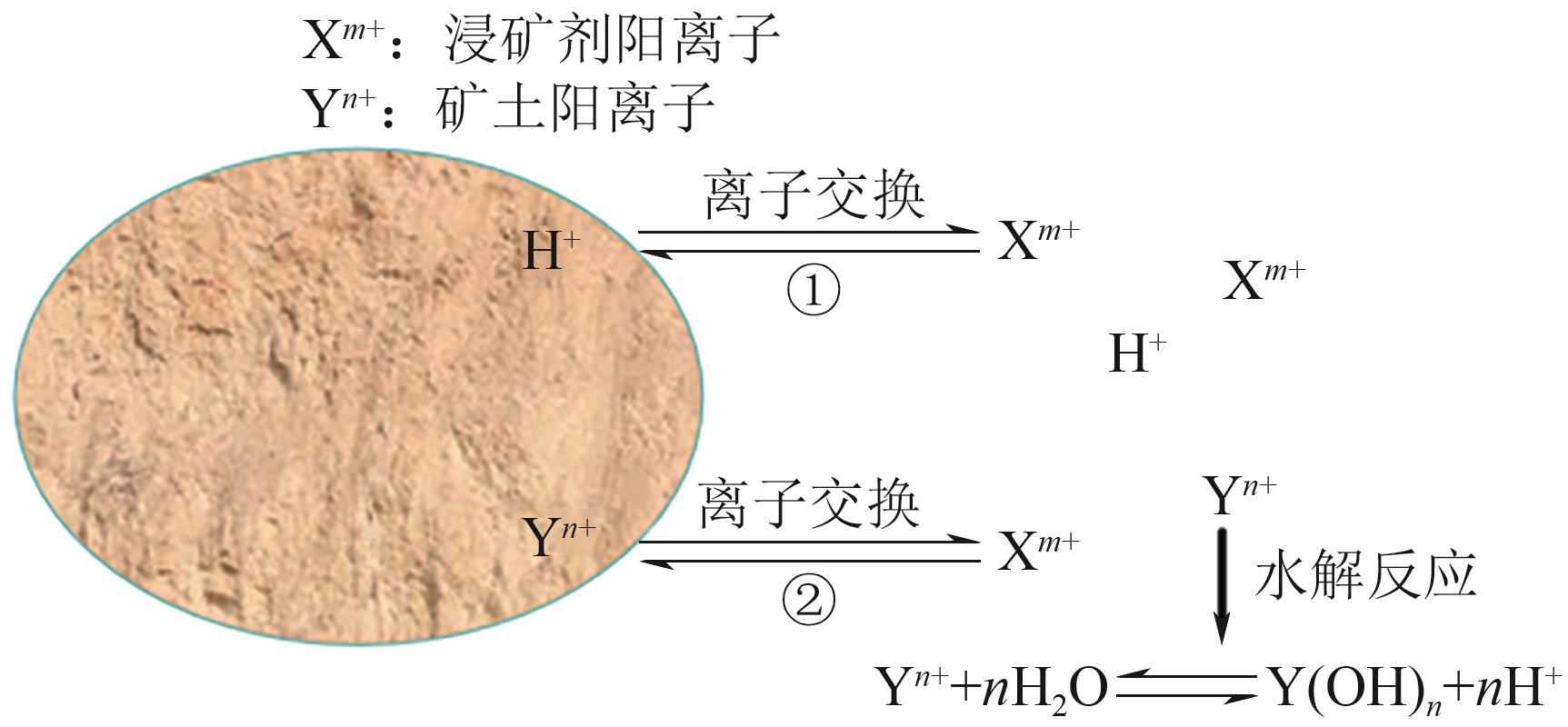
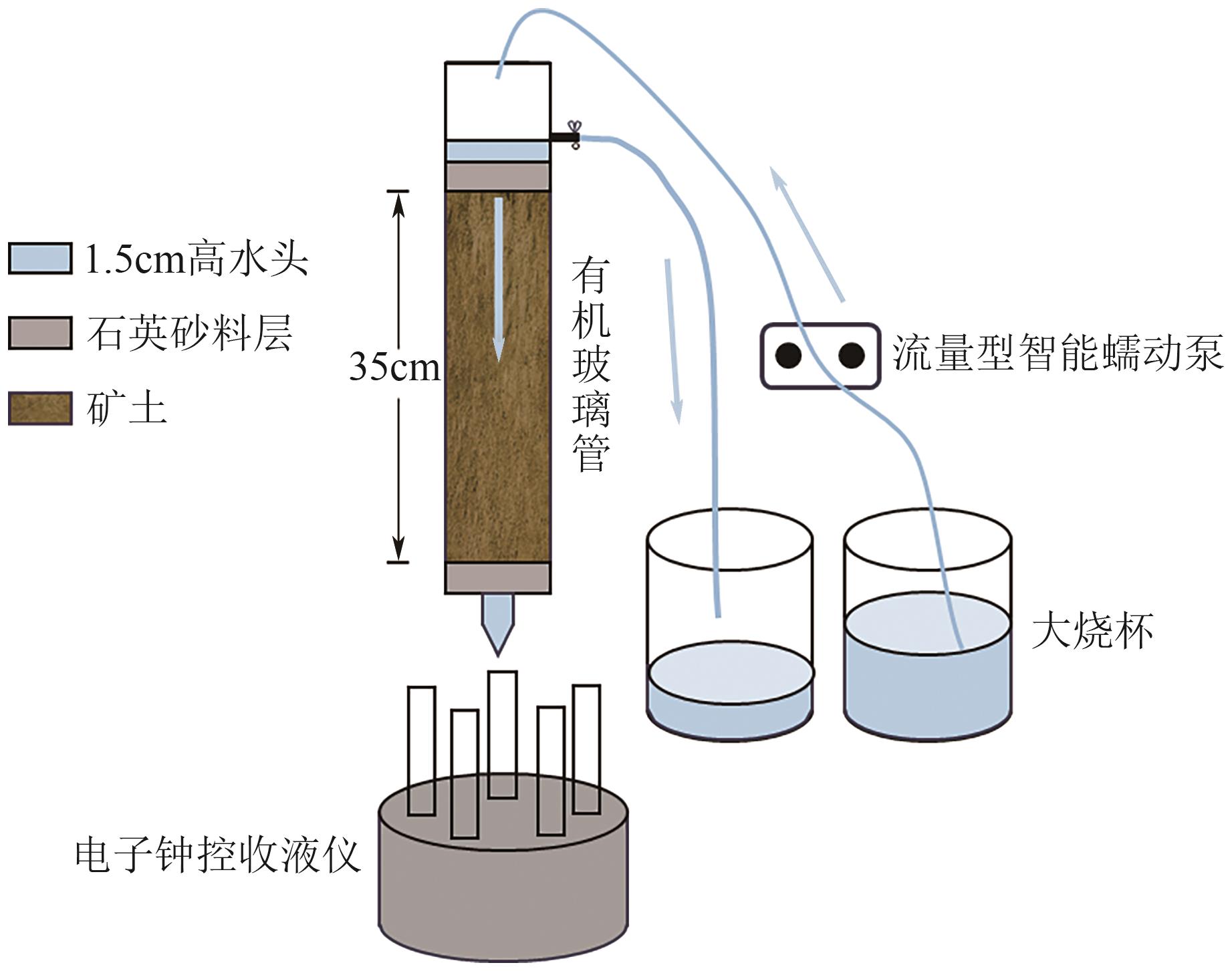
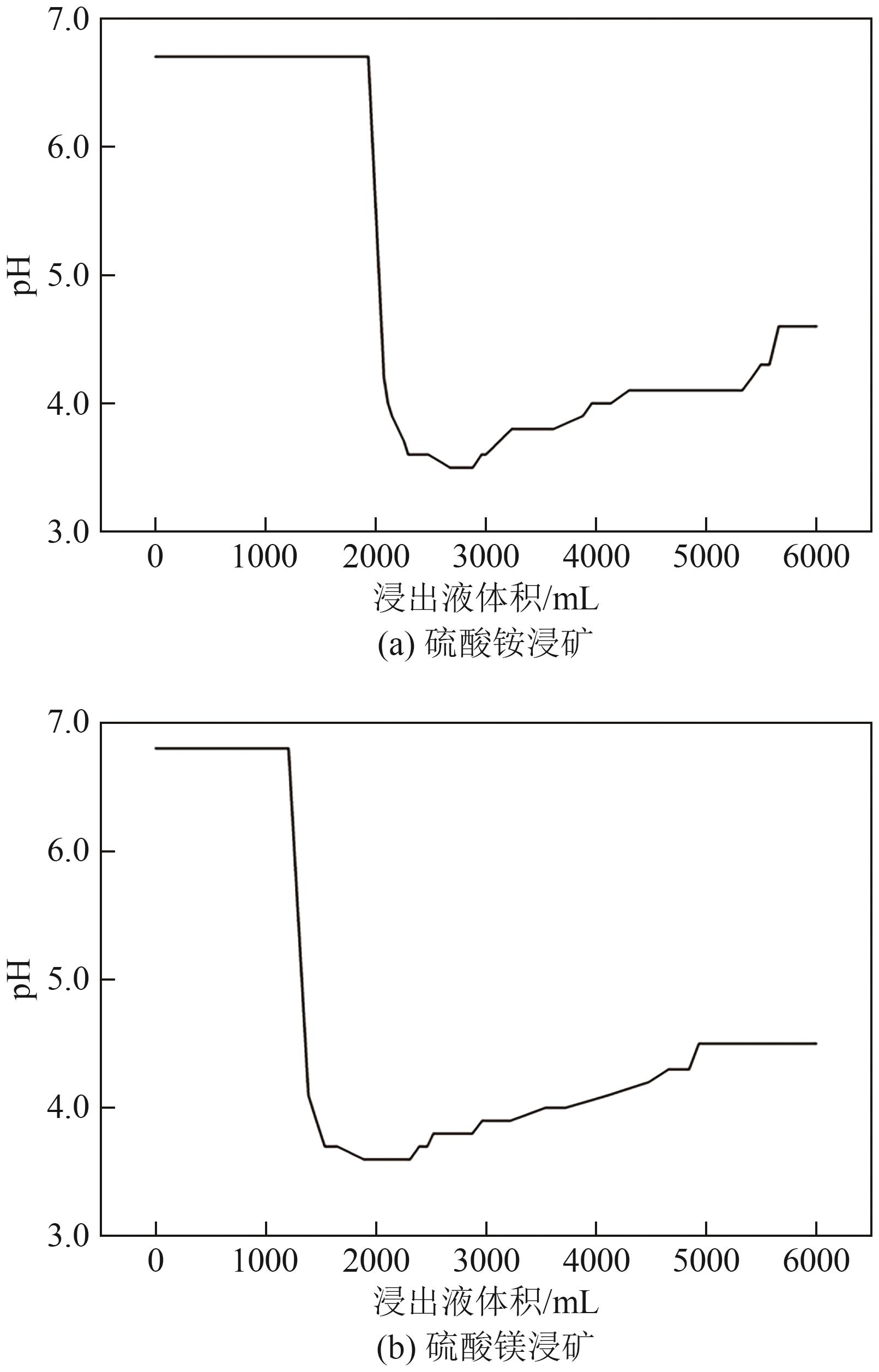
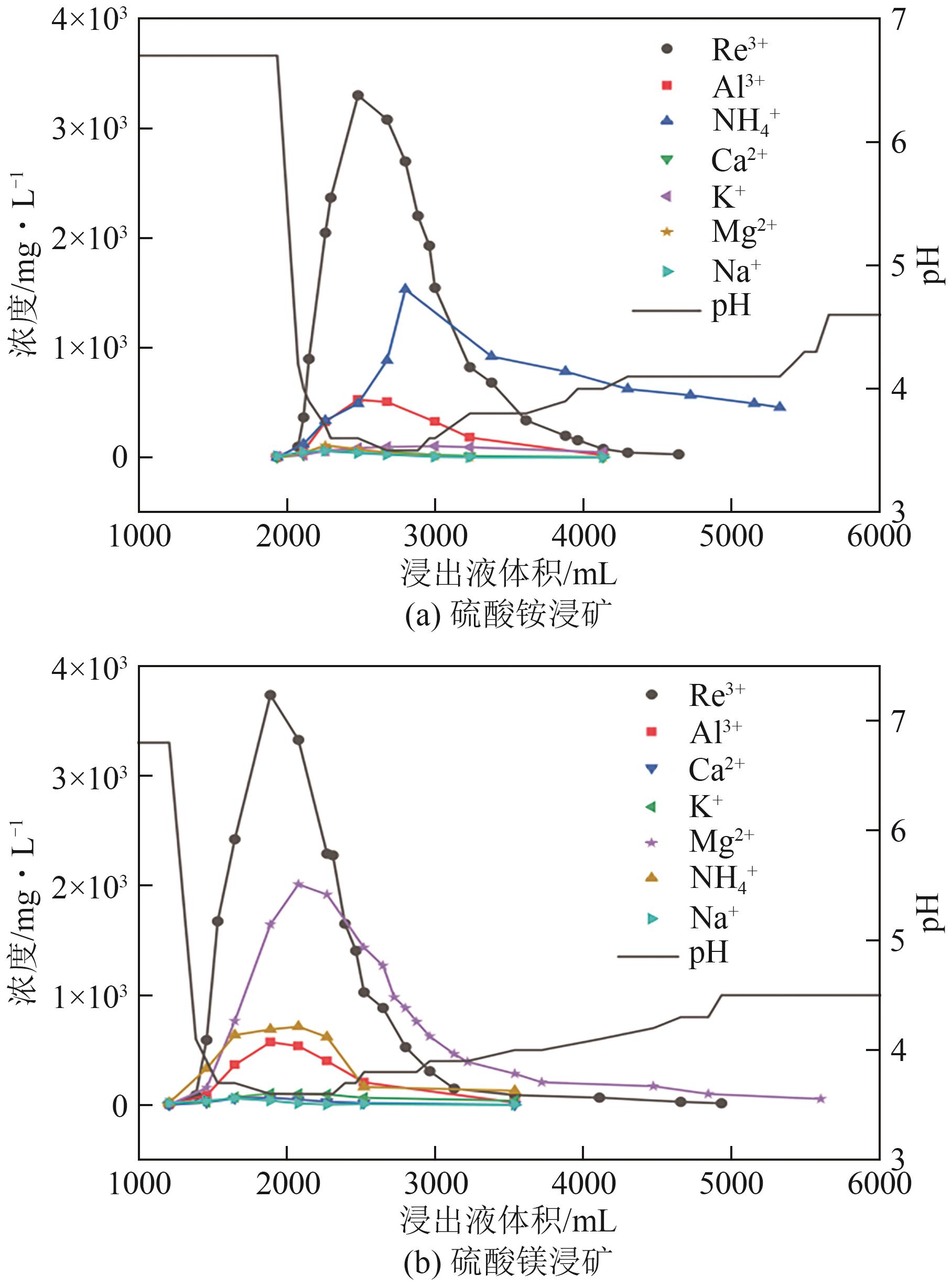
Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (7): 4267-4273.DOI: 10.16085/j.issn.1000-6613.2024-0786
• Resources and environmental engineering • Previous Articles
Factors affecting the concentration of H+ in the leaching solution of ionic rare earth ore
HU Sitao1( ), QIN Lei1(
), QIN Lei1( ), WANG Guanshi1, CAI Longxiang1, LUO Sihai2, PENG Chenliang1, LONG Ping1
), WANG Guanshi1, CAI Longxiang1, LUO Sihai2, PENG Chenliang1, LONG Ping1
- 1.School of Civil and Surveying & Mapping Engineering, Jiangxi University of Science and Technology, Ganzhou 341000, Jiangxi, China
2.School of Infrastructure Engineering, Nanchang University, Nanchang 330031, Jiangxi, China
-
Received:2024-05-10Revised:2024-08-29Online:2025-08-04Published:2025-07-25 -
Contact:QIN Lei
影响离子型稀土矿浸出液H+浓度的因素
胡思涛1( ), 秦磊1(
), 秦磊1( ), 王观石1, 蔡隆祥1, 罗嗣海2, 彭陈亮1, 龙平1
), 王观石1, 蔡隆祥1, 罗嗣海2, 彭陈亮1, 龙平1
- 1.江西理工大学土木与测绘工程学院,江西 赣州 341000
2.南昌大学工程建设学院,江西 南昌 330031
-
通讯作者:秦磊 -
作者简介:胡思涛(2000—),男,硕士研究生,研究方向为离子型稀土资源绿色提取。E-mail:1344862821@qq.com。 -
基金资助:国家自然科学基金(52164008);国家自然科学基金(52364015);江西省03专项及5G项目(20224ABC03A11);江西省“千人计划”科技创新高端人才项目(jxsq2023201013);江西省高等学校井冈学者特聘教授岗位资助项目(205201200003);江西省自然科学基金(20212BAB211012)
CLC Number:
Cite this article
HU Sitao, QIN Lei, WANG Guanshi, CAI Longxiang, LUO Sihai, PENG Chenliang, LONG Ping. Factors affecting the concentration of H+ in the leaching solution of ionic rare earth ore[J]. Chemical Industry and Engineering Progress, 2025, 44(7): 4267-4273.
胡思涛, 秦磊, 王观石, 蔡隆祥, 罗嗣海, 彭陈亮, 龙平. 影响离子型稀土矿浸出液H+浓度的因素[J]. 化工进展, 2025, 44(7): 4267-4273.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-0786
| 阳离子 | 含量/mg·g-1 |
|---|---|
| Re3+ | 0.37 |
| Al3+ | 0.087 |
| Na+ | 0.059 |
| K+ | 0.061 |
| Mg2+ | 0.043 |
| Ca2+ | 1.47 |
| NH4+ | 0.12 |
| 阳离子 | 含量/mg·g-1 |
|---|---|
| Re3+ | 0.37 |
| Al3+ | 0.087 |
| Na+ | 0.059 |
| K+ | 0.061 |
| Mg2+ | 0.043 |
| Ca2+ | 1.47 |
| NH4+ | 0.12 |
| 矿样稀土离子相 | 质量分数/% |
|---|---|
| Nd2O3 | 27.12 |
| La2O3 | 29.99 |
| Eu2O3 | 0.82 |
| Dy2O3 | 2.58 |
| Pr6O11 | 6.81 |
| Gd2O3 | 3.29 |
| Lu2O3 | 0.22 |
| CeO2 | 4.06 |
| Er2O3 | 1.51 |
| Sm2O3 | 5.91 |
| Yb2O3 | 1.52 |
| Tb4O7 | 0.59 |
| Ho2O3 | 0.53 |
| Tm2O3 | 0.22 |
| Y2O3 | 14.82 |
| 矿样稀土离子相 | 质量分数/% |
|---|---|
| Nd2O3 | 27.12 |
| La2O3 | 29.99 |
| Eu2O3 | 0.82 |
| Dy2O3 | 2.58 |
| Pr6O11 | 6.81 |
| Gd2O3 | 3.29 |
| Lu2O3 | 0.22 |
| CeO2 | 4.06 |
| Er2O3 | 1.51 |
| Sm2O3 | 5.91 |
| Yb2O3 | 1.52 |
| Tb4O7 | 0.59 |
| Ho2O3 | 0.53 |
| Tm2O3 | 0.22 |
| Y2O3 | 14.82 |
| 项目 | 硫酸铵浸矿前 | 硫酸镁浸矿前 | ||||
|---|---|---|---|---|---|---|
| 浸矿剂 | 顶水 | 矿土滞留水 | 浸矿剂 | 顶水 | 矿土滞留水 | |
| 体积/L | 0.335 | 8.154 | 0.669 | 0.587 | 8.111 | 0.669 |
| H+浓度/g·L-1 | 10-5.30 | 10-5 | 10-6.80 | 10-5.77 | 10-5 | 10-6.80 |
| H+质量/g | 10-5.78 | 10-4.09 | 10-6.98 | 10-6.00 | 10-4.09 | 10-6.98 |
| 项目 | 硫酸铵浸矿前 | 硫酸镁浸矿前 | ||||
|---|---|---|---|---|---|---|
| 浸矿剂 | 顶水 | 矿土滞留水 | 浸矿剂 | 顶水 | 矿土滞留水 | |
| 体积/L | 0.335 | 8.154 | 0.669 | 0.587 | 8.111 | 0.669 |
| H+浓度/g·L-1 | 10-5.30 | 10-5 | 10-6.80 | 10-5.77 | 10-5 | 10-6.80 |
| H+质量/g | 10-5.78 | 10-4.09 | 10-6.98 | 10-6.00 | 10-4.09 | 10-6.98 |
| 样品编号 | 硫酸铵浸矿后 | 样品编号 | 硫酸镁浸矿后 | ||
|---|---|---|---|---|---|
| 体积/L | H+浓度/g·L-1 | 体积/L | H+浓度/g·L-1 | ||
| A-1 | 0.052 | 10-6.80 | M-1 | 0.080 | 10-6.79 |
| A-2 | 0.056 | 10-6.80 | M-2 | 0.084 | 10-6.70 |
| ︙ | ︙ | ︙ | ︙ | ︙ | ︙ |
| A-88 | 0.079 | 10-4.30 | M-85 | 0.086 | 10-4.68 |
| 矿土滞留水 | 1.927 | 10-4.60 | 矿土滞留水 | 2.141 | 10-4.50 |
| 样品编号 | 硫酸铵浸矿后 | 样品编号 | 硫酸镁浸矿后 | ||
|---|---|---|---|---|---|
| 体积/L | H+浓度/g·L-1 | 体积/L | H+浓度/g·L-1 | ||
| A-1 | 0.052 | 10-6.80 | M-1 | 0.080 | 10-6.79 |
| A-2 | 0.056 | 10-6.80 | M-2 | 0.084 | 10-6.70 |
| ︙ | ︙ | ︙ | ︙ | ︙ | ︙ |
| A-88 | 0.079 | 10-4.30 | M-85 | 0.086 | 10-4.68 |
| 矿土滞留水 | 1.927 | 10-4.60 | 矿土滞留水 | 2.141 | 10-4.50 |
| 样品编号 | 浸矿前矿土pH | 硫酸铵浸矿后矿土pH | 硫酸镁浸矿后矿土pH |
|---|---|---|---|
| K-1 | 5.4 | 4.1 | 4.5 |
| K-2 | 5.4 | 4.4 | 4.3 |
| K-3 | 5.1 | 4.4 | 4.6 |
| K-4 | 5.4 | 4.2 | 4.4 |
| K-5 | 5.2 | 4.4 | 4.2 |
| 平均值 | 5.3 | 4.3 | 4.4 |
| 样品编号 | 浸矿前矿土pH | 硫酸铵浸矿后矿土pH | 硫酸镁浸矿后矿土pH |
|---|---|---|---|
| K-1 | 5.4 | 4.1 | 4.5 |
| K-2 | 5.4 | 4.4 | 4.3 |
| K-3 | 5.1 | 4.4 | 4.6 |
| K-4 | 5.4 | 4.2 | 4.4 |
| K-5 | 5.2 | 4.4 | 4.2 |
| 平均值 | 5.3 | 4.3 | 4.4 |
| 试样号 | A | B | C |
|---|---|---|---|
| 1 | 500 | 50 | 500 |
| 2 | 800 | 200 | 800 |
| 3 | 1000 | 300 | 1000 |
| 4 | 1200 | 500 | 1200 |
| 试样号 | A | B | C |
|---|---|---|---|
| 1 | 500 | 50 | 500 |
| 2 | 800 | 200 | 800 |
| 3 | 1000 | 300 | 1000 |
| 4 | 1200 | 500 | 1200 |
| 试样号 | A | B | C | pH |
|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 3.695 |
| 2 | 1 | 2 | 2 | 3.226 |
| 3 | 1 | 3 | 3 | 3.102 |
| 4 | 1 | 4 | 4 | 3.066 |
| 5 | 2 | 1 | 2 | 3.317 |
| 6 | 2 | 2 | 1 | 3.092 |
| 7 | 2 | 3 | 4 | 3.554 |
| 8 | 2 | 4 | 3 | 3.184 |
| 9 | 3 | 1 | 3 | 3.507 |
| 10 | 3 | 2 | 4 | 3.396 |
| 11 | 3 | 3 | 1 | 3.016 |
| 12 | 3 | 4 | 2 | 3.285 |
| 13 | 4 | 1 | 4 | 3.237 |
| 14 | 4 | 2 | 3 | 3.412 |
| 15 | 4 | 3 | 2 | 3.311 |
| 16 | 4 | 4 | 1 | 3.284 |
| 试样号 | A | B | C | pH |
|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 3.695 |
| 2 | 1 | 2 | 2 | 3.226 |
| 3 | 1 | 3 | 3 | 3.102 |
| 4 | 1 | 4 | 4 | 3.066 |
| 5 | 2 | 1 | 2 | 3.317 |
| 6 | 2 | 2 | 1 | 3.092 |
| 7 | 2 | 3 | 4 | 3.554 |
| 8 | 2 | 4 | 3 | 3.184 |
| 9 | 3 | 1 | 3 | 3.507 |
| 10 | 3 | 2 | 4 | 3.396 |
| 11 | 3 | 3 | 1 | 3.016 |
| 12 | 3 | 4 | 2 | 3.285 |
| 13 | 4 | 1 | 4 | 3.237 |
| 14 | 4 | 2 | 3 | 3.412 |
| 15 | 4 | 3 | 2 | 3.311 |
| 16 | 4 | 4 | 1 | 3.284 |
| 试样号 | A | B | C |
|---|---|---|---|
| K1 | 13.089 | 13.756 | 13.087 |
| K2 | 13.147 | 13.126 | 13.139 |
| K3 | 13.204 | 12.983 | 13.205 |
| K4 | 13.244 | 12.819 | 13.253 |
| K12 | 171.322 | 189.228 | 171.270 |
| K22 | 172.844 | 172.292 | 172.633 |
| K32 | 174.346 | 168.558 | 174.372 |
| K42 | 175.404 | 164.327 | 175.642 |
| R | 1.020 | 6.225 | 1.093 |
| 试样号 | A | B | C |
|---|---|---|---|
| K1 | 13.089 | 13.756 | 13.087 |
| K2 | 13.147 | 13.126 | 13.139 |
| K3 | 13.204 | 12.983 | 13.205 |
| K4 | 13.244 | 12.819 | 13.253 |
| K12 | 171.322 | 189.228 | 171.270 |
| K22 | 172.844 | 172.292 | 172.633 |
| K32 | 174.346 | 168.558 | 174.372 |
| K42 | 175.404 | 164.327 | 175.642 |
| R | 1.020 | 6.225 | 1.093 |
| 方差来源 | 平方和 | 自由度 | 均方 | F | 显著性 |
|---|---|---|---|---|---|
| A | 0.003 | 3 | 0.001 | 14.14 | 显著 |
| B | 0.126 | 3 | 0.042 | 519.07 | 特别显著 |
| C | 0.004 | 3 | 0.001 | 16.45 | 显著 |
| 误差 | 0.001 | 6 | 0.00008 | — | — |
| 总和 | 0.134 | 15 | — | — | — |
| 方差来源 | 平方和 | 自由度 | 均方 | F | 显著性 |
|---|---|---|---|---|---|
| A | 0.003 | 3 | 0.001 | 14.14 | 显著 |
| B | 0.126 | 3 | 0.042 | 519.07 | 特别显著 |
| C | 0.004 | 3 | 0.001 | 16.45 | 显著 |
| 误差 | 0.001 | 6 | 0.00008 | — | — |
| 总和 | 0.134 | 15 | — | — | — |
| 样品编号 | 浓度/mol·L-1 | 实际测量pH | 理论计算pH | 绝对值 | ||
|---|---|---|---|---|---|---|
| Re3+ | Al3+ | NH4+ | ||||
| A-42 | 0 | 0 | 0 | 6.70 | 7.00 | 0.30 |
| A-46 | 14.53×10-3 | 11.96×10-3 | 18.61×10-3 | 3.72 | 3.64 | 0.08 |
| A-50 | 23.40×10-3 | 19.48×10-3 | 27.17×10-3 | 3.51 | 3.56 | 0.05 |
| A-51 | 21.84×10-3 | 18.70×10-3 | 49.17×10-3 | 3.53 | 3.57 | 0.04 |
| A-55 | 10.96×10-3 | 12.04×10-3 | 61.00×10-3 | 3.64 | 3.63 | 0.01 |
| A-60 | 0.55×10-3 | 0.73×10-3 | 33.33×10-3 | 4.13 | 4.06 | 0.07 |
| 样品编号 | 浓度/mol·L-1 | 实际测量pH | 理论计算pH | 绝对值 | ||
|---|---|---|---|---|---|---|
| Re3+ | Al3+ | NH4+ | ||||
| A-42 | 0 | 0 | 0 | 6.70 | 7.00 | 0.30 |
| A-46 | 14.53×10-3 | 11.96×10-3 | 18.61×10-3 | 3.72 | 3.64 | 0.08 |
| A-50 | 23.40×10-3 | 19.48×10-3 | 27.17×10-3 | 3.51 | 3.56 | 0.05 |
| A-51 | 21.84×10-3 | 18.70×10-3 | 49.17×10-3 | 3.53 | 3.57 | 0.04 |
| A-55 | 10.96×10-3 | 12.04×10-3 | 61.00×10-3 | 3.64 | 3.63 | 0.01 |
| A-60 | 0.55×10-3 | 0.73×10-3 | 33.33×10-3 | 4.13 | 4.06 | 0.07 |
| [1] | LONG Ping, WANG Guanshi, TIAN Jun, et al. Simulation of one-dimensional column leaching of weathered crust elution-deposited rare earth ore[J]. Transactions of Nonferrous Metals Society of China, 2019, 29(3): 625-633. |
| [2] | 池汝安, 刘雪梅. 风化壳淋积型稀土矿开发的现状及展望[J]. 中国稀土学报, 2019, 37(2): 129-140. |
| CHI Ru’an, LIU Xuemei. Prospect and development of weathered crust elution-deposited rare earth ore[J]. Journal of the Chinese Society of Rare Earths, 2019, 37(2): 129-140. | |
| [3] | HUANG Ying, LONG Ping, WANG Guanshi, et al. Ion-exchange model for the leaching process of ion-adsorption-type rare-earth ores considering the influence of anions[J]. Minerals, 2023, 13(12): 1475. |
| [4] | 郭钟群, 赵奎, 金解放, 等. 离子型稀土开发面临的问题与绿色提取研究进展[J]. 化工进展, 2019, 38(7): 3425-3433. |
| GUO Zhongqun, ZHAO Kui, JIN Jiefang, et al. Problems facing ion adsorption type rare earth exploitation and research progresses on green extraction[J]. Chemical Industry and Engineering Progress, 2019, 38(7): 3425-3433. | |
| [5] | HE Zhengyan, ZHANG Zhenyue, YU Junxia, et al. Column leaching process of rare earth and aluminum from weathered crust elution-deposited rare earth ore with ammonium salts[J]. Transactions of Nonferrous Metals Society of China, 2016, 26(11): 3024-3033. |
| [6] | 魏明远, 秦磊, 王观石, 等. 原地浸矿经验注液下离子型稀土残留规律[J]. 中国有色金属学报, 2023, 33(4): 1287-1296. |
| WEI Mingyuan, QIN Lei, WANG Guanshi, et al. Law of ionic rare earth residue under in-situ leaching empirical injection[J]. The Chinese Journal of Nonferrous Metals, 2023, 33(4): 1287-1296. | |
| [7] | XIAO Yanfei, CHEN Yingying, FENG Zongyu, et al. Leaching characteristics of ion-adsorption type rare earths ore with magnesium sulfate[J]. Transactions of Nonferrous Metals Society of China, 2015, 25(11): 3784-3790. |
| [8] | 池汝安, 田君. 风化壳淋积型稀土矿化工冶金[M]. 北京: 科学出版社, 2006: 119-121. |
| CHI Ru’an, TIAN Jun. Chemical metallurgy of weathering crust eluvial rare earth ore[M]. Beijing: Science Press, 2006: 119-121. | |
| [9] | FENG Jian, ZHOU Fang, CHI Ruan, et al. Effect of a novel compound on leaching process of weathered crust elution-deposited rare earth ore[J]. Minerals Engineering, 2018, 129: 63-70. |
| [10] | LI Kaizhong, LIU Huiping, LAI Fuguo, et al. Migration of natural radionuclides in the extraction process of the ion-adsorption type rare earths ore[J]. Hydrometallurgy, 2017, 171: 236-244. |
| [11] | 李永绣, 张玲, 周新木. 南方离子型稀土的资源和环境保护性开采模式[J]. 稀土, 2010, 31(2): 80-85. |
| LI Yongxiu, ZHANG Ling, ZHOU Xinmu. Resource and environment protected exploitation model for ion-type rare earth deposit in southern of China[J]. Chinese Rare Earths, 2010, 31(2): 80-85. | |
| [12] | 郭钟群, 金解放, 赵奎, 等. 离子吸附型稀土开采工艺与理论研究现状[J]. 稀土, 2018, 39(1): 132-141. |
| GUO Zhongqun, JIN Jiefang, ZHAO Kui, et al. Status of leaching technology and theory of ion adsorption type rare earth ores[J]. Chinese Rare Earths, 2018, 39(1): 132-141. | |
| [13] | 郭小斌, 王明. 含稀土高岭土精矿回收离子相稀土工艺研究[J]. 有色金属科学与工程, 2016, 7(6): 124-128. |
| GUO Xiaobin, WANG Ming. Recovery of ionic type rare earth from Kaolin concentrate[J]. Nonferrous Metals Science and Engineering, 2016, 7(6): 124-128. | |
| [14] | 李博, 赵琼, 毛兵, 等. 我国东部主要类型土壤酸缓冲能力的影响因素[J]. 生态学杂志, 2021, 40(12): 3901-3910. |
| LI Bo, ZHAO Qiong, MAO Bing, et al. Factors influencing acid buffering capacity of main soil types in Eastern China[J]. Chinese Journal of Ecology, 2021, 40(12): 3901-3910. | |
| [15] | 陈志峰, 李金辉, 申邦坡, 等. 离子吸附型稀土矿除铝技术研究进展[J]. 有色金属科学与工程, 2017, 8(2): 112-118. |
| CHEN Zhifeng, LI Jinhui, SHEN Bangpo, et al. Research progress on technology of removing aluminum from ion adsorption type rare earth ore[J]. Nonferrous Metals Science and Engineering, 2017, 8(2): 112-118. | |
| [16] | WU Wenyuan, LI Dong, ZHAO Zhihua, et al. Formation mechanism of micro emulsion on aluminum and lanthanum extraction in P507-HCl system[J]. Journal of Rare Earths, 2010, 28: 174-178. |
| [17] | Roberto GONZÁLEZ-MENDOZA, Hilario LÓPEZ-GONZÁLEZ, Alberto ROJAS-HERNÁNDEZ. Spectrophotometric determination of the first hydrolysis constant of praseodymium (Ⅲ)[J]. Journal of the Mexican Chemical Society, 2019, 54(1). |
| [18] | CACECI Marco S, CHOPPIN Gregory R. The determination of the first hydrolysis constant of Eu(Ⅲ) and Am(Ⅲ)[J]. ract, 1983, 33(2/3): 101-104. |
| [19] | 杨幼明, 王莉, 肖敏, 等. 离子型稀土矿浸出过程主要物质浸出规律研究[J]. 有色金属科学与工程, 2016, 7(3): 125-130. |
| YANG Youming, WANG Li, XIAO Min, et al. Study of leaching behaviors of main materials in ionic rare earth ore[J]. Nonferrous Metals Science and Engineering, 2016, 7(3): 125-130. | |
| [20] | 齐晋. 风化壳淋积型稀土矿浸矿剂注液优化及铵残留分布研究[D]. 赣州: 江西理工大学, 2018: 13-15. |
| QI Jin. Study on optimization of leaching agent injection and distribution of ammonium residue in weathered crust leaching rare earth ore[D]. Ganzhou: Jiangxi University of Science and Technology, 2018: 13-15. | |
| [21] | 生态环境部. 土壤 pH值的测定 电位法: [S]. 北京: 中国环境科学出版社, 2018. |
| Ministry of Ecology and Environment of the People’s Republic of China. Soil—Determination of pH—Potentiometry: [S]. Beijing: China Environmental Science Press, 2018. | |
| [22] | 杜全斌, 陈超, 张黎燕, 等. 基于正交试验的低温电镀铁工艺探讨[J]. 新技术新工艺, 2020(8): 31-34. |
| DU Quanbin, CHEN Chao, ZHANG Liyan, et al. Discussion of low-temperature iron plating process based on orthogonal test[J]. New Technology & New Process, 2020(8): 31-34. | |
| [23] | ARAMLI Mohammad Sadegh, SARVI MOGHANLOU Kourosh, IMANI Ahmad. Effect of dietary antioxidant supplements (selenium forms, alpha-tocopherol, and coenzyme Q10) on growth performance, immunity, and physiological responses in rainbow trout (oncorhynchus mykiss) using orthogonal array design[J]. Fish & Shellfish Immunology, 2023, 134: 108615. |
| [24] | VILD Andrew, TEIXEIRA Sara, Klaus KÜHN, et al. Orthogonal experimental design of titanium dioxide—Poly(methyl methacrylate) electrospun nanocomposite membranes for photocatalytic applications[J]. Journal of Environmental Chemical Engineering, 2016, 4(3): 3151-3158. |
| [25] | 韩阳, 李潜, 赵云升, 等. 三因素及其交互作用对植物叶片多角度偏振高光谱特征的影响[J]. 红外与毫米波学报, 2010, 29(4): 316-320. |
| HAN Yang, LI Qian, ZHAO Yunsheng, et al. Effects of three interactive factors on the multi-angle polarized hyperspectrum of vegetation leaves[J]. Journal of Infrared and Millimeter Waves, 2010, 29(4): 316-320. |
| [1] | YANG Wenming, XIE Linsheng, WANG Yu, MA Yulu, LI Guo. Application of SPH-DEM coupling simulation method in meshing twin-screw extruder [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 3748-3756. |
| [2] | SUN Jinlei, LIAO Dankui, CHEN Xiaopeng, TONG Zhangfa. Preparation of spheroidal nano-calcium carbonate via high gravity-microinterface method [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 3757-3769. |
| [3] | SHI Qinchuan, WANG Shiyuan, LI Peiya, LU Shuhan, WANG Bo, WANG Jiahui, YANG Fusheng, WANG Bin, YANG Shengchun, FANG Tao. Research on the solubilities of hydrogen in liquid organic hydrogen carriers [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 3816-3827. |
| [4] | CHEN Yang, LUO Jin, WANG Dianlin, YANG Zuguo, YANG Peng, CHEN Yong, ZHONG Xiang, TANG Li. Experimental study on the effect of surfactant complex systems with different molecular structures on emulsification and viscosity reduction of extra-heavy oil [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 3838-3849. |
| [5] | WANG Jintao, ZHANG Hongzhen, LIANG Bo. Preparation of dual superlyophobic filter paper and its separation to oil in alkaline salt water [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 4006-4012. |
| [6] | LI Dan, KONG Chuilong, HU Bo, WANG Shengyan, LIU Dongyan, LIU Lihua. Preparation of phosphorescent carbon nanoparticles powder and its application in the non-fluorescent interference development of latent fingerprints [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 4013-4021. |
| [7] | JIANG Rongyuan, LI Simin, CHEN Zhiqiang, WANG Guilong, CHEN Juntao, LIN Guanfeng, LU Beili, HUANG Biao, CHEN Yandan. Preparation of phosphorus-doped hard carbon/MnO x composite and its electrochemical properties [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 4039-4049. |
| [8] | DONG Zhiyun, Ruidan SHI, ZHOU Jiali, LEI Xinxing, XI Fugui. A naphthalimide-phenanthroimidazole-based fluorescent sensor for the colorimetric and ratiometric detection of Cu2+ [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 4078-4088. |
| [9] | CHEN Juhui, ZHANG Qian, LI Dan, LI Weikang, CHEN Ke, ZHOU Huan, ZHURAVKOV Michael, LAPATSIN Siarhel, JIANG Wenrui. Flow characterization of non-spherical particles based on DEM-PPM method [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3382-3392. |
| [10] | CHUN Liang, LIAO Zicheng, WANG Guoqiang, XIAO Yao, HUO Jinpeng, LIU Dong. Performance evaluation of PV/T-driven desiccant-wheel-coupled vacuum membrane dehumidification cooling system [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3457-3467. |
| [11] | XIAO Li, QI Shaopeng, ZHOU Kun, BO Yanan, WANG Xiulin, YAO Huichao, DAI Ruoyun, SUI Yiyan. Composite structure design of defect state TiO2-x -Au clusters for efficient visible light driven CO2 reduction [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3062-3071. |
| [12] | WANG Yuting, WANG Mengxiang, LI Wenwen, LI Gang, WANG Yajun. Photo-Fenton synergistic degradation of tetracycline by Fe(Ⅲ)/3D conjugated carbon nitride system [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3072-3083. |
| [13] | ZHOU Meimei, HE Jiahui, XIANG Wanting, SHANG Jiaxin, WEI Xinyu, SUN Mimi, ZOU Wei, LUO Pingping. Electrospun PVA/SiO2 nanofibers loaded with A-TiO2/BiOBr for enhanced visible light photocatalytic activity [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3084-3092. |
| [14] | XIE Wuqiang, ZHANG Ling, HE Gang, JIANG Lifeng, ZHENG Xirui, ZHANG Hepeng. Electrocatalytic CO2 reduction to methane by CoTBrPP-PTAB-Cu catalyst [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3093-3100. |
| [15] | FU Jiang, SUN Jiaoxia, FU Junjie, ZHU Min, SONG Pinxue, ZHOU Yining, FAN Jianxin. Self-cleaning effect and oil-water separation performance of hydrophobic modified polyester fiber fabric [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3121-3131. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||