Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (6): 3324-3332.DOI: 10.16085/j.issn.1000-6613.2021-1356
• Resources and environmental engineering • Previous Articles Next Articles
Highly efficient adsorption of Basic Violet 3 dye by composite material derived from graphene oxide intercalated bentonite
CHEN Yong1( ), CHENG Ning2(
), CHENG Ning2( ), YANG Yubing1, LU Kailing1, LUO Ying1, YI Hui1
), YANG Yubing1, LU Kailing1, LUO Ying1, YI Hui1
- 1.Department of Food and Chemical Engineering, Liuzhou Institute of Technology, Liuzhou 545616, Guangxi, China
2.School of Environment and Food Engineering, Liuzhou Vocational and Technical College, Liuzhou 545000, Guangxi, China
-
Received:2021-06-28Revised:2021-08-25Online:2022-06-21Published:2022-06-10 -
Contact:CHENG Ning
氧化石墨烯插层膨润土复合材料高效吸附碱性紫3染料
陈勇1( ), 程宁2(
), 程宁2( ), 杨育兵1, 卢凯玲1, 罗应1, 易慧1
), 杨育兵1, 卢凯玲1, 罗应1, 易慧1
- 1.柳州工学院食品与化学工程学院,广西 柳州 545616
2.柳州职业技术学院环境与食品工程学院,广西 柳州 545000
-
通讯作者:程宁 -
作者简介:陈勇(1986—),男,硕士,讲师,研究方向为化工材料。E-mail:chenyong2009biye@163.com 。 -
基金资助:广西高校中青年教师科研基础能力提升项目(2022KY1035);柳州工学院科学基金(2021KXJJ05);广西高校中青年教师科研基础能力提升项目(2018KY0882)
CLC Number:
Cite this article
CHEN Yong, CHENG Ning, YANG Yubing, LU Kailing, LUO Ying, YI Hui. Highly efficient adsorption of Basic Violet 3 dye by composite material derived from graphene oxide intercalated bentonite[J]. Chemical Industry and Engineering Progress, 2022, 41(6): 3324-3332.
陈勇, 程宁, 杨育兵, 卢凯玲, 罗应, 易慧. 氧化石墨烯插层膨润土复合材料高效吸附碱性紫3染料[J]. 化工进展, 2022, 41(6): 3324-3332.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2021-1356
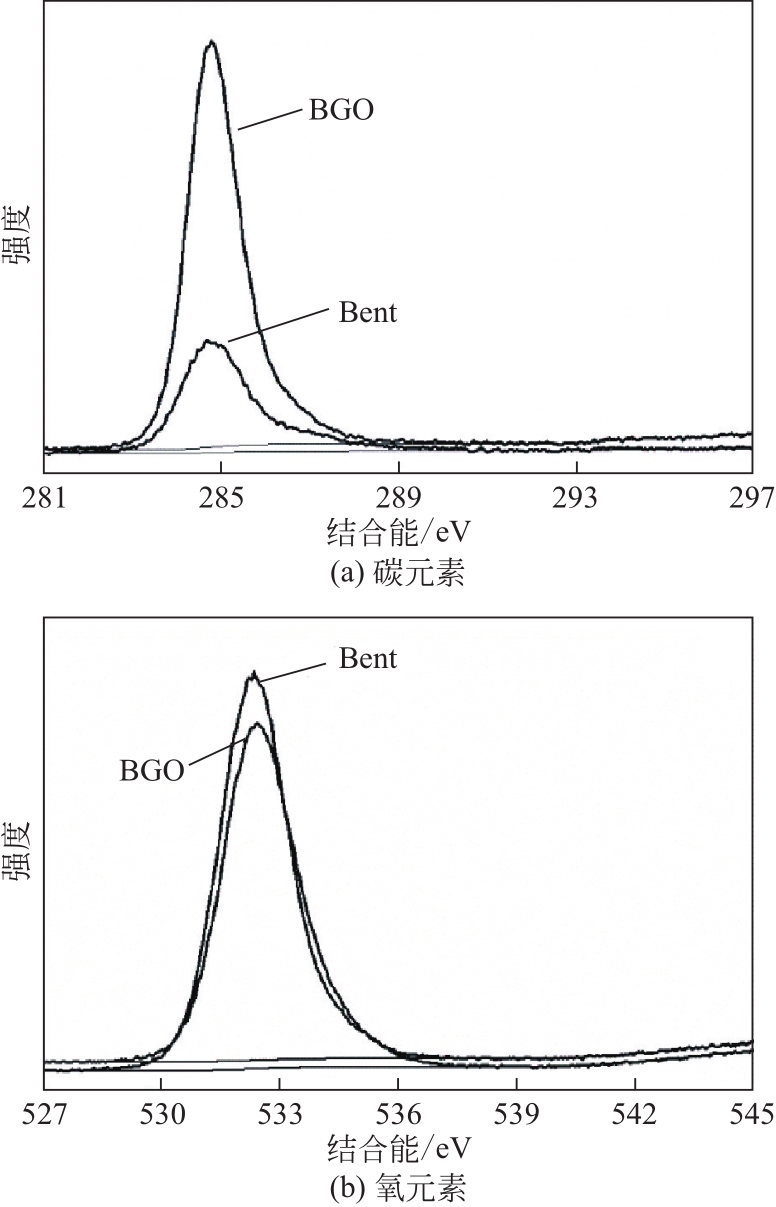
| 样品 | 元素质量分数/% | ||||
|---|---|---|---|---|---|
| C | O | N | Al | Si | |
| Bent | 20.71 | 51.54 | 0.93 | 8.64 | 18.17 |
| BGO | 40.09 | 35.71 | 0.58 | 6.05 | 13.57 |
| 样品 | 元素质量分数/% | ||||
|---|---|---|---|---|---|
| C | O | N | Al | Si | |
| Bent | 20.71 | 51.54 | 0.93 | 8.64 | 18.17 |
| BGO | 40.09 | 35.71 | 0.58 | 6.05 | 13.57 |
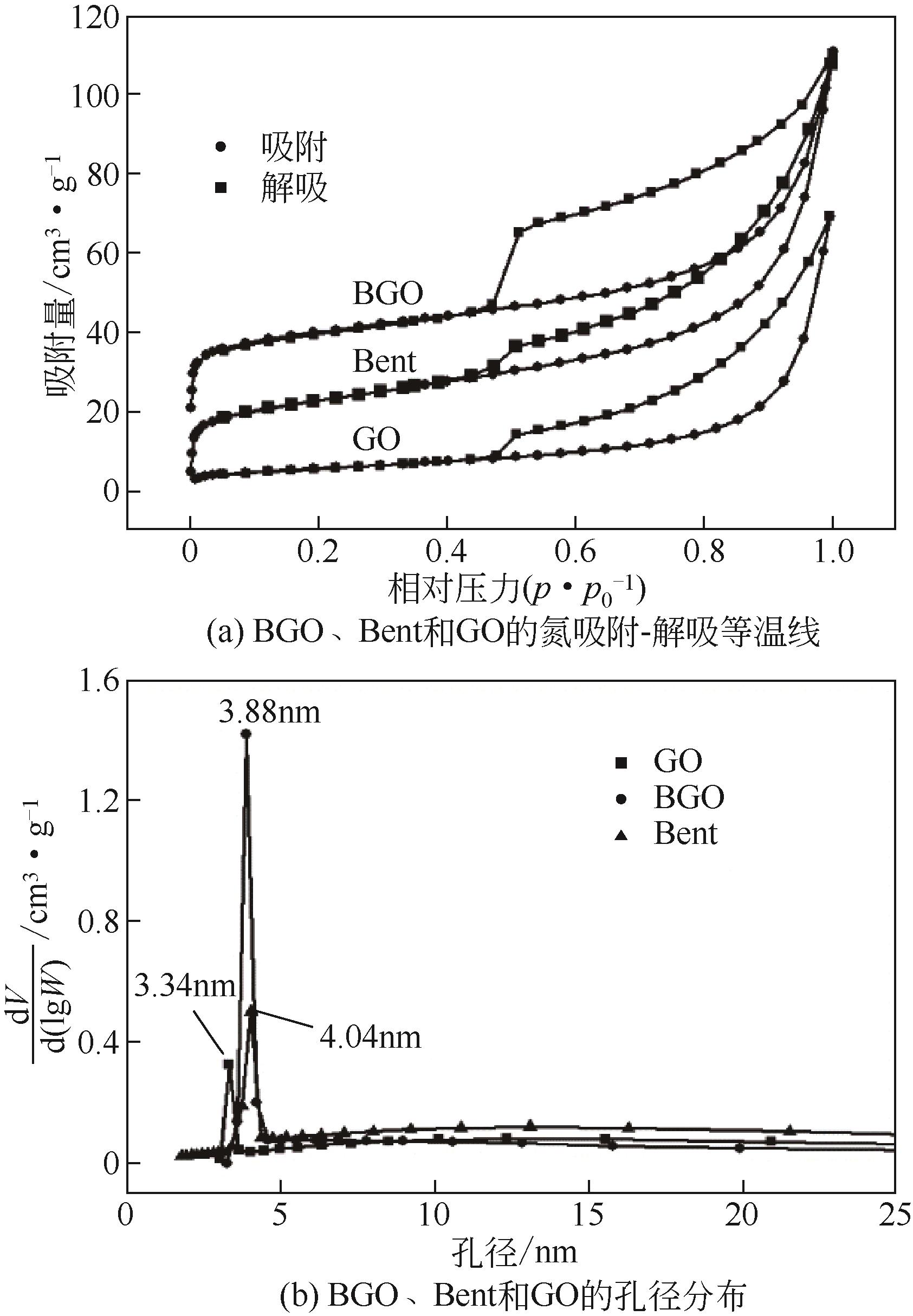
| 样品 | 总比表面积SBET/m2·g-1 | 孔径DBJH/nm | 孔容/cm3·g-1 |
|---|---|---|---|
| Bent | 79.28 | 4.04 | 0.1513 |
| GO | 44.80 | 3.34 | 0.1069 |
| BGO | 95.79 | 3.88 | 0.1637 |
| 样品 | 总比表面积SBET/m2·g-1 | 孔径DBJH/nm | 孔容/cm3·g-1 |
|---|---|---|---|
| Bent | 79.28 | 4.04 | 0.1513 |
| GO | 44.80 | 3.34 | 0.1069 |
| BGO | 95.79 | 3.88 | 0.1637 |
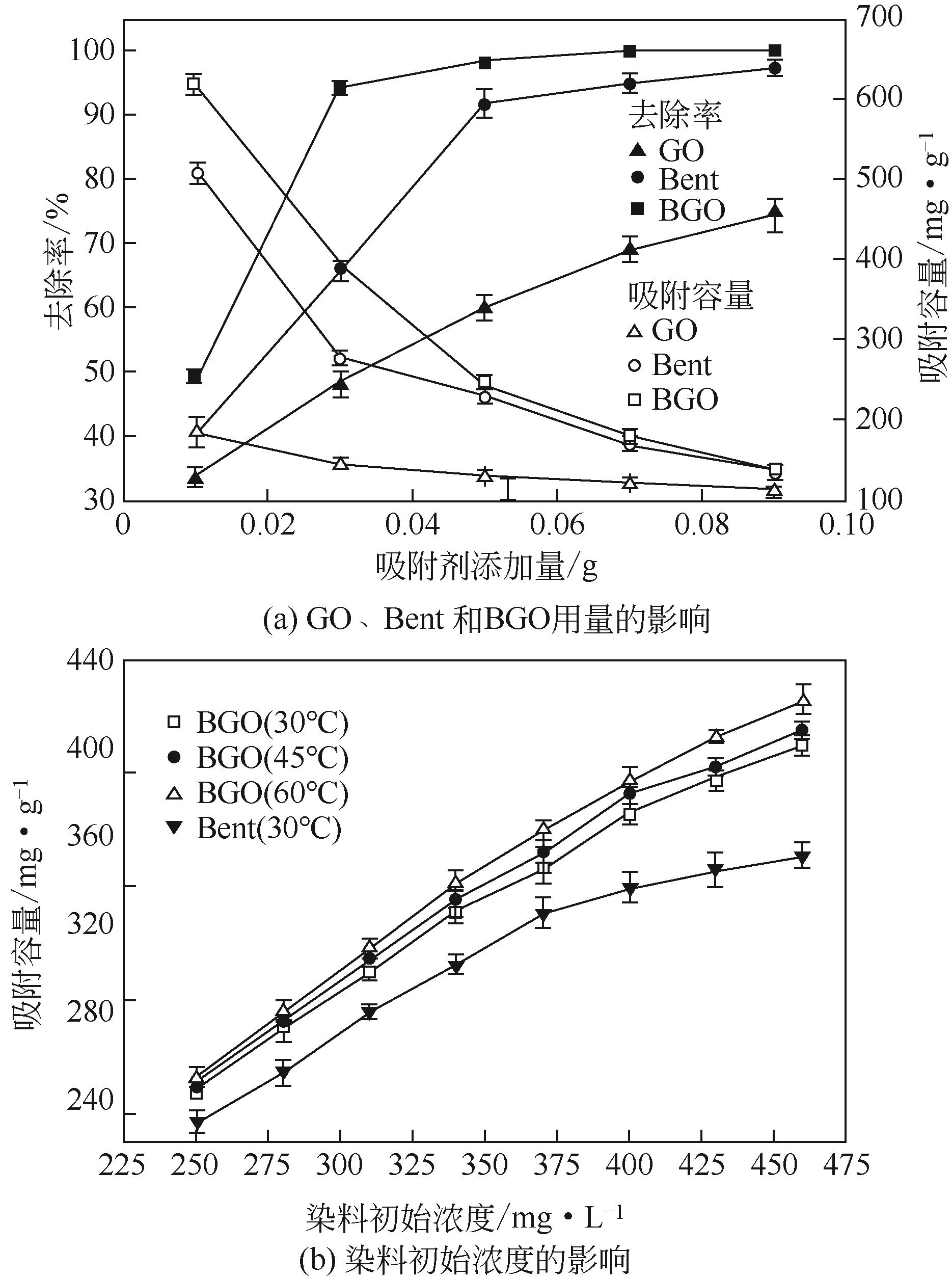
初始浓度 /mg·L-1 | 伪一级反应速率模型 | 伪二级反应速率模型 | ||||
|---|---|---|---|---|---|---|
| qe/mg·g-1 | k1/min-1 | r12 | qe/mg·g-1 | k2/g·mg-1·min-1 | r22 | |
| 200 | 10.22 | 0.04 | 0.8691 | 199.60 | 0.290 | 0.99999 |
| 250 | 3.30 | 0.08 | 0.9640 | 248.76 | 0.010 | 0.99999 |
| 300 | 29.52 | 0.06 | 0.8951 | 299.40 | 0.007 | 0.99998 |
初始浓度 /mg·L-1 | 伪一级反应速率模型 | 伪二级反应速率模型 | ||||
|---|---|---|---|---|---|---|
| qe/mg·g-1 | k1/min-1 | r12 | qe/mg·g-1 | k2/g·mg-1·min-1 | r22 | |
| 200 | 10.22 | 0.04 | 0.8691 | 199.60 | 0.290 | 0.99999 |
| 250 | 3.30 | 0.08 | 0.9640 | 248.76 | 0.010 | 0.99999 |
| 300 | 29.52 | 0.06 | 0.8951 | 299.40 | 0.007 | 0.99998 |
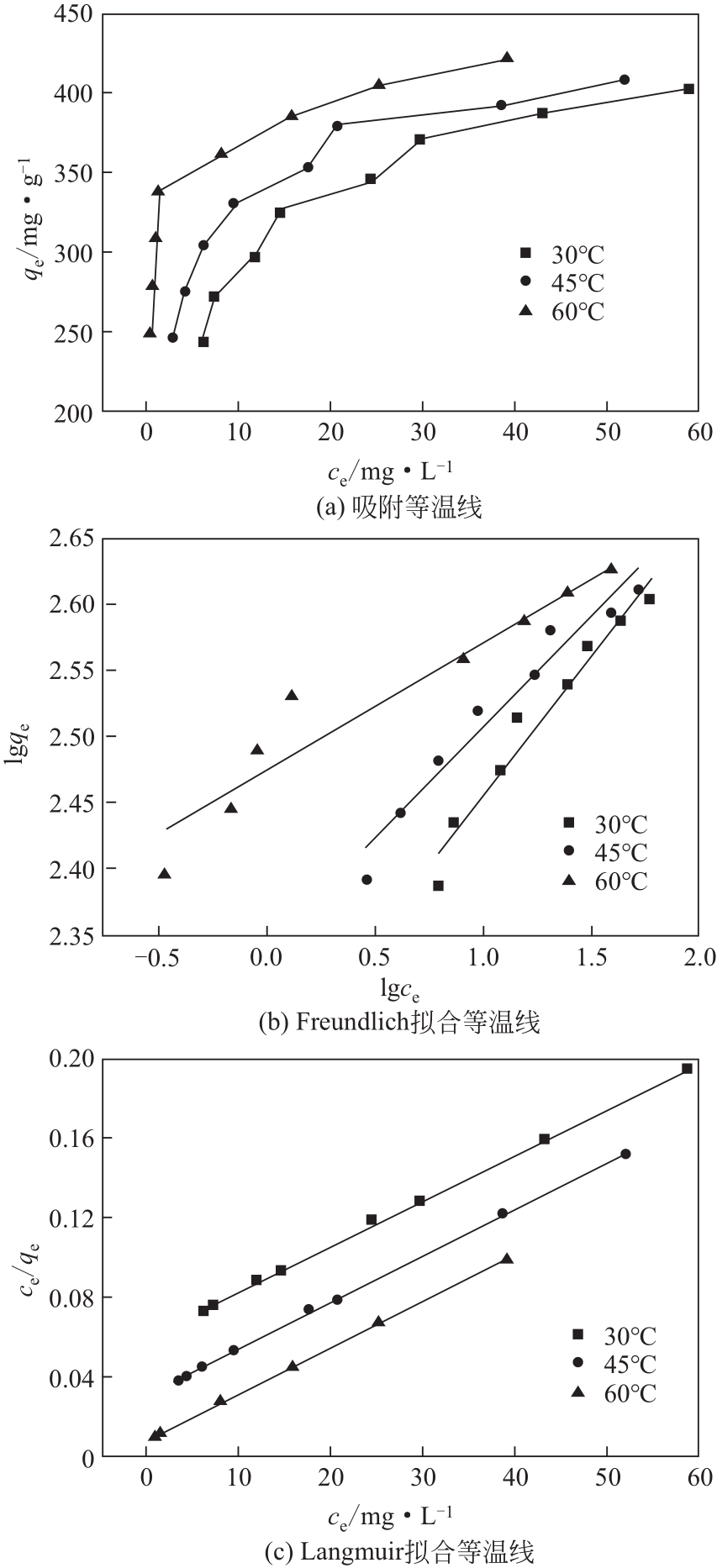
吸附温度 /℃ | Langmuir方程 | Freundlich方程 | |||||
|---|---|---|---|---|---|---|---|
| qmax/mg·g-1 | kL/L·mg-1 | RL2 | kF/L·g-1 | 1/n | RF2 | ||
| 30 | 420.17 | 0.20 | 0.9989 | 176.86 | 0.21 | 0.95494 | |
| 45 | 421.94 | 0.40 | 0.9989 | 218.70 | 0.17 | 0.95244 | |
| 60 | 431.03 | 1.64 | 0.9983 | 298.63 | 0.097 | 0.90636 | |
吸附温度 /℃ | Langmuir方程 | Freundlich方程 | |||||
|---|---|---|---|---|---|---|---|
| qmax/mg·g-1 | kL/L·mg-1 | RL2 | kF/L·g-1 | 1/n | RF2 | ||
| 30 | 420.17 | 0.20 | 0.9989 | 176.86 | 0.21 | 0.95494 | |
| 45 | 421.94 | 0.40 | 0.9989 | 218.70 | 0.17 | 0.95244 | |
| 60 | 431.03 | 1.64 | 0.9983 | 298.63 | 0.097 | 0.90636 | |
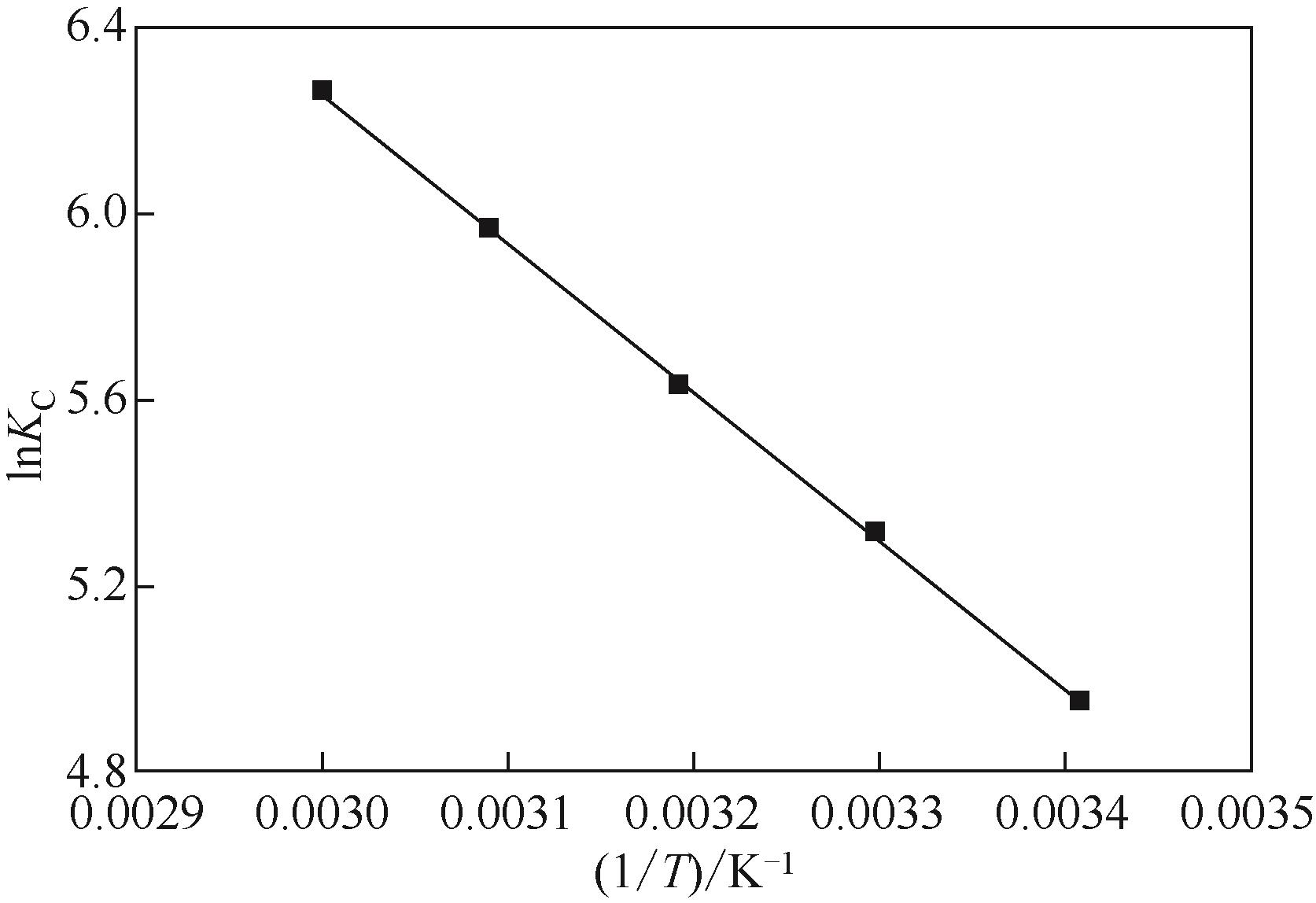
| 温度/K | ?G/kJ·mol-1 | ?S/J·mol-1·K-1 | ?H/kJ·mol-1 |
|---|---|---|---|
| 293 | -12.06 | 130.78 | 26.29 |
| 303 | -12.39 | ||
| 313 | -14.63 | ||
| 323 | -16.01 | ||
| 333 | -17.33 |
| 温度/K | ?G/kJ·mol-1 | ?S/J·mol-1·K-1 | ?H/kJ·mol-1 |
|---|---|---|---|
| 293 | -12.06 | 130.78 | 26.29 |
| 303 | -12.39 | ||
| 313 | -14.63 | ||
| 323 | -16.01 | ||
| 333 | -17.33 |
| 1 | KARIMIFARD S, ALAVI MOGHADDAM M R. Application of response surface methodology in physicochemical removal of dyes from wastewater: a critical review[J]. Science of the Total Environment, 2018, 640/641: 772-797. |
| 2 | LI W, MU B N, YANG Y Q. Feasibility of industrial-scale treatment of dye wastewater via bio-adsorption technology[J]. Bioresource Technology, 2019, 277: 157-170. |
| 3 | ZHANG P, LO I, O'CONNOR D, et al. High efficiency removal of methylene blue using SDS surface-modified ZnFe2O4 nanoparticles[J]. Journal of Colloid and Interface Science, 2017, 508: 39-48. |
| 4 | THAKUR S, PANDEY S, AROTIBA O A. Development of a sodium alginate-based organic/inorganic superabsorbent composite hydrogel for adsorption of methylene blue[J]. Carbohydrate Polymers, 2016, 153: 34-46. |
| 5 | LIU C, OMER A M, OUYANG X K. Adsorptive removal of cationic methylene blue dye using carboxymethyl cellulose/k-carrageenan/activated montmorillonite composite beads: isotherm and kinetic studies[J]. International Journal of Biological Macromolecules, 2018, 106: 823-833. |
| 6 | LIU C, ZHU G L, SONG S X, et al. Flotation separation of smithsonite from quartz using calcium lignosulphonate as a depressant and sodium oleate as a collector[J]. Minerals Engineering, 2019, 131: 385-391. |
| 7 | ZHU Y, FAN W H, ZHOU T T, et al. Removal of chelated heavy metals from aqueous solution: a review of current methods and mechanisms[J]. Science of the Total Environment, 2019, 678: 253-266. |
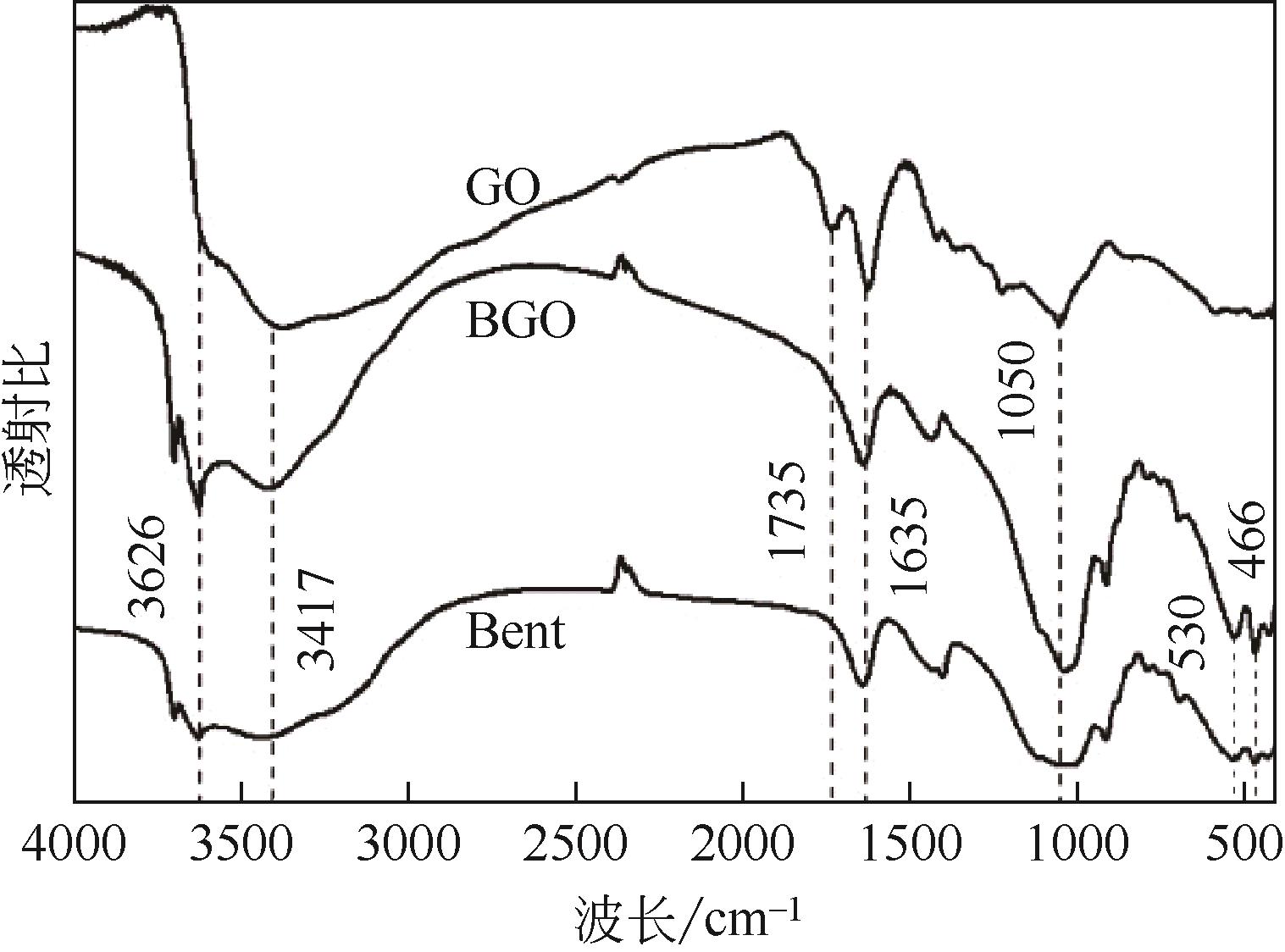
| 8 | 宋晓东, 程昌敬, 余海溶. 阴离子聚电解质修饰磁性氧化石墨烯对碱性品红的吸附性能[J]. 化工进展, 2016, 35(9): 2973-2981. |
| SONG Xiaodong, CHENG Changjing, YU Hairong. Adsorption performance of an anionic polyelectrolyte-functionalized magnetic graphene oxide for basic fuchsin[J]. Chemical Industry and Engineering Progress, 2016, 35(9): 2973-2981. | |
| 9 | BAI H Y, ZHAO Y L, ZHANG X, et al. Correlation of exfoliation performance with interlayer cations of montmorillonite in the preparation of two-dimensional nanosheets[J]. Journal of the American Ceramic Society, 2019, 102(7): 3908-3922. |
| 10 | ZHAO Y L, KANG S C, QIN L, et al. Self-assembled gels of Fe-chitosan/montmorillonite nanosheets: dye degradation by the synergistic effect of adsorption and photo-Fenton reaction[J]. Chemical Engineering Journal, 2020, 379: 122322. |
| 11 | FARROKHI-RAD M, MOHAMMADALIPOUR M, SHAHRABI T. Electrophoretically deposited halloysite nanotubes coating as the adsorbent for the removal of methylene blue from aqueous solution[J]. Journal of the European Ceramic Society, 2018, 38(10): 3650-3659. |
| 12 | LI Y H, DU Q J, LIU T H, et al. Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes[J]. Chemical Engineering Research and Design, 2013, 91(2): 361-368. |
| 13 | KYZAS G Z, FU J, MATIS K A. The change from past to future for adsorbent materials in treatment of dyeing wastewaters[J]. Materials, 2013, 6(11): 5131-5158. |
| 14 | 杜夕铭, 卢钧, 陈泉源. 氧化石墨对活性红X-3B的吸附特性及再生性能[J]. 水处理技术, 2019, 45(8): 35-39, 44. |
| DU Ximing, LU Jun, CHEN Quanyuan. Adsorption characteristics of reactive red X-3B by graphite oxide and its regeneration[J]. Technology of Water Treatment, 2019, 45(8): 35-39, 44. | |
| 15 | 王希越, 明明, 连丽丽, 等. 磁性氧化石墨烯纳米粒子的制备及其对大红染料的去除[J]. 应用化工, 2019, 48(10): 2324-2327. |
| WANG Xiyue, MING Ming, LIAN Lili, et al. Preparation of magnetic graphene oxide nanoparticles and its application for red dye removal[J]. Applied Chemical Industry, 2019, 48(10): 2324-2327. | |
| 16 | LIU L, ZHANG Y R, HE Y J, et al. Preparation of montmorillonite-pillared graphene oxide with increased single- and co-adsorption towards lead ions and methylene blue[J]. RSC Advances, 2015, 5(6): 3965-3973. |
| 17 | ZHANG Z L, LUO H J, JIANG X L, et al. Synthesis of reduced graphene oxide-montmorillonite nanocomposite and its application in hexavalent chromium removal from aqueous solutions[J]. RSC Advances, 2015, 5(59): 47408-47417. |
| 18 | 王志明, 吕宪俊, 渠美云. 膨润土提纯工艺研究[J]. 现代矿业, 2013, 29(3): 26-29, 50. |
| WANG Zhiming, Xianjun LYU, QU Meiyun. Research on purification technology of montmorillonite[J]. Modern Mining, 2013, 29(3): 26-29, 50. | |
| 19 | MUNIR M, AHMAD M, SAEED M, et al. Sustainable production of bioenergy from novel non-edible seed oil (Prunus cerasoides) using bimetallic impregnated montmorillonite clay catalyst[J]. Renewable and Sustainable Energy Reviews, 2019, 109: 321-332. |
| 20 | WANG J Q, HAN Z D. The combustion behavior of polyacrylate ester/graphite oxide composites[J]. Polymers for Advanced Technologies, 2006, 17(4): 335-340. |
| 21 | TYAGI B, CHUDASAMA C D, JASRA R V. Determination of structural modification in acid activated montmorillonite clay by FT-IR spectroscopy[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2006, 64(2): 273-278. |
| 22 | ZHANG X L, CHENG L P, WU X P, et al. Activated carbon coated palygorskite as adsorbent by activation and its adsorption for methylene blue[J]. Journal of Environmental Sciences, 2015, 33: 97-105. |
| 23 | BRADDER P, LING S K, WANG S B, et al. Dye adsorption on layered graphite oxide[J]. Journal of Chemical & Engineering Data, 2011, 56(1): 138-141. |
| 24 | TEKIN N, ŞAFAKLI A, BINGÖL D. Process modeling and thermodynamics and kinetics evaluation of Basic Yellow 28 adsorption onto sepiolite[J]. Desalination and Water Treatment, 2015, 54(7): 2023-2035. |
| 25 | DERAKHSHAN Z, BAGHAPOUR M A, RANJBAR M, et al. Adsorption of methylene blue dye from aqueous solutions by modified pumice stone: kinetics and equilibrium studies[J]. Health Scope, 2013, 2(3): 136-144. |
| [1] | XU Chunshu, YAO Qingda, LIANG Yongxian, ZHOU Hualong. Effects of graphene oxide/carbon nanotubes on the properties of several typical polymer materials [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3012-3028. |
| [2] | HE Yang, LI Siying, LI Chuanqiang, YUAN Xiaoya, ZHENG Xuxu. Anticorrosion performance of thermal reduction graphene oxide /epoxy resin composite coating [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1983-1994. |
| [3] | ZU Mei, XU Haitao, XIE Wei, CHENG Haifeng. Progress in water stable and water absorption applications of metal-organic frameworks [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4254-4267. |
| [4] | DUAN Zhengyang, HU Ningmeng, LI Tianguo. Preparation of xanthate-functionalized cross-linked baker's yeast and its adsorption characteristics for Pb(Ⅱ) [J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3925-3937. |
| [5] | XIONG Jian, XIA Liufen, YU Lei, FEI Anjie, XU Chu, CHEN Shengya, JIANG Guodong. Preparation and electrochromic properties of graphene oxide and TiO2-B composite films [J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3794-3800. |
| [6] | YANG Honghai, ZHANG Miao, LIU Liwei, ZHOU Yi, SHEN Junjie, SHI Weigang, YIN Yong. Heat transfer performance enhancement and prediction in GO/water pulsating heat pipe [J]. Chemical Industry and Engineering Progress, 2022, 41(4): 1725-1734. |
| [7] | MAO Menglei, SUN Danyang, MENG Zihui, LIU Wenfang. Enzyme immobilization on graphene oxide and transition metal carbon/nitrogen compounds [J]. Chemical Industry and Engineering Progress, 2022, 41(4): 1941-1955. |
| [8] | FAN Xiangru, YANG Yijin, GUO Xujing, ZHANG Quanbi. Study on the adsorption characteristics of methylene blue by soft manganese ore-oil sludge-based activated carbon [J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6664-6671. |
| [9] | CHEN Jing, SHEN Yanqin, YAO Yijun, HU Chengmeng, WU Hailiang. Research progress of superabsorbent polymer materials [J]. Chemical Industry and Engineering Progress, 2022, 41(11): 5925-5935. |
| [10] | ZHENG Anda, YANG Chenggong, WANG Dong’e, TIAN Zhijian. Highly active MoS2/reduced graphene oxide catalyst for anthracene hydrogenation [J]. Chemical Industry and Engineering Progress, 2022, 41(1): 244-252. |
| [11] | YANG Xiaofang, WEI Ming, SUN Li. Preparation and research of PAM/CQDs/GO composite hydrogel [J]. Chemical Industry and Engineering Progress, 2021, 40(S2): 301-308. |
| [12] | HU Huimin, FANG Xiaofeng, LOU Mengmeng, LIU Shuai, XU Chenye, LI Fang. Research process on performance regulation and mass transfer mechanism of graphene oxide separation membrane [J]. Chemical Industry and Engineering Progress, 2021, 40(S1): 291-300. |
| [13] | JIANG Bolong, SHI Shunjie, JIANG Hailin, FENG Xin, SUN Haofen. Research progress in phenol adsorption mechanism over metal-organic framework from wastewater [J]. Chemical Industry and Engineering Progress, 2021, 40(8): 4525-4539. |
| [14] | MA Jun, SUN Dong, ZHANG Mingshuang, ZHANG Lanhe, CHEN Zicheng. Preparation of graphene oxide modified epoxy resin coating and research on its anti-corrosive performance [J]. Chemical Industry and Engineering Progress, 2021, 40(8): 4456-4462. |
| [15] | LIU Qin, ZHOU Xintao, HUANG Jing, LUO Zhongqiu, SHAO Zhoujun, WANG Luxing, WEI Yu, LUO Yunlong. Research statue on adsorption properties and mechanism of heavy metal ions using red mud [J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3455-3465. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||