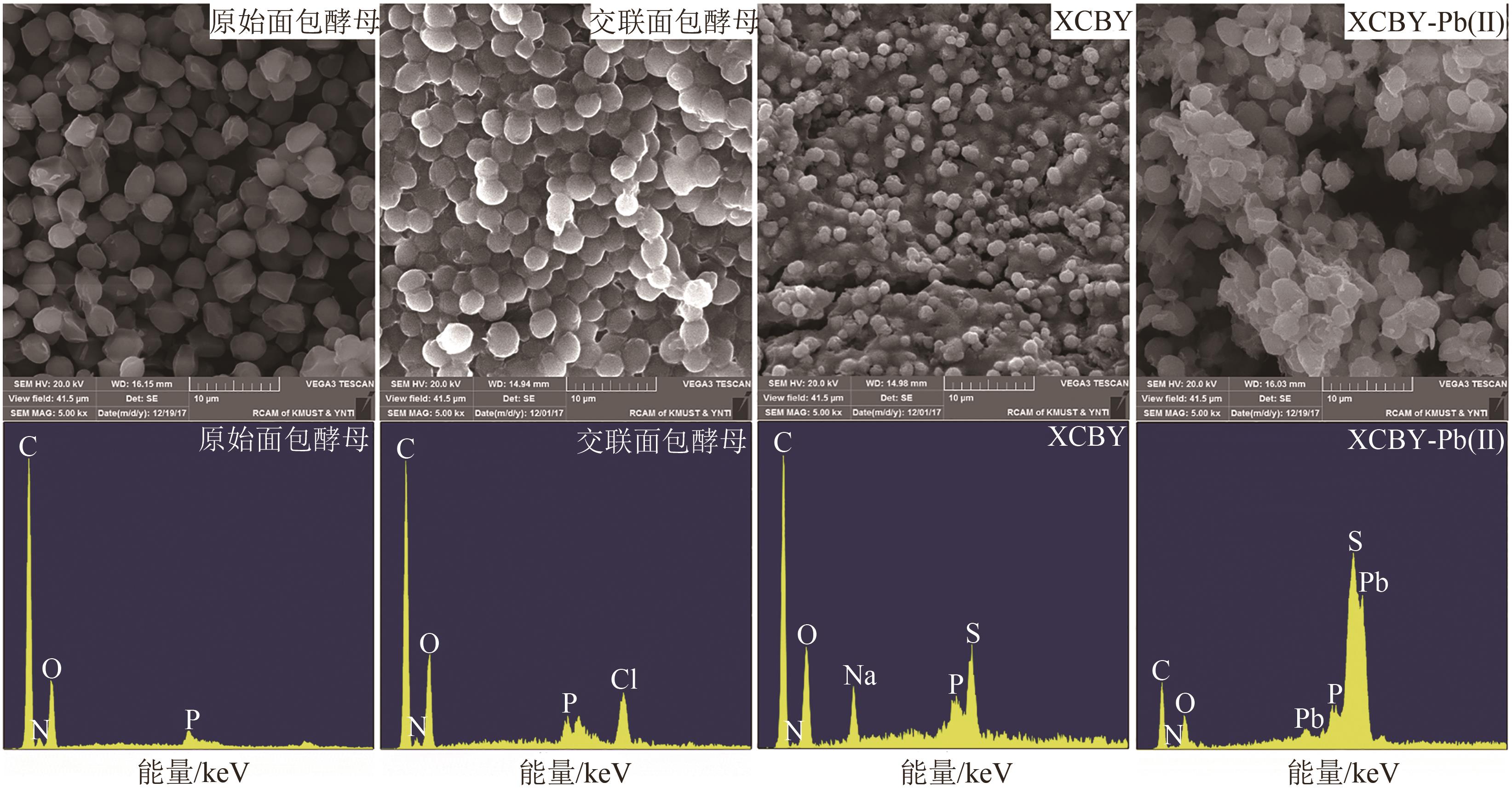
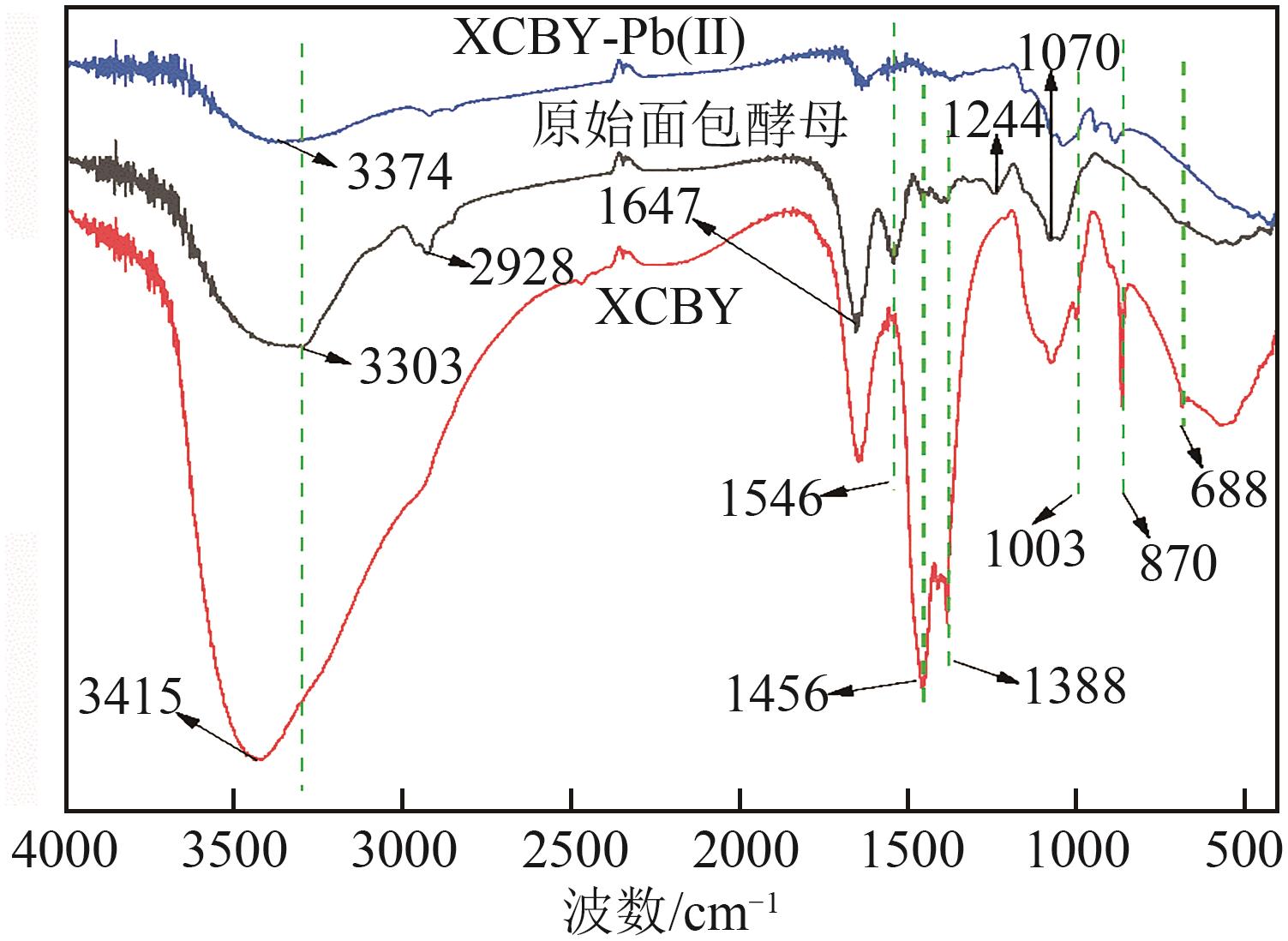
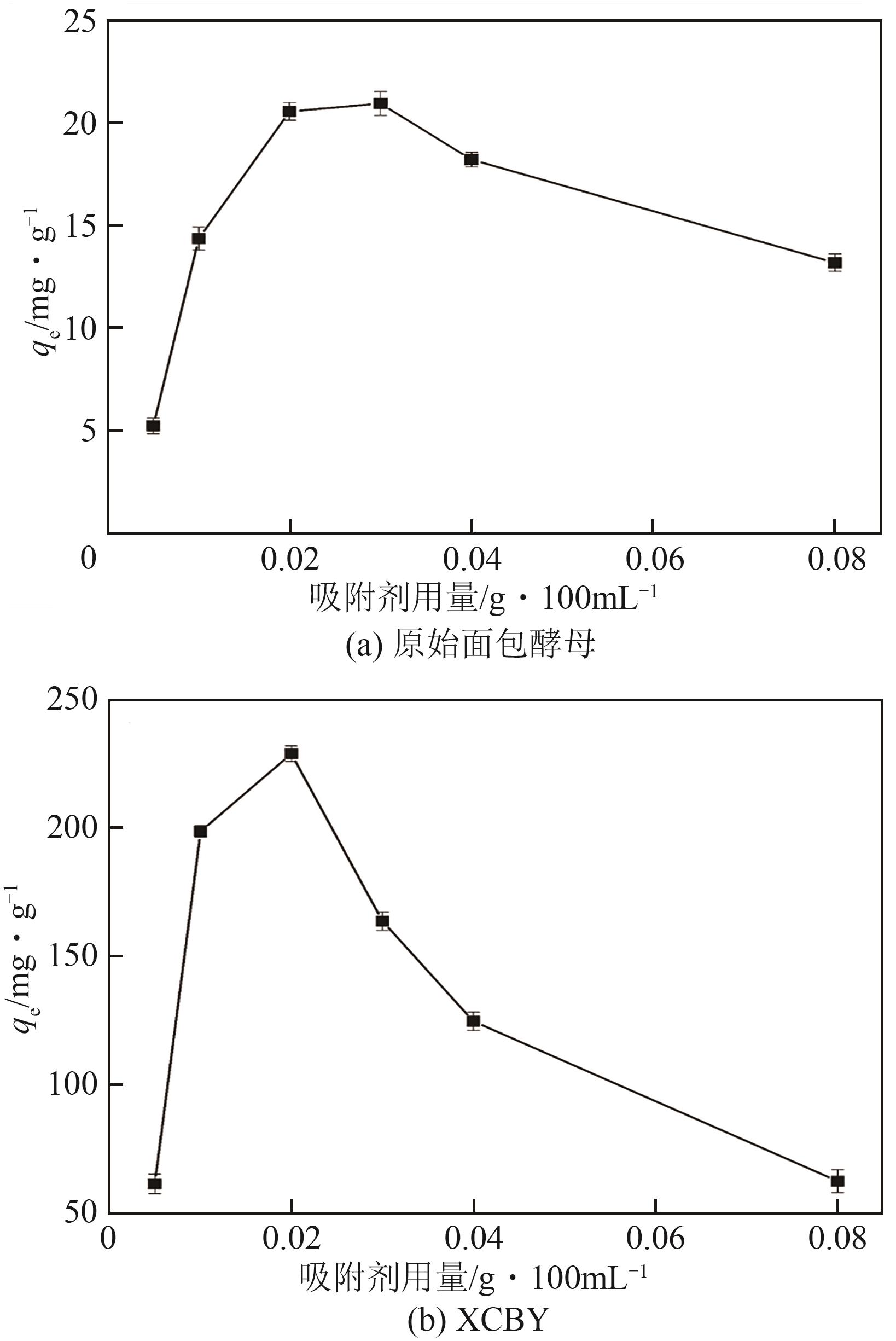
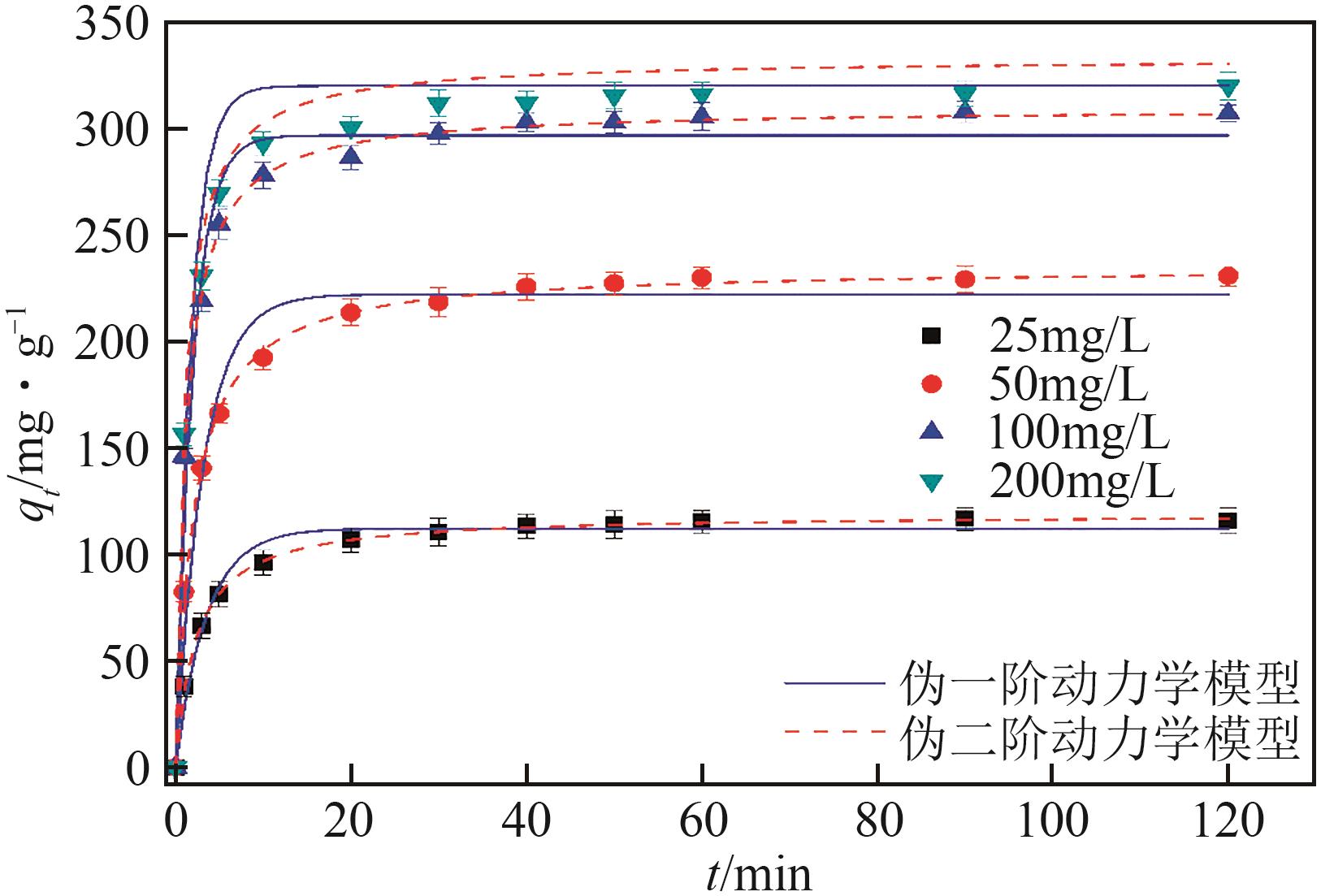
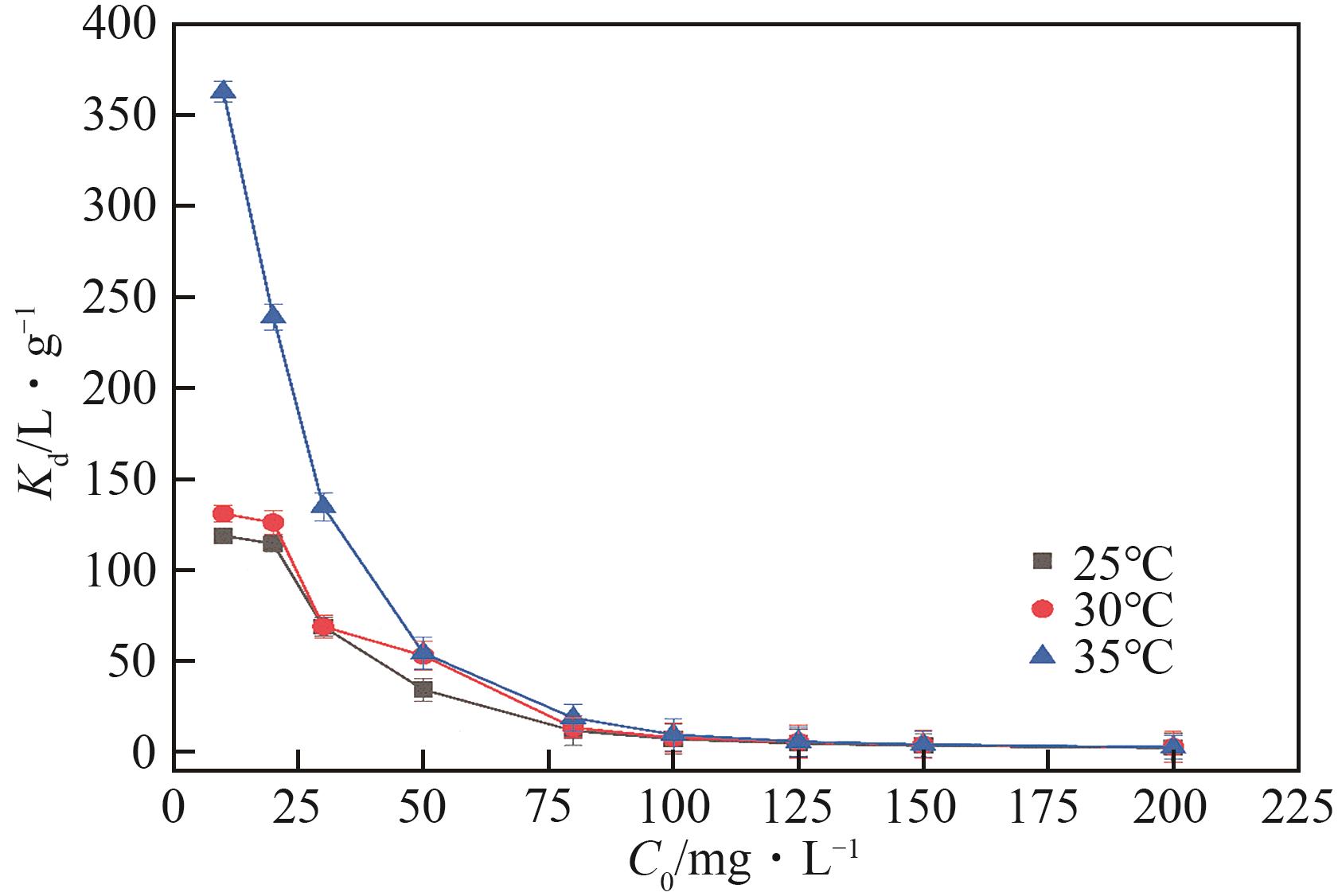
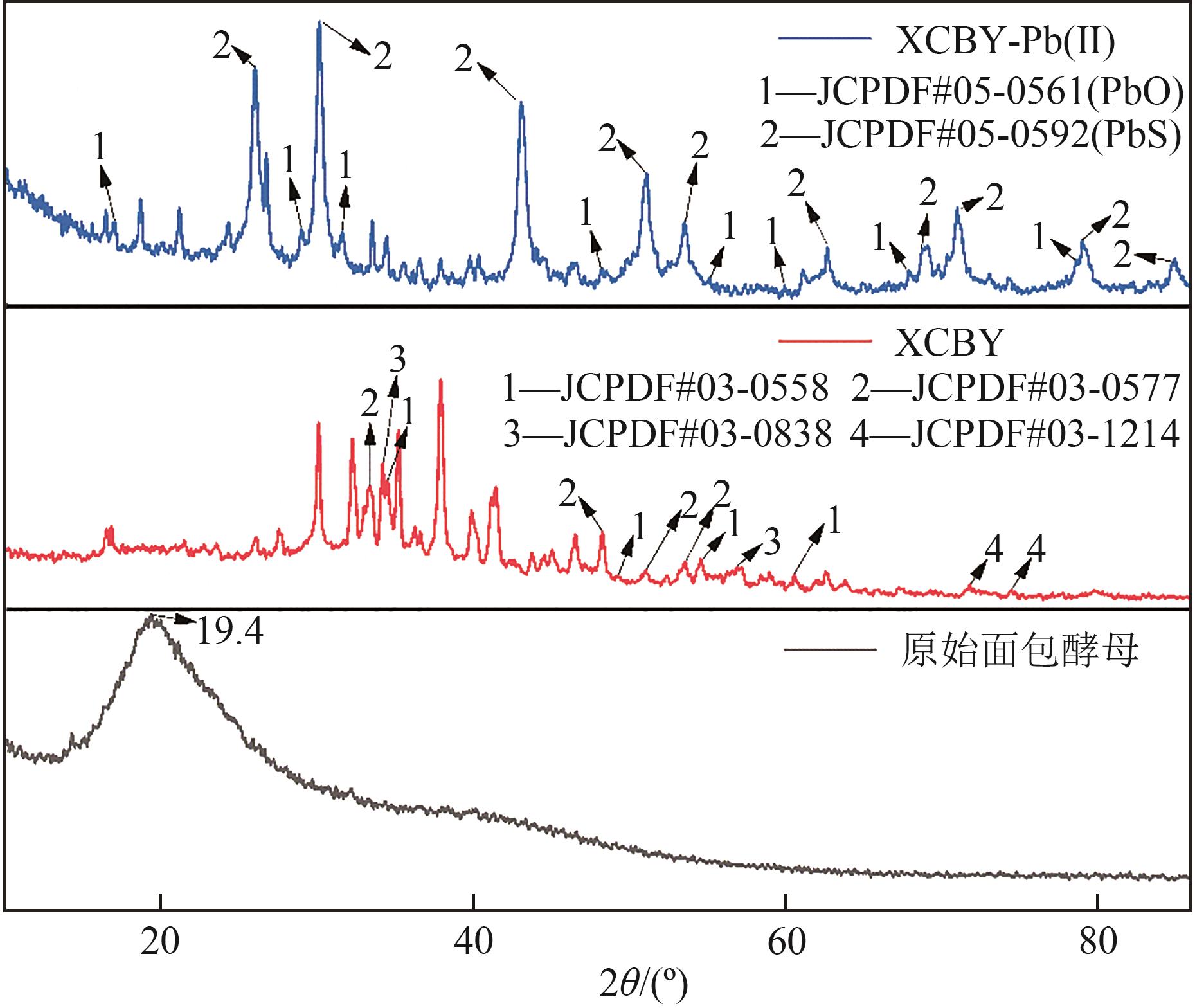
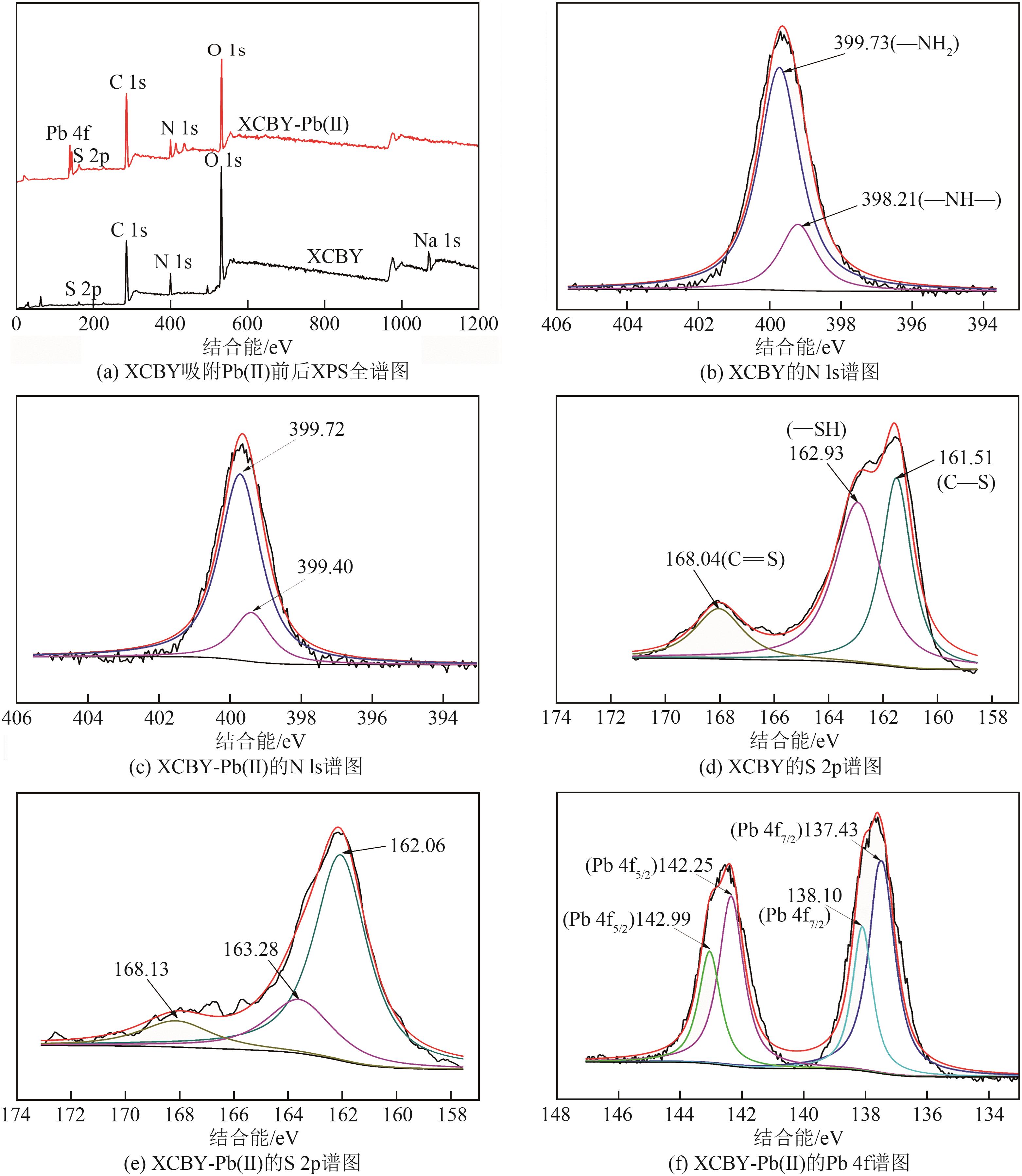
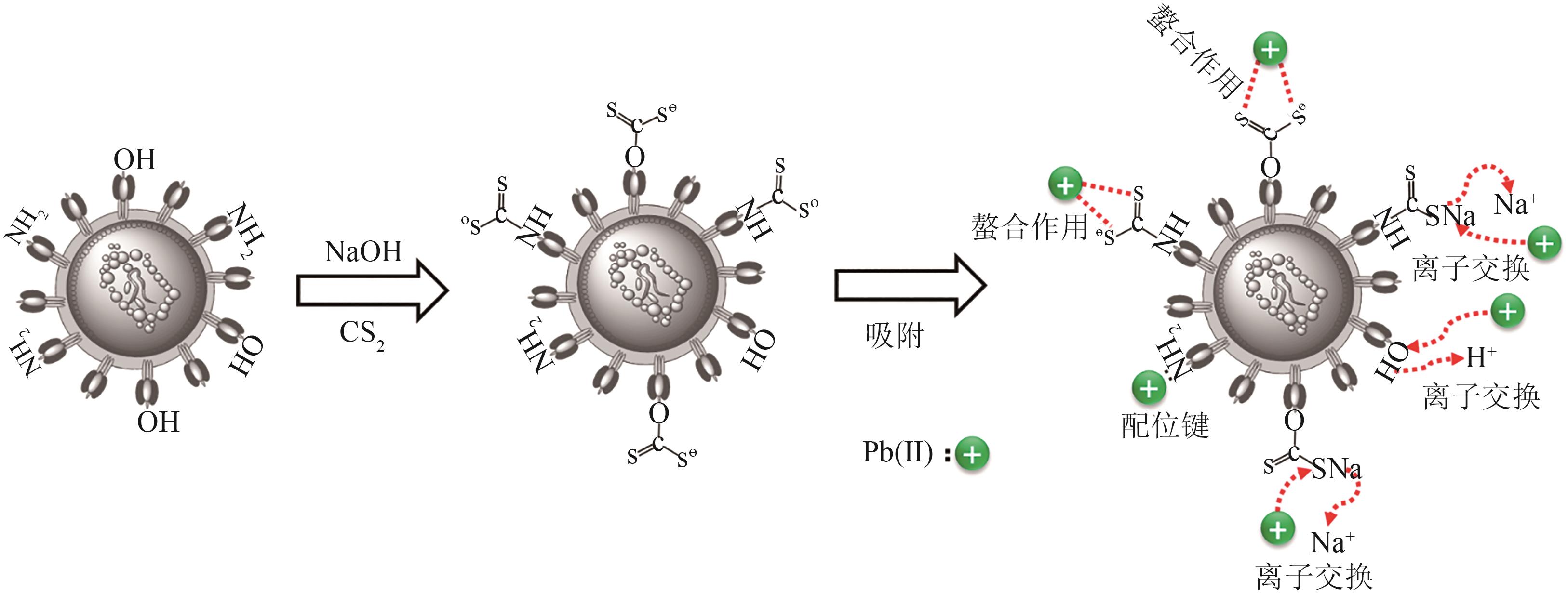
| 1 |
WANG N N, XU X J, LI H Y, et al. Enhanced selective adsorption of Pb(Ⅱ) from aqueous solutions by one-pot synthesis of xanthate-modified chitosan sponge: behaviors and mechanisms[J]. Industrial & Engineering Chemistry Research, 2016, 55(47): 12222-12231.
|
| 2 |
WANG N N, XU X J, LI H Y, et al. Preparation and application of a xanthate-modified thiourea chitosan sponge for the removal of Pb(Ⅱ) from aqueous solutions[J]. Industrial & Engineering Chemistry Research, 2016, 55(17): 4960-4968.
|
| 3 |
JIA Q J, ZHANG W T, LI D P, et al. Hydrazinolyzed cellulose-g-polymethyl acrylate as adsorbent for efficient removal of Cd(Ⅱ) and Pb(Ⅱ) ions from aqueous solution[J]. Water Science and Technology, 2017, 75(5): 1051-1058.
|
| 4 |
徐大勇, 张苗, 杨伟伟, 等. 氧化铝改性污泥生物炭粒制备及其对Pb(Ⅱ)的吸附特性[J]. 化工进展, 2020, 39(3): 1153-1166.
|
|
XU Dayong, ZHANG Miao, YANG Weiwei, et al. Preparation of alumina modified sludge biocharcoal particles and their adsorption characteristics for Pb(Ⅱ)[J]. Chemical Industry and Engineering Progress, 2020, 39(3): 1153-1166.
|
| 5 |
PENG X L, XU F, ZHANG W Z, et al. Magnetic Fe3O4@silica-xanthan gum composites for aqueous removal and recovery of Pb2+ [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2014, 443: 27-36.
|
| 6 |
刘江龙, 郭焱, 席艺慧. FeCl3和十六烷基三甲基溴化铵改性赤泥对水中铜离子的吸附性能和机理[J]. 化工进展, 2020, 39(2): 776-789.
|
|
LIU Jianglong, GUO Yan, XI Yihui. Adsorption and mechanism of copper ions in water by red mud modified with FeCl3 and hexadecyl trimethyl ammonium bromide (CTAB)[J]. Chemical Industry and Engineering Progress, 2020, 39(2): 776-789.
|
| 7 |
ZONG P F, CAO D L, WANG S F, et al. Synthesis of Fe3O4/CD magnetic nanocomposite via low temperature plasma technique with high enrichment of Ni(Ⅱ) from aqueous solution[J]. Journal of the Taiwan Institute of Chemical Engineers, 2017, 70: 134-140.
|
| 8 |
ZHOU G Y, LUO J M, LIU C B, et al. A highly efficient polyampholyte hydrogel sorbent based fixed-bed process for heavy metal removal in actual industrial effluent[J]. Water Research, 2016, 89: 151-160.
|
| 9 |
YUAN M L, XIE T F, YAN G J, et al. Effective removal of Pb2+ from aqueous solutions by magnetically modified zeolite[J]. Powder Technology, 2018, 332: 234-241.
|
| 10 |
KUL A R, KOYUNCU H. Adsorption of Pb(Ⅱ) ions from aqueous solution by native and activated bentonite: Kinetic, equilibrium and thermodynamic study[J]. Journal of Hazardous Materials, 2010, 179(1/2/3): 332-339.
|
| 11 |
OKOLI C P, DIAGBOYA P N, ANIGBOGU I O, et al. Competitive biosorption of Pb(Ⅱ) and Cd(Ⅱ) ions from aqueous solutions using chemically modified moss biomass (Barbula lambarenensis)[J]. Environmental Earth Sciences, 2016, 76(1): 33-42.
|
| 12 |
TONG M, YU J X, SUN X M, et al. Polymer modified biomass of baker’s yeast for treating simulated wastewater containing nickel and lead[J]. Polymers for Advanced Technologies, 2007, 18(10): 829-834.
|
| 13 |
XIA Y X, MENG L Y, JIANG Y J, et al. Facile preparation of MnO2 functionalized baker’s yeast composites and their adsorption mechanism for Cadmium[J]. Chemical Engineering Journal, 2015, 259: 927-935.
|
| 14 |
ZHANG W, MENG L Y, MU G Q, et al. A facile strategy for fabrication of nano-ZnO/yeast composites and their adsorption mechanism towards lead (Ⅱ) ions[J]. Applied Surface Science, 2016, 378: 196-206.
|
| 15 |
GÖKSUNGUR Y, ÜREN S, GÜVENÇ U. Biosorption of cadmium and lead ions by ethanol treated waste baker's yeast biomass[J]. Bioresource Technology, 2005, 96(1): 103-109.
|
| 16 |
LI T T, LIU Y G, PENG Q Q, et al. Removal of lead(Ⅱ) from aqueous solution with ethylenediamine-modified yeast biomass coated with magnetic chitosan microparticles: kinetic and equilibrium modeling[J]. Chemical Engineering Journal, 2013, 214: 189-197.
|
| 17 |
XU M, ZHANG Y S, ZHANG Z M, et al. Study on the adsorption of Ca2+, Cd2+ and Pb2+ by magnetic Fe3O4 yeast treated with EDTA dianhydride[J]. Chemical Engineering Journal, 2011, 168(2): 737-745.
|
| 18 |
BEDIAKO J K, WEI W, KIM S, et al. Removal of heavy metals from aqueous phases using chemically modified waste Lyocell fiber[J]. Journal of Hazardous Materials, 2015, 299: 550-561.
|
| 19 |
VIEIRA M G A, NETO A F A, GIMENES M L, et al. Removal of nickel on Bofe bentonite calcined clay in porous bed[J]. Journal of Hazardous Materials, 2010, 176(1/2/3): 109-118.
|
| 20 |
ÁLVAREZ-AYUSO E, GARCÍA-SÁNCHEZ A. Removal of heavy metals from waste waters by natural and Na-exchanged bentonites[J]. Clays and Clay Minerals, 2003, 51(5): 475-480.
|
| 21 |
OUARDI Y EL, LENOBLE V, BRANGER C, et al. Enhancing clay adsorption properties: a comparison between chemical and combined chemical/thermal treatments[J]. Groundwater for Sustainable Development, 2021, 12: 100544.
|
| 22 |
ZHU Y H, HU J, WANG J L. Competitive adsorption of Pb(Ⅱ), Cu(Ⅱ) and Zn(Ⅱ) onto xanthate-modified magnetic chitosan[J]. Journal of Hazardous Materials, 2012, 221/222: 155-161.
|
| 23 |
DENG S B, TING Y P. Characterization of PEI-modified biomass and biosorption of Cu(Ⅱ), Pb(Ⅱ) and Ni(Ⅱ)[J]. Water Research, 2005, 39(10): 2167-2177.
|
| 24 |
XIA L, HU Y X, ZHANG B H. Kinetics and equilibrium adsorption of copper(Ⅱ) and nickel(Ⅱ) ions from aqueous solution using sawdust xanthate modified with ethanediamine[J]. Transactions of Nonferrous Metals Society of China, 2014, 24(3): 868-875.
|
| 25 |
ZHENG L C, MENG P P. Preparation, characterization of corn stalk xanthates and its feasibility for Cd (Ⅱ) removal from aqueous solution[J]. Journal of the Taiwan Institute of Chemical Engineers, 2016, 58: 391-400.
|
| 26 |
LIANG S, GUO X Y, FENG N C, et al. Application of orange peel xanthate for the adsorption of Pb2+ from aqueous solutions[J]. Journal of Hazardous Materials, 2009, 170(1): 425-429.
|
| 27 |
KANNAMBA B, REDDY K L, APPARAO B V. Removal of Cu(Ⅱ) from aqueous solutions using chemically modified chitosan[J]. Journal of Hazardous Materials, 2010, 175(1/2/3): 939-948.
|
| 28 |
LAGERGREN S. Zur theorie der sogenannten adsorption gelöster stoffe, Kungliga Svenska Vetenskapsakademiens[J]. Handlingar, 1898, 24(4): 1-39.
|
| 29 |
HO Y S, MCKAY G. Pseudo-second order model for sorption processes[J]. Process Biochemistry, 1999, 34(5): 451-465.
|
| 30 |
CHEN Y W, WANG J L. The characteristics and mechanism of Co(Ⅱ) removal from aqueous solution by a novel xanthate-modified magnetic chitosan[J]. Nuclear Engineering and Design, 2012, 242: 452-457.
|
| 31 |
KAMARI A, NGAH W S W. Isotherm, kinetic and thermodynamic studies of lead and copper uptake by H2SO4 modified chitosan[J]. Colloids and Surfaces B: Biointerfaces, 2009, 73(2): 257-266.
|
| 32 |
LI Z L, KONG Y, GE Y Y. Synthesis of porous lignin xanthate resin for Pb2+ removal from aqueous solution[J]. Chemical Engineering Journal, 2015, 270: 229-234.
|
| 33 |
LANGMUIR I. The adsorption of gases on plane surfaces of glass, mica and platinum[J]. Journal of the American Chemical Society, 1918, 40(9): 1361-1403.
|
| 34 |
FREUNDLICH H. Of the adsorption of gases. Section Ⅱ. Kinetics and energetics of gas adsorption. Introductory paper to section Ⅱ[J]. Transactions of the Faraday Society, 1932, 28: 195.
|
| 35 |
SIPS R. Combined form of Langmuir and freundlich equations[J]. Journal of Chemical Physics, 1948, 16: 490-495.
|
| 36 |
GUERRA D J L, MELLO I, RESENDE R, et al. Application as absorbents of natural and functionalized Brazilian bentonite in Pb2+ adsorption: equilibrium, kinetic, pH, and thermodynamic effects[J]. Water Resources and Industry, 2013, 4: 32-50.
|
| 37 |
邹雪, 龚正君. 黄原酸功能化蛋壳膜及其对Pb(Ⅱ)的吸附研究[J]. 西南大学学报(自然科学版), 2020, 42(11): 141-146.
|
|
ZOU Xue, GONG Zhengjun. Study on adsorption removal of Pb(Ⅱ) by xanthated eggshell membrane[J]. Journal of Southwest University (Natural Science Edition), 2020, 42(11): 141-146.
|
| 38 |
FENG K, WEN G H. Absorbed Pb2+ and Cd2+ ions in water by cross-linked starch xanthate[J]. International Journal of Polymer Science, 2017, 2017: 1-9.
|
| 39 |
LV L, CHEN N, FENG C P, et al. Heavy metal ions removal from aqueous solution by xanthate-modified cross-linked magnetic chitosan/poly(vinyl alcohol) particles[J]. RSC Advances, 2017, 7(45): 27992-28000.
|
| 40 |
GAO T T, YU J G, ZHOU Y, et al. Performance of xanthate-modified multi-walled carbon nanotubes on adsorption of lead ions[J]. Water, Air, & Soil Pollution, 2017, 228(5): 172-183.
|
| 41 |
WANG C Q, WANG H, GU G H. Ultrasound-assisted xanthation of cellulose from lignocellulosic biomass optimized by response surface methodology for Pb(Ⅱ) sorption[J]. Carbohydrate Polymers, 2018, 182: 21-28.
|
| 42 |
CHEN M, WANG X F, ZHANG H. Comparative research on selective adsorption of Pb(Ⅱ) by biosorbents prepared by two kinds of modifying waste biomass: highly-efficient performance, application and mechanism[J]. Journal of Environmental Management, 2021, 288: 112388.
|
| 43 |
EVERETT D H. The thermodynamics of adsorption. Part Ⅱ.—Thermodynamics of monolayers on solids[J]. Transactions of the Faraday Society, 1950, 46: 942-957.
|
| 44 |
DENG J Q, LIU Y G, LIU S B, et al. Competitive adsorption of Pb(Ⅱ), Cd(Ⅱ) and Cu(Ⅱ) onto chitosan-pyromellitic dianhydride modified biochar[J]. Journal of Colloid and Interface Science, 2017, 506: 355-364.
|
| 45 |
GÖK Ö, ÖZCAN A, ERDEM B, et al. Prediction of the kinetics, equilibrium and thermodynamic parameters of adsorption of copper(Ⅱ) ions onto 8-hydroxy quinoline immobilized bentonite[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2008, 317(1/2/3): 174-185.
|
| 46 |
QIN F, BAI B, JING D W, et al. CdS nanoparticles anchored on the surface of yeast via a hydrothermal processes for environmental applications[J]. RSC Advances, 2014, 4(66): 34864-34872.
|
| 47 |
CHANG Y K, LEU M H, CHANG J E, et al. Combined two-stage xanthate processes for the treatment of copper-containing wastewater[J]. Engineering in Life Sciences, 2007, 7(1): 75-80.
|
| 48 |
HE J, LU Y C, LUO G S. Ca(Ⅱ) imprinted chitosan microspheres: an effective and green adsorbent for the removal of Cu(II), Cd(Ⅱ) and Pb(Ⅱ) from aqueous solutions[J]. Chemical Engineering Journal, 2014, 244: 202-208.
|
| 49 |
ZHAO F P, REPO E, SONG Y, et al. Polyethylenimine-cross-linked cellulose nanocrystals for highly efficient recovery of rare earth elements from water and a mechanism study[J]. Green Chemistry, 2017, 19(20): 4816-4828.
|
| 50 |
ODIO O F, LARTUNDO-ROJAS L, PALACIOS E G, et al. Synthesis of a novel poly-thiolated magnetic nano-platform for heavy metal adsorption. Role of thiol and carboxyl functions[J]. Applied Surface Science, 2016, 386: 160-177.
|
| 51 |
GEDAM N, NETI N R, KORMUNDA M, et al. Novel lead dioxide-graphite-polymer composite anode for electrochemical chlorine generation[J]. Electrochimica Acta, 2015, 169: 109-116.
|
| 52 |
ZINGG D S, HERCULES D M. ChemInform abstract: electron spectroscopy for chemical analysis studies of lead sulfide oxidation[J]. Chemischer Informationsdienst, 1978, 9(50): 1992-1995.
|
| 53 |
LIANG X F, XU Y M, SUN G H, et al. Preparation, characterization of thiol-functionalized silica and application for sorption of Pb2+ and Cd2+ [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2009, 349(1/2/3): 61-68.
|
 ), HU Ningmeng1, LI Tianguo2(
), HU Ningmeng1, LI Tianguo2( )
)