Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (10): 5689-5696.DOI: 10.16085/j.issn.1000-6613.2024-1319
• Energy processes and technology • Previous Articles
Hydrogen storage and release performances of N-heterocyclic hydrogen storage carriers
LI Peiya1( ), YANG Fusheng1,2, WANG Bin1,2(
), YANG Fusheng1,2, WANG Bin1,2( ), FANG Tao1,2(
), FANG Tao1,2( )
)
- 1.School of Chemical Engineering, Xi’an Jiaotong University, Shaanxi Key Laboratory of Energy Chemical Process Intensification, Engineering Research Center of New Energy System Engineering and Equipment, Xi’an 710049, Shaanxi, China
2.Hydrotransformer Energy Technology Co. , Ltd. , Xi’an 712000, Shaanxi, China
-
Received:2024-08-12Revised:2024-10-12Online:2025-11-10Published:2025-10-25 -
Contact:WANG Bin, FANG Tao
N-杂环储氢载体的储放氢性能
李培雅1( ), 杨福胜1,2, 王斌1,2(
), 杨福胜1,2, 王斌1,2( ), 方涛1,2(
), 方涛1,2( )
)
- 1.西安交通大学化工学院,陕西能源化工过程强化重点实验室,新能源系统与装备工程研究中心,陕西 西安 710049
2.陕西氢易能源科技有限公司,陕西 西安 712000
-
通讯作者:王斌,方涛 -
作者简介:李培雅(1998—),女,博士研究生,研究方向为有机液态储氢。E-mail:18236902757@stu.xjtu.edu.cn。 -
基金资助:国家自然科学基金(22005236);中国博士后科研项目(22020T130508);陕西省基础科学研究重点项目(2022JZ-07);陕西省“两链融合”重点研发专项(2022QCY-LL-16)
CLC Number:
Cite this article
LI Peiya, YANG Fusheng, WANG Bin, FANG Tao. Hydrogen storage and release performances of N-heterocyclic hydrogen storage carriers[J]. Chemical Industry and Engineering Progress, 2025, 44(10): 5689-5696.
李培雅, 杨福胜, 王斌, 方涛. N-杂环储氢载体的储放氢性能[J]. 化工进展, 2025, 44(10): 5689-5696.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-1319
| 储氢体系 | 熔点/℃ | 沸点/℃ | 加氢温度/℃ | 脱氢温度/℃ | 储氢量(质量分数)/% | 反应焓/kJ·mol-1 | 价格/CNY·kg-1 |
|---|---|---|---|---|---|---|---|
| 苯/环己烷 | 6/7 | 80/81 | 约150 | >300 | 7.13 | -68.8 | 约6 |
| 甲苯/甲基环己烷 | -95/-126 | 111/101 | 约150 | 300~350 | 6.12 | -68.3 | 约8 |
| 二苄基甲苯/全氢二苄基甲苯 | -39/-45 | 390/354 | 约150 | 260~310 | 6.21 | -65.4 | 约40 |
| 苄基甲苯/全氢苄基甲苯 | -30/<-30 | 280/270 | 约150 | 250~270 | 6.18 | -63.5 | 约30 |
| 萘/十氢萘 | 80/-30 | 218/187 | 约200 | 320~340 | 7.25 | -66.3 | 约8 |
| 储氢体系 | 熔点/℃ | 沸点/℃ | 加氢温度/℃ | 脱氢温度/℃ | 储氢量(质量分数)/% | 反应焓/kJ·mol-1 | 价格/CNY·kg-1 |
|---|---|---|---|---|---|---|---|
| 苯/环己烷 | 6/7 | 80/81 | 约150 | >300 | 7.13 | -68.8 | 约6 |
| 甲苯/甲基环己烷 | -95/-126 | 111/101 | 约150 | 300~350 | 6.12 | -68.3 | 约8 |
| 二苄基甲苯/全氢二苄基甲苯 | -39/-45 | 390/354 | 约150 | 260~310 | 6.21 | -65.4 | 约40 |
| 苄基甲苯/全氢苄基甲苯 | -30/<-30 | 280/270 | 约150 | 250~270 | 6.18 | -63.5 | 约30 |
| 萘/十氢萘 | 80/-30 | 218/187 | 约200 | 320~340 | 7.25 | -66.3 | 约8 |
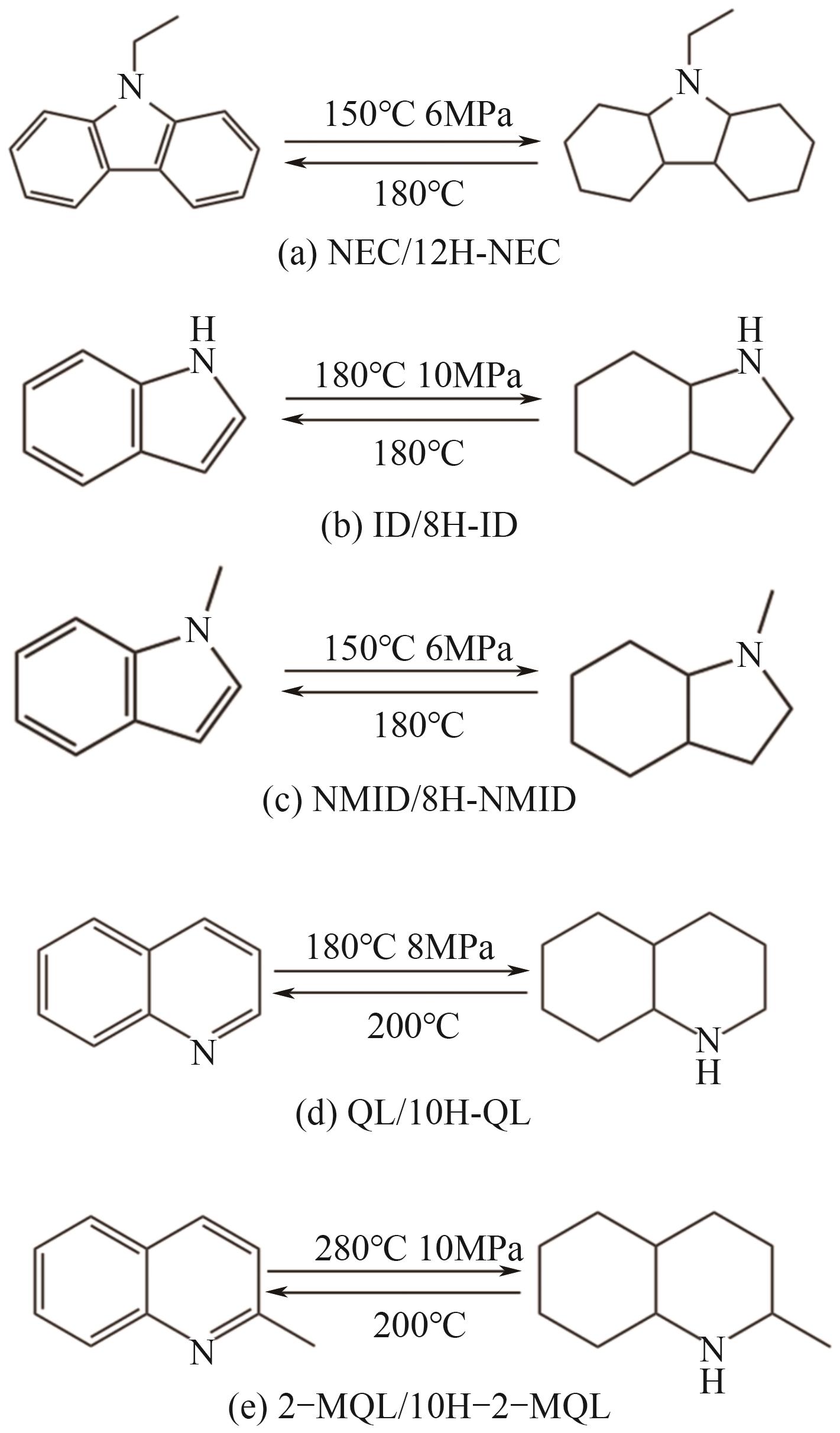
| 储氢体系 | 熔点/℃ | 沸点/℃ | 加氢反应条件 | 脱氢温度/℃ | 储氢量(质量分数)/% | 反应焓/kJ·mol-1 | 价格/CNY·kg-1 |
|---|---|---|---|---|---|---|---|
| 咔唑/12H-咔唑 | 248/58 | 352/267 | 150~180℃,6~8MPa | 170~200 | 6.70 | -52.57 | 约80 |
| N-乙基咔唑/12H-N-乙基咔唑 | 68/-13 | 270/245 | 150~180℃,6~8MPa | 140~180 | 5.79 | -50.5 | 约200 |
| 吲哚/8H-吲哚 | 54/<0 | 254/186 | 180~200℃,8~10MPa | 约180 | 6.39 | -58.48 | 约80 |
| N-甲基吲哚/8H-N-甲基吲哚 | 4/-20 | 238/185 | 130~150℃,6~8MPa | 150~180 | 5.76 | -53.5 | 约500 |
| 喹啉/10H-喹啉 | -16/45 | 237/200 | 150~180℃,6~8MPa | >200 | 7.20 | -61.5 | 约90 |
| 2-甲基喹啉/10H-2-甲基喹啉 | -2/35 | 248/225 | 250~300℃,8~10MPa | 约200 | 6.53 | -59.5 | 约300 |
| 吩嗪/14H-吩嗪 | 175/97 | 360/305 | 250~300℃,8~12MPa | 约200 | 7.20 | -61.3 | 约800 |
| 储氢体系 | 熔点/℃ | 沸点/℃ | 加氢反应条件 | 脱氢温度/℃ | 储氢量(质量分数)/% | 反应焓/kJ·mol-1 | 价格/CNY·kg-1 |
|---|---|---|---|---|---|---|---|
| 咔唑/12H-咔唑 | 248/58 | 352/267 | 150~180℃,6~8MPa | 170~200 | 6.70 | -52.57 | 约80 |
| N-乙基咔唑/12H-N-乙基咔唑 | 68/-13 | 270/245 | 150~180℃,6~8MPa | 140~180 | 5.79 | -50.5 | 约200 |
| 吲哚/8H-吲哚 | 54/<0 | 254/186 | 180~200℃,8~10MPa | 约180 | 6.39 | -58.48 | 约80 |
| N-甲基吲哚/8H-N-甲基吲哚 | 4/-20 | 238/185 | 130~150℃,6~8MPa | 150~180 | 5.76 | -53.5 | 约500 |
| 喹啉/10H-喹啉 | -16/45 | 237/200 | 150~180℃,6~8MPa | >200 | 7.20 | -61.5 | 约90 |
| 2-甲基喹啉/10H-2-甲基喹啉 | -2/35 | 248/225 | 250~300℃,8~10MPa | 约200 | 6.53 | -59.5 | 约300 |
| 吩嗪/14H-吩嗪 | 175/97 | 360/305 | 250~300℃,8~12MPa | 约200 | 7.20 | -61.3 | 约800 |
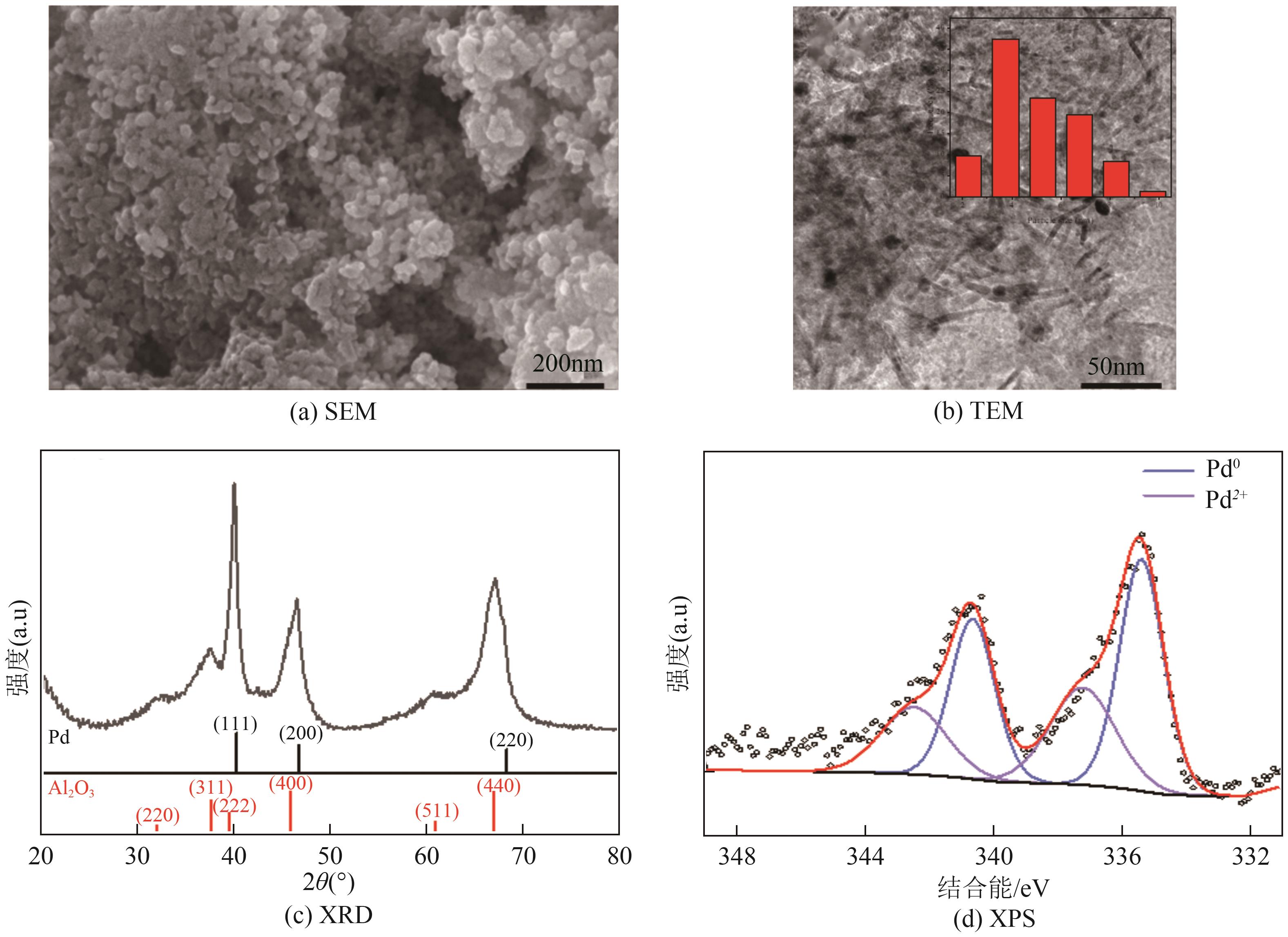
| 催化剂 | 金属负载量(质量分数)/% | 比表面积/m2·g-1 | 孔径/nm | 孔容/cm3·g-1 | 平均颗粒直径/μm | 活性金属平均粒径/nm |
|---|---|---|---|---|---|---|
| Ru/Al2O3 | 5.00 | 156.88 | 9.17 | 0.36 | 70 | — |
| Pd/Al2O3 | 5.00 | 155.08 | 8.72 | 0.33 | 28~34 | 4.95 |
| 催化剂 | 金属负载量(质量分数)/% | 比表面积/m2·g-1 | 孔径/nm | 孔容/cm3·g-1 | 平均颗粒直径/μm | 活性金属平均粒径/nm |
|---|---|---|---|---|---|---|
| Ru/Al2O3 | 5.00 | 156.88 | 9.17 | 0.36 | 70 | — |
| Pd/Al2O3 | 5.00 | 155.08 | 8.72 | 0.33 | 28~34 | 4.95 |
| 储氢体系 | 加氢温度/℃ | 加氢压力/MPa | 反应时间/d | 全氢产物收率/% |
|---|---|---|---|---|
| N-乙基咔唑/12H-N-乙基咔唑 | 150 | 6 | 约12 | >98 |
| 吲哚/8H-吲哚 | 180 | 10 | 约15 | >99 |
| N-甲基吲哚/8H-N-甲基吲哚 | 150 | 6 | 约10 | >98 |
| 喹啉/10H-喹啉 | 180 | 8 | 约10 | >99 |
| 2-甲基喹啉/10H-2-甲基喹啉 | 280 | 10 | >20 | >97 |
| 储氢体系 | 加氢温度/℃ | 加氢压力/MPa | 反应时间/d | 全氢产物收率/% |
|---|---|---|---|---|
| N-乙基咔唑/12H-N-乙基咔唑 | 150 | 6 | 约12 | >98 |
| 吲哚/8H-吲哚 | 180 | 10 | 约15 | >99 |
| N-甲基吲哚/8H-N-甲基吲哚 | 150 | 6 | 约10 | >98 |
| 喹啉/10H-喹啉 | 180 | 8 | 约10 | >99 |
| 2-甲基喹啉/10H-2-甲基喹啉 | 280 | 10 | >20 | >97 |
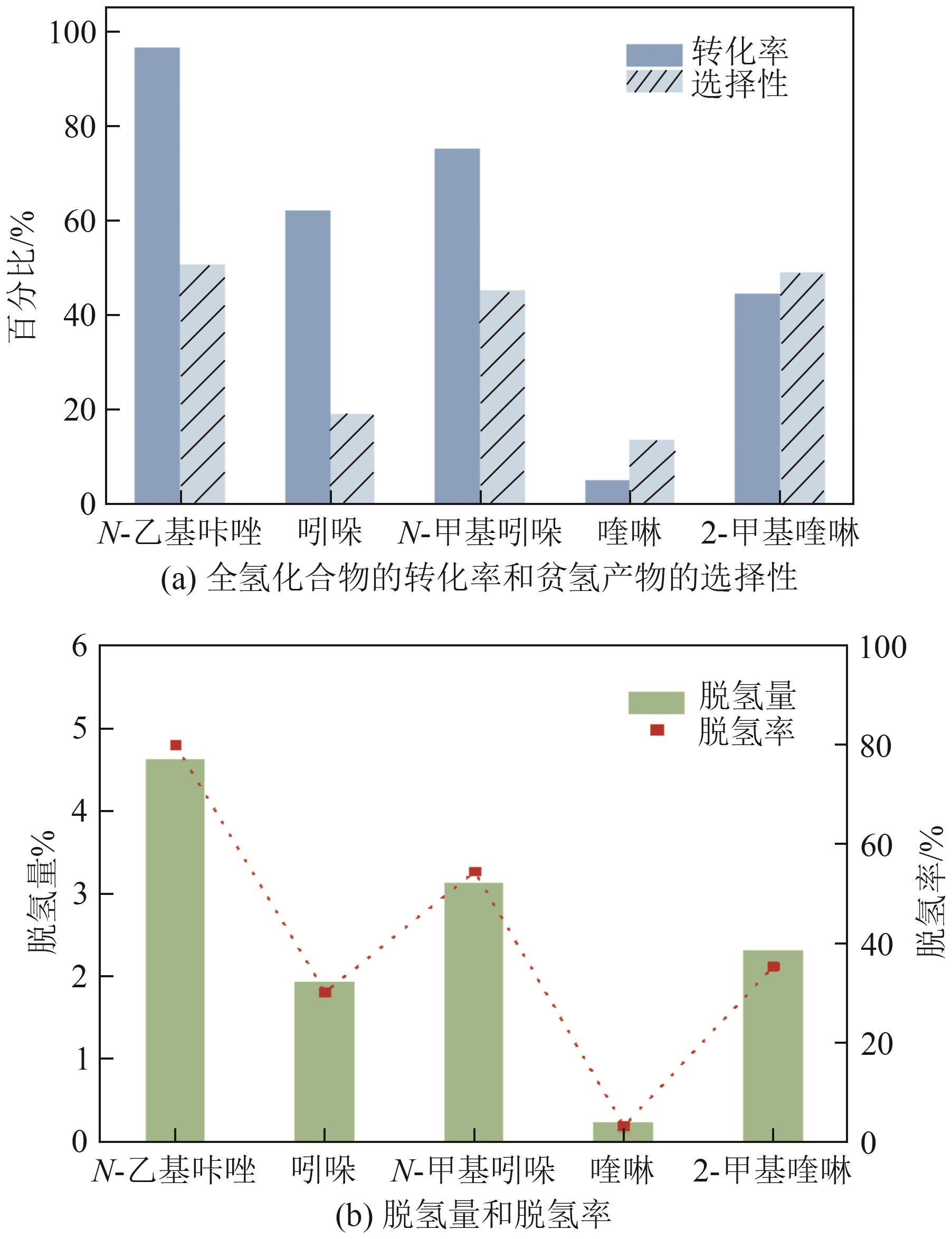
| 储氢体系 | 脱氢量(质量分数)/% | 全氢化合物的转化率/% | 脱氢率/% | 贫氢产物的选择性/% |
|---|---|---|---|---|
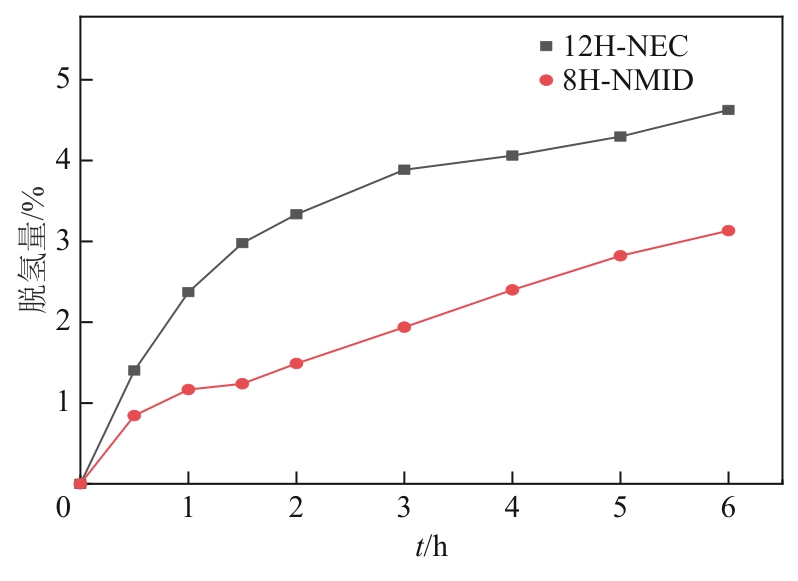
| N-乙基咔唑/12H-N-乙基咔唑 | 4.62 | 96.54 | 79.88 | 50.58 |
| 吲哚/8H-吲哚 | 1.93 | 61.98 | 30.15 | 18.94 |
| N-甲基吲哚/8H-N-甲基吲哚 | 3.13 | 75.11 | 54.47 | 45.05 |
| 喹啉/10H-喹啉 | 0.23 | 4.92 | 3.22 | 13.44 |
| 2-甲基喹啉/10H-2-甲基喹啉 | 2.31 | 44.42 | 35.35 | 48.96 |
| 储氢体系 | 脱氢量(质量分数)/% | 全氢化合物的转化率/% | 脱氢率/% | 贫氢产物的选择性/% |
|---|---|---|---|---|
| N-乙基咔唑/12H-N-乙基咔唑 | 4.62 | 96.54 | 79.88 | 50.58 |
| 吲哚/8H-吲哚 | 1.93 | 61.98 | 30.15 | 18.94 |
| N-甲基吲哚/8H-N-甲基吲哚 | 3.13 | 75.11 | 54.47 | 45.05 |
| 喹啉/10H-喹啉 | 0.23 | 4.92 | 3.22 | 13.44 |
| 2-甲基喹啉/10H-2-甲基喹啉 | 2.31 | 44.42 | 35.35 | 48.96 |
| [1] | PREUSTER Patrick, PAPP Christian, WASSERSCHEID Peter. Liquid organic hydrogen carriers (LOHCs): Toward a hydrogen-free hydrogen economy[J]. Accounts of Chemical Research, 2017, 50(1): 74-85. |
| [2] | MODISHA Phillimon M, OUMA Cecil N M, GARIDZIRAI Rudaviro, et al. The prospect of hydrogen storage using liquid organic hydrogen carriers[J]. Energy & Fuels, 2019, 33(4): 2778-2796. |
| [3] | MARKIEWICZ M, ZHANG Y Q, BÖSMANN A, et al. Environmental and health impact assessment of liquid organic hydrogen carrier (LOHC) systems-challenges and preliminary results[J]. Energy & Environmental Science, 2015, 8(3): 1035-1045. |
| [4] | NIERMANN Matthias, BECKENDORFF Alexander, KALTSCHMITT Martin, et al. Liquid organic hydrogen carrier (LOHC)-Assessment based on chemical and economic properties[J]. International Journal of Hydrogen Energy, 2019, 44(13): 6631-6654. |
| [5] | RAO Purna, YOON Minyoung. Potential liquid-organic hydrogen carrier (LOHC) systems: A review on recent progress[J]. Energies, 2020, 13(22): 6040. |
| [6] | 周一鸣, 齐随涛, 周宇亮, 等. 多环芳烃类液体有机氢载体储放氢技术研究进展[J]. 化工进展, 2023, 42(2): 1000-1007. |
| ZHOU Yiming, QI Suitao, ZHOU Yuliang, et al. Research progress in the hydrogenation and dehydrogenation technology of polycyclic aromatic hydrocarbon liquid organic hydrogen carriers[J]. Chemical Industry and Engineering Progress, 2023, 42(2): 1000-1007. | |
| [7] | 李佳豪, 杨锦, 潘伦, 等. 含氮有机液体储放氢催化体系研究进展[J]. 化工进展, 2023, 42(12): 6325-6344. |
| LI Jiahao, YANG Jin, PAN Lun, et al. Research progress in catalytic system for hydrogen storage and release from nitrogen-containing liquid organic carriers[J]. Chemical Industry and Engineering Progress, 2023, 42(12): 6325-6344. | |
| [8] | HU Peng, FOGLER Eran, Yael DISKIN-POSNER, et al. A novel liquid organic hydrogen carrier system based on catalytic peptide formation and hydrogenation[J]. Nature Communications, 2015, 6: 6859. |
| [9] | TAN Khai Chen, YU Yang, CHEN Ruting, et al. Metallo-N-heterocycles—A new family of hydrogen storage material[J]. Energy Storage Materials, 2020, 26: 198-202. |
| [10] | Nicole BRÜCKNER, OBESSER Katharina, Andreas BÖSMANN, et al. Evaluation of industrially applied heat-transfer fluids as liquid organic hydrogen carrier systems[J]. ChemSusChem, 2014, 7(1): 229-235. |
| [11] | ZHOU Guilin, LI Tao, CHEN Jiongyi, et al. Nano-Pd/CeO2 catalysts for hydrogen storage by reversible benzene hydrogenation/dehydrogenation reactions[J]. International Journal of Hydrogen Energy, 2021, 46(27): 14540-14555. |
| [12] | SEDMINEK Anja, LIKOZAR Blaž, GYERGYEK Sašo. Electrification of selective catalytic liquid organic hydrogen carriers: Hydrogenation and dehydrogenation reactions[J]. ACS Omega, 2024, 9(6): 6027-6035. |
| [13] | Guido Peter PEZ, SCOTT Aaron RAymond, COOPER Alan Charles, et al. Hydrogen storage by reversible hydrogenation of pi-conjugated substrates: US7351395[P]. 2008-04-01. |
| [14] | SOTOODEH Farnaz, SMITH Kevin J. An overview of the kinetics and catalysis of hydrogen storage on organic liquids[J]. The Canadian Journal of Chemical Engineering, 2013, 91(9): 1477-1490. |
| [15] | SAFRONOV Sergey P, VOSTRIKOV Sergey V, SAMAROV Artemiy A, et al. Reversible storage and release of hydrogen with LOHC: Evaluation of thermochemical data for methyl-quinolines with complementary experimental and computational methods[J]. Fuel, 2022, 317: 123501. |
| [16] | VOSTRIKOV Sergey V, KONNOVA Maria E, TUROVTSEV Vladimir V, et al. Thermodynamics of hydrogen storage: LOHC system 1-alkyl-indole/octahydro-1-alkyl-indole[J]. Fuel, 2023, 344: 128079. |
| [17] | SAFRONOV Sergey P, VOSTRIKOV Sergey V, SAMAROV Artemiy A, et al. Comprehensive thermodynamic study of substituted indoles/perhydro indoles as potential liquid organic hydrogen carrier system[J]. Fuel, 2023, 331: 125764. |
| [18] | GONG Xiang, LI Linsen, SHI Renyi, et al. Novel liquid organic hydrogen carriers with high hydrogen performance: NPhCZ/18H-NPhCZ[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(7): 3085-3092. |
| [19] | WANG Bin, YAN Ting, CHANG Tieyan, et al. Palladium supported on reduced graphene oxide as a high-performance catalyst for the dehydrogenation of dodecahydro-N-ethylcarbazole[J]. Carbon, 2017, 122: 9-18. |
| [20] | YANG Ming, CHENG Guoe, XIE Dandan, et al. Study of hydrogenation and dehydrogenation of 1-methylindole for reversible onboard hydrogen storage application[J]. International Journal of Hydrogen Energy, 2018, 43(18): 8868-8876. |
| [21] | AAKKO-SAKSA Päivi T, COOK Chris, KIVIAHO Jari, et al. Liquid organic hydrogen carriers for transportation and storing of renewable energy—Review and discussion[J]. Journal of Power Sources, 2018, 396: 803-823. |
| [22] | CAMPANATI M, VACCARI A, PICCOLO O. Mild hydrogenation of quinoline 1. Role of reaction parameters[J]. Journal of Molecular Catalysis A: Chemical, 2002, 179(1/2): 287-292. |
| [23] | Jinho OH, BATHULA Hari Babu, PARK Ji Hoon, et al. A sustainable mesoporous palladium-alumina catalyst for efficient hydrogen release from N-heterocyclic liquid organic hydrogen carriers[J]. Communications Chemistry, 2019, 2: 68. |
| [24] | WANG Bin, WANG Shiyuan, LU Shuhan, et al. Trace ultrafine Ru nanoparticles on Ni/Al hydrotalcite-derived oxides supports as extremely active catalysts for N-ethylcarbazole hydrogenation[J]. Fuel, 2023, 339: 127338. |
| [25] | GONG Xiang, JIANG Zhao, FANG Tao. Enhancing selectivity and reducing cost for dehydrogenation of dodecahydro-N-ethylcarbazole by supporting platinum on titanium dioxide[J]. International Journal of Hydrogen Energy, 2020, 45(11): 6838-6847. |
| [26] | STEPANENKO Sergey A, SHIVTSOV Danil M, KOSKIN Anton P, et al. N-heterocyclic molecules as potential liquid organic hydrogen carriers: Reaction routes and dehydrogenation efficacy[J]. Catalysts, 2022, 12(10): 1260. |
| [27] | JING Zijun, YUAN Qinqin, YU Yang, et al. Developing ideal metalorganic hydrides for hydrogen storage: From theoretical prediction to rational fabrication[J]. ACS Materials Letters, 2021, 3(9): 1417-1425. |
| [28] | László HEGEDÜS, Tibor MÁTHÉ. Hydrogenation of pyrrole derivatives Part Ⅴ. Poisoning effect of nitrogen on precious metal on carbon catalysts[J]. Applied Catalysis A: General, 2002, 226(1/2): 319-322. |
| [29] | 李欣雨, 唐鋆磊, 李佳奇, 等. 有机液体储氢体系研究进展[J]. 低碳化学与化工, 2023, 48(6): 107-119. |
| LI Xinyu, TANG Junlei, LI Jiaqi, et al. Research progress in hydrogen storage system of organic liquid[J]. Low-Carbon Chemistry and Chemical Engineering, 2023, 48(6): 107-119. | |
| [30] | RYABCHUK Pavel, AGAPOVA Anastasiya, KREYENSCHULTE Carsten, et al. Heterogeneous nickel-catalysed reversible, acceptorless dehydrogenation of N-heterocycles for hydrogen storage[J]. Chemical Communications, 2019, 55(34): 4969-4972. |
| [31] | LI Qingqing, SUN Zhiwei, WEI Yilin, et al. Acceptorless ambient-temperature dehydrogenation and reversible hydrogenation of N-heterocycles over single-atom Co-N-C catalysts[J]. Applied Catalysis B: Environment and Energy, 2024, 351: 123959. |
| [32] | CAMPANATI M, CASAGRANDE M, FAGIOLINO I, et al. Mild hydrogenation of quinoline 2. A novel Rh-containing pillared layered clay catalyst[J]. Journal of Molecular Catalysis A: Chemical, 2002, 184(1/2): 267-272. |
| [33] | FISH Richard H, BARALT Eduardo, SMITH Sandra J. Homogeneous catalytic hydrogenation. 5. Regioselective reductions of mono- and polynuclear heteroaromatic model coal compounds with the (η 5-pentamethylcyclopentadienyl)rhodium tris(acetonitrile) dication as the catalyst precursor[J]. Organometallics, 1991, 10(1): 54-56. |
| [34] | VEREVKIN Sergey P, SAFRONOV Sergey P, SAMAROV Artemiy A, et al. Hydrogen storage: Thermodynamic analysis of alkyl-quinolines and alkyl-pyridines as potential liquid organic hydrogen carriers (LOHC)[J]. Applied Sciences, 2021, 11(24): 11758. |
| [35] | SOTOODEH Farnaz, SMITH Kevin J. Analysis of H2 release from organic polycyclics over Pd catalysts using DFT[J]. The Journal of Physical Chemistry C, 2013, 117(1): 194-204. |
| [36] | DEAN Darrell, DAVIS Boyd, JESSOP Philip G. The effect of temperature, catalyst and sterics on the rate of N-heterocycle dehydrogenation for hydrogen storage[J]. New Journal of Chemistry, 2011, 35(2): 417-422. |
| [1] | XU Cong, FENG Yingjie, LIU Dongbing, XIE Zaiku. Review of zeolite confined Pt-based catalysts for propane dehydrogenation [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 4954-4967. |
| [2] | CHEN Zizhao, HE Fangshu, HU Qiang, YANG Yang, CHEN Hanping, YANG Haiping. Research progress on anti-carbon deposition Ni-based catalysts for dry reforming of methane [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 4968-4978. |
| [3] | WANG Zhen, ZHANG Yaoyuan, WU Qin, SHI Daxin, CHEN Kangcheng, LI Hansheng. Development of Ni/Al2O3-based catalysts for the dry reforming of methane [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 4979-4998. |
| [4] | ZHANG Haipeng, QIN Shanshan, WANG Yuxuan, YU Haibiao. Preparation of 3.0F-Ag x Co catalysts for N2O decomposition [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 4999-5005. |
| [5] | SUN Mengyuan, LU Shijian, LIU Ling, XUE Yanyang, ZHANG Yunrong, DONG Qi, KANG Guojun. Research progress of MOF and their derivatives in carbon capture [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5339-5350. |
| [6] | WANG Wenjun, LIU Ruixin, WANG Jun, ZHANG Qinglei, HOU Li’an. Research progress of visible light degradation of indoor VOCs by titanium dioxide materials [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5351-5362. |
| [7] | ZENG Jin, GAO Yan, WANG Zhaopeng, XIE Yuyun, LIU Jun, LIANG Qi, WANG Chunying. Degradation mechanism of 2,4-dichlorophenoxyacetic acid by NaYF4:Yb,Tm composite TiO2/Bi2WO6 photocatalyst and evaluation of products toxicity [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5416-5431. |
| [8] | LI Zeng, ZHAO Yunpeng, LI Yuhui, LIU Nan, ZHU Chunmeng, SHI Xiaogang, GAO Jinsen, LAN Xingying. Abnormal diagnosis of catalyst loss for FCC disengager based on CFD simulation [J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4430-4442. |
| [9] | ZHAO Yongming, BU Yifeng, WANG Tao, DU Bing, MEN Zhuowu. Integrated optimization of catalyst dynamic replacement and steady-state Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4536-4544. |
| [10] | YANG Jiacong, CHENG Guangxu, JIA Tonghua, JIANG Zhao. Simulation and techno-economic analysis of new efficient coupling processes between coal to methanol and green hydrogen [J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4657-4668. |
| [11] | SHI Qinchuan, WANG Shiyuan, LI Peiya, LU Shuhan, WANG Bo, WANG Jiahui, YANG Fusheng, WANG Bin, YANG Shengchun, FANG Tao. Research on the solubilities of hydrogen in liquid organic hydrogen carriers [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 3816-3827. |
| [12] | ZHANG Wei, LIANG Yaocheng, WU Qiao, FU Yehao, YIN Yanshan, CHENG Shan, RUAN Min, LIU Tao, ZHOU Zhaoyi, ZHANG Kaikai, LI Dancong. Metal ion modified Cu-SSZ-13 catalyst for NH3-selective catalytic reduction of NO x [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 3879-3891. |
| [13] | LU Peng, ZHANG Di, LIU Yaoyao, YU Wanjin, LIU Wucan, ZHANG Jianjun. Research progress of catalysts for gas-phase dehydrofluorination to synthesize C2 hydrofluoroolefins [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 3907-3916. |
| [14] | GAO Jiaojiao, YAN Shiyu, YANG Taishun, XIE Shangzhi, YANG Yanjuan, XU Jing. Effect of alumina support crystal structure of Ru-based catalysts on polyethylene hydrogenolysis performance [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 3917-3927. |
| [15] | CHEN Dongjian, SUN Yuqian, YIN Fengxiang. Preparation of FeNi3-Fe3O4/CN electrocatalysts and their electrocatalytic oxygen evolution performance [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 3928-3937. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||