight into the electron transfer, radical oxidation and degradation pathways[J]. Chemical Engineering Journal, 2019, 362: 561-569.
|
| 94 | HUANG Baocheng, JIANG Jun, HUANG Guixiang, et al. Sludge biochar-based catalyst for improved pollutant degradation by activating peroxymonosulfate[J]. Journal of Materials Chemistry A, 2018, 6: 8978-8985. |
| 95 | 陈丹丹, 窦昱昊, 卢平. 等. 污泥深度脱水技术研究进展[J]. 化工进展, 2019, 38(10): 4722-4746. |
| CHEN Dandan, DOU Yuhao, LU Ping, et al. A review on sludge deep dewatering technology[J]. Chemical Industry and Engineering Progress, 2019, 38(10): 4722-4746. | |
| 96 | WU Yan, ZHANG Panyue, ZENG Guangming, et al. Enhancing sewage sludge dewaterability by a skeleton builder: biochar produced from sludge cake conditioned with rice husk flour and FeCl3[J]. ACS Sustainable Chemistry & Engineering, 2016, 4(10): 5711-5717. |
| 97 | WU Yan, ZHANG Panyue, ZHANG Haibo, et al. Possibility of sludge conditioning and dewatering with rice husk biochar modified by ferric chloride[J]. Bioresource Technology, 2016, 205: 258-263. |
| 98 | HE Dongqin, WANG Longfei, JIANG Hong, et al. A Fenton-like process for the enhanced activated sludge dewatering[J]. Chemical Engineering Journal, 2015, 272: 128-134. |
| 99 | TAO Shuangyi, YANG Jiakuan, HUO Huijie, et al. Enhanced sludge dewatering via homogeneous and heterogeneous Fenton reactions initiated by Fe-rich biochar derived from sludge[J]. Chemical Engineering Journal, 2019, 372: 966-977. |
| 100 | RUALES-LONFAT C, BARONA J F, SIENKIEWICZ A, et al. Iron oxides semiconductors are efficients for solar water disinfection: a comparison with photo-Fenton processes at neutral pH[J]. Applied Catalysis B: Environmental, 2015, 166/167: 497-508. |
| [1] | WANG Shengyan, DENG Shuai, ZHAO Ruikai. Research progress on carbon dioxide capture technology based on electric swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 233-245. |
| [2] | DAI Huantao, CAO Lingyu, YOU Xinxiu, XU Haoliang, WANG Tao, XIANG Wei, ZHANG Xueyang. Adsorption properties of CO2 on pomelo peel biochar impregnated by lignin [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 356-363. |
| [3] | CUI Shoucheng, XU Hongbo, PENG Nan. Simulation analysis of two MOFs materials for O2/He adsorption separation [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 382-390. |
| [4] | CHEN Chongming, CHEN Qiu, GONG Yunqian, CHE Kai, YU Jinxing, SUN Nannan. Research progresses on zeolite-based CO2 adsorbents [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 411-419. |
| [5] | WANG Ying, HAN Yunping, LI Lin, LI Yanbo, LI Huili, YAN Changren, LI Caixia. Research status and future prospects of the emission characteristics of virus aerosols in urban wastewater treatment plants [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 439-446. |
| [6] | XU Chunshu, YAO Qingda, LIANG Yongxian, ZHOU Hualong. Research progress on functionalization strategies of covalent organic frame materials and its adsorption properties for Hg(Ⅱ) and Cr(Ⅵ) [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 461-478. |
| [7] | GU Yongzheng, ZHANG Yongsheng. Dynamic behavior and kinetic model of Hg0 adsorption by HBr-modified fly ash [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 498-509. |
| [8] | GUO Qiang, ZHAO Wenkai, XIAO Yonghou. Numerical simulation of enhancing fluid perturbation to improve separation of dimethyl sulfide/nitrogen via pressure swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 64-72. |
| [9] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [10] | YANG Ying, HOU Haojie, HUANG Rui, CUI Yu, WANG Bing, LIU Jian, BAO Weiren, CHANG Liping, WANG Jiancheng, HAN Lina. Coal tar phenol-based carbon nanosphere prepared by Stöber method for adsorption of CO2 [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5011-5018. |
| [11] | WANG Haoran, YIN Quanyu, FANG Ming, HOU Jianlin, LI Jun, HE Bin, ZHANG Mingyue. Optimization of near critical-water treatment process of tobacco stems [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5019-5027. |
| [12] | ZHANG Zhen, LI Dan, CHEN Chen, WU Jinglan, YING Hanjie, QIAO Hao. Separation and purification of salivary acids with adsorption resin [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4153-4158. |
| [13] | JIANG Jing, CHEN Xiaoyu, ZHANG Ruiyan, SHENG Guangyao. Research progress of manganese-loaded biochar preparation and its application in environmental remediation [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4385-4397. |
| [14] | YU Jingwen, SONG Luna, LIU Yanchao, LYU Ruidong, WU Mengmeng, FENG Yu, LI Zhong, MI Jie. An indole-bearing hypercrosslinked polymer In-HCP for iodine adsorption from water [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3674-3683. |
| [15] | LI Yanling, ZHUO Zhen, CHI Liang, CHEN Xi, SUN Tanglei, LIU Peng, LEI Tingzhou. Research progress on preparation and application of nitrogen-doped biochar [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3720-3735. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||