Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (7): 3532-3549.DOI: 10.16085/j.issn.1000-6613.2022-1682
• Industrial catalysis • Previous Articles Next Articles
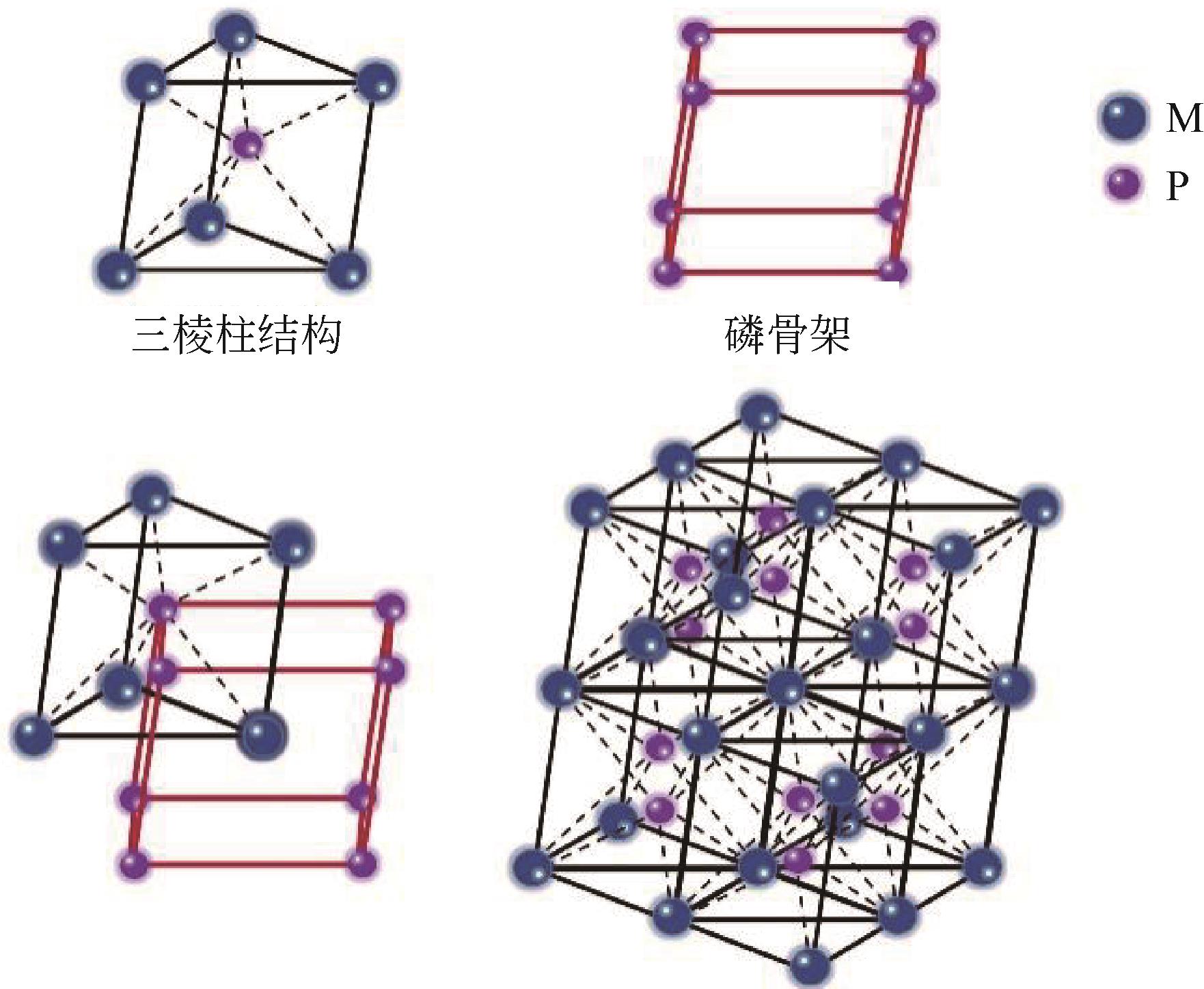
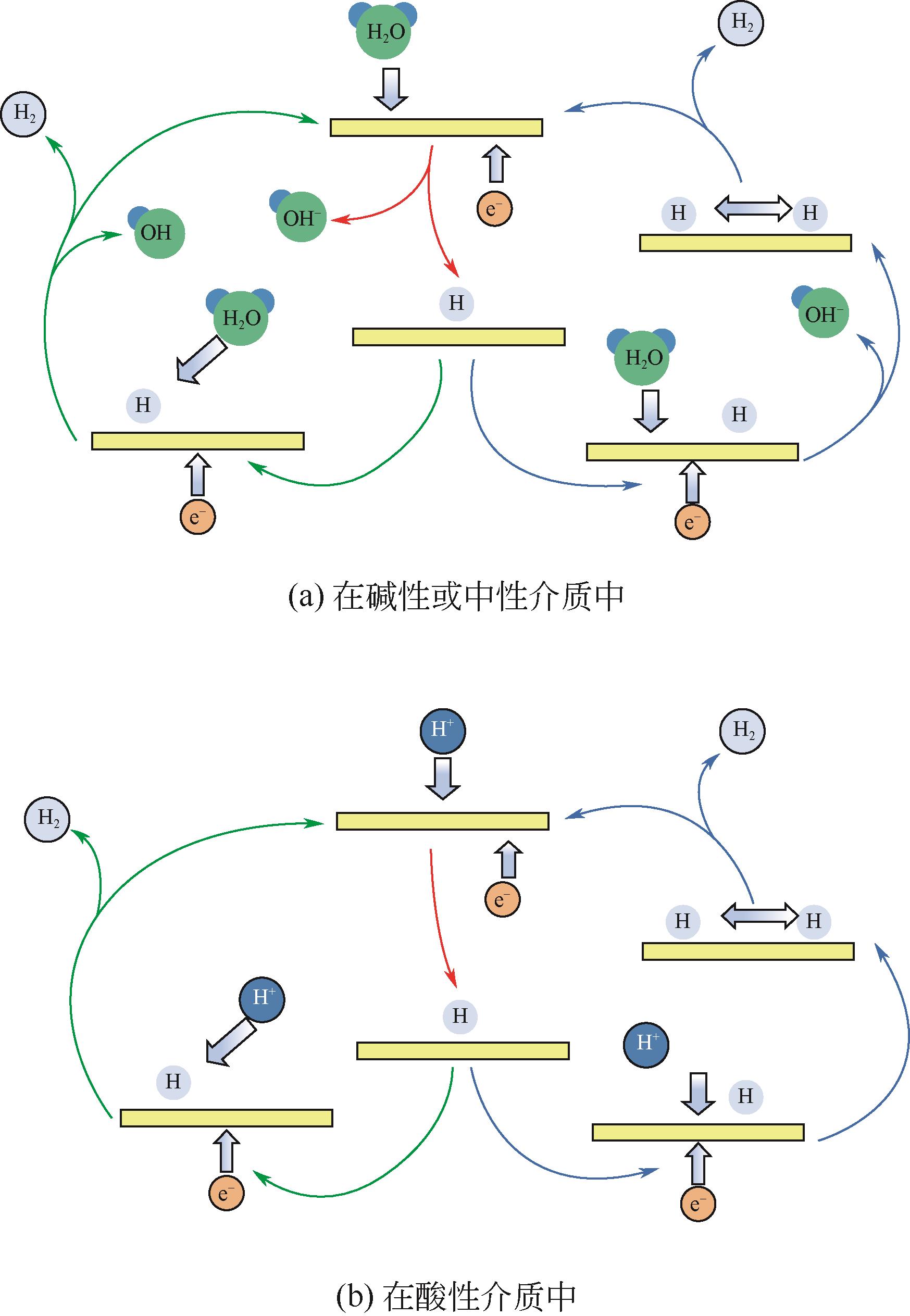
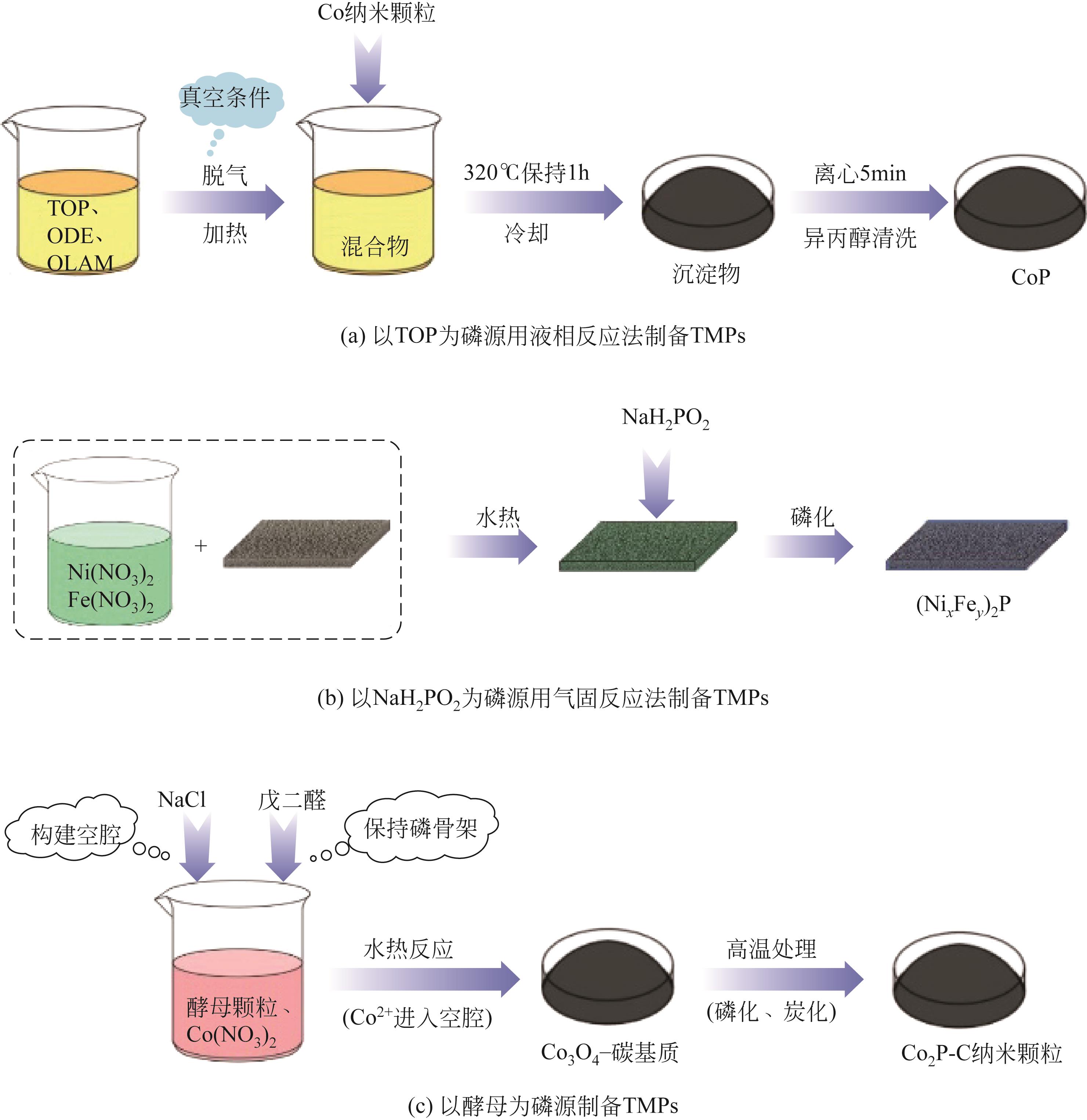
Transition metal phosphide modification and its applications in electrochemical hydrogen evolution reaction
WANG Yunqing1,2( ), YANG Guorui3(
), YANG Guorui3( ), YAN Wei1,2(
), YAN Wei1,2( )
)
- 1.Xi’an Key Laboratory of Solid Waste Recycling and Resource Recovery, Xi’an 710049, Shaanxi, China
2.Department of Environmental Engineering, Xi’an Jiaotong University, Xi’an 710049, Shaanxi, China
3.Department of Applied Chemistry, Xi’an Jiaotong University, Xi’an 710049, Shaanxi, China
-
Received:2022-09-09Revised:2023-02-28Online:2023-08-14Published:2023-07-15 -
Contact:YANG Guorui, YAN Wei
过渡金属磷化物的改性方法及其在电化学析氢中的应用
- 1.西安市固体废物资源再生与循环利用重点实验室,陕西 西安 710049
2.西安交通大学环境工程系,陕西 西安 710049
3.西安交通大学应用化学系,陕西 西安 710049
-
通讯作者:杨国锐,延卫 -
作者简介:王蕴青(1999—),女,硕士研究生,研究方向为电解水制氢。E-mail:1410950142@qq.com。 -
基金资助:国家自然科学基金(51978569)
CLC Number:
Cite this article
WANG Yunqing, YANG Guorui, YAN Wei. Transition metal phosphide modification and its applications in electrochemical hydrogen evolution reaction[J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3532-3549.
王蕴青, 杨国锐, 延卫. 过渡金属磷化物的改性方法及其在电化学析氢中的应用[J]. 化工进展, 2023, 42(7): 3532-3549.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-1682
| 催化剂 | 电解质 | 过电位η10/mV | Tafel斜率/mV·dec-1 | 电化学稳定性 | 参考文献 |
|---|---|---|---|---|---|
| NiP2 NS/CC | 0.5mol/L H2SO4 | 75 | 51 | 3000CV | [ |
| NiP/RGO | 0.5mol/L H2SO4 | 89(η起始) | 135.1 | 500CV | [ |
| 1mol/L KOH | 116(η起始) | 122.4 | |||
| NiP x /TNAs | 1mol/L KOH | 104 | 70.1 | — | [ |
| NiP@C | 1mol/L KOH | 23(η100) | 12.4 | 10/20mA/cm2(10h) | [ |
| NiP/NF | 1mol/L KOH | 102 | 90 | 1000CV | [ |
| Ni2P/Ni/NF | 1mol/L KOH | 94 | 72 | 10mA/cm2(20h) | [ |
| V-Ni2P/NF | 1mol/L KOH | 55 | 48 | 10mA/cm2(50h) | [ |
| Ni-Ni3P@NPC | 0.5mol/L H2SO4 | 73 | 57.93 | 30/60mA/cm2(25h) | [ |
| Ni3P PHNs | 0.5mol/L H2SO4 | 85 | 50 | 2000CV | [ |
| 1mol/L KOH | 338(η20) | 190 | |||
| N-Ni5P4 | 1mol/L KOH | 96 | 62.2 | 1000CV | [ |
| Ni5P4/NF | 1mol/L KOH | 64 | 64 | 3000CV | [ |
| Cuf@Ni5P4 | 0.5mol/L H2SO4 | 90 | 49 | 10/160mA/cm2(84h) | [ |
| Ni12P5/CNT | 0.5mol/L H2SO4 | 129 | 56 | 1000CV | [ |
| Ni12P5 NCs | 0.5mol/L H2SO4 | 118 | 42 | 500CV | [ |
| 催化剂 | 电解质 | 过电位η10/mV | Tafel斜率/mV·dec-1 | 电化学稳定性 | 参考文献 |
|---|---|---|---|---|---|
| NiP2 NS/CC | 0.5mol/L H2SO4 | 75 | 51 | 3000CV | [ |
| NiP/RGO | 0.5mol/L H2SO4 | 89(η起始) | 135.1 | 500CV | [ |
| 1mol/L KOH | 116(η起始) | 122.4 | |||
| NiP x /TNAs | 1mol/L KOH | 104 | 70.1 | — | [ |
| NiP@C | 1mol/L KOH | 23(η100) | 12.4 | 10/20mA/cm2(10h) | [ |
| NiP/NF | 1mol/L KOH | 102 | 90 | 1000CV | [ |
| Ni2P/Ni/NF | 1mol/L KOH | 94 | 72 | 10mA/cm2(20h) | [ |
| V-Ni2P/NF | 1mol/L KOH | 55 | 48 | 10mA/cm2(50h) | [ |
| Ni-Ni3P@NPC | 0.5mol/L H2SO4 | 73 | 57.93 | 30/60mA/cm2(25h) | [ |
| Ni3P PHNs | 0.5mol/L H2SO4 | 85 | 50 | 2000CV | [ |
| 1mol/L KOH | 338(η20) | 190 | |||
| N-Ni5P4 | 1mol/L KOH | 96 | 62.2 | 1000CV | [ |
| Ni5P4/NF | 1mol/L KOH | 64 | 64 | 3000CV | [ |
| Cuf@Ni5P4 | 0.5mol/L H2SO4 | 90 | 49 | 10/160mA/cm2(84h) | [ |
| Ni12P5/CNT | 0.5mol/L H2SO4 | 129 | 56 | 1000CV | [ |
| Ni12P5 NCs | 0.5mol/L H2SO4 | 118 | 42 | 500CV | [ |
| 催化剂 | 电解质 | 过电位η10/mV | Tafel斜率/mV·dec-1 | 电化学稳定性 | 参考文献 |
|---|---|---|---|---|---|
| CoP/GF | 1mol/L KOH | 130(η20) | 80.1 | 50h | [ |
| CoP | 0.5mol/L H2SO4 | 84 | 61 | 1000CV | [ |
| 1mol/L KOH | 94 | 67 | |||
| CoP/Co2P | 0.5mol/L H2SO4 | 87 | 58 | 10mA/cm2(24h) | [ |
| 1mol/L KOH | 133 | 60 | |||
| CoP-NC | 0.5mol/L H2SO4 | 145 | 55 | 2000CV | [ |
| 1mol/L KOH | 167 | 57 | |||
| 1mol/L PBS | 252 | 110 | |||
| CoP/CN/Ni | 0.5mol/L H2SO4 | 66 | 39.5 | 10mA/cm2(12h) | [ |
| 1mol/L KOH | 106 | 53.4 | |||
| CoP | 1mol/L KOH | 71 | 60.75 | 3000CV | [ |
| CoP/Co2P | 0.5mol/L H2SO4 | 81 | 36.2 | 2000CV | [ |
| 1mol/L KOH | 109 | 78.9 | |||
| 1mol/L PBS | 227 | 190.1 | |||
| Co2P NF | 0.5mol/L H2SO4 | 178 | 32 | 1000CV | [ |
| 1mol/L KOH | 190 | 61 |
| 催化剂 | 电解质 | 过电位η10/mV | Tafel斜率/mV·dec-1 | 电化学稳定性 | 参考文献 |
|---|---|---|---|---|---|
| CoP/GF | 1mol/L KOH | 130(η20) | 80.1 | 50h | [ |
| CoP | 0.5mol/L H2SO4 | 84 | 61 | 1000CV | [ |
| 1mol/L KOH | 94 | 67 | |||
| CoP/Co2P | 0.5mol/L H2SO4 | 87 | 58 | 10mA/cm2(24h) | [ |
| 1mol/L KOH | 133 | 60 | |||
| CoP-NC | 0.5mol/L H2SO4 | 145 | 55 | 2000CV | [ |
| 1mol/L KOH | 167 | 57 | |||
| 1mol/L PBS | 252 | 110 | |||
| CoP/CN/Ni | 0.5mol/L H2SO4 | 66 | 39.5 | 10mA/cm2(12h) | [ |
| 1mol/L KOH | 106 | 53.4 | |||
| CoP | 1mol/L KOH | 71 | 60.75 | 3000CV | [ |
| CoP/Co2P | 0.5mol/L H2SO4 | 81 | 36.2 | 2000CV | [ |
| 1mol/L KOH | 109 | 78.9 | |||
| 1mol/L PBS | 227 | 190.1 | |||
| Co2P NF | 0.5mol/L H2SO4 | 178 | 32 | 1000CV | [ |
| 1mol/L KOH | 190 | 61 |
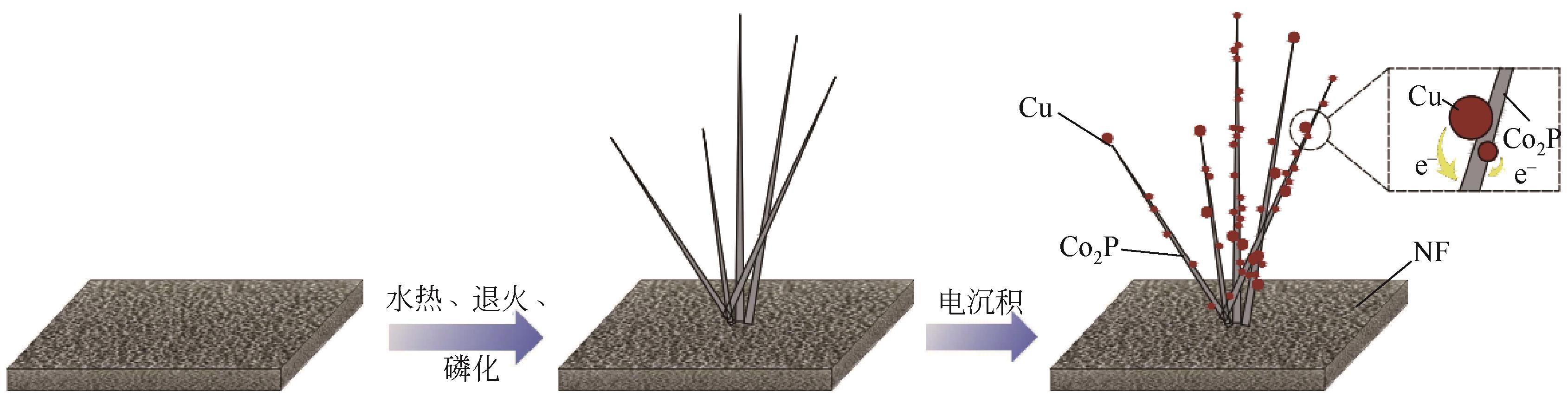
| 催化剂 | 电解质 | 过电位η10/mV | Tafel斜率/mV·dec-1 | 电化学稳定性 | 参考文献 |
|---|---|---|---|---|---|
| FeP@PPy/CTs | 0.5mol/L H2SO4 | 103.1 | 49.2 | 46h | [ |
| FeP HNPs | 0.5mol/L H2SO4 | 76 | 55 | 10mA/cm2(5h) | [ |
| FeP | 0.5mol/L H2SO4 | 154 | 65 | 160mV(6000s) | [ |
| Vc-FeP | 0.5mol/L H2SO4 | 65 | 49 | 10mA/cm2(7d) | [ |
| 1mol/L KOH | 108 | 62 | |||
| CFP-FeP HNA | 0.5mol/L H2SO4 | 45(η20) | 53 | 12h | [ |
| 1mol/L KOH | 221(η20) | 134 | |||
| FeP NPs | 0.5mol/L H2SO4 | 76 | 60 | 10mA/cm2(12h) | [ |
| Fe2P/GCS | 0.5mol/L H2SO4 | 88 | 49 | 2000CV | [ |
| Fe2P-ND/FG | 0.5mol/L H2SO4 | 91 | 47 | 4000CV | [ |
| 1mol/L KOH | 168 | 74 | |||
| 1mol/L PBS | 349 | 113 | |||
| Fe3P | 0.5mol/L H2SO4 | 49 | 57 | 120mV(20h) | [ |
| Fe3P | 0.5mol/L H2SO4 | 160 | 149 | 5h | [ |
| 催化剂 | 电解质 | 过电位η10/mV | Tafel斜率/mV·dec-1 | 电化学稳定性 | 参考文献 |
|---|---|---|---|---|---|
| FeP@PPy/CTs | 0.5mol/L H2SO4 | 103.1 | 49.2 | 46h | [ |
| FeP HNPs | 0.5mol/L H2SO4 | 76 | 55 | 10mA/cm2(5h) | [ |
| FeP | 0.5mol/L H2SO4 | 154 | 65 | 160mV(6000s) | [ |
| Vc-FeP | 0.5mol/L H2SO4 | 65 | 49 | 10mA/cm2(7d) | [ |
| 1mol/L KOH | 108 | 62 | |||
| CFP-FeP HNA | 0.5mol/L H2SO4 | 45(η20) | 53 | 12h | [ |
| 1mol/L KOH | 221(η20) | 134 | |||
| FeP NPs | 0.5mol/L H2SO4 | 76 | 60 | 10mA/cm2(12h) | [ |
| Fe2P/GCS | 0.5mol/L H2SO4 | 88 | 49 | 2000CV | [ |
| Fe2P-ND/FG | 0.5mol/L H2SO4 | 91 | 47 | 4000CV | [ |
| 1mol/L KOH | 168 | 74 | |||
| 1mol/L PBS | 349 | 113 | |||
| Fe3P | 0.5mol/L H2SO4 | 49 | 57 | 120mV(20h) | [ |
| Fe3P | 0.5mol/L H2SO4 | 160 | 149 | 5h | [ |
金属磷酸盐 种类 | 温度/℃(在N2氛围中, H2体积分数5%) | 组成(Rietveld分析) | |
|---|---|---|---|
| 目标磷化物 | 其他相/结构 | ||
| Ni | 800 | 约51% Ni2P | 49% Ni12P5 |
| Co | 800 | 85%~95% Co2P | Co2P2O7 |
| W | 1000 | 85% WP | 15% WC |
| Mo | 800 | 100% MoP | — |
| Cr | 1000 | 40.5% CrP | 59.5% Cr12P7 |
金属磷酸盐 种类 | 温度/℃(在N2氛围中, H2体积分数5%) | 组成(Rietveld分析) | |
|---|---|---|---|
| 目标磷化物 | 其他相/结构 | ||
| Ni | 800 | 约51% Ni2P | 49% Ni12P5 |
| Co | 800 | 85%~95% Co2P | Co2P2O7 |
| W | 1000 | 85% WP | 15% WC |
| Mo | 800 | 100% MoP | — |
| Cr | 1000 | 40.5% CrP | 59.5% Cr12P7 |
| 1 | ZOU Xiaoxin, ZHANG Yu. Noble metal-free hydrogen evolution catalysts for water splitting[J]. Chemical Society Reviews, 2015, 44(15): 5148-5180. |
| 2 | HU Congling, ZHANG Lei, GONG Jinlong. Recent progress made in the mechanism comprehension and design of electrocatalysts for alkaline water splitting[J]. Energy & Environmental Science, 2019, 12(9): 2620-2645. |
| 3 | LEDENDECKER Marc, KRICK CALDERÓN Sandra, PAPP Christian, et al. The synthesis of nanostructured Ni5P4Films and their use as a non-noble bifunctional electrocatalyst for full water splitting[J]. Angewandte Chemie International Edition, 2015, 54(42): 12361-12365. |
| 4 | YAN Ya, XIA Baoyu, ZHAO Bin, et al. A review on noble-metal-free bifunctional heterogeneous catalysts for overall electrochemical water splitting[J]. Journal of Materials Chemistry A, 2016, 4(45): 17587-17603. |
| 5 | KIBSGAARD Jakob, TSAI Charlie, CHAN Karen, et al. Designing an improved transition metal phosphide catalyst for hydrogen evolution using experimental and theoretical trends[J]. Energy & Environmental Science, 2015, 8(10): 3022-3029. |
| 6 | SULTAN Siraj, TIWARI Jitendra N, SINGH Aditya Narayan, et al. Single atoms and clusters based nanomaterials for hydrogen evolution, oxygen evolution reactions, and full water splitting[J]. Advanced Energy Materials, 2019, 9(22): 1900624. |
| 7 | LI Yingjie, ZHANG Haichuan, JIANG Mingyan, et al. 3D self-supported Fe-doped Ni2P nanosheet arrays as bifunctional catalysts for overall water splitting[J]. Advanced Functional Materials, 2017, 27(37): 1702513. |
| 8 | SHENG Qiang, LI Xiang, ROEL Prins, et al. Understanding the reduction of transition-metal phosphates to transition-metal phosphides by combining temperature-programmed reduction and infrared spectroscopy[J]. Angewandte Chemie (International Ed in English), 2021, 60(20): 11180-11183. |
| 9 | PU Zonghua, LIU Tingting, AMIINU Ibrahim Saana, et al. Transition-metal phosphides: Activity origin, energy-related electrocatalysis applications, and synthetic strategies[J]. Advanced Functional Materials, 2020, 30(45): 2004009. |
| 10 | LI Xin, ELSHAHAWY Abdelnaby M, GUAN Cao, et al. Metal phosphides and phosphates-based electrodes for electrochemical supercapacitors[J]. Small (Weinheim an Der Bergstrasse, Germany), 2017, 13(39): 1701530. |
| 11 | WENG Chenchen, REN Jintao, YUAN Zhongyong. Transition metal phosphide-based materials for efficient electrochemical hydrogen evolution: A critical review[J]. ChemSusChem, 2020, 13(13): 3357-3375. |
| 12 | 季小好, 王祖民, 陈晓煜, 等. 过渡金属磷化物的制备及电催化析氢性能提升策略[J]. 高等学校化学学报, 2021, 42(5): 1377-1394. |
| JI Xiaohao, WANG Zumin, CHEN Xiaoyu, et al. Overview of transition metal phosphide catalysts and hydrogen production by electrolyzed water[J]. Chemical Journal of Chinese Universities, 2021, 42(5): 1377-1394. | |
| 13 | CARENCO Sophie, PORTEHAULT David, Cédric BOISSIÉRE, et al. Nanoscaled metal borides and phosphides: Recent developments and perspectives[J]. Chemical Reviews, 2013, 113(10): 7981-8065. |
| 14 | 蒙阳, 杨婵, 彭娟. 基于铁、钴、镍金属磷化物纳米催化剂的碱性条件下电解水制氢的研究进展[J]. 应用化学, 2020, 37(7): 733-745. |
| MENG Yang, YANG Chan, PENG Juan. Progress in iron, cobalt and nickel-based metal phosphide nano-catalysts for hydrogen production under alkaline conditions[J]. Chinese Journal of Applied Chemistry, 2020, 37(7): 733-745. | |
| 15 | WANG Jing, XU Fan, JIN Haiyan, et al. Non-noble metal-based carbon composites in hydrogen evolution reaction: Fundamentals to applications[J]. Advanced Materials (Deerfield Beach, Fla), 2017, 29(14): 1605838. |
| 16 | HU Cun, Chao LYU, LIU Shuai, et al. Nickel phosphide electrocatalysts for hydrogen evolution reaction[J]. Catalysts, 2020, 10(2): 188. |
| 17 | EL-REFAEI S, RUSSO P, SCHULTZ T, et al. Dual doping of MoP with M(Mn, Fe) and S to achieve high hydrogen evolution reaction activity in both acidic and alkaline media[J]. ChemCatChem, 2021, 13(20): 4392-4402. |
| 18 | 杜迎晨, 雷浩, 钱余海. 电解水制氢技术概述及发展现状[J]. 上海节能, 2021(8): 824-831. |
| DU Yingchen, LEI Hao, QIAN Yuhai. Technology overview and development status of hydrogen production from water electrolysis[J]. Shanghai Energy Conservation, 2021(8): 824-831. | |
| 19 | THEERTHAGIRI Jayaraman, MURTHY Arun Prasad, LEE Seung Jun, et al. Recent progress on synthetic strategies and applications of transition metal phosphides in energy storage and conversion[J]. Ceramics International, 2021, 47(4): 4404-4425. |
| 20 | OJHA Kasinath, SAHA Soumen, DAGAR Preeti, et al. Nanocatalysts for hydrogen evolution reactions[J]. Physical Chemistry Chemical Physics: PCCP, 2018, 20(10): 6777-6799. |
| 21 | SCHIPPER Desmond E, ZHAO Zhenhuan, THIRUMALAI Hari, et al. Effects of catalyst phase on the hydrogen evolution reaction of water splitting: Preparation of phase-pure films of FeP, Fe2P, and Fe3P and their relative catalytic activities[J]. Chemistry of Materials, 2018, 30(10): 3588-3598. |
| 22 | CALLEJAS Juan F, READ Carlos G, POPCZUN Eric J, et al. Nanostructured Co2P electrocatalyst for the hydrogen evolution reaction and direct comparison with morphologically equivalent CoP[J]. Chemistry of Materials, 2015, 27(10): 3769-3774. |
| 23 | Asheli RAY, SULTANA Sabiha, PARAMANIK Lekha, et al. Recent advances in phase, size, and morphology-oriented nanostructured nickel phosphide for overall water splitting[J]. Journal of Materials Chemistry A, 2020, 8(37): 19196-19245. |
| 24 | JIANG Ping, LIU Qian, SUN Xuping. NiP₂ nanosheet arrays supported on carbon cloth: An efficient 3D hydrogen evolution cathode in both acidic and alkaline solutions[J]. Nanoscale, 2014, 6(22): 13440-13445. |
| 25 | LI Zuopeng, SHANG Jianpeng, SU Caina, et al. Preparation of amorphous NiP-based catalysts for hydrogen evolution reactions[J]. Journal of Fuel Chemistry and Technology, 2018, 46(4): 473-478. |
| 26 | GAO Shiyuan, ZAVABETI Ali, WANG Bin, et al. Nickel phosphides electrodeposited on TiO2 nanotube arrays as electrocatalysts for hydrogen evolution[J]. ACS Applied Nano Materials, 2021, 4(5): 4542-4551. |
| 27 | 陈健鑫, 盛楠, 朱春宇, 等. 生物质碳负载镍基纳米颗粒及其电解水析氢性能[J]. 储能科学与技术, 2022, 11(5): 1350-1357. |
| CHEN Jianxin, SHENG Nan, ZHU Chunyu, et al. Study on nickel-based nanoparticles supported by biomass carbon for electrocatalytic hydrogen evolution[J]. Energy Storage Science and Technology, 2022, 11(5): 1350-1357. | |
| 28 | REN Jintao, HU Zhongpan, CHEN Chong, et al. Integrated Ni2P nanosheet arrays on three-dimensional Ni foam for highly efficient water reduction and oxidation[J]. Journal of Energy Chemistry, 2017, 26(6): 1196-1202. |
| 29 | YOU Bo, JIANG Nan, SHENG Meili, et al. Hierarchically porous urchin-like Ni2P superstructures supported on nickel foam as efficient bifunctional electrocatalysts for overall water splitting[J]. ACS Catalysis, 2016, 6(2): 714-721. |
| 30 | ZHANG Wenzhuo, CHEN Guangyi, ZHAO Jian, et al. Self-growth Ni2P nanosheet arrays with cationic vacancy defects as a highly efficient bifunctional electrocatalyst for overall water splitting[J]. Journal of Colloid and Interface Science, 2020, 561: 638-646. |
| 31 | LI Guixiang, WANG Jingang, YU Jiayuan, et al. Ni-Ni3P nanoparticles embedded into N, P-doped carbon on 3D graphene frameworks via in situ phosphatization of Saccharomycetes with multifunctional electrodes for electrocatalytic hydrogen production and anodic degradation[J]. Applied Catalysis B: Environmental, 2020, 261: 118147. |
| 32 | JIN Lihuang, XIA Han, HUANG Zhipeng, et al. Phase separation synthesis of trinickel monophosphide porous hollow nanospheres for efficient hydrogen evolution[J]. Journal of Materials Chemistry A, 2016, 4(28): 10925-10932. |
| 33 | ZHOU Guangyao, MA Yaru, WU Xiaomei, et al. Electronic modulation by N incorporation boosts the electrocatalytic performance of urchin-like Ni5P4 hollow microspheres for hydrogen evolution[J]. Chemical Engineering Journal, 2020, 402: 126302. |
| 34 | LAI Changgan, LIU Xianbin, DENG Yiqun, et al. Rice-shape nanocrystalline Ni5P4: A promising bifunctional electrocatalyst for hydrogen evolution reaction and oxygen evolution reaction[J]. Inorganic Chemistry Communications, 2018, 97: 98-102. |
| 35 | Manisha DAS, JENA Nityasagar, PURKAIT Taniya, et al. Single-phase Ni5P4-copper foam superhydrophilic and aerophobic core-shell nanostructures for efficient hydrogen evolution reaction[J]. Journal of Materials Chemistry A, 2019, 7(41): 23989-23999. |
| 36 | WANG Chunde, DING Tao, SUN Yuan, et al. Ni₁₂P₅ nanoparticles decorated on carbon nanotubes with enhanced electrocatalytic and lithium storage properties[J]. Nanoscale, 2015, 7(45): 19241-19249. |
| 37 | PAN Yuan, LIU Yanru, ZHAO Jinchong, et al. Monodispersed nickel phosphide nanocrystals with different phases: Synthesis, characterization and electrocatalytic properties for hydrogen evolution[J]. Journal of Materials Chemistry A, 2015, 3(4): 1656-1665. |
| 38 | ZHANG Wenxiu, CUI Liang, LIU Jingquan. Recent advances in cobalt-based electrocatalysts for hydrogen and oxygen evolution reactions[J]. Journal of Alloys and Compounds, 2020, 821: 153542. |
| 39 | REN Yuchun, LI Zerong, DENG Biao, et al. Superior hydrogen evolution electrocatalysis enabled by CoP nanowire array on graphite felt[J]. International Journal of Hydrogen Energy, 2022, 47(6): 3580-3586. |
| 40 | GENG Shuo, TIAN Fenyang, LI Menggang, et al. Hole-rich CoP nanosheets with an optimized d-band center for enhancing pH-universal hydrogen evolution electrocatalysis[J]. Journal of Materials Chemistry A, 2021, 9(13): 8561-8567. |
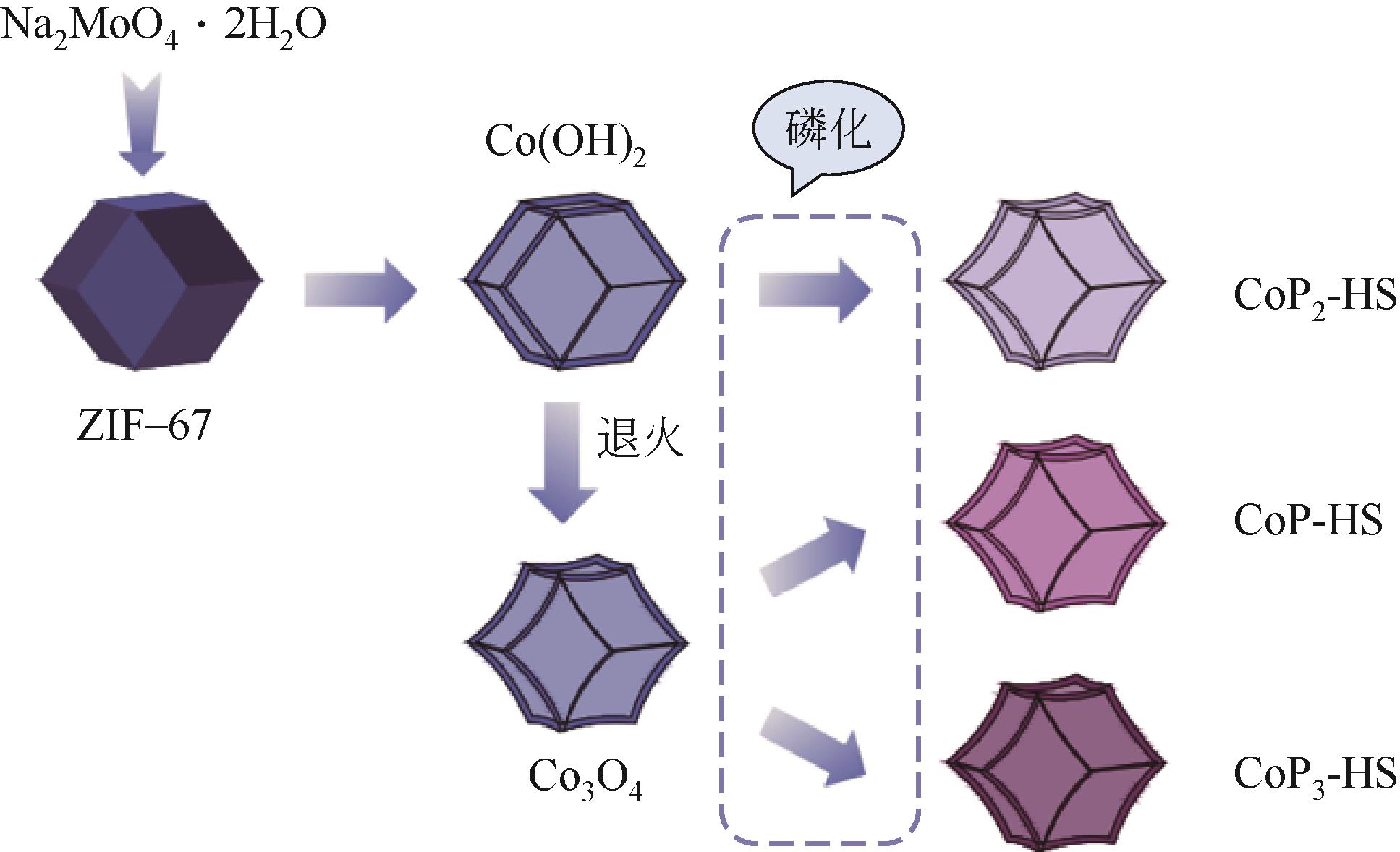
| 41 | LIU Guangbo, WANG Min, XU Yingshuang, et al. Porous CoP/Co2P heterostructure for efficient hydrogen evolution and application in magnesium/seawater battery[J]. Journal of Power Sources, 2021, 486: 229351. |
| 42 | LAI Yue, XIA Wei, LI Jingjing, et al. A confinement strategy for stabilizing two-dimensional carbon/CoP hybrids with enhanced hydrogen evolution[J]. Electrochimica Acta, 2021, 375: 137966. |
| 43 | CHEN Teng, MA Jun, CHEN Shanyong, et al. Construction of heterostructured CoP/CN/Ni: Electron redistribution towards effective hydrogen generation and oxygen reduction[J]. Chemical Engineering Journal, 2021, 415: 129031. |
| 44 | XU Tingting, YANG Liu, LI Jing, et al. NH4F-induced morphology control of CoP nanostructures to enhance the hydrogen evolution reaction[J]. Inorganic Chemistry, 2021, 60(14): 10781-10790. |
| 45 | CHEN Lei, REN Jintao, YUAN Zhongyong. Interface engineering for boosting electrocatalytic performance of CoP-Co2P polymorphs for all-pH hydrogen evolution reaction and alkaline overall water splitting[J]. Science China Materials, 2022, 65(9): 2433-2444. |
| 46 | JEBASLINHEPZYBAI Balasingh Thangadurai, PARTHEEBAN Thamodaran, GAVALI Deepak S, et al. One-pot solvothermal synthesis of Co2P nanoparticles: An efficient HER and OER electrocatalysts[J]. International Journal of Hydrogen Energy, 2021, 46(42): 21924-21938. |
| 47 | XU Siran, ZHAO Haitao, LI Tingshuai, et al. Iron-based phosphides as electrocatalysts for the hydrogen evolution reaction: Recent advances and future prospects[J]. Journal of Materials Chemistry A, 2020, 8(38): 19729-19745. |
| 48 | GE Zhenhua, FU Bin, ZHAO Jinping, et al. A review of the electrocatalysts on hydrogen evolution reaction with an emphasis on Fe, Co and Ni-based phosphides[J]. Journal of Materials Science, 2020, 55(29): 14081-14104. |
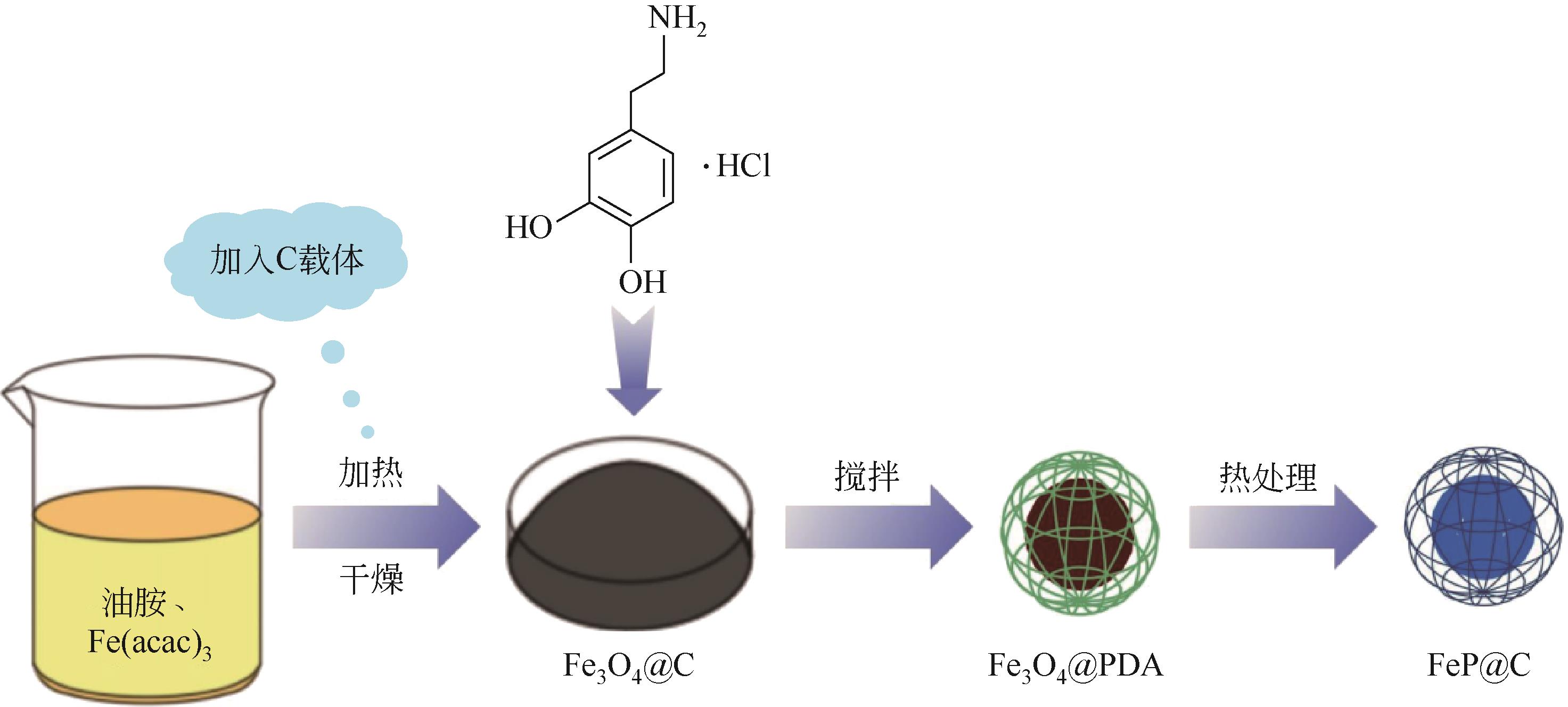
| 49 | PEI Haijiao, ZHANG Limin, ZHI Gang, et al. Rational construction of hierarchical porous FeP nanorod arrays encapsulated in polypyrrole for efficient and durable hydrogen evolution reaction[J]. Chemical Engineering Journal, 2022, 433: 133643. |
| 50 | WANG Yongsheng, WANG Xinyu, ZHANG Lipeng, et al. Insights into the effect of precursors on the FeP-catalyzed hydrogen evolution reaction[J]. Inorganic Chemistry, 2022, 61(6): 2954-2961. |
| 51 | TIAN Lihong, YAN Xiaodong, CHEN Xiaobo. Electrochemical activity of iron phosphide nanoparticles in hydrogen evolution reaction[J]. ACS Catalysis, 2016, 6(8): 5441-5448. |
| 52 | KWONG Wai Ling, Eduardo GRACIA-ESPINO, LEE Cheng Choo, et al. Cationic vacancy defects in iron phosphide: A promising route toward efficient and stable hydrogen evolution by electrochemical water splitting[J]. ChemSusChem, 2017, 10(22): 4544-4551. |
| 53 | Cuncai LYU, PENG Zhen, ZHAO Yaoxing, et al. The hierarchical nanowires array of iron phosphide integrated on a carbon fiber paper as an effective electrocatalyst for hydrogen generation[J]. Journal of Materials Chemistry A, 2016, 4(4): 1454-1460. |
| 54 | PARK Yoonsu, KANG Hyeri, HONG Yunkun, et al. Influence of the phosphorus source on iron phosphide nanoparticle synthesis for hydrogen evolution reaction catalysis[J]. International Journal of Hydrogen Energy, 2020, 45(57): 32780-32788. |
| 55 | ZHANG Yan, ZHANG Huijuan, FENG Yangyang, et al. Unique Fe2P nanoparticles enveloped in sandwichlike graphited carbon sheets as excellent hydrogen evolution reaction catalyst and lithium-ion battery anode[J]. ACS Applied Materials & Interfaces, 2015, 7(48): 26684-26690. |
| 56 | HUANG Huawei, YU Chang, YANG Juan, et al. Ultrasmall diiron phosphide nanodots anchored on graphene sheets with enhanced electrocatalytic activity for hydrogen production via high-efficiency water splitting[J]. Journal of Materials Chemistry A, 2016, 4(41): 16028-16035. |
| 57 | CHOUKI Takwa, MACHREKI Manel, EMIN Saim. Solvothermal synthesis of iron phosphides and their application for efficient electrocatalytic hydrogen evolution[J]. International Journal of Hydrogen Energy, 2020, 45(41): 21473-21482. |
| 58 | PRAMANIK Malay, TOMINAKA Satoshi, WANG Zhongli, et al. Mesoporous semimetallic conductors: Structural and electronic properties of cobalt phosphide systems[J]. Angewandte Chemie International Edition, 2017, 56(43): 13508-13512. |
| 59 | Andres PARRA-PUERTO, Kai Ling NG, FAHY Kieran, et al. Supported transition metal phosphides: Activity survey for HER, ORR, OER, and corrosion resistance in acid and alkaline electrolytes[J]. ACS Catalysis, 2019, 9(12): 11515-11529. |
| 60 | ZHOU Zheng, PEI Zengxia, WEI Li, et al. Electrocatalytic hydrogen evolution under neutral pH conditions: Current understandings, recent advances, and future prospects[J]. Energy & Environmental Science, 2020, 13(10): 3185-3206. |
| 61 | SU Jinzhan, ZHOU Jinglan, WANG Lu, et al. Synthesis and application of transition metal phosphides as electrocatalyst for water splitting[J]. Science Bulletin, 2017, 62(9): 633-644. |
| 62 | SHI Yanmei, ZHANG Bin. Recent advances in transition metal phosphide nanomaterials: Synthesis and applications in hydrogen evolution reaction[J]. Chemical Society Reviews, 2016, 45(6): 1529-1541. |
| 63 | DU Huitong, KONG Rongmei, GUO Xiaoxi, et al. Recent progress in transition metal phosphides with enhanced electrocatalysis for hydrogen evolution[J]. Nanoscale, 2018, 10(46): 21617-21624. |
| 64 | CHEN Jiahui, LIU Jianwen, XIE Jinqi, et al. Co-Fe-P nanotubes electrocatalysts derived from metal-organic frameworks for efficient hydrogen evolution reaction under wide pH range[J]. Nano Energy, 2019, 56: 225-233. |
| 65 | KUCERNAK Anthony R J, NARANAMMALPURAM SUNDARAM Venkata N. Nickel phosphide: The effect of phosphorus content on hydrogen evolution activity and corrosion resistance in acidic medium[J]. Journal of Materials Chemistry A, 2014, 2(41): 17435-17445. |
| 66 | ZHANG Yanan, LI Lei, CHEN Junlei, et al. MOFs template derived Co/Fe binary phosphide nanocomposite embedded in ternary-doped carbon matrix for efficient water splitting[J]. Ceramics International, 2021, 47(9): 12843-12850. |
| 67 | LIN Mengting, LU Ruihu, LUO Wen, et al. Active site identification and interfacial design of a MoP/N-doped carbon catalyst for efficient hydrogen evolution reaction[J]. ACS Applied Energy Materials, 2021, 4(6): 5486-5492. |
| 68 | SUN Hongming, YAN Zhenhua, LIU Fangming, et al. Self-supported transition-metal-based electrocatalysts for hydrogen and oxygen evolution[J]. Advanced Materials, 2020, 32(3): 1806326. |
| 69 | ZHOU Haiqing, YU Fang, SUN Jingying, et al. Highly active catalyst derived from a 3D foam of Fe(PO3)2/Ni2P for extremely efficient water oxidation[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(22): 5607-5611. |
| 70 | HUANG Zhipeng, CHEN Zhongzhong, CHEN Zhibo, et al. Cobalt phosphide nanorods as an efficient electrocatalyst for the hydrogen evolution reaction[J]. Nano Energy, 2014, 9: 373-382. |
| 71 | SUN Meng, LIU Huijuan, QU Jiuhui, et al. Earth-rich transition metal phosphide for energy conversion and storage[J]. Advanced Energy Materials, 2016, 6(13): 1600087. |
| 72 | 王钟, 刘家岐, 刘琛, 等. 过渡金属磷化物用于电解水析氢反应的研究进展[J]. 中国有色金属学报, 2021, 31(11): 3344-3361. |
| WANG Zhong, LIU Jiaqi, LIU Chen, et al. Recent progress of transition metal phosphides in hydrogen evolution reaction of electrolyzed water[J]. The Chinese Journal of Nonferrous Metals, 2021, 31(11): 3344-3361. | |
| 73 | POPCZUN Eric J, READ Carlos G, ROSKE Christopher W, et al. Highly active electrocatalysis of the hydrogen evolution reaction by cobalt phosphide nanoparticles[J]. Angewandte Chemie International Edition, 2014, 53(21): 5427-5430. |
| 74 | JIANG Jun, WANG Chunde, ZHANG Jiajia, et al. Synthesis of FeP2/C nanohybrids and their performance for hydrogen evolution reaction[J]. Journal of Materials Chemistry A, 2015, 3(2): 499-503. |
| 75 | EL-REFAEI Sayed M, RUSSO Patrícia A, NICOLA Pinna. Recent advances in multimetal and doped transition-metal phosphides for the hydrogen evolution reaction at different pH values[J]. ACS Applied Materials & Interfaces, 2021, 13(19): 22077-22097. |
| 76 | LI Yang, DONG Zihao, JIAO Lifang. Multifunctional transition metal-based phosphides in energy-related electrocatalysis[J]. Advanced Energy Materials, 2020, 10(11): 1902104. |
| 77 | ROY Sanjib Baran, TRUONG Linh, JEON Jae Ho, et al. Highly desirable platform for efficient hydrogen generation: Electrodeposited CoP on N-doped vertical graphene[J]. ACS Applied Energy Materials, 2021, 4(6): 5697-5705. |
| 78 | ZHANG Tianqi, LIU Jian, HUANG Linbo, et al. Microbial-phosphorus-enabled synthesis of phosphide nanocomposites for efficient electrocatalysts[J]. Journal of the American Chemical Society, 2017, 139(32): 11248-11253. |
| 79 | ZHANG Chengtian, PU Zonghua, AMIINU Ibrahim Saana, et al. Co2P quantum dot embedded N,P dual-doped carbon self-supported electrodes with flexible and binder-free properties for efficient hydrogen evolution reactions[J]. Nanoscale, 2018, 10(6): 2902-2907. |
| 80 | YU Peng, WANG Fengmei, SHIFA Tofik Ahmed, et al. Earth abundant materials beyond transition metal dichalcogenides: A focus on electrocatalyzing hydrogen evolution reaction[J]. Nano Energy, 2019, 58: 244-276. |
| 81 | YANG Miao, JIANG Yimin, QU Meijiao, et al. Strong electronic couple engineering of transition metal phosphides-oxides heterostructures as multifunctional electrocatalyst for hydrogen production[J]. Applied Catalysis B: Environmental, 2020, 269: 118803. |
| 82 | DANILOVIC N, SUBBARAMAN Ram, STRMCNIK D, et al. Enhancing the alkaline hydrogen evolution reaction activity through the bifunctionality of Ni(OH)2/metal catalysts[J]. Angewandte Chemie International Edition, 2012, 51(50): 12495-12498. |
| 83 | ZHAO Guoqiang, RUI Kun, DOU Shi xue, et al. Heterostructures for electrochemical hydrogen evolution reaction: A review[J]. Advanced Functional Materials, 2018, 28(43): 1803291. |
| 84 | ZHANG Xiangyong, GUO Ting, LIU Tianying, et al. Tungsten phosphide (WP) nanoparticles with tunable crystallinity, W vacancies, and electronic structures for hydrogen production[J]. Electrochimica Acta, 2019, 323: 134798. |
| 85 | DUAN Jingjing, CHEN Sheng, ORTÍZ-LEDÓN César A, et al. Phosphorus vacancies that boost electrocatalytic hydrogen evolution by two orders of magnitude[J]. Angewandte Chemie International Edition, 2020, 59(21): 8181-8186. |
| 86 | KUMARAVEL Sangeetha, KARTHICK Kannimuthu, SANKAR Selvasundarasekar SAM, et al. Recent progresses in engineering of Ni and Co based phosphides for effective electrocatalytic water splitting[J]. ChemElectroChem, 2021, 8(24): 4638-4685. |
| 87 | WANG Pengcheng, LIU Xuefeng, YAN Yaotian, et al. Exploring CoP core-shell nanosheets by Fe and Zn dual cation doping as efficient electrocatalysts for overall water splitting[J]. Catalysis Science & Technology, 2020, 10(5): 1395-1400. |
| 88 | KITCHIN J R, NØRSKOV J K, BARTEAU M A, et al. Modification of the surface electronic and chemical properties of Pt(111) by subsurface 3d transition metals[J]. The Journal of Chemical Physics, 2004, 120(21): 10240-10246. |
| 89 | ZHOU Binghui, GAO Ruijie, ZOU Jijun, et al. Surface design strategy of catalysts for water electrolysis[J]. Small (Weinheim an Der Bergstrasse, Germany), 2022, 18(27): e2202336. |
| 90 | XIN Hongliang, VOJVODIC Aleksandra, VOSS Johannes, et al. Effects of d-band shape on the surface reactivity of transition-metal alloys[J]. Physical Review B, 2014, 89(11): 115114. |
| 91 | Yana MEN, LI Peng, ZHOU Juanhua, et al. Trends in alkaline hydrogen evolution activity on cobalt phosphide electrocatalysts doped with transition metals[J]. Cell Reports Physical Science, 2020, 1(8): 100136. |
| 92 | MAN Ho-Wing, TSANG Chui-Shan, LI Molly Meng-Jung, et al. Transition metal-doped nickel phosphide nanoparticles as electro- and photocatalysts for hydrogen generation reactions[J]. Applied Catalysis B: Environmental, 2019, 242: 186-193. |
| 93 | SUN Shanfu, ZHOU Xin, CONG Bowen, et al. Tailoring the d-band centers endows (Ni x Fe1– x )2P nanosheets with efficient oxygen evolution catalysis[J]. ACS Catalysis, 2020, 10(16): 9086-9097. |
| 94 | PAN Yuan, SUN Kaian, LIN Yan, et al. Electronic structure and d-band center control engineering over M-doped CoP (M=Ni, Mn, Fe) hollow polyhedron frames for boosting hydrogen production[J]. Nano Energy, 2019, 56: 411-419. |
| 95 | CAO Erping, CHEN Zhimin, WU Hao, et al. Boron-induced electronic-structure reformation of CoP nanoparticles drives enhanced pH-universal hydrogen evolution[J]. Angewandte Chemie International Edition, 2020, 59(10): 4154-4160. |
| 96 | WANG Yang, KONG Biao, ZHAO Dongyuan, et al. Strategies for developing transition metal phosphides as heterogeneous electrocatalysts for water splitting[J]. Nano Today, 2017, 15: 26-55. |
| 97 | KIBSGAARD Jakob, JARAMILLO Thomas F. Molybdenum phosphosulfide: An active, acid-stable, earth-abundant catalyst for the hydrogen evolution reaction[J]. Angewandte Chemie International Edition, 2014, 53(52): 14433-14437. |
| 98 | YAN Xuecheng, JIA Yi, YAO Xiangdong. Defective structures in metal compounds for energy-related electrocatalysis[J]. Small Structures, 2021, 2(2): 2000067. |
| 99 | LI Guowei, BLAKE Graeme R, PALSTRA Thomas T M. Vacancies in functional materials for clean energy storage and harvesting: The perfect imperfection[J]. Chemical Society Reviews, 2017, 46(6): 1693-1706. |
| 100 | YANG Mengru, WANG Yuanqiang, GU Yanfang, et al. Electro-deposited copper nanoclusters on leaf-shaped cobalt phosphide for boosting hydrogen evolution reaction[J]. Journal of Alloys and Compounds, 2022, 902: 163771. |
| 101 | YU Yang, QIU Xiaoyu, ZHANG Xinxin, et al. Metal-organic frameworks derived bundled N-doped carbon nanowires confined cobalt phosphide nanocrystals as a robust electrocatalyst for hydrogen production[J]. Electrochimica Acta, 2019, 299: 423-429. |
| 102 | ZHANG Xing, YU Xiaolu, ZHANG Linjie, et al. Molybdenum phosphide/carbon nanotube hybrids as pH-universal electrocatalysts for hydrogen evolution reaction[J]. Advanced Functional Materials, 2018, 28(16): 1706523. |
| 103 | CHUNG Dong Young, Samuel Woojoo JUN, YOON Gabin, et al. Large-scale synthesis of carbon-shell-coated FeP nanoparticles for robust hydrogen evolution reaction electrocatalyst[J]. Journal of the American Chemical Society, 2017, 139(19): 6669-6674. |
| 104 | ZHAO Di, SUN Kaian, CHEONG Weng-Chon, et al. Synergistically interactive pyridinic-N-MoP sites: Identified active centers for enhanced hydrogen evolution in alkaline solution[J]. Angewandte Chemie International Edition, 2020, 59(23): 8982-8990. |
| 105 | WANG Minqiang, YE Cui, LIU Heng, et al. Nanosized metal phosphides embedded in nitrogen-doped porous carbon nanofibers for enhanced hydrogen evolution at all pH values[J]. Angewandte Chemie International Edition, 2018, 57(7): 1963-1967. |
| 106 | KIM Dongwon, QIN Xinyu, YAN Bingyi, et al. Nano/microscale integrated mushroom-shaped hydrophilic CoP@Ni-CoP with optimized gas bubble release for high-performance water splitting catalysis[J]. ACS Applied Energy Materials, 2020, 3(10): 9769-9784. |
| 107 | WANG Xiang, YANG Linlin, XING Congcong, et al. MOF-derived ultrathin cobalt molybdenum phosphide nanosheets for efficient electrochemical overall water splitting[J]. Nanomaterials (Basel, Switzerland), 2022, 12(7): 1098. |
| 108 | ZHANG Wei, HAN Ning, LUO Jiangshui, et al. Critical role of phosphorus in hollow structures cobalt-based phosphides as bifunctional catalysts for water splitting[J]. Small (Weinheim an Der Bergstrasse, Germany), 2022, 18(4): e2103561. |
| 109 | WANG Qin, LIU Zhengqing, ZHAO Hongyang, et al. MOF-derived porous Ni2P nanosheets as novel bifunctional electrocatalysts for the hydrogen and oxygen evolution reactions[J]. Journal of Materials Chemistry A, 2018, 6(38): 18720-18727. |
| 110 | LIU Meijun, YANG Liming, LIU Tian, et al. Fe2P/reduced graphene oxide/Fe2P sandwich-structured nanowall arrays: A high-performance non-noble-metal electrocatalyst for hydrogen evolution[J]. Journal of Materials Chemistry A, 2017, 5(18): 8608-8615. |
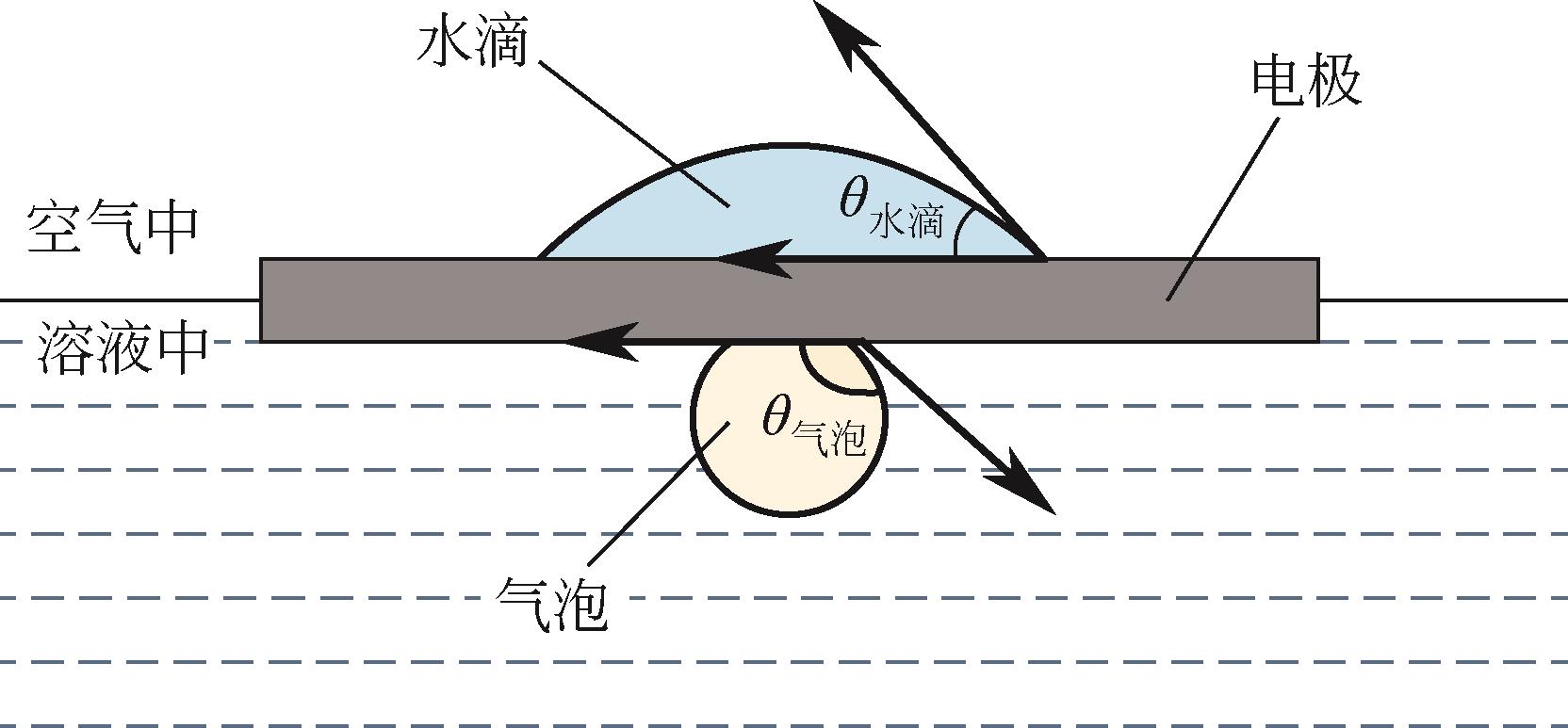
| 111 | LONG Zhiyun, ZHAO Yuyan, ZHANG Chunhui, et al. A multi-bioinspired dual-gradient electrode for microbubble manipulation toward controllable water splitting[J]. Advanced Materials (Deerfield Beach, Fla), 2020, 32(17): e1908099. |
| 112 | YI Xinli, SONG Lizhu, OUYANG Shuxin, et al. Structural and componential engineering of Co2P&CoP@N-C nanoarrays for energy-efficient hydrogen production from water electrolysis[J]. ACS Applied Materials & Interfaces, 2021, 13(47): 56064-56072. |
| 113 | CHEN Xibang, SHENG Lang, LI Shuangxiao, et al. Facile syntheses and in situ study on electrocatalytic properties of superaerophobic Co x P-nanoarray in hydrogen evolution reaction[J]. Chemical Engineering Journal, 2021, 426: 131029. |
| 114 | JIN Song. Are metal chalcogenides, nitrides, and phosphides oxygen evolution catalysts or bifunctional catalysts?[J]. ACS Energy Letters, 2017, 2(8): 1937-1938. |
| 115 | LAURSEN Anders B, WEXLER Robert B, WHITAKER Marianna J, et al. Climbing the volcano of electrocatalytic activity while avoiding catalyst corrosion: Ni3P, a hydrogen evolution electrocatalyst stable in both acid and alkali[J]. ACS Catalysis, 2018, 8(5): 4408-4419. |
| 116 | SU Liang, CUI Xiangzhi, HE Ting, et al. Surface reconstruction of cobalt phosphide nanosheets by electrochemical activation for enhanced hydrogen evolution in alkaline solution[J]. Chemical Science, 2018, 10(7): 2019-2024. |
| 117 | ZHANG Linlin, DING Xin, CONG Meiyu, et al. Self-adaptive amorphous Co2P@Co2P/Co-polyoxometalate/nickel foam as an effective electrode for electrocatalytic water splitting in alkaline electrolyte[J]. International Journal of Hydrogen Energy, 2019, 44(18): 9203-9209. |
| 118 | YOU Bo, TANG Michael T, CHARLIE Tsai, et al. Enhancing electrocatalytic water splitting by strain engineering[J]. Advanced Materials (Deerfield Beach, Fla), 2019, 31(17): e1807001. |
| 119 | ZHANG Chenyun, XIN Bingwei, XI Zhucong, et al. Phosphonium-based ionic liquid: A new phosphorus source toward microwave-driven synthesis of nickel phosphide for efficient hydrogen evolution reaction[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(1): 1468-1477. |
| 120 | POPCZUN Eric J, MCKONE James R, READ Carlos G, et al. Nanostructured nickel phosphide as an electrocatalyst for the hydrogen evolution reaction[J]. Journal of the American Chemical Society, 2013, 135(25): 9267-9270. |
| 121 | 杨环环, 喻彬璐, 王佳宏, 等. 二维黑磷的制备、表面功能化与光电催化[J]. 无机盐工业, 2021, 53(5): 13-20. |
| YANG Huanhuan, YU Binlu, WANG Jiahong, et al. Preparation, surface functionalization and photoelectrocatalysis of two-dimensional black phosphorus[J]. Inorganic Chemicals Industry, 2021, 53(5): 13-20. | |
| 122 | 曹福臣, 袁振东. 黑磷的发现及其制备和应用发展史[J]. 化学通报, 2021, 84(2): 185-191. |
| CAO Fuchen, YUAN Zhendong. The discovery, preparation and application history of black phosphorus[J]. Chemistry, 2021, 84(2): 185-191. |
| [1] | ZHANG Mingyan, LIU Yan, ZHANG Xueting, LIU Yake, LI Congju, ZHANG Xiuling. Research progress of non-noble metal bifunctional catalysts in zinc-air batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 276-286. |
| [2] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [3] | XIE Luyao, CHEN Songzhe, WANG Laijun, ZHANG Ping. Platinum-based catalysts for SO2 depolarized electrolysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 299-309. |
| [4] | YANG Xiazhen, PENG Yifan, LIU Huazhang, HUO Chao. Regulation of active phase of fused iron catalyst and its catalytic performance of Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 310-318. |
| [5] | ZHANG Jie, BAI Zhongbo, FENG Baoxin, PENG Xiaolin, REN Weiwei, ZHANG Jingli, LIU Eryong. Effect of PEG and its compound additives on post-treatment of electrolytic copper foils [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 374-381. |
| [6] | WANG Lele, YANG Wanrong, YAO Yan, LIU Tao, HE Chuan, LIU Xiao, SU Sheng, KONG Fanhai, ZHU Canghai, XIANG Jun. Influence of spent SCR catalyst blending on the characteristics and deNO x performance for new SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 489-497. |
| [7] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [8] | CHENG Tao, CUI Ruili, SONG Junnan, ZHANG Tianqi, ZHANG Yunhe, LIANG Shijie, PU Shi. Analysis of impurity deposition and pressure drop increase mechanisms in residue hydrotreating unit [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4616-4627. |
| [9] | WANG Peng, SHI Huibing, ZHAO Deming, FENG Baolin, CHEN Qian, YANG Da. Recent advances on transition metal catalyzed carbonylation of chlorinated compounds [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4649-4666. |
| [10] | ZHANG Qi, ZHAO Hong, RONG Junfeng. Research progress of anti-toxicity electrocatalysts for oxygen reduction reaction in PEMFC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4677-4691. |
| [11] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| [12] | WANG Weitao, BAO Tingyu, JIANG Xulu, HE Zhenhong, WANG Kuan, YANG Yang, LIU Zhaotie. Oxidation of benzene to phenol over aldehyde-ketone resin based metal-free catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4706-4715. |
| [13] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [14] | WU Haibo, WANG Xilun, FANG Yanxiong, JI Hongbing. Progress of the development and application of 3D printing catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3956-3964. |
| [15] | XIANG Yang, HUANG Xun, WEI Zidong. Recent progresses in the activity and selectivity improvement of electrocatalytic organic synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4005-4014. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||