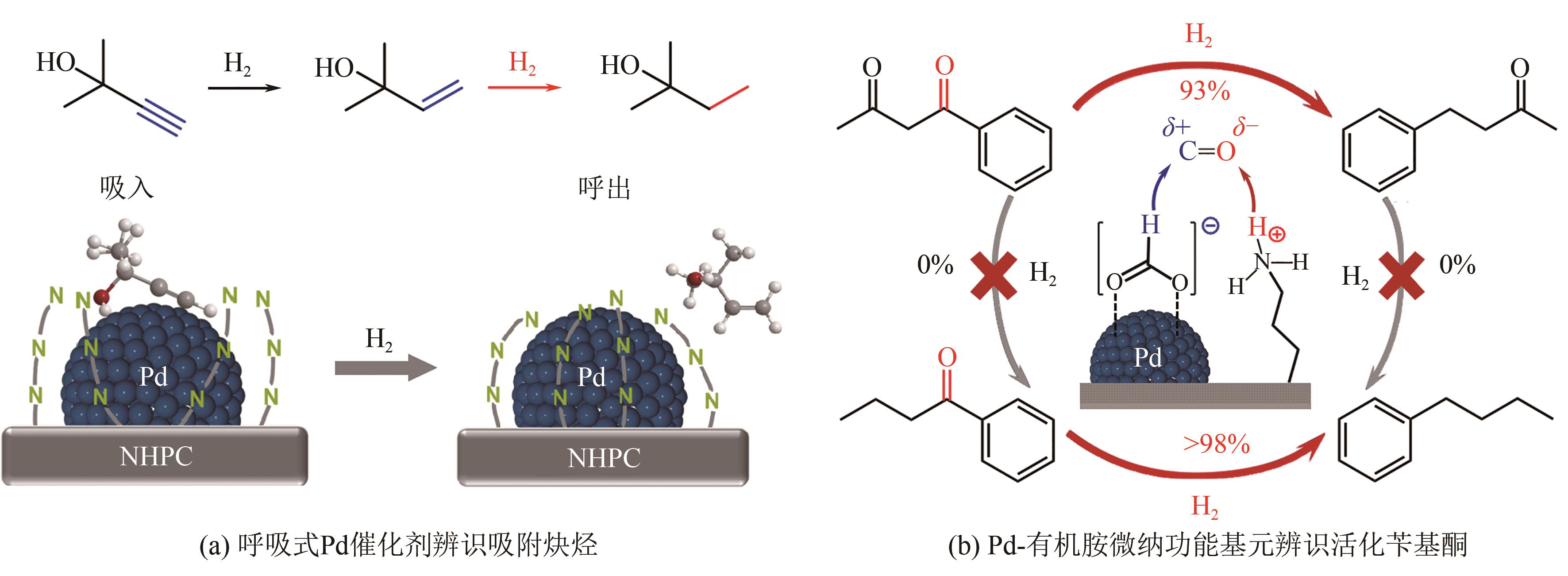
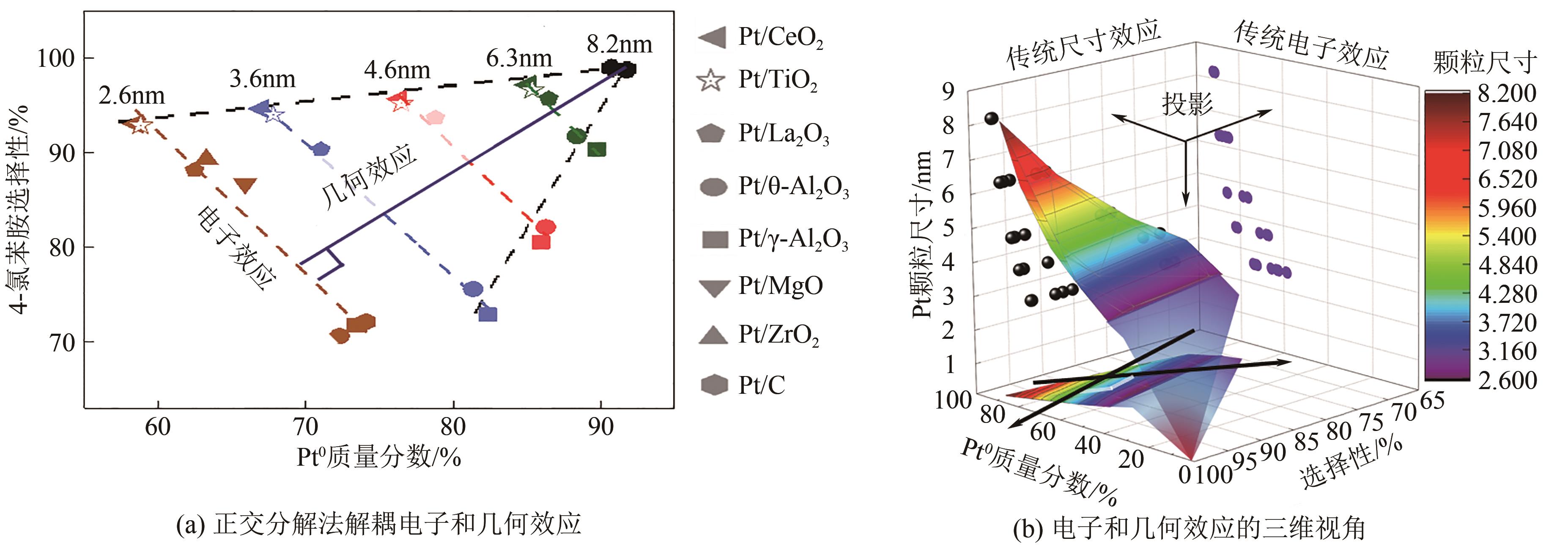
| [1] |
JIN Yong, CHENG Yi, BAI Dingrong, ZHANG Chenxi, WEI Fei.
Fluidization research and development in China
[J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2761-2780.
|
| [2] |
XIAO Yaoxin, ZHANG Jun, HU Sheng, SHAN Rui, YUAN Haoran, CHEN Yong.
Cu-Zn catalyzed hydrogenation of furfural with methanol as hydrogen donor
[J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1341-1352.
|
| [3] |
LI Bin, PAN Qinggang, JIANG Shuang, ZHANG Tianyong.
Green synthesis of fluopyram intermediates in high yield
[J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 469-476.
|
| [4] |
SHAO Bin, SUN Zheyi, ZHANG Yun, PAN Fenghongkang, ZHAO Kaiqing, HU Jun, LIU Honglai.
Recent progresses in CO2 to syngas and high value-added products
[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1136-1151.
|
| [5] |
ZHU Feifei, MA Lei, LONG Huimin.
Research progresses on the preparation and application of PdxSy catalysts
[J]. Chemical Industry and Engineering Progress, 2022, 41(2): 740-749.
|
| [6] |
WANG Zitan, ZHANG Minqing, ZHANG Jinli, LI Wenpeng.
Liquid-liquid biphase mass transfer and reaction selectivity in a micropore-jet capillary reactor
[J]. Chemical Industry and Engineering Progress, 2022, 41(1): 52-59.
|
| [7] |
ZHENG Anda, YANG Chenggong, WANG Dong’e, TIAN Zhijian.
Highly active MoS2/reduced graphene oxide catalyst for anthracene hydrogenation
[J]. Chemical Industry and Engineering Progress, 2022, 41(1): 244-252.
|
| [8] |
WANG Minjia, ZHOU Shaodong, RUAN Jiancheng, LI Rongrong, CHEN Xinzhi.
Progress in the research of chloronitrobenzene selective hydrogenation catalysts
[J]. Chemical Industry and Engineering Progress, 2021, 40(8): 4223-4230.
|
| [9] |
FAN Tao.
Industrial application progress of lignite pyrolysis technology in eastern area of Inner Mongolia, China
[J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1362-1370.
|
| [10] |
Yitao LIU, Minghui ZHU, Zixu YANG, Bo MENG, Weifeng TU, Yifan HAN.
Advances of catalysts for direct synthesis of lower olefins from syngas
[J]. Chemical Industry and Engineering Progress, 2021, 40(2): 594-604.
|
| [11] |
Zhongli JI, Xin LUAN, Linfeng MIAO.
Overview of hot-gas filtration technology and equipment development
[J]. Chemical Industry and Engineering Progress, 2020, 39(6): 2304-2311.
|
| [12] |
Rui WANG,Xue YANG,Ping MIAO.
Progress in preparation research and industrial application of SO42-/ZrO2catalysts for light paraffin isomerization
[J]. Chemical Industry and Engineering Progress, 2019, 38(11): 4930-4940.
|
| [13] |
Bin DONG, Jie WU, Jianting ZHANG, Chunxin WU, Ningren JIN, Deming ZHAO.
Synthesis of trans-PBO AA type monomer 2,5-diamino-hydroquinone hydrochloride
[J]. Chemical Industry and Engineering Progress, 2019, 38(06): 2947-2952.
|
| [14] |
Qijiang LIAO,Hongyun QIN,Mingliang ZHOU,Minqing ZHANG,Jinli ZHANG.
Progress of researches and applications for high shear mixers
[J]. Chemical Industry and Engineering Progress, 2019, 38(03): 1160-1175.
|
| [15] |
GUO Zhengdong, SU Mengjun, LIU Hanxiao, LI Yajun, QING Yizhao, LUO Yong, CHU Guangwen, CHEN Jianfeng.
States-of-the-arts progress on fundamental research and industrial applications of rotating packed bed
[J]. Chemical Industry and Engineering Progress, 2018, 37(04): 1335-1346.
|
 ), WANG Zhe, WANG Yong(
), WANG Zhe, WANG Yong( )
)