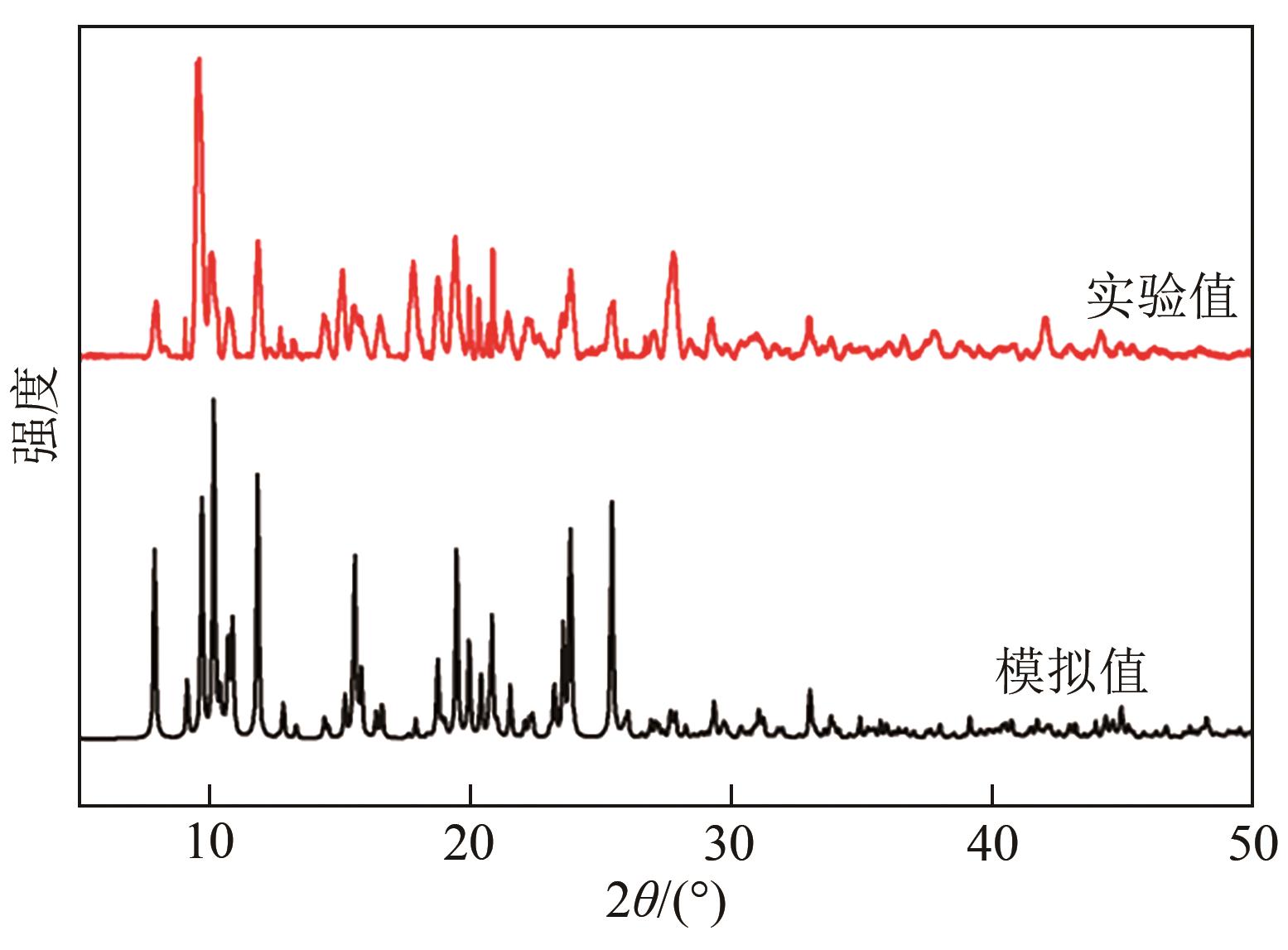
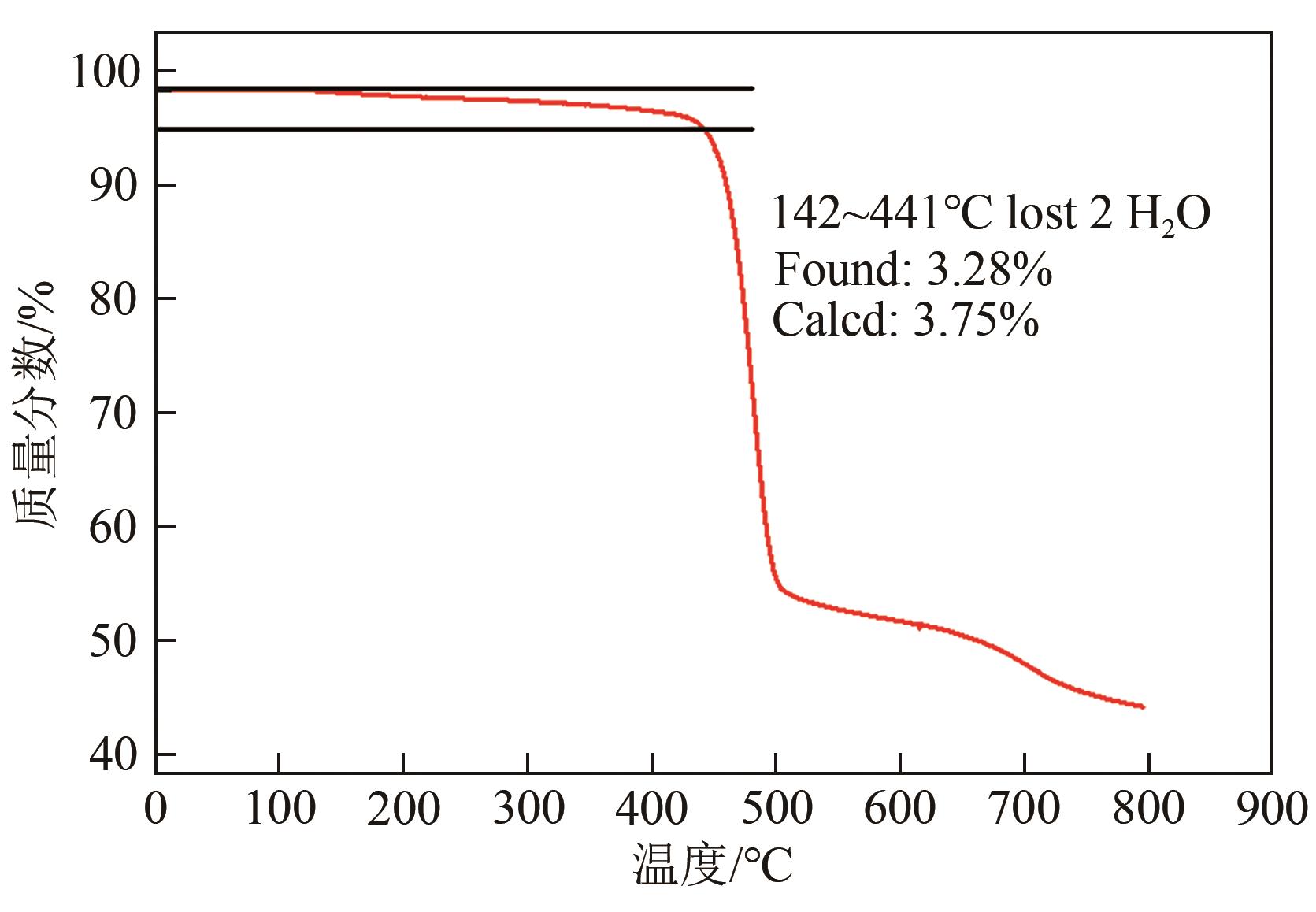
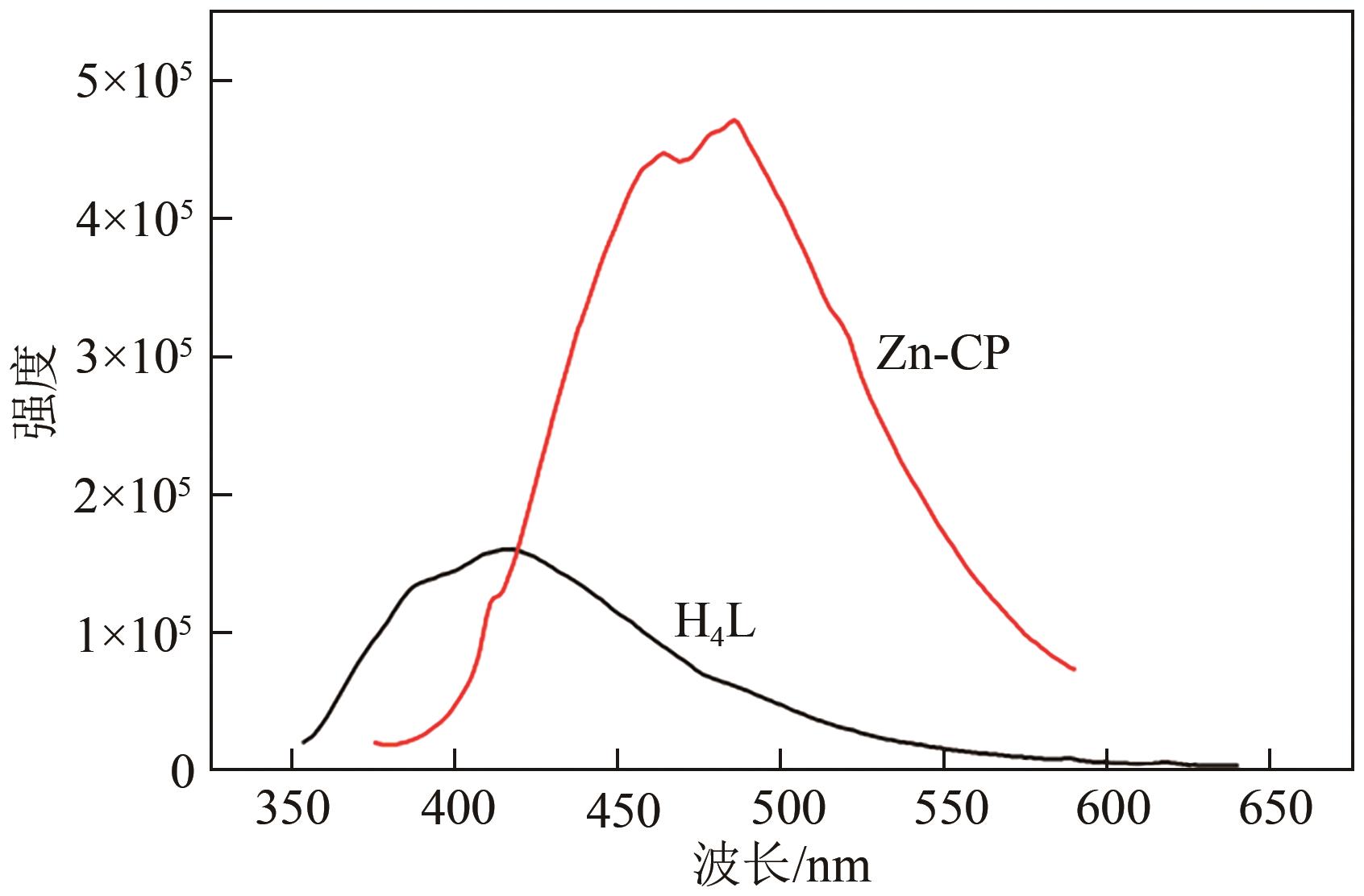
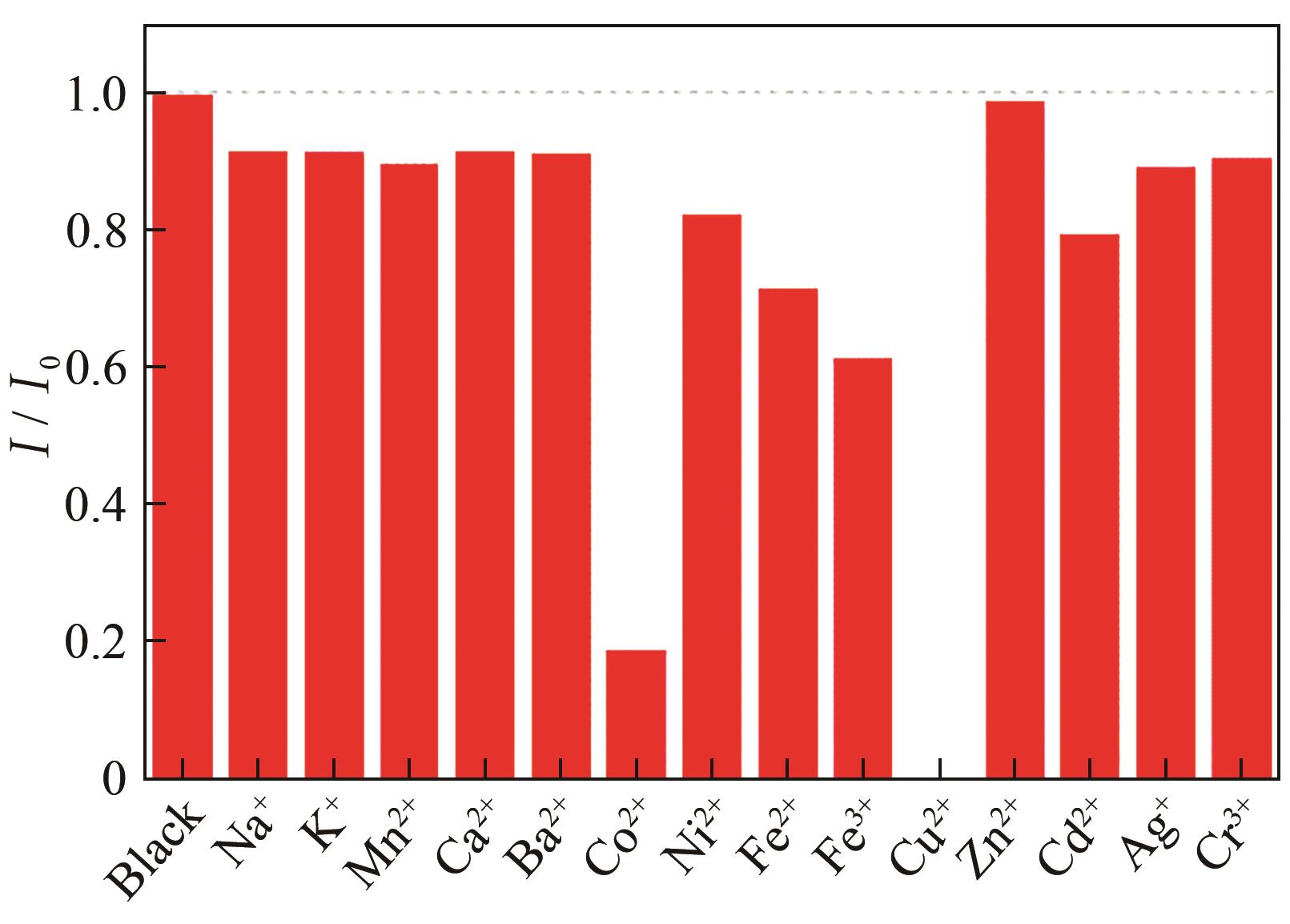
| 1 |
ZHANG Meng, ZHANG Jianhong, CHE Xin, et al. Biomimetic mineralization-based in situ growth of AuNCs@ZIF-8 on paper fibers for visual detection of copper ions[J]. Talanta, 2024, 268: 125364.
|
| 2 |
ZHANG Yibin, Jia MIU, WANG Boling, et al. A novel near-infrared fluorescent probe based on the dicyanoisophorone for the selective detection of Cu2+ in real water samples[J]. Journal of Molecular Structure, 2023, 1286: 135632.
|
| 3 |
PARK Seonmin, BONG So Yeon, SHARMA Shilpa, et al. Simple turn-on fluorescent chemosensor for ultrafast and highly selective trace-level detection of Cu2+ ions in aqueous solutions[J]. Spectrochimica Acta A: Molecular and Biomolecular Spectroscopy, 2024, 305: 123555.
|
| 4 |
MA Ting, LIU Mingzhu, SUN Jingran, et al. N-doped molybdenum oxide quantum dots as fluorescent probes for the quantitative detection of copper ions in environmental samples[J]. Analytical Methods, 2023, 15(45): 6239-6244.
|
| 5 |
董佳佳, 薛松松. 新型三苯胺类Cu2+荧光探针的合成及性能研究[J]. 化工新型材料, 2023, 51(S2): 659-664.
|
|
DONG Jiajia, XUE Songsong. Synthesis and properties of novel triphenylamine Cu2+ fluorescent probes[J]. New Chemical Materials, 2023, 51(S2): 659-664.
|
| 6 |
LI Xiangqian, YANG Peiying, ZHAO Yuxin, et al. Antibiotic identification in aqueous solution by a two-dimensional cadmium-based coordination polymer with excellent fluorescence properties[J]. Polyhedron, 2024, 250: 116818.
|
| 7 |
WANG Yuna, ZHANG Xiaofeng, ZHAO Yanru, et al. Three novel Zn-based coordination polymers: Synthesis, structure, and effective detection of Al3+ and S2- ions[J]. Molecules, 2020, 25(2): 382.
|
| 8 |
WANG Dan, DU Linhuan, LI Long, et al. Zn(Ⅱ)-based mixed-ligand-bearing coordination polymers as multi-responsive fluorescent sensors for detecting dichromate, iodide, nitenpyram, and imidacloprid[J]. Polymers, 2023, 15(11): 2570.
|
| 9 |
张艳. 一例由BMIP和1, 4-BDF混合配体构筑的Zn(Ⅱ)配位聚合物、晶体结构及其性能[J]. 化学研究与应用, 2024, 36(2): 323-330.
|
|
ZHANG Yan. A Zn(Ⅱ)coordination polymer constructed by mixed ligands of BMIP and 1,4-BDF, crystal structure and properties[J]. Chemical Research and Application, 2024, 36(2): 323-330.
|
| 10 |
KLONGDEE Fatima, YOUNGME Sujittra, BOONMAK Jaursup. A luminescent sensor based on zinc(Ⅱ) 1D chain coordination polymer for effective acetone detection[J]. Polyhedron, 2020, 180: 114437.
|
| 11 |
ZHANG Yatong, WANG Ai, HUANG Bing, et al. A Zn-coordination polymer for the quantitative and selective colorimetric detection of residual tetracycline in aqueous solution and urine[J]. Spectrochimica Acta A: Molecular and Biomolecular Spectroscopy, 2023, 294: 122470.
|
| 12 |
YU Xiaoyan, DONG Wenwen, HAN Huimin, et al. A water-stable Zn (Ⅱ) coordination polymer as fluorescent sensor for selective and sensitive detection of antibiotics and Fe3+ [J]. Journal of Solid State Chemistry, 2021, 296: 122032.
|
| 13 |
QI Shengsheng, LI Zhang, JIA Yuejiao, et al. A Zn-coordination polymer as a multifunctional fluorescent probe for the detection of V2O7 4-, Fe3+, and p-nitrotoluene[J]. Physical Chemistry Chemical Physics, 2023, 25(14): 10090-10096.
|
| 14 |
SUN Zan, SUN Juan, XI Lu, et al. Two novel lanthanide metal organic frameworks: Selective luminescent sensing for nitrobenzene, Cu2+, and MnO4 - [J]. Crystal Growth & Design, 2020, 20(8): 5225-5234.
|
| 15 |
ZHANG Xiaofei, FENG Lihui, MA Shiyu, et al. A microporous Tb-based MOF for multifunctional detection of the α-CHC, Cu2+ and Fe3+ [J]. Journal of Solid State Chemistry, 2022, 312: 123232.
|
| 16 |
LUO Jun, LIU Baoshu, ZHANG Yunchang, et al. A new fluorescent probe constructed by europium(Ⅲ)-organic framework (Eu-MOF) for detecting Cu2+ selectively and sensitively[J]. Journal of Molecular Structure, 2023, 1274: 134460.
|
| 17 |
WAN Xiaoyu, ZHANG Yifan, WANG Huaiwei, et al. One amino-functionalized luminescence sensor demonstrating high sensitivity and selectivity for detecting Al3+ and Cu2+ as well as its luminescent mixed matrix membranes and test papers[J]. Journal of Solid State Chemistry, 2022, 305: 122705.
|
| 18 |
ZHANG Ensheng, JIANG Long, Ruijiang LYU, et al. The design, synthesis and fluorescent sensing applications of a thermo-sensitive Zn-MOF[J]. Journal of Solid State Chemistry, 2021, 303: 122476.
|
| 19 |
HAN Meirong, DONG Wenxia, FENG Sisi, et al. An ultra-sensitive selective fluorescent sensor based on a 3D zinc-tetracarboxylic framework for the detection and enrichment of trace Cu2+ in aqueous media[J]. Dalton Transactions, 2021, 50(14): 4944-4951.
|
| 20 |
WANG Ruidong, ZHANG Wenqian, ZHOU Sihan, et al. A novel dual-functional coordination polymer for detection and ultra-effectively removal of Fe(Ⅲ) in water[J]. Journal of Molecular Liquids, 2022, 355: 118942.
|
| 21 |
MI Xiuna, SHENG Dafei, YU Yu’e, et al. Tunable light emission and multiresponsive luminescent sensitivities in aqueous solutions of two series of lanthanide metal-organic frameworks based on structurally related ligands[J]. ACS Applied Materials Interfaces, 2019, 11(8): 7914-7926.
|
| 22 |
YAO Bin, WEI Yuan, LI Ceng, et al. A mixed-ligand Zn(Ⅱ)-coordination polymer: Selective detection of Fe3+ ion and promotion activity on the flap transplantation via increasing the vascular endothelial cells proliferation[J]. Journal of the Iranian Chemical Society, 2021, 18(10): 2597-2604.
|
| 23 |
WANG Cong, REN Guojian, TAN Qinyue, et al. Detection of organic arsenic based on acid-base stable coordination polymer[J]. Spectrochimica Acta A: Molecular and Biomolecular Spectroscopy, 2023, 299: 122812.
|
 ), WEI Xiaoyang2, YOU Xuerui1, ZHANG Zhichao1, HANG Meirong3, GUO Xiang4, ZHAO Yiyan4(
), WEI Xiaoyang2, YOU Xuerui1, ZHANG Zhichao1, HANG Meirong3, GUO Xiang4, ZHAO Yiyan4( )
)
 ), 魏晓阳2, 尤雪瑞1, 张志超1, 韩美荣3, 郭湘4, 赵艺妍4(
), 魏晓阳2, 尤雪瑞1, 张志超1, 韩美荣3, 郭湘4, 赵艺妍4( )
)