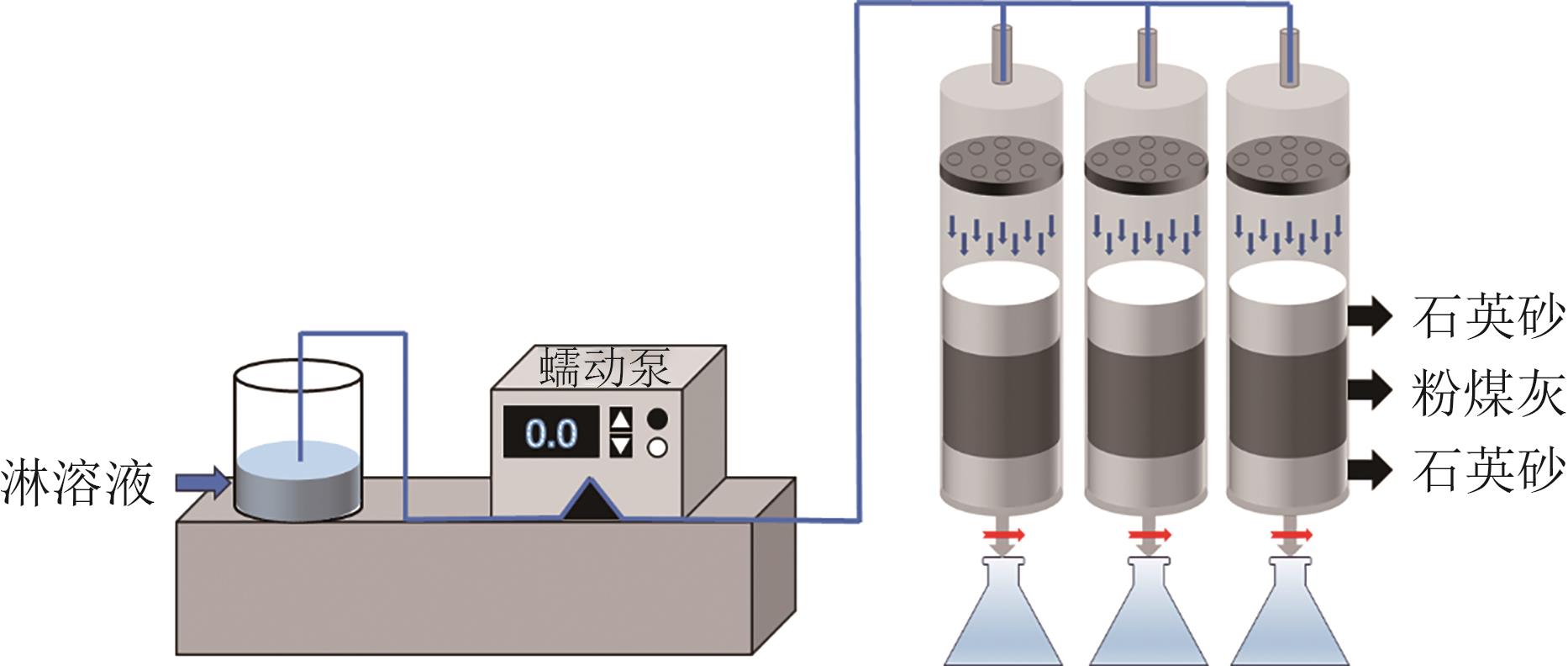
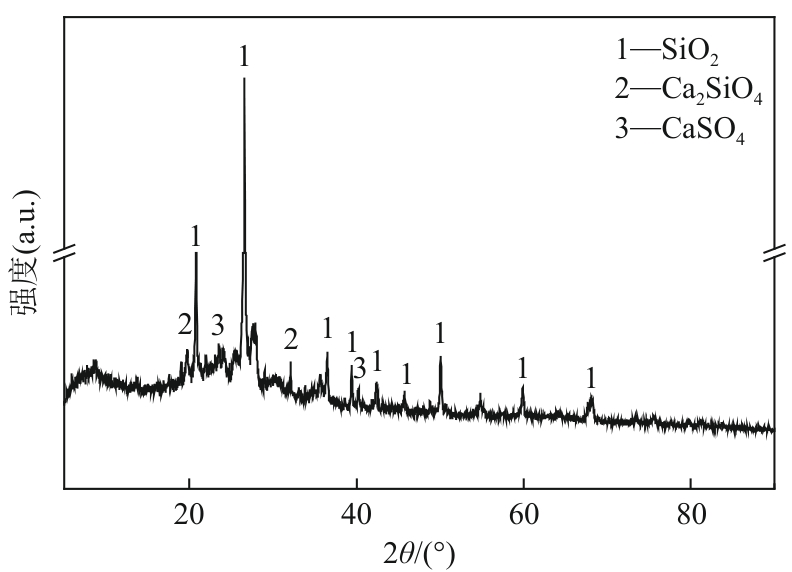
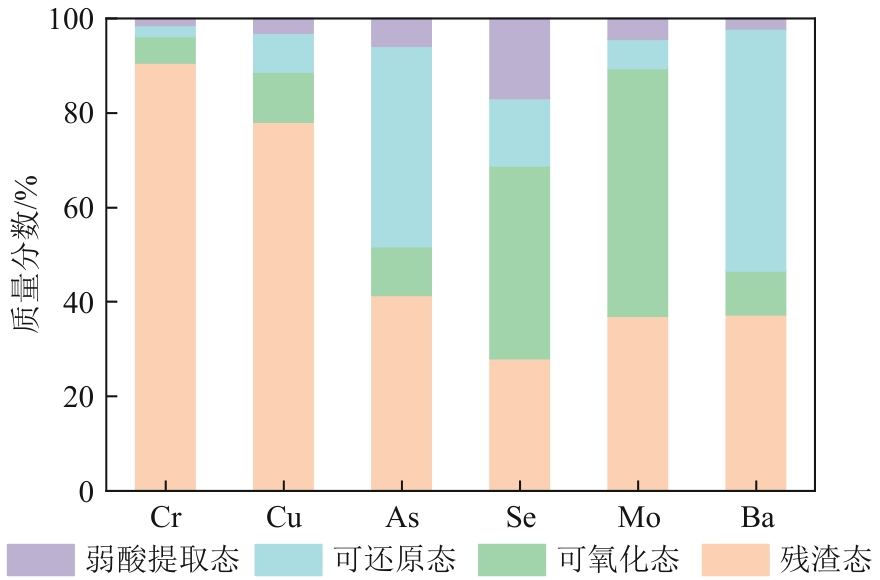
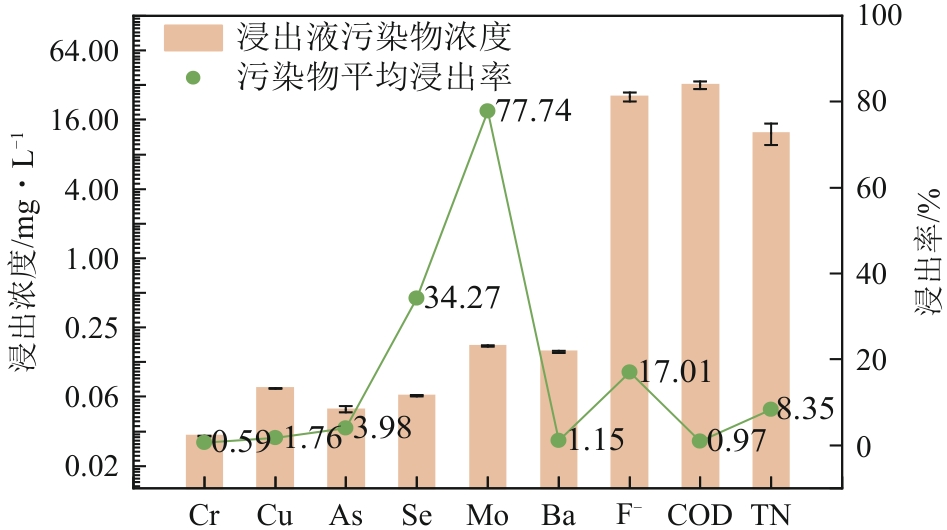
Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (9): 5479-5490.DOI: 10.16085/j.issn.1000-6613.2025-0056
• Resources and environmental engineering • Previous Articles
Pollutants release characteristics and environmental risk assessment in long-term leaching process of stockpiled coal fly ash
LU Yongqi1,2( ), XIAO Jianing3, DIE Qingqi1,2, XU Siqi1,2,4, HUANG Ruixiao1,2, KONG Xiangrui1(
), XIAO Jianing3, DIE Qingqi1,2, XU Siqi1,2,4, HUANG Ruixiao1,2, KONG Xiangrui1( ), YANG Yufei1,2(
), YANG Yufei1,2( )
)
- 1.State Key Laboratory of Environmental Criteria and Risk Assessment, Chinese Research Academy of Environment Sciences, Beijing 100012, China
2.National Joint Research Center for Ecological Conservation and High Quality Development of the Yellow River Basin, Beijing 100012, China
3.Beijing No. 66 High School, Beijing 100053, China
4.College of Water Sciences, Beijing Normal University, Beijing 100085, China
-
Received:2025-01-09Revised:2025-04-22Online:2025-09-30Published:2025-09-25 -
Contact:KONG Xiangrui, YANG Yufei
堆存粉煤灰长期淋溶过程污染物释放特征与环境风险评估
卢永琦1,2( ), 肖嘉宁3, 迭庆杞1,2, 徐思琪1,2,4, 黄瑞潇1,2, 孔祥蕊1(
), 肖嘉宁3, 迭庆杞1,2, 徐思琪1,2,4, 黄瑞潇1,2, 孔祥蕊1( ), 杨玉飞1,2(
), 杨玉飞1,2( )
)
- 1.中国环境科学研究院环境基准标准与风险管控全国重点实验室,北京 100012
2.国家黄河流域生态保护和 高质量发展联合研究中心,北京 100012
3.北京市第六十六中学,北京 100053
4.北京师范大学水科学研究院,北京 100085
-
通讯作者:孔祥蕊,杨玉飞 -
作者简介:卢永琦(1998—),女,硕士研究生,研究方向为固体废物环境风险控制。E-mail:yongqi0224@163.com。 -
基金资助:黄河流域生态保护和高质量发展联合研究项目(2022-YRUC-01-0301)
CLC Number:
Cite this article
LU Yongqi, XIAO Jianing, DIE Qingqi, XU Siqi, HUANG Ruixiao, KONG Xiangrui, YANG Yufei. Pollutants release characteristics and environmental risk assessment in long-term leaching process of stockpiled coal fly ash[J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5479-5490.
卢永琦, 肖嘉宁, 迭庆杞, 徐思琪, 黄瑞潇, 孔祥蕊, 杨玉飞. 堆存粉煤灰长期淋溶过程污染物释放特征与环境风险评估[J]. 化工进展, 2025, 44(9): 5479-5490.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2025-0056
| 步骤 | 重金属提取形态 | 提取方法 |
|---|---|---|
| 1 | 弱酸提取态 | 加入40mL 0.11mol/L的HOAc,于25℃、180r/min下水平振荡16h |
| 2 | 可还原态 | 剩余残渣中加入40mL 0.5mol/L的NH2OH·HCl,调节pH=2,于25℃、180r/min下水平振荡16h |
| 3 | 可氧化态 | 剩余残渣中加入10mL 30% H2O2,室温下消解1h,后放入85℃水浴锅消解1h,加入50mL 1mol/L的NH4Ac,pH=2,于25℃、180r/min下水平振荡16h |
| 4 | 残渣态 | 剩余残渣转入消解管中,依次添加4mL HNO3、1mL HCl、1mL HF、1mL H2O2,转入微波消解仪中消解 |
| 步骤 | 重金属提取形态 | 提取方法 |
|---|---|---|
| 1 | 弱酸提取态 | 加入40mL 0.11mol/L的HOAc,于25℃、180r/min下水平振荡16h |
| 2 | 可还原态 | 剩余残渣中加入40mL 0.5mol/L的NH2OH·HCl,调节pH=2,于25℃、180r/min下水平振荡16h |
| 3 | 可氧化态 | 剩余残渣中加入10mL 30% H2O2,室温下消解1h,后放入85℃水浴锅消解1h,加入50mL 1mol/L的NH4Ac,pH=2,于25℃、180r/min下水平振荡16h |
| 4 | 残渣态 | 剩余残渣转入消解管中,依次添加4mL HNO3、1mL HCl、1mL HF、1mL H2O2,转入微波消解仪中消解 |
| 指标 | 方法 | 仪器 | |
|---|---|---|---|
| 固相 | 金属元素 | 《固体废物 金属元素测定 电感耦合等离子体质谱法》(HJ 766—2015) | 电感耦合等离子体质谱仪(Agilent 7500a,安捷伦公司,美国) |
| 氟化物 | 《固体废物 氟的测定 碱熔-离子选择电极法》(HJ 999—2018) | 离子计(PXSJ-227L,上海雷磁) | |
| TOC | 《土壤 有机碳的测定 重铬酸钾氧化-分光光度法》(HJ 615—2011) | 紫外可见分光光度计(TU-1901) | |
| TN | 《元素分析仪分析方法通则》(JY/T 0580—2020) | 元素分析仪(德国Elementar Vario MACRO cube) | |
| 液相 | F- | 《水质 无机阴离子(F-、Cl-、NO2-、Br-、NO3-、PO42-、SO32-、SO42-)的测定 离子色谱法》(HJ 84—2016) | 离子色谱仪(ICS3000,美国戴安) |
| COD | 《水质 化学需氧量的测定 快速消解分光光度法》(HJ/T 399—2007) | 紫外可见分光光度计(TU-1901) | |
| TN | 《水质 总氮的测定 碱性过硫酸钾消解紫外分光光度法》(HJ 636—2012) | 紫外可见分光光度计(TU-1901) |
| 指标 | 方法 | 仪器 | |
|---|---|---|---|
| 固相 | 金属元素 | 《固体废物 金属元素测定 电感耦合等离子体质谱法》(HJ 766—2015) | 电感耦合等离子体质谱仪(Agilent 7500a,安捷伦公司,美国) |
| 氟化物 | 《固体废物 氟的测定 碱熔-离子选择电极法》(HJ 999—2018) | 离子计(PXSJ-227L,上海雷磁) | |
| TOC | 《土壤 有机碳的测定 重铬酸钾氧化-分光光度法》(HJ 615—2011) | 紫外可见分光光度计(TU-1901) | |
| TN | 《元素分析仪分析方法通则》(JY/T 0580—2020) | 元素分析仪(德国Elementar Vario MACRO cube) | |
| 液相 | F- | 《水质 无机阴离子(F-、Cl-、NO2-、Br-、NO3-、PO42-、SO32-、SO42-)的测定 离子色谱法》(HJ 84—2016) | 离子色谱仪(ICS3000,美国戴安) |
| COD | 《水质 化学需氧量的测定 快速消解分光光度法》(HJ/T 399—2007) | 紫外可见分光光度计(TU-1901) | |
| TN | 《水质 总氮的测定 碱性过硫酸钾消解紫外分光光度法》(HJ 636—2012) | 紫外可见分光光度计(TU-1901) |
| 成分 | 质量分数/% | 成分 | 质量分数/% |
|---|---|---|---|
| SiO2 | 54.23±0.35 | K2O | 2.14±0.04 |
| Al2O3 | 19.84±0.72 | MgO | 1.03±0.01 |
| Fe2O3 | 8.59±0.18 | Na2O | 1.15±0.03 |
| CaO | 8.13±0.35 | Cl | 0.13±0.01 |
| SO3 | 3.08±0.1 | P2O5 | 0.54±0.02 |
| TiO2 | 1.53±0.04 | MnO | 0.09±0.02 |
| 成分 | 质量分数/% | 成分 | 质量分数/% |
|---|---|---|---|
| SiO2 | 54.23±0.35 | K2O | 2.14±0.04 |
| Al2O3 | 19.84±0.72 | MgO | 1.03±0.01 |
| Fe2O3 | 8.59±0.18 | Na2O | 1.15±0.03 |
| CaO | 8.13±0.35 | Cl | 0.13±0.01 |
| SO3 | 3.08±0.1 | P2O5 | 0.54±0.02 |
| TiO2 | 1.53±0.04 | MnO | 0.09±0.02 |
| 污染物 | 含量/mg·kg-1 | 污染物 | 含量/mg·kg-1 |
|---|---|---|---|
| Cr | 48.6±1.99 | Ba | 134.16±0.99 |
| Cu | 42.04±1.11 | F | 1491±26.51 |
| As | 12.21±1.01 | TOC | 32949.61±109.02 |
| Se | 1.86±0.05 | TN | 1467±32.08 |
| Mo | 2.21±0.15 |
| 污染物 | 含量/mg·kg-1 | 污染物 | 含量/mg·kg-1 |
|---|---|---|---|
| Cr | 48.6±1.99 | Ba | 134.16±0.99 |
| Cu | 42.04±1.11 | F | 1491±26.51 |
| As | 12.21±1.01 | TOC | 32949.61±109.02 |
| Se | 1.86±0.05 | TN | 1467±32.08 |
| Mo | 2.21±0.15 |
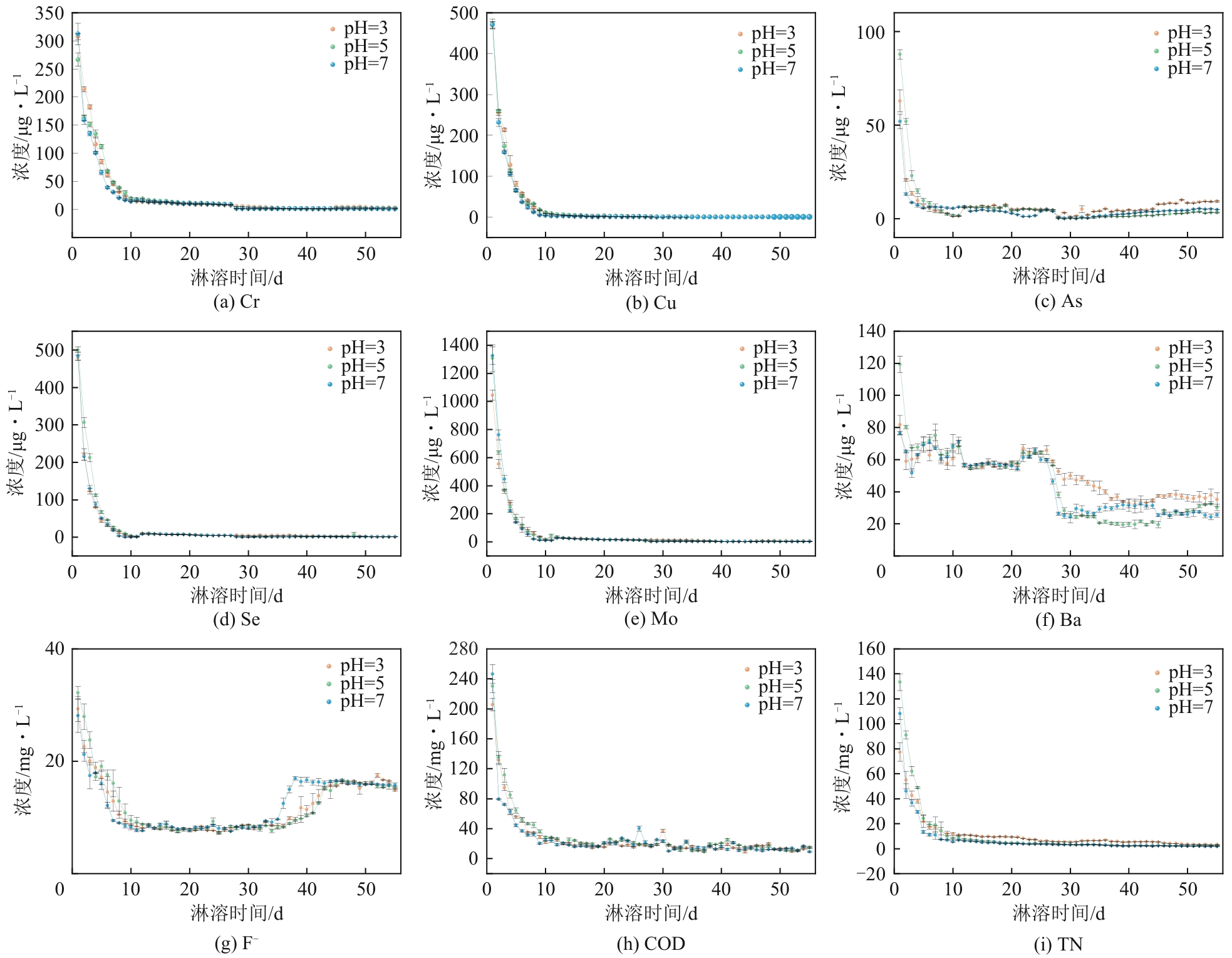
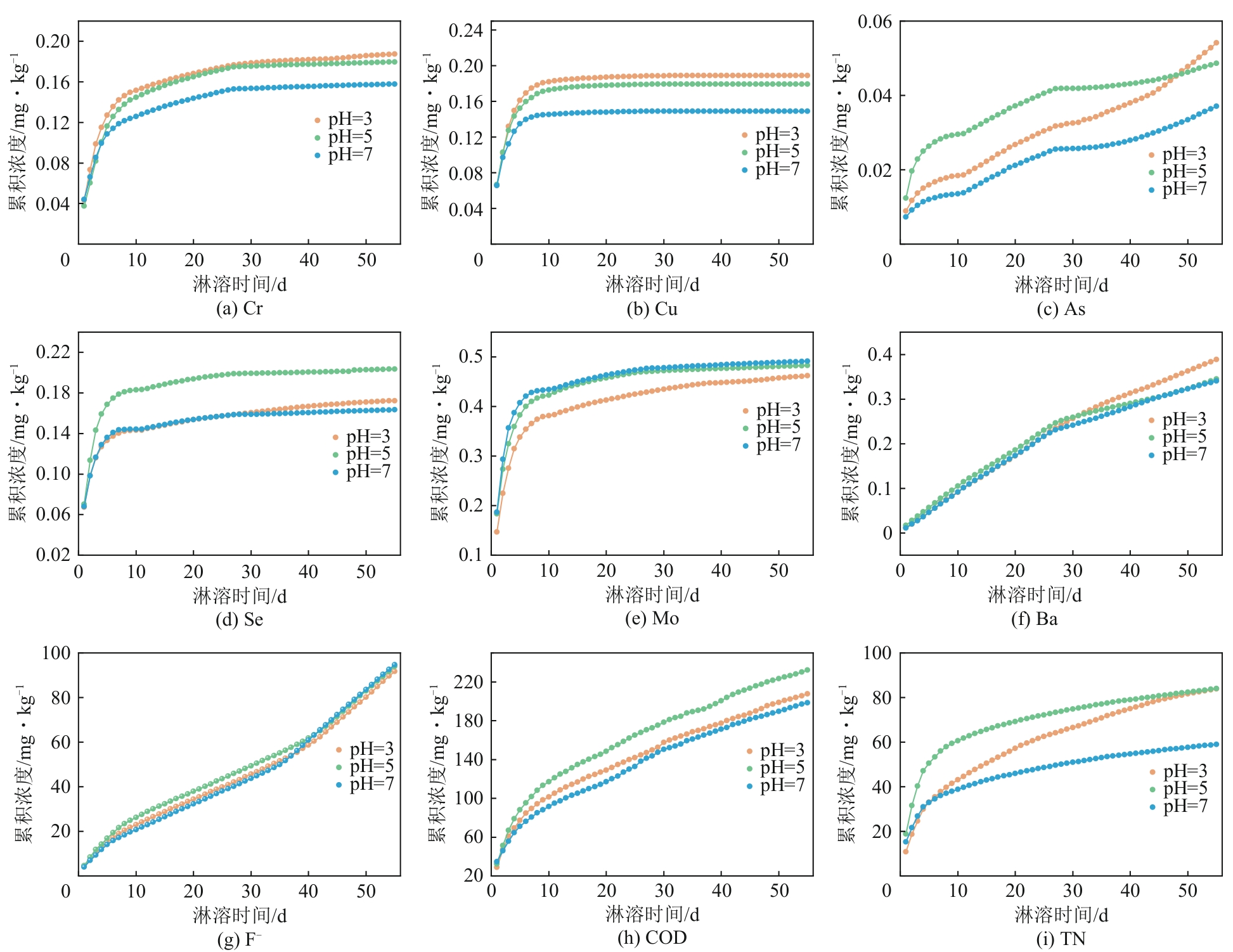
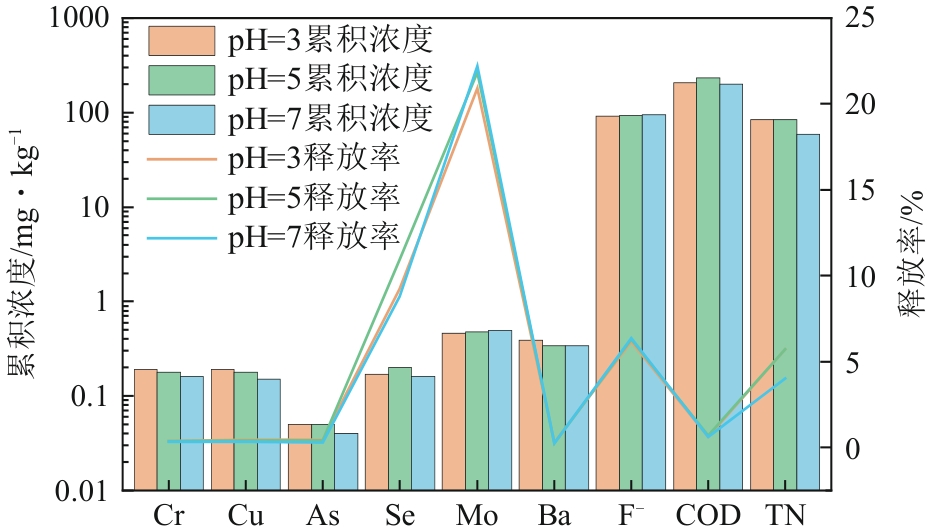
| 污染物 | 淋溶条件 | 符合模型 | 模型表达式 | R2 | a | b | c | 5年预测释放量/mg·kg-1 | 陕西土壤背景值/mg·kg-1 |
|---|---|---|---|---|---|---|---|---|---|
| Cr | pH=3 | 二级动力学方程 | Q=t/(at+b) | 0.9946 | 5.11±0.01 | 15.17±0.26 | 0.19 | 62.5 | |
| pH=5 | 二级动力学方程 | Q=t/(at+b) | 0.9928 | 5.16±0.02 | 18.37±0.38 | 0.19 | |||
| pH=7 | 二级动力学方程 | Q=t/(at+b) | 0.9939 | 6.01±0.02 | 17.73±0.32 | 0.17 | |||
| Cu | pH=3 | 二级动力学方程 | Q=t/(at+b) | 0.9455 | 5.05±0.03 | 6.95±0.37 | 0.20 | 21.4 | |
| pH=5 | 二级动力学方程 | Q=t/(at+b) | 0.9552 | 5.34±0.02 | 6.96±0.33 | 0.19 | |||
| pH=7 | 二级动力学方程 | Q=t/(at+b) | 0.9437 | 6.48±0.03 | 6.42±0.32 | 0.15 | |||
| As | pH=3 | 抛物线方程 | Q=at2+bt+c | 0.9858 | 1.42×10-6±8.33×10-7 | 6.3×10-4±4.81×10-5 | 0.01±5.84×10-4 | 0.42 | 11.1 |
| pH=5 | Freundlich方程 | lnQ=alnt+b | 0.9807 | 0.27±0.01 | -4.11±0.02 | 0.08 | |||
| pH=7 | Freundlich方程 | lnQ=alnt+b | 0.9741 | 0.49±0.01 | -5.34±0.05 | 0.09 | |||
| Se | pH=3 | 二级动力学方程 | Q=t/(at+b) | 0.9536 | 5.84±0.03 | 9.56±0.44 | 0.17 | 0.115 | |
| pH=5 | 二级动力学方程 | Q=t/(at+b) | 0.9821 | 4.79±0.01 | 7.06±0.21 | 0.21 | |||
| pH=7 | 二级动力学方程 | Q=t/(at+b) | 0.9845 | 6.04±0.02 | 7.95±0.21 | 0.16 | |||
| Mo | pH=3 | 二级动力学方程 | Q=t/(at+b) | 0.9857 | 2.13±0.01 | 4.67±0.13 | 0.47 | — | |
| pH=5 | 二级动力学方程 | Q=t/(at+b) | 0.9973 | 2.02 | 3.22±0.04 | 0.49 | |||
| pH=7 | 二级动力学方程 | Q=t/(at+b) | 0.9844 | 2±0.01 | 2.74±0.07 | 0.50 | |||
| Ba | pH=3 | 二级动力学方程 | Q=t/(at+b) | 0.9985 | 0.8±0.03 | 96.72±1.04 | 0.94 | 515 | |
| pH=5 | 二级动力学方程 | Q=t/(at+b) | 0.9954 | 1.5±0.04 | 74.23±1.37 | 0.59 | |||
| pH=7 | 二级动力学方程 | Q=t/(at+b) | 0.9972 | 1.3±0.03 | 88.79±1.28 | 0.65 | |||
| F- | pH=3 | Freundlich方程 | lnQ=alnt+b | 0.9731 | 0.88±0.03 | 0.9±0.1 | 436.93 | 497 | |
| pH=5 | Freundlich方程 | lnQ=alnt+b | 0.9736 | 0.8±0.02 | 1.26±0.09 | 391.07 | |||
| pH=7 | Freundlich方程 | lnQ=alnt+b | 0.9744 | 1.01±0.03 | 0.42±0.12 | 581.12 | |||
| COD | pH=3 | Freundlich方程 | lnQ=alnt+b | 0.9961 | 0.43 | 3.6±0.02 | 459.91 | — | |
| pH=5 | Freundlich方程 | lnQ=alnt+b | 0.9969 | 0.42 | 3.76±0.01 | 508.86 | |||
| pH=7 | Freundlich方程 | lnQ=alnt+b | 0.9949 | 0.46±0.01 | 3.43±0.02 | 463.87 | |||
| TN | pH=3 | Freundlich方程 | lnQ=alnt+b | 0.9964 | 0.41 | 2.82±0.01 | 187.41 | — | |
| pH=5 | Freundlich方程 | lnQ=alnt+b | 0.9554 | 0.23±0.01 | 3.52±0.03 | 131.21 | |||
| pH=7 | Freundlich方程 | lnQ=alnt+b | 0.9892 | 0.26 | 3.04±0.02 | 96.58 |
| 污染物 | 淋溶条件 | 符合模型 | 模型表达式 | R2 | a | b | c | 5年预测释放量/mg·kg-1 | 陕西土壤背景值/mg·kg-1 |
|---|---|---|---|---|---|---|---|---|---|
| Cr | pH=3 | 二级动力学方程 | Q=t/(at+b) | 0.9946 | 5.11±0.01 | 15.17±0.26 | 0.19 | 62.5 | |
| pH=5 | 二级动力学方程 | Q=t/(at+b) | 0.9928 | 5.16±0.02 | 18.37±0.38 | 0.19 | |||
| pH=7 | 二级动力学方程 | Q=t/(at+b) | 0.9939 | 6.01±0.02 | 17.73±0.32 | 0.17 | |||
| Cu | pH=3 | 二级动力学方程 | Q=t/(at+b) | 0.9455 | 5.05±0.03 | 6.95±0.37 | 0.20 | 21.4 | |
| pH=5 | 二级动力学方程 | Q=t/(at+b) | 0.9552 | 5.34±0.02 | 6.96±0.33 | 0.19 | |||
| pH=7 | 二级动力学方程 | Q=t/(at+b) | 0.9437 | 6.48±0.03 | 6.42±0.32 | 0.15 | |||
| As | pH=3 | 抛物线方程 | Q=at2+bt+c | 0.9858 | 1.42×10-6±8.33×10-7 | 6.3×10-4±4.81×10-5 | 0.01±5.84×10-4 | 0.42 | 11.1 |
| pH=5 | Freundlich方程 | lnQ=alnt+b | 0.9807 | 0.27±0.01 | -4.11±0.02 | 0.08 | |||
| pH=7 | Freundlich方程 | lnQ=alnt+b | 0.9741 | 0.49±0.01 | -5.34±0.05 | 0.09 | |||
| Se | pH=3 | 二级动力学方程 | Q=t/(at+b) | 0.9536 | 5.84±0.03 | 9.56±0.44 | 0.17 | 0.115 | |
| pH=5 | 二级动力学方程 | Q=t/(at+b) | 0.9821 | 4.79±0.01 | 7.06±0.21 | 0.21 | |||
| pH=7 | 二级动力学方程 | Q=t/(at+b) | 0.9845 | 6.04±0.02 | 7.95±0.21 | 0.16 | |||
| Mo | pH=3 | 二级动力学方程 | Q=t/(at+b) | 0.9857 | 2.13±0.01 | 4.67±0.13 | 0.47 | — | |
| pH=5 | 二级动力学方程 | Q=t/(at+b) | 0.9973 | 2.02 | 3.22±0.04 | 0.49 | |||
| pH=7 | 二级动力学方程 | Q=t/(at+b) | 0.9844 | 2±0.01 | 2.74±0.07 | 0.50 | |||
| Ba | pH=3 | 二级动力学方程 | Q=t/(at+b) | 0.9985 | 0.8±0.03 | 96.72±1.04 | 0.94 | 515 | |
| pH=5 | 二级动力学方程 | Q=t/(at+b) | 0.9954 | 1.5±0.04 | 74.23±1.37 | 0.59 | |||
| pH=7 | 二级动力学方程 | Q=t/(at+b) | 0.9972 | 1.3±0.03 | 88.79±1.28 | 0.65 | |||
| F- | pH=3 | Freundlich方程 | lnQ=alnt+b | 0.9731 | 0.88±0.03 | 0.9±0.1 | 436.93 | 497 | |
| pH=5 | Freundlich方程 | lnQ=alnt+b | 0.9736 | 0.8±0.02 | 1.26±0.09 | 391.07 | |||
| pH=7 | Freundlich方程 | lnQ=alnt+b | 0.9744 | 1.01±0.03 | 0.42±0.12 | 581.12 | |||
| COD | pH=3 | Freundlich方程 | lnQ=alnt+b | 0.9961 | 0.43 | 3.6±0.02 | 459.91 | — | |
| pH=5 | Freundlich方程 | lnQ=alnt+b | 0.9969 | 0.42 | 3.76±0.01 | 508.86 | |||
| pH=7 | Freundlich方程 | lnQ=alnt+b | 0.9949 | 0.46±0.01 | 3.43±0.02 | 463.87 | |||
| TN | pH=3 | Freundlich方程 | lnQ=alnt+b | 0.9964 | 0.41 | 2.82±0.01 | 187.41 | — | |
| pH=5 | Freundlich方程 | lnQ=alnt+b | 0.9554 | 0.23±0.01 | 3.52±0.03 | 131.21 | |||
| pH=7 | Freundlich方程 | lnQ=alnt+b | 0.9892 | 0.26 | 3.04±0.02 | 96.58 |
| 项目 | pH | Cr | Cu | As | Se | Mo | Ba | F- | COD | TN |
|---|---|---|---|---|---|---|---|---|---|---|
| 地下水Ⅲ类水体标准浓度/mg·L-1 | 6.5~8.5 | 0.05(Cr6+) | 1.00 | 0.01 | 0.01 | 0.07 | 0.70 | 1.0 | 3.0 | — |
| 超标倍数 | 1.23 | — | — | 4.86 | 6.37 | 2.45 | — | 25.36 | 10.16 | — |
| 地表水Ⅲ类水体标准浓度/mg·L-1 | 6~9 | 0.05(Cr6+) | 1.00 | 0.05 | 0.01 | — | — | 1.0 | 20 | 1.0 |
| 超标倍数 | 1.16 | — | — | — | 6.37 | — | — | 25.36 | 1.60 | 12.25 |
| 项目 | pH | Cr | Cu | As | Se | Mo | Ba | F- | COD | TN |
|---|---|---|---|---|---|---|---|---|---|---|
| 地下水Ⅲ类水体标准浓度/mg·L-1 | 6.5~8.5 | 0.05(Cr6+) | 1.00 | 0.01 | 0.01 | 0.07 | 0.70 | 1.0 | 3.0 | — |
| 超标倍数 | 1.23 | — | — | 4.86 | 6.37 | 2.45 | — | 25.36 | 10.16 | — |
| 地表水Ⅲ类水体标准浓度/mg·L-1 | 6~9 | 0.05(Cr6+) | 1.00 | 0.05 | 0.01 | — | — | 1.0 | 20 | 1.0 |
| 超标倍数 | 1.16 | — | — | — | 6.37 | — | — | 25.36 | 1.60 | 12.25 |
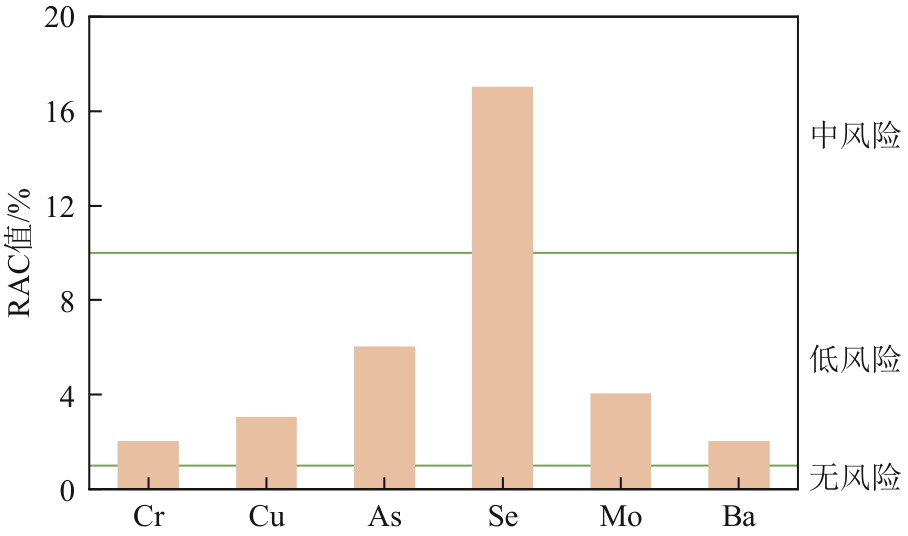
| 项目 | RAC值 | ||||
|---|---|---|---|---|---|
| <1% | 1%~10% | 10%~30% | 30%~50% | ≥50% | |
| 污染程度 | 无 | 轻度 | 中度 | 重度 | 极严重 |
| 风险等级 | 无风险 | 低风险 | 中等风险 | 高风险 | 极高风险 |
| 项目 | RAC值 | ||||
|---|---|---|---|---|---|
| <1% | 1%~10% | 10%~30% | 30%~50% | ≥50% | |
| 污染程度 | 无 | 轻度 | 中度 | 重度 | 极严重 |
| 风险等级 | 无风险 | 低风险 | 中等风险 | 高风险 | 极高风险 |
| CI范围 | 单个元素污染程度 | Er范围 | 潜在生态风险程度 | RI范围 | 综合潜在生态风险程度 |
|---|---|---|---|---|---|
| CI<0.7 | 清洁 | Er<40 | 轻微 | RI<150 | 轻微 |
| 0.7≤CI<1.0 | 尚清洁 | 40≤Er<80 | 中等 | 150≤RI<300 | 中等 |
| 1.0≤CI<2.0 | 轻污染 | 80≤Er<160 | 强 | 300≤RI<600 | 较强 |
| 2.0≤CI<3.0 | 中污染 | 160≤Er<320 | 很强 | 600≤RI | 极强 |
| 3.0≤CI | 重污染 | 320≤Er | 极强 |
| CI范围 | 单个元素污染程度 | Er范围 | 潜在生态风险程度 | RI范围 | 综合潜在生态风险程度 |
|---|---|---|---|---|---|
| CI<0.7 | 清洁 | Er<40 | 轻微 | RI<150 | 轻微 |
| 0.7≤CI<1.0 | 尚清洁 | 40≤Er<80 | 中等 | 150≤RI<300 | 中等 |
| 1.0≤CI<2.0 | 轻污染 | 80≤Er<160 | 强 | 300≤RI<600 | 较强 |
| 2.0≤CI<3.0 | 中污染 | 160≤Er<320 | 很强 | 600≤RI | 极强 |
| 3.0≤CI | 重污染 | 320≤Er | 极强 |
| 项目 | Cr | Cu | As |
|---|---|---|---|
| 单一元素污染指数(CI) | 0.78 | 1.96 | 1.1 |
| 单一元素污染程度 | 尚清洁 | 轻污染 | 轻污染 |
| 潜在生态风险指数(Er) | 1.56 | 9.8 | 11 |
| 潜在生态风险程度 | 轻微污染 | 轻微污染 | 轻微污染 |
| 综合风险指数(RI) | 22.36 | ||
| 综合潜在生态风险程度 | 轻微污染 | ||
| 项目 | Cr | Cu | As |
|---|---|---|---|
| 单一元素污染指数(CI) | 0.78 | 1.96 | 1.1 |
| 单一元素污染程度 | 尚清洁 | 轻污染 | 轻污染 |
| 潜在生态风险指数(Er) | 1.56 | 9.8 | 11 |
| 潜在生态风险程度 | 轻微污染 | 轻微污染 | 轻微污染 |
| 综合风险指数(RI) | 22.36 | ||
| 综合潜在生态风险程度 | 轻微污染 | ||
| [1] | 张国卿, 宋舒波, 王兴瑞, 等. 煤固废基分子筛的制备及其应用进展[J]. 化工进展, 2024, 43(5): 2311-2323. |
| ZHANG Guoqing, SONG Shubo, WANG Xingrui, et al. Recent advances in the synthesis and application of zeolites from coal-based solid wastes[J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2311-2323. | |
| [2] | YANG Zili, CHANG Guohui, XIA Yangchao, et al. Utilization of waste cooking oil for highly efficient recovery of unburned carbon from coal fly ash[J]. Journal of Cleaner Production, 2021, 282: 124547. |
| [3] | ZHANG Jiqiang, YANG Ke, HE Xiang, et al. Research status of comprehensive utilization of coal-based solid waste (CSW) and key technologies of filling mining in China: A review[J]. Science of the Total Environment, 2024, 926: 171855. |
| [4] | ZIEROLD Kristina M, ODOH Chisom. A review on fly ash from coal-fired power plants: Chemical composition, regulations, and health evidence[J]. Reviews on Environmental Health, 2020, 35(4): 401-418. |
| [5] | IZQUIERDO Maria, QUEROL Xavier. Leaching behaviour of elements from coal combustion fly ash: An overview[J]. International Journal of Coal Geology, 2012, 94: 54-66. |
| [6] | DENG Shuang, SHU Yun, LI Songgeng, et al. Chemical forms of the fluorine, chlorine, oxygen and carbon in coal fly ash and their correlations with mercury retention[J]. Journal of Hazardous Materials, 2016, 301: 400-406. |
| [7] | 王雅男, 肖长来, 刘婷. 粉煤灰淋滤液中氮对水环境影响的实验研究[J]. 人民长江, 2014, 45(3): 94-96, 100. |
| WANG Yanan, XIAO Changlai, LIU Ting. Experimental research on nitrogen pollution of fly-ash leachate to water environment[J]. Yangtze River, 2014, 45(3): 94-96, 100. | |
| [8] | RIBEIRO J, SILVA T F, MENDONÇA FILHO J G, et al. Fly ash from coal combustion—An environmental source of organic compounds[J]. Applied Geochemistry, 2014, 44: 103-110. |
| [9] | RIVERA Nelson, KAUR Navdeep, HESTERBERG Dean, et al. Chemical composition, speciation, and elemental associations in coal fly ash samples related to the Kingston ash spill[J]. Energy & Fuels, 2015, 29(2): 954-967. |
| [10] | 白继红. 火电厂干贮灰场灰水淋溶物的渗透特性研究[D]. 太原: 太原理工大学, 2008. |
| BAI Jihong. Study on permeability characteristics of ash water leachate in dry ash storage yard of thermal power plant[D]. Taiyuan: Taiyuan University of Technology, 2008. | |
| [11] | 耿方方, 王延辉, 饶虎, 等. 粉煤灰浸出试验在灰场地下水环评中的应用[J]. 电力勘测设计, 2019(12): 34-39. |
| GENG Fangfang, WANG Yanhui, RAO Hu, et al. Application of fly ash leaching test in groundwater environmental impact assessment of ash field[J]. Electric Power Survey & Design, 2019(12): 34-39. | |
| [12] | 祁鹏飞, 康静文. 粉煤灰作为灌浆材料对地下水的影响[J]. 能源与节能, 2015(5): 184-186. |
| QI Pengfei, KANG Jingwen. Influence of fly ash as grouting material on groundwater[J]. Energy and Energy Conservation, 2015(5): 184-186. | |
| [13] | 袁霄梅, 赵营刚, 尹国勋, 等. 某粉煤灰堆放场对周围岩溶地下水的影响分析[J]. 环境监测管理与技术, 2014, 26(2): 48-51, 55. |
| YUAN Xiaomei, ZHAO Yinggang, YIN Guoxun, et al. Influence of fly ash dumping site on Karst groundwater[J]. The Administration and Technique of Environmental Monitoring, 2014, 26(2): 48-51, 55. | |
| [14] | WANG Nannan, SUN Xiyu, ZHAO Qiang, et al. Leachability and adverse effects of coal fly ash: A review[J]. Journal of Hazardous Materials, 2020, 396: 122725. |
| [15] | WANG Wenfeng, QIN Yong, SONG Dangyu, et al. Column leaching of coal and its combustion residues, Shizuishan, China[J]. International Journal of Coal Geology, 2008, 75(2): 81-87. |
| [16] | 王琦, 张文静, 胡琳, 等. 陕西省酸雨时空分布特征研究[C]//第28届中国气象学会年会论文集, 厦门, 2011: 415-423. |
| WANG Qi, ZHANG Wenjing, HU Lin, et al. Research on the spatio temporal distribution characteristics of acid rain in Shaanxi Province[C]//Proceeding of the 28th Annual Meeting of the chinese Meteorological Society, Xiamen, 2011:415-423. | |
| [17] | 王亚平, 黄毅, 王苏明, 等. 土壤和沉积物中元素的化学形态及其顺序提取法[J]. 地质通报, 2005, 24(8): 728-734. |
| WANG Yaping, HUANG Yi, WANG Suming, et al. Chemical speciation of elements in sediments and soils and their sequential extraction process[J]. Regional Geology of China, 2005, 24(8): 728-734. | |
| [18] | 马志斌, 张学里, 郭彦霞, 等. 循环流化床粉煤灰理化特性及元素溶出行为研究进展[J]. 化工进展, 2021, 40(6): 3058-3071. |
| MA Zhibin, ZHANG Xueli, GUO Yanxia, et al. Research progress on characteristics and element dissolution behaviors of circulating gluidized bed-derived fly ash[J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3058-3071. | |
| [19] | AHMAD Shaz, SINGH Reena, ARFIN Tanvir, et al. Fluoride contamination, consequences and removal techniques in water: A review[J]. Environmental Science: Advances, 2022, 1(5): 620-661. |
| [20] | GUPTA Dharmendra K, Upendra N RAI, TRIPATHI Rudra D, et al. Impacts of fly-ash on soil and plant responses[J]. Journal of Plant Research, 2002, 115(6): 401-409. |
| [21] | 窦红宾, 郭唯. 重金属污染及其对水土的危害[J]. 生态经济, 2022, 38(11): 5-8. |
| DOU Hongbin, GUO Wei. Heavy metal pollution and its harm to water and soil[J]. Ecological Economy, 2022, 38(11): 5-8. | |
| [22] | LI Tianru, WANG Baomin. Effect and mechanism of nano-alumina on early hydration properties and heavy metals solidification/stabilization of alkali-activated MSWI fly ash solidified body[J]. Journal of Hazardous Materials, 2023, 452: 131327. |
| [23] | 张卫国, 李焕同, 王峰, 等. 陕南石煤及煤灰中钼元素在固液间迁移规律[J]. 煤田地质与勘探, 2020, 48(2): 64-70. |
| ZHANG Weiguo, LI Huantong, WANG Feng, et al. Solid-liquid migration of molybdenum in stone coal and coal ash in southern Shaanxi[J]. Coal Geology & Exploration, 2020, 48(2): 64-70. | |
| [24] | 杨慧芬, 党春阁, 肖晶晶. 钢渣对酸性含Cd2+废水的中和-吸附作用[C]//环境污染与大众健康学术会议论文集. 武汉: 美国科研出版社, 2010. |
| YANG Huifen, DANG Chunge, XIAO Jingjing. Steel slag as neutralization-adsorption material for treatment of acidic Cd2+-containing wastewater[C]//Proceedings of Conference on Environmental Pollution and Public Health. Wuhan: Scientific Research Publishing, 2010. | |
| [25] | 刘学文. 改性粉煤灰除磷效果及其免烧结陶粒制备工艺研究[D]. 南昌: 南昌大学, 2015. |
| LIU Xuewen. Study on phosphorus removal effect of modified fly ash and preparation technology of sintering-free ceramsite[D]. Nanchang: Nanchang University, 2015. | |
| [26] | 周思雨, 张世文, 李恩伟, 等. 不同pH值条件下煤基固废重金属浸出特征及环境风险分析[J]. 安徽工程大学学报, 2024, 39(1): 53-62. |
| ZHOU Siyu, ZHANG Shiwen, LI Enwei, et al. Characteristics and environmental risk analysis of heavy metal leaching from coal-based solid waste under different pH values[J]. Journal of Anhui Polytechnic University, 2024, 39(1): 53-62. | |
| [27] | HABERL Jasmin, SCHUSTER Michael. Solubility of elements in waste incineration fly ash and bottom ash under various leaching conditions studied by a sequential extraction procedure[J]. Waste Management, 2019, 87: 268-278. |
| [28] | LINH Ho Nhut, TAMURA Hiroto, KOMIYA Teppei, et al. Simulating the impact of heavy rain on leaching behavior of municipal solid waste incineration bottom ash (MSWI BA) in semi-aerobic landfill[J]. Waste Management, 2020, 113: 280-293. |
| [29] | NEUPANE Ghanashyam, DONAHOE Rona J. Leachability of elements in alkaline and acidic coal fly ash samples during batch and column leaching tests[J]. Fuel, 2013, 104: 758-770. |
| [30] | 张晴. 粉煤灰复垦土壤中重金属元素的淋溶实验研究[D]. 合肥: 合肥工业大学, 2017. |
| ZHANG Qing. Experimental study on leaching of heavy metals in reclaimed soil with fly ash[D]. Hefei: Hefei University of Technology, 2017. | |
| [31] | GOODARZI F, HUGGINS F E, SANEI H. Assessment of elements, speciation of As, Cr, Ni and emitted Hg for a Canadian power plant burning bituminous coal[J]. International Journal of Coal Geology, 2008, 74(1): 1-12. |
| [32] | TIAN Quanzhi, GUO Binglin, NAKAMA Shingo, et al. Distributions and leaching behaviors of toxic elements in fly ash[J]. ACS Omega, 2018, 3(10): 13055-13064. |
| [33] | ZHOU Chuncai, LIU Guijian, XU Zhongyu, et al. Effect of ash composition on the partitioning of arsenic during fluidized bed combustion[J]. Fuel, 2017, 204: 91-97. |
| [34] | RIVERA Nelson, HESTERBERG Dean, KAUR Navdeep, et al. Chemical speciation of potentially toxic trace metals in coal fly ash associated with the Kingston fly ash spill[J]. Energy & Fuels, 2017, 31(9): 9652-9659. |
| [35] | NARUKAWA Tomohiro, TAKATSU Akiko, CHIBA Koichi, et al. Investigation on chemical species of arsenic, selenium and antimony in fly ash from coal fuel thermal power stations[J]. Journal of Environmental Monitoring, 2005, 7(12): 1342-1348. |
| [36] | JONES D R. The leaching of major and trace elements from coal ash[M]//Environmental aspects of trace elements in coal. Dordrecht: Springer Netherlands, 1995: 221-262. |
| [37] | LI Zhiyong, CHEN Lan, LIU Songtao, et al. Characterization of PAHs and PCBs in fly ashes of eighteen coal-fired power plants[J]. Aerosol and Air Quality Research, 2016, 16(12): 3175-3186. |
| [38] | KIM Ann G, KAZONICH George, DAHLBERG Michael. Relative solubility of cations in Class F fly ash[J]. Environmental Science & Technology, 2003, 37(19): 4507-4511. |
| [39] | 朱晓敏, 姚波, 江熙, 等. 云南某钢渣磁选尾矿堆场重金属在模拟降雨下溶出特性[J]. 昆明冶金高等专科学校学报, 2024, 40(2): 63-72. |
| ZHU Xiaomin, YAO Bo, JIANG Xi, et al. Study on leaching characteristics of heavy metals from a magnetic separation tailings yard of steel slag in Yunnan Province under simulated rainfall[J]. Journal of Kunming Metallurgy College, 2024, 40(2): 63-72. | |
| [40] | 刘博, 梁宇廷, 孟棒棒, 等. 电解锰渣-赤泥路面砖中锰的浸出行为研究及长期释放预测[J]. 环境科学研究, 2023, 36(10): 2000-2010. |
| LIU Bo, LIANG Yuting, MENG Bangbang, et al. Study on leaching behavior of manganese in electrolytic manganese residue and red mud paving bricks and long-term release prediction[J]. Research of Environmental Sciences, 2023, 36(10): 2000-2010. | |
| [41] | 王希尹. 固废生产建材中重金属浸出方法研究[D]. 重庆: 重庆交通大学, 2018. |
| WANG Xiyin. Study on leaching method of heavy metals in building materials produced by solid waste[D]. Chongqing: Chongqing Jiaotong University, 2018. | |
| [42] | 邓凯沣, 杨熙斌, 黄小凤, 等. 酸雨条件下锡尾矿中砷、锰的释放特征[J]. 环境科学与技术, 2024, 47(6): 18-26. |
| DENG Kaifeng, YANG Xibin, HUANG Xiaofeng, et al. Study on As and Mn release characteristics of a tin-tailing pond under simulated condition of acid rain[J]. Environmental Science & Technology, 2024, 47(6): 18-26. | |
| [43] | 陈伸. 赤泥-磷石膏稳定碎石基层力学性能及其重金属迁移规律研究[D]. 重庆: 重庆交通大学, 2024. |
| CHEN Shen. Study on mechanical properties and heavy metal migration law of red mud-phosphogypsum stabilized macadam base[D]. Chongqing: Chongqing Jiaotong University, 2024. | |
| [44] | 纪东如, 钱建平, 张璇, 等. 模拟降雨淋洗对土壤重金属释放规律的研究[J]. 环境污染与防治, 2024, 46(7): 927-932, 940. |
| JI Dongru, QIAN Jianping, ZHANG Xuan, et al. Study on the release of heavy metals from soil by simulated rainfall leaching[J]. Environmental Pollution & Control, 2024, 46(7): 927-932, 940. | |
| [45] | 国家环境保护局, 中国环境监测总站. 中国土壤元素背景值[M]. 北京: 中国环境科学出版社, 1990. |
| State Environmental Protection Administration, China Environmental Monitoring Station. Background values of soil elements in China [M]. Beijing: China Environmental Science Press, 1990. | |
| [46] | SPREADBURY Chad J, CLAVIER Kyle A, LIN Ashley M, et al. A critical analysis of leaching and environmental risk assessment for reclaimed asphalt pavement management[J]. Science of the Total Environment, 2021, 775: 145741. |
| [47] | 李万鹏. 淋滤作用下煤矸石中污染物质的释放机理及潜在生态风险评价[D]. 西安: 长安大学, 2017. |
| LI Wanpeng. Release mechanism and potential ecological risk assessment of pollutants in coal gangue under leaching[D]. Xi’an: Changan University, 2017. | |
| [48] | 马建华, 韩昌序, 姜玉玲. 潜在生态风险指数法应用中的一些问题[J]. 地理研究, 2020, 39(6): 1233-1241. |
| MA Jianhua, HAN Changxu, JIANG Yuling. Some problems in the application of potential ecological risk index[J]. Geographical Research, 2020, 39(6): 1233-1241. | |
| [49] | 王春光, 彭景颂, 李婉莹, 等. 黄河中游重要生产煤矿山土壤重金属特征分析与评价[J]. 矿业安全与环保, 2022, 49(5): 124-130. |
| WANG Chunguang, PENG Jingsong, LI Wanying, et al. The analysis and evaluation on the features of the heavy metals in coal mining soil in the middle reaches of the Yellow River[J]. Mining Safety & Environmental Protection, 2022, 49(5): 124-130. |
| [1] | DU Xuan, WANG Zhanhong, ZHENG Bin, XU Wei, WANG Shuo, SHI Peng, GAO Guo. Progress on separation of cobalt-iron acid leaching solution and battery grade iron phosphate recovery technology [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5327-5338. |
| [2] | WU Min, LIAO Yalong, JIA Xiaobao, YANG Shuangyu. Research progress on enhancing bacterial leaching methods of chalcopyrite [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3618-3629. |
| [3] | GAO Jiawei, HUANG Yaji, WANG Sheng, ZHU Zhicheng, XIAO Yixuan, SONG Huikang, LIU Jun, QI Shuaijie, ZHANG Yuyao, ZHAO Jiaqi. Effects of silicon-aluminum mineral components on the melting characteristics and the solidification of heavy metal of municipal solid waste incineration fly ash [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1716-1725. |
| [4] | ZHAO Jiaqi, HUANG Yaji, LI Zhiyuan, ZHU Zhicheng, QI Shuaijie, GAO Jiawei, LIU Jun, ZHANG Yuyao. Characteristics of heavy metal migration and transformation during co-pyrolysis of sludge with agroforestry wastes [J]. Chemical Industry and Engineering Progress, 2025, 44(2): 1064-1075. |
| [5] | JIANG Liping, ZHANG Xueqiao, ZHONG Xiaojuan, WEI Yufan, XIAO Li, GUO Xujing, YANG Yijin. Optimization of acid leaching process of iron from vanadium slag and preparation of composite photocatalysts [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 538-548. |
| [6] | LI Lin, HUANG Guoyong, XU Shengming, YU Fengshan, WENG Yaqing, CAO Caifang, WEN Jiawei, WANG Chunxia, WANG Junlian, GU Bintao, ZHANG Yuanhua, LIU Bin, WANG Caiping, PAN Jianming, XU Zeliang, WANG Chong, WANG Ke. Recovery and regeneration preparation of aluminum-based spent catalyst support [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 640-649. |
| [7] | LI Zihan, SHU Jiancheng, CAO Wenxing, YANG Huimin, CHEN Mengjun. Physicochemical properties of rhodochrosite before and after leaching and the changing law of interface hydration behavior [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5320-5328. |
| [8] | QUAN Cui, GAO Ningbo, ZHANG Guangtao, SUO Haojie. Leaching characteristics of heavy metals and polycyclic aromatic hydrocarbons from permeable bricks prepared by pyrolysis residue of oily sludge [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5226-5233. |
| [9] | LI Weijie, LU Leilei, LI Deke, WANG Chunhang, ZHANG Zuming, TAN Qiang. Lithium-ion battery disassembly and recycling technology and progress [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4601-4613. |
| [10] | YIN Junquan, WU Yinkai, LI Weihua, SUN Yingjie, ZHANG Wenxuan, ZHANG Qingjian, MA Xiaoteng, BIAN Rongxing, WANG Huawei. Physicochemical characteristics and environmental risk of ash/slag in typical sections of MSW incineration [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4714-4725. |
| [11] | GUO Peng, LI Hongwei, LI Guixian, JI Dong, WANG Dongliang, ZHAO Xinhong. Mechanisms and coping strategies on deactivation of anode catalysts for direct methanol fuel cells [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3812-3823. |
| [12] | KONG Xiangrui, DONG Yuecen, ZHANG Mengyu, WANG Biao, YIN Shui′e, CHEN Bing, LU Jiawei, ZHANG Yuan, FENG Lele, WANG Hongtao, XU Haiyun. Treatment technologies of fly ash from municipal solid waste incineration [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 4102-4117. |
| [13] | MA Jiahui, WANG Yibin, FENG Jingwu, TAN Houzhang, LIN Chi. Experimental of CO2 mineralization by industrial containing calcium solid wastes [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3440-3449. |
| [14] | ZHOU Mingxian, YE Xiaozhou. Optimization of preferential lithium extraction from waste ternary lithium ion batteries by carbothermal reduction [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 2174-2182. |
| [15] | MA Wenjun, ZHANG Xu, LIU Mengshun, LIANG Zhiyuan. Research progress of novel hydrometallurgy in recycling cathode materials from spent lithium-ion batteries [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 2077-2090. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||