Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (11): 6301-6315.DOI: 10.16085/j.issn.1000-6613.2024-1560
• Industrial catalysis • Previous Articles
Advances in catalysts for electrochemical ammonia oxidation
HAN Yan( ), HU Xinli(
), HU Xinli( ), ZHENG Xiaoqin(
), ZHENG Xiaoqin( )
)
- Suqian Unitech Co. , Ltd. , Suqian 223809, Jiangsu, China
-
Received:2024-09-25Revised:2025-03-31Online:2025-12-08Published:2025-11-25
用于电化学氨氧化的催化剂研究进展
- 宿迁联盛科技股份有限公司,江苏 宿迁 223809
-
作者简介:韩炎(1993—),男,硕士,工程师,研究方向为有机电化学合成。E-mail:1047522239@qq.com
胡新利(1986—),男,硕士,高级工程师,研究方向为有机电化学。E-mail:huxinli2006@126.com
郑晓芹(1998—),女,硕士,研究方向为有机电化学。E-mail:zhengxiaoqinnjt@163.com。
第一联系人:所有作者对本文贡献相同。
CLC Number:
Cite this article
HAN Yan, HU Xinli, ZHENG Xiaoqin. Advances in catalysts for electrochemical ammonia oxidation[J]. Chemical Industry and Engineering Progress, 2025, 44(11): 6301-6315.
韩炎, 胡新利, 郑晓芹. 用于电化学氨氧化的催化剂研究进展[J]. 化工进展, 2025, 44(11): 6301-6315.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-1560
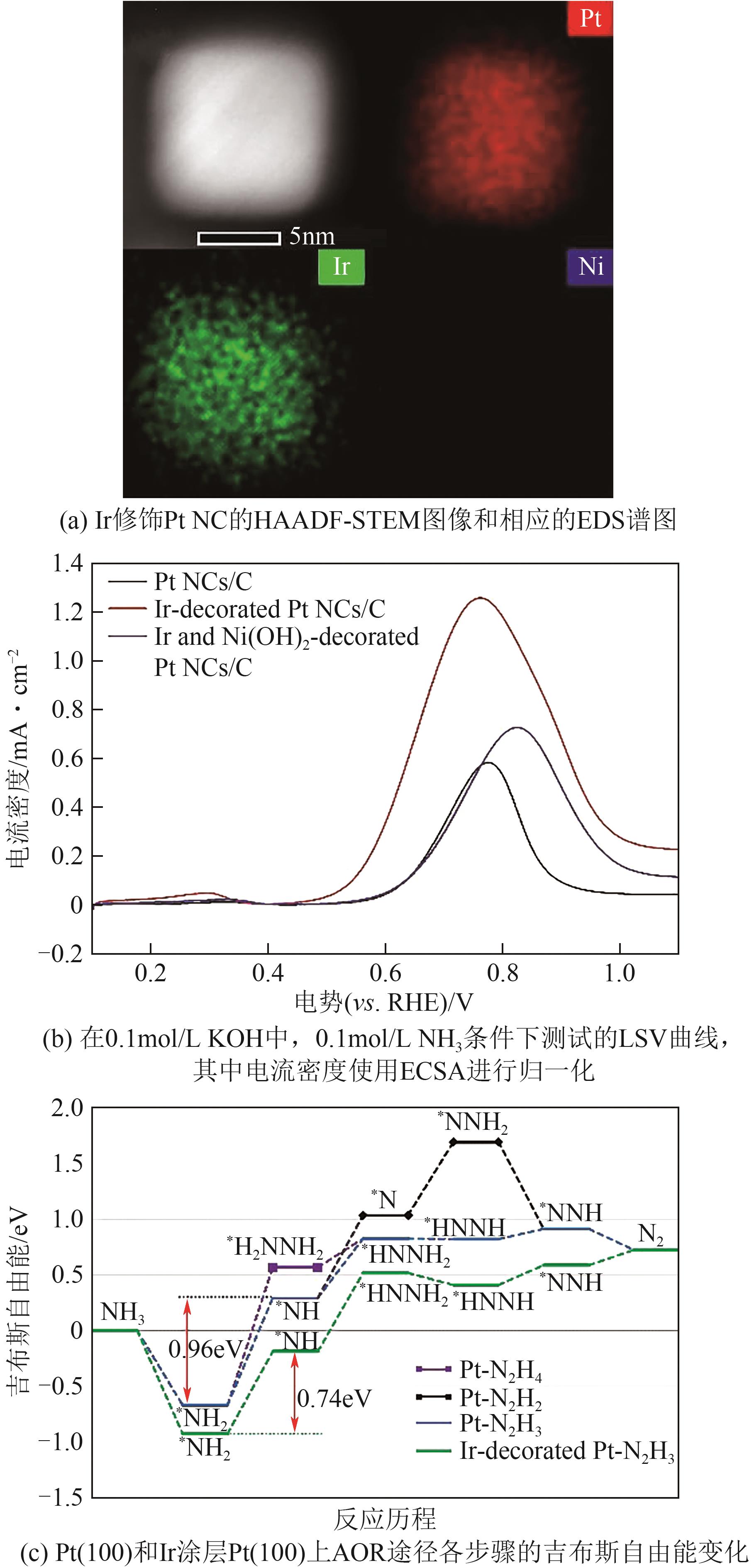
| 催化剂 | 电解液 | 起始电位(相对于RHE)/V | 峰值电流密度/mA·cm-2 | 参考文献 |
|---|---|---|---|---|
| 500CV-Pt | 1mol/L NH3+5mol/L KOH | 0.42 | 0.27 | [ |
| Pt纳米片 | 0.1mol/L NH3+1mol/L KOH | 0.57 | 0.32 | [ |
| PtZn | 0.1mol/L NH4OH+0.5mol/L KOH | 0.42 | 0.6 | [ |
| Pt薄膜 | 0.1mol/L NH3+0.2mol/L NaOH | 0.5 | 0.212 | [ |
| 花状Pt | 0.1mol/L NH3+1mol/L KOH | 0.5 | 0.48 | [ |
| Pt纳米立方体(Pt-NC) | 0.1mol/L NH4OH+1mol/L KOH | 0.5 | 5.1 | [ |
| Pt纳米颗粒(Pt NP) | 0.1mol/L NH3+0.2mol/L NaOH | 0.55 | 1.96 | [ |
| Ir-修饰Pt | 0.1mol/L NH3 +0.1mol/L KOH | 0.43 | 1.26 | [ |
| PtIr纳米颗粒 | 0.5mol/L NH4OH+1mol/L KOH | 0.4 | 0.11 | [ |
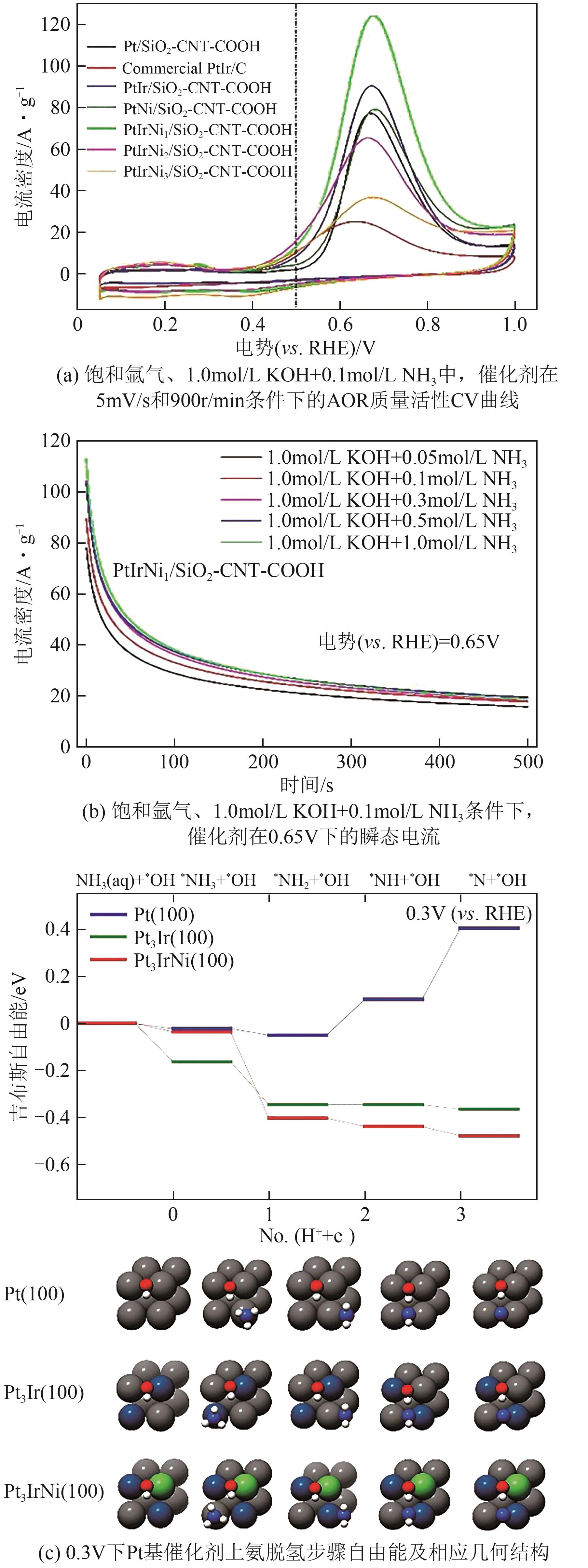
| PtIrNi/SiO2-CNT-COOH | 0.1mol/L NH3+1mol/L KOH | 0.4 | 2.48 | [ |
| PtNC/C | 0.1mol/L NH3+1mol/L KOH | 0.48 | 3.89 | [ |
| C-Pt/SnO2 | 0.1mol/L NH3+1mol/L KOH | 0.45 | 1.6 | [ |
| Pt/PBI/MWNT-CeO2 | 0.1mol/L NH3+1mol/L KOH | 0.45 | 0.26 | [ |
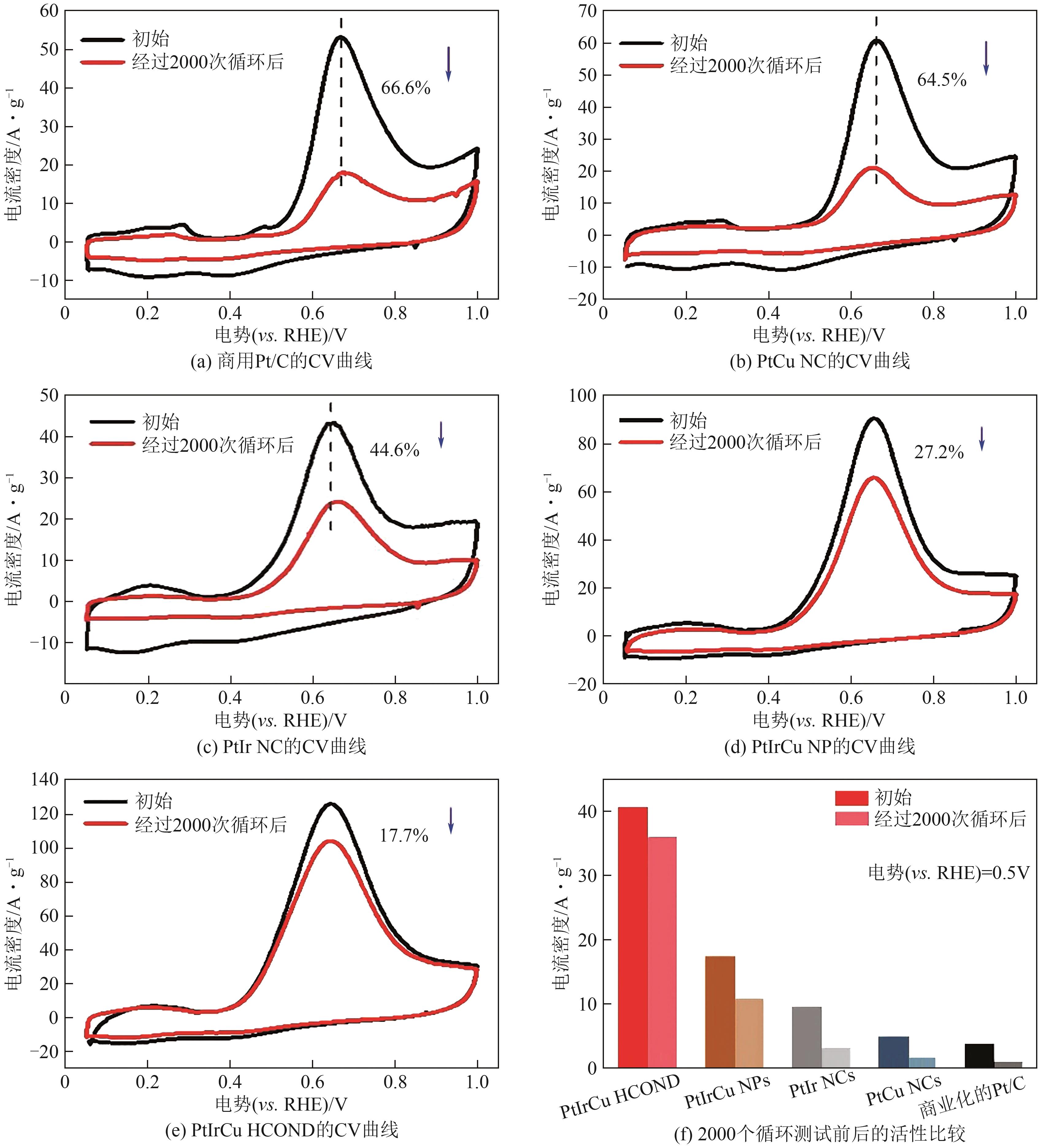
| PtIrCu HCOND | 0.1 mol/L NH3+1mol/L KOH | 0.35 | 122.9A/g | [ |
| PtIrZn2/CeO2-ZIF-8 | 0.1mol/L NH3+1mol/L KOH | 0.35 | 0.64 | [ |
| Pt85Pd15/rGO | 0.1mol/L NH3+1mol/L KOH | 0.47 | 1.46 | [ |
| Pt/Ir/MWCNT | 0.1mol/L NH3+0.1mol/L KOH | 0.38 | — | [ |
| Pt-修饰Ni NP | 0.1mol/L NH3+1mol/L KOH | 0.5 | 5.32A/g | [ |
| PtIrZn | 0.1mol/L NH4OH+0.5mol/L KOH | 0.3 | 0.56 | [ |
| PtPb/C | 1mol/L KOH+0.1mol/L NH3 | — | 191A/g | [ |
| Pt x Ru | 0.1mol/L NH3+1mol/L KOH | 0.5 | 92A/g | [ |
| Au@Pt NP | 1mol/L NaOH+0.05mol/L NH3 | 0.4 | 1.19~1.06 | [ |
| 催化剂 | 电解液 | 起始电位(相对于RHE)/V | 峰值电流密度/mA·cm-2 | 参考文献 |
|---|---|---|---|---|
| 500CV-Pt | 1mol/L NH3+5mol/L KOH | 0.42 | 0.27 | [ |
| Pt纳米片 | 0.1mol/L NH3+1mol/L KOH | 0.57 | 0.32 | [ |
| PtZn | 0.1mol/L NH4OH+0.5mol/L KOH | 0.42 | 0.6 | [ |
| Pt薄膜 | 0.1mol/L NH3+0.2mol/L NaOH | 0.5 | 0.212 | [ |
| 花状Pt | 0.1mol/L NH3+1mol/L KOH | 0.5 | 0.48 | [ |
| Pt纳米立方体(Pt-NC) | 0.1mol/L NH4OH+1mol/L KOH | 0.5 | 5.1 | [ |
| Pt纳米颗粒(Pt NP) | 0.1mol/L NH3+0.2mol/L NaOH | 0.55 | 1.96 | [ |
| Ir-修饰Pt | 0.1mol/L NH3 +0.1mol/L KOH | 0.43 | 1.26 | [ |
| PtIr纳米颗粒 | 0.5mol/L NH4OH+1mol/L KOH | 0.4 | 0.11 | [ |
| PtIrNi/SiO2-CNT-COOH | 0.1mol/L NH3+1mol/L KOH | 0.4 | 2.48 | [ |
| PtNC/C | 0.1mol/L NH3+1mol/L KOH | 0.48 | 3.89 | [ |
| C-Pt/SnO2 | 0.1mol/L NH3+1mol/L KOH | 0.45 | 1.6 | [ |
| Pt/PBI/MWNT-CeO2 | 0.1mol/L NH3+1mol/L KOH | 0.45 | 0.26 | [ |
| PtIrCu HCOND | 0.1 mol/L NH3+1mol/L KOH | 0.35 | 122.9A/g | [ |
| PtIrZn2/CeO2-ZIF-8 | 0.1mol/L NH3+1mol/L KOH | 0.35 | 0.64 | [ |
| Pt85Pd15/rGO | 0.1mol/L NH3+1mol/L KOH | 0.47 | 1.46 | [ |
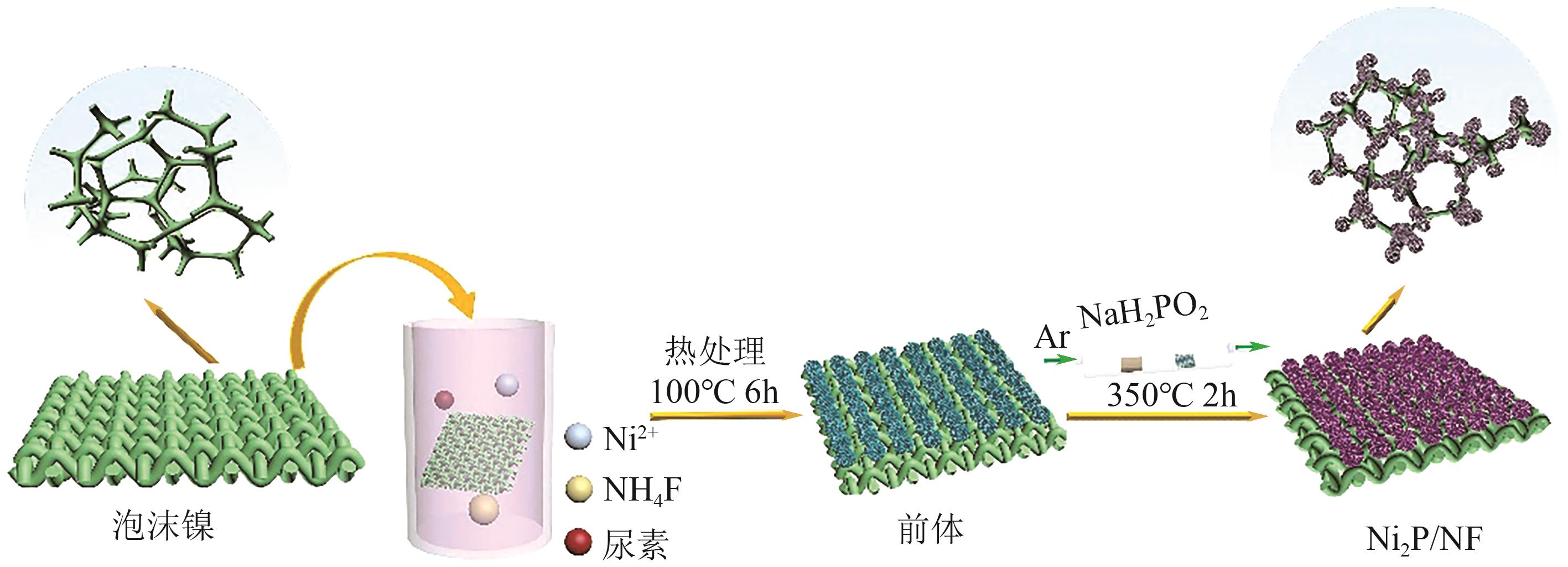
| Pt/Ir/MWCNT | 0.1mol/L NH3+0.1mol/L KOH | 0.38 | — | [ |
| Pt-修饰Ni NP | 0.1mol/L NH3+1mol/L KOH | 0.5 | 5.32A/g | [ |
| PtIrZn | 0.1mol/L NH4OH+0.5mol/L KOH | 0.3 | 0.56 | [ |
| PtPb/C | 1mol/L KOH+0.1mol/L NH3 | — | 191A/g | [ |
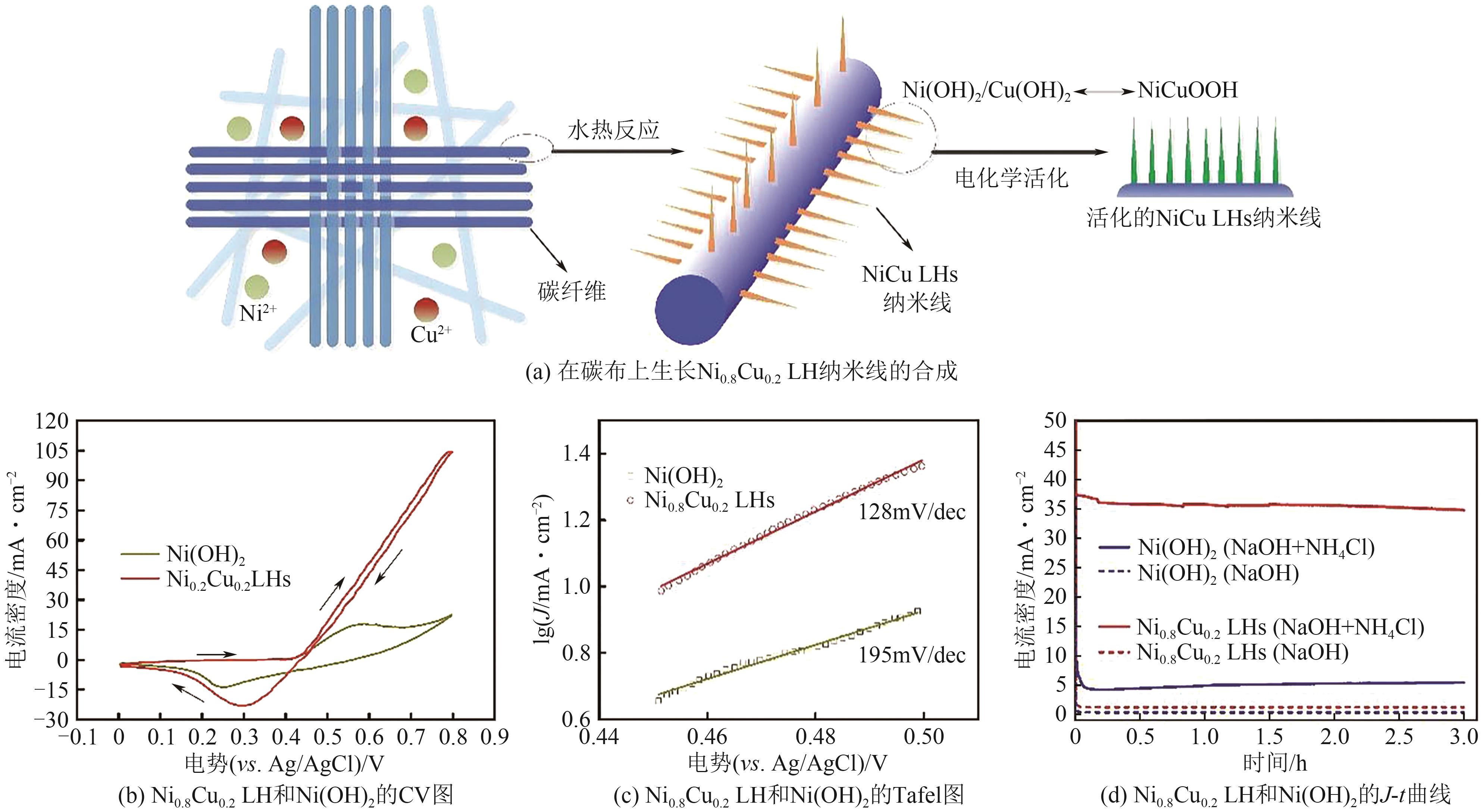
| Pt x Ru | 0.1mol/L NH3+1mol/L KOH | 0.5 | 92A/g | [ |
| Au@Pt NP | 1mol/L NaOH+0.05mol/L NH3 | 0.4 | 1.19~1.06 | [ |
| 催化剂 | 电解液 | 起始电位 | 参考文献 |
|---|---|---|---|
| Ag/Ni | 0.5mol/L NH3+1.5mol/L NaOH | 1.37V vs. RHE | [ |
| Ni(OH)2-Cu2O@CuO | 1mol/L NH3+1mol/L KOH | 1.37V vs. RHE | [ |
| NiCu/BDD | 0.5mol/L NH3+0.5mol/L NaOH | 1.35V vs. RHE | [ |
| NiCuCo-S-T/CP | 0.2mol/L NH4Cl+1mol/L NaOH | 1.24V vs. RHE | [ |
| Ni(OH)2/NiOOH | 3mmol/L NH3+0.1mol/L Na2SO4 | 0.65V vs. Hg/HgO | [ |
| Ni/NiOOH | 3mmol/L NH3+0.01mol/L Na2SO4 | 0.6V vs. Hg/HgO | [ |
| NiCu DHT | 0.05mol/L NH4OH+0.1mol/L NaOH | 1.31V vs. RHE | [ |
| Ni0.8Cu0.2 LH | 55mmol/L NH4Cl+0.5mol/L NaOH | 0.4V vs. Ag/AgCl | [ |
| NiCu/CP | 1mol/L NaOH+55mmol/L NH4Cl | 0.47V vs. Ag/AgCl | [ |
| Ni0.8Cu0.2氢氧化物 | 1 mmol/L NH4++0.1mol/L KOH | 1.4V vs. RHE | [ |
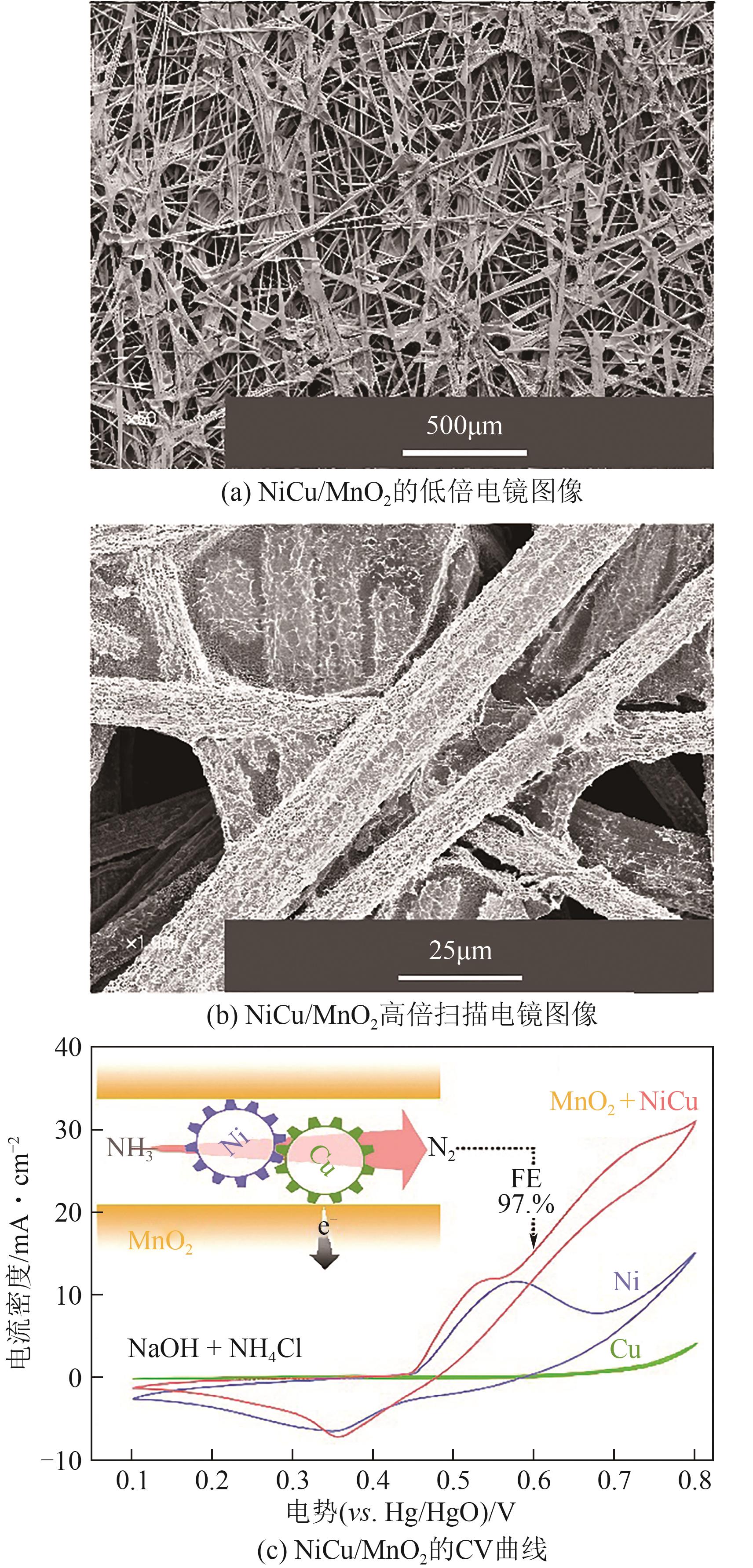
| NiCu/MnO2 | 0.5mol/L NaOH+55mmol/L NH4Cl | 0.65V vs.Hg/HgO | [ |
| NiCo2N | NH3饱和的0.1mol/L KPF6 | 0.55V vs. NHE | [ |
| NiO-TiO2 | 0.2mol/L NH4++0.1mmol/L NaNO3 | 0.5V vs. Hg/HgO | [ |
| Ni(OH)2 | 0.2mol/L NH3+0.1mol/L NaOH | 1.4V vs. RHE | [ |
| Ni1Cu3-S-T/CP | 1mol/L NaOH+0.2mol/L NH4Cl | 1.37V vs. RHE | [ |
| Ni(OH)2/SnO2 | 0.5mol/L K2SO4+10 mmol/L NH3 | 1.39V vs. RHE | [ |
| Co10/Ni-C | 0.5mol/L KOH+0.3mmol/L NH4+ | 0.35V vs. Hg/HgO | [ |
| NiCu/NF | 1mol/L KOH+0.3mol/L NH3 | 0.8V vs. Hg/HgO | [ |
| 催化剂 | 电解液 | 起始电位 | 参考文献 |
|---|---|---|---|
| Ag/Ni | 0.5mol/L NH3+1.5mol/L NaOH | 1.37V vs. RHE | [ |
| Ni(OH)2-Cu2O@CuO | 1mol/L NH3+1mol/L KOH | 1.37V vs. RHE | [ |
| NiCu/BDD | 0.5mol/L NH3+0.5mol/L NaOH | 1.35V vs. RHE | [ |
| NiCuCo-S-T/CP | 0.2mol/L NH4Cl+1mol/L NaOH | 1.24V vs. RHE | [ |
| Ni(OH)2/NiOOH | 3mmol/L NH3+0.1mol/L Na2SO4 | 0.65V vs. Hg/HgO | [ |
| Ni/NiOOH | 3mmol/L NH3+0.01mol/L Na2SO4 | 0.6V vs. Hg/HgO | [ |
| NiCu DHT | 0.05mol/L NH4OH+0.1mol/L NaOH | 1.31V vs. RHE | [ |
| Ni0.8Cu0.2 LH | 55mmol/L NH4Cl+0.5mol/L NaOH | 0.4V vs. Ag/AgCl | [ |
| NiCu/CP | 1mol/L NaOH+55mmol/L NH4Cl | 0.47V vs. Ag/AgCl | [ |
| Ni0.8Cu0.2氢氧化物 | 1 mmol/L NH4++0.1mol/L KOH | 1.4V vs. RHE | [ |
| NiCu/MnO2 | 0.5mol/L NaOH+55mmol/L NH4Cl | 0.65V vs.Hg/HgO | [ |
| NiCo2N | NH3饱和的0.1mol/L KPF6 | 0.55V vs. NHE | [ |
| NiO-TiO2 | 0.2mol/L NH4++0.1mmol/L NaNO3 | 0.5V vs. Hg/HgO | [ |
| Ni(OH)2 | 0.2mol/L NH3+0.1mol/L NaOH | 1.4V vs. RHE | [ |
| Ni1Cu3-S-T/CP | 1mol/L NaOH+0.2mol/L NH4Cl | 1.37V vs. RHE | [ |
| Ni(OH)2/SnO2 | 0.5mol/L K2SO4+10 mmol/L NH3 | 1.39V vs. RHE | [ |
| Co10/Ni-C | 0.5mol/L KOH+0.3mmol/L NH4+ | 0.35V vs. Hg/HgO | [ |
| NiCu/NF | 1mol/L KOH+0.3mol/L NH3 | 0.8V vs. Hg/HgO | [ |
| [1] | BUSHUYEV Oleksandr S, DE LUNA Phil, DINH Cao Thang, et al. What should we make with CO2 and how can we make it?[J]. Joule, 2018, 2(5): 825-832. |
| [2] | DE LUNA Phil, HAHN Christopher, HIGGINS Drew, et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes?[J]. Science, 2019, 364(6438): eaav3506. |
| [3] | GUO Wenhan, ZHANG Kexin, LIANG Zibin, et al. Electrochemical nitrogen fixation and utilization: Theories, advanced catalyst materials and system design[J]. Chemical Society Reviews, 2019, 48(24): 5658-5716. |
| [4] | TURNER John A. Sustainable hydrogen production[J]. Science, 2004, 305(5686): 972-974. |
| [5] | SIDDIQUI O, DINCER I. Experimental investigation and assessment of direct ammonia fuel cells utilizing alkaline molten and solid electrolytes[J]. Energy, 2019, 169: 914-923. |
| [6] | TRENERRY Michael J, WALLEN Christian M, BROWN Tristan R, et al. Spontaneous N2 formation by a diruthenium complex enables electrocatalytic and aerobic oxidation of ammonia[J]. Nature Chemistry, 2021, 13(12): 1221-1227. |
| [7] | CHRISTENSEN Claus Hviid, SØRENSEN Rasmus Zink, JOHANNESSEN Tue, et al. Metal ammine complexes for hydrogen storage[J]. Journal of Materials Chemistry, 2005, 15(38): 4106-4108. |
| [8] | XI Xiaoshuang, FAN Yunying, ZHANG Kai, et al. Carbon-free sustainable energy technology: Electrocatalytic ammonia oxidation reaction[J]. Chemical Engineering Journal, 2022, 435: 134818. |
| [9] | BODIRSKY Benjamin Leon, POPP Alexander, Hermann LOTZE-CAMPEN, et al. Reactive nitrogen requirements to feed the world in 2050 and potential to mitigate nitrogen pollution[J]. Nature Communications, 2014, 5: 3858. |
| [10] | CRUZ Heidy, Ying Yu LAW, GUEST Jeremy S, et al. Mainstream ammonium recovery to advance sustainable urban wastewater management[J]. Environmental Science & Technology, 2019, 53(19): 11066-11079. |
| [11] | XUE Runmiao, DONOVAN Ariel, ZHANG Haiting, et al. Simultaneous removal of ammonia and N-nitrosamine precursors from high ammonia water by zeolite and powdered activated carbon[J]. Journal of Environmental Sciences, 2018, 64: 82-91. |
| [12] | ZHANG Zhiyong, AI Huiying, FU Minglai, et al. A new insight into catalytic ozonation of ammonia by MgO/Co3O4 composite: The effects, reaction kinetics and mechanism[J]. Chemical Engineering Journal, 2021, 418: 129461. |
| [13] | YANG Hongxin, HU Jinling, JIANG Xuesong, et al. Study on ammonia nitrogen in pesticide wastewater by breakpoint chlorination method[J]. Modern Agrochemicals, 2018, 17(5): 19-21. |
| [14] | CHANG Mingdong, LIANG Baorui, ZHANG Kuo, et al. Simultaneous shortcut nitrification and denitrification in a hybrid membrane aerated biofilms reactor (H-MBfR) for nitrogen removal from low COD/N wastewater[J]. Water Research, 2022, 211: 118027. |
| [15] | HERRON Jeffrey A, FERRIN Peter, MAVRIKAKIS Manos. Electrocatalytic oxidation of ammonia on transition-metal surfaces: A first-principles study[J]. The Journal of Physical Chemistry C, 2015, 119(26): 14692-14701. |
| [16] | KATAYAMA Yu, OKANISHI Takeou, MUROYAMA Hiroki, et al. Enhancement of ammonia oxidation activity over Y2O3-modified platinum surface: Promotion of NH2, ad dimerization process[J]. Journal of Catalysis, 2016, 344: 496-506. |
| [17] | GERISCHER H, MAUERER A. Untersuchungen zur anodischen oxidation von ammoniak an platin-elektroden[J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1970, 25(3): 421-433. |
| [18] | JIN Yongzhen, KE Changming, LIU Yang, et al. Enhancing electrocatalytic ammonia oxidation using super-concentrated electrolytes[J/OL]. ChemRxiv, 2025. . |
| [19] | JACOB S I, CHAKRABORTY A, CHAMAS A, et al. Rapid aqueous ammonia oxidation to N2 using a molecular Ru electrocatalyst[J]. ACS Energy Letters, 2023, 8(9): 3760-3766. |
| [20] | AHMED M E, STAPLES R J, UNDARI T R C, et al. Electrocatalytic ammonia oxidation by pyridyl-substituted ferrocenes[J]. Journal of the American Chemical Society, 2025, 147(8): 6514-6522. |
| [21] | OSWIN H G, SALOMON M. The anodic oxidation of ammonia at platinum black electrodes in aqueous KOH electrolyte[J]. Canadian Journal of Chemistry, 1963, 41(7): 1686-1694. |
| [22] | VIDAL-IGLESIAS F J, SOLLA-GULLÓN J, PÉREZ J M, et al. Evidence by SERS of azide anion participation in ammonia electrooxidation in alkaline medium on nanostructured Pt electrodes[J]. Electrochemistry Communications, 2006, 8(1): 102-106. |
| [23] | DE VOOYS A C A, MROZEK F, KOPER T M, et al. The nature of chemisorbates formed from ammonia on gold and palladium electrodes as discerned from surface-enhanced Raman spectroscopy[J]. Electrochemistry Communications, 2001, 3(6): 293-298. |
| [24] | WASMUS S, VASINI E J, KRAUSA M, et al. DEMS-cyclic voltammetry investigation of the electrochemistry of nitrogen compounds in 0.5mol/L potassium hydroxide[J]. Electrochimica Acta, 1994, 39(1): 23-31. |
| [25] | GOOTZEN J F E, WONDERS A H, VISSCHER W, et al. A DEMS and cyclic voltammetry study of NH3 oxidation on platinized platinum[J]. Electrochimica Acta, 1998, 43(12/13): 1851-1861. |
| [26] | ENDO Kazuki, KATAYAMA Yasushi, MIURA Takashi. A rotating disk electrode study on the ammonia oxidation[J]. Electrochimica Acta, 2005, 50(11): 2181-2185. |
| [27] | DIAZ Luis A, BOTTE Gerardine G. Mathematical modeling of ammonia electrooxidation kinetics in a polycrystalline Pt rotating disk electrode[J]. Electrochimica Acta, 2015, 179: 519-528. |
| [28] | FINKELSTEIN David A, BERTIN Erwan, GARBARINO Sébastien, et al. Mechanistic similarity in catalytic N2 production from NH3 and NO2 - at Pt(100) thin films: Toward a universal catalytic pathway for simple N-containing species, and its application to in situ removal of NH3 poisons[J]. The Journal of Physical Chemistry C, 2015, 119(18): 9860-9878. |
| [29] | PILLAI Hemanth Somarajan, XIN Hongliang. New insights into electrochemical ammonia oxidation on Pt(100) from first principles[J]. Industrial & Engineering Chemistry Research, 2019, 58(25): 10819-10828. |
| [30] | JANG Ji Hee, PARK So Young, YOUN Duck Hyun, et al. Recent advances in electrocatalysts for ammonia oxidation reaction[J]. Catalysts, 2023, 13(5): 803. |
| [31] | Erich MÜLLER, SPITZER Fritz. Über die elektrolytische oxydation des ammoniaks und ihre abhängigkeit vom anodenmaterial[J]. Zeitschrift Für Elektrochemie und Angewandte Physikalische Chemie, 1905, 11(50): 917-931. |
| [32] | Zhenhua LYU, FU Jiaju, TANG Tang, et al. Design of ammonia oxidation electrocatalysts for efficient direct ammonia fuel cells[J]. EnergyChem, 2023, 5(3): 100093. |
| [33] | KIM Hyunki, HONG Seokjin, KIM Hedam, et al. Recent progress in Pt-based electrocatalysts for ammonia oxidation reaction[J]. Applied Materials Today, 2022, 29: 101640. |
| [34] | VIDAL-IGLESIAS F J, GARCÍA-ARÁEZ N, MONTIEL V, et al. Selective electrocatalysis of ammonia oxidation on Pt(100) sites in alkaline medium[J]. Electrochemistry Communications, 2003, 5(1): 22-26. |
| [35] | VIDAL-IGLESIAS Francisco J, José SOLLA-GULLÓN, MONTIEL Vicente, et al. Ammonia selective oxidation on Pt(100) sites in an alkaline medium[J]. The Journal of Physical Chemistry B, 2005, 109(26): 12914-12919. |
| [36] | NOVELL-LERUTH G, VALCÁRCEL A, CLOTET A, et al. DFT characterization of adsorbed NH x species on Pt(100) and Pt(111) surfaces[J]. The Journal of Physical Chemistry B, 2005, 109(38): 18061-18069. |
| [37] | ROSCA Victor, KOPER Marc T M. Electrocatalytic oxidation of ammonia on Pt(111) and Pt(100) surfaces[J]. Physical Chemistry Chemical Physics, 2006, 8(21): 2513-2524. |
| [38] | SUN Huiying, XU Guangrui, LI Fumin, et al. Hydrogen generation from ammonia electrolysis on bifunctional platinum nanocubes electrocatalysts[J]. Journal of Energy Chemistry, 2020, 47: 234-240. |
| [39] | FU Gengtao, LIU Chang, WU Rui, et al. L-lysine mediated synthesis of platinum nanocuboids and their electrocatalytic activity towards ammonia oxidation[J]. Journal of Materials Chemistry A, 2014, 2(42): 17883-17888. |
| [40] | YANG Yejin, KIM Jeongwon, Hyoi JO, et al. A rigorous electrochemical ammonia electrolysis protocol with in operando quantitative analysis[J]. Journal of Materials Chemistry A, 2021, 9(19): 11571-11579. |
| [41] | LIU Jie, HU Wenbin, ZHONG Cheng, et al. Surfactant-free electrochemical synthesis of hierarchical platinum particle electrocatalysts for oxidation of ammonia[J]. Journal of Power Sources, 2013, 223: 165-174. |
| [42] | LOMOCSO Thegy L, BARANOVA Elena A. Electrochemical oxidation of ammonia on carbon-supported bi-metallic PtM (M=Ir, Pd, SnO x ) nanoparticles[J]. Electrochimica Acta, 2011, 56(24): 8551-8558. |
| [43] | BOGGS Bryan K, BOTTE Gerardine G. Optimization of Pt-Ir on carbon fiber paper for the electro-oxidation of ammonia in alkaline media[J]. Electrochimica Acta, 2010, 55(19): 5287-5293. |
| [44] | ENDO Kazuki, NAKAMURA Kyoko, KATAYAMA Yasushi, et al. Pt-Me (Me=Ir, Ru, Ni) binary alloys as an ammonia oxidation anode[J]. Electrochimica Acta, 2004, 49(15): 2503-2509. |
| [45] | XUE Qi, ZHAO Yue, ZHU Jingyi, et al. PtRu nanocubes as bifunctional electrocatalysts for ammonia electrolysis[J]. Journal of Materials Chemistry A, 2021, 9(13): 8444-8451. |
| [46] | SILVA Júlio César M, SILVA Sirlane G DA, DE SOUZA Rodrigo F B, et al. PtAu/C electrocatalysts as anodes for direct ammonia fuel cell[J]. Applied Catalysis A: General, 2015, 490: 133-138. |
| [47] | WEI Ruilin, LIU Yue, CHEN Zhen, et al. Ammonia oxidation on iridium electrode in alkaline media: An in situ ATR-SEIRAS study[J]. Journal of Electroanalytical Chemistry, 2021, 896: 115254. |
| [48] | SIDDHARTH Kumar, HONG Youngmin, QIN Xueping, et al. Surface engineering in improving activity of Pt nanocubes for ammonia electrooxidation reaction[J]. Applied Catalysis B: Environmental, 2020, 269: 118821. |
| [49] | LIN Xu, ZHANG Xiaoran, WANG Zhen, et al. Hyperbranched concave octahedron of PtIrCu nanocrystals with high-index facets for efficiently electrochemical ammonia oxidation reaction[J]. Journal of Colloid and Interface Science, 2021, 601: 1-11. |
| [50] | LI Yi, LI Xing, PILLAI Hemanth Somarajan, et al. Ternary PtIrNi catalysts for efficient electrochemical ammonia oxidation[J]. ACS Catalysis, 2020, 10(7): 3945-3957. |
| [51] | JIANG Junhua. Promotion of PtIr and Pt catalytic activity towards ammonia electrooxidation through the modification of Zn[J]. Electrochemistry Communications, 2017, 75: 52-55. |
| [52] | BERTIN Erwan, GARBARINO Sébastien, GUAY Daniel, et al. Electrodeposited platinum thin films with preferential (100) orientation: Characterization and electrocatalytic properties for ammonia and formic acid oxidation[J]. Journal of Power Sources, 2013, 225: 323-329. |
| [53] | LIU J, ZHONG C, YANG Y, et al. Electrochemical preparation and characterization of Pt particles on ITO substrate: Morphological effect on ammonia oxidation[J]. International Journal of Hydrogen Energy, 2012, 37(11): 8981-8987. |
| [54] | MARTÍNEZ-RODRÍGUEZ Roberto A, VIDAL-IGLESIAS Francisco J, José SOLLA-GULLÓN, et al. Synthesis of Pt nanoparticles in water-in-oil microemulsion: Effect of HCl on their surface structure[J]. Journal of the American Chemical Society, 2014, 136(4): 1280-1283. |
| [55] | ALLAGUI Anis, OUDAH Mohamed, TUAEV Xenia, et al. Ammonia electro-oxidation on alloyed PtIr nanoparticles of well-defined size[J]. International Journal of Hydrogen Energy, 2013, 38(5): 2455-2463. |
| [56] | ZHANG Changlin, HWANG Sang Youp, PENG Zhenmeng. Shape-enhanced ammonia electro-oxidation property of a cubic platinum nanocrystal catalyst prepared by surfactant-free synthesis[J]. Journal of Materials Chemistry A, 2013, 1(45): 14402-14408. |
| [57] | OKANISHI Takeou, KATAYAMA Yu, MUROYAMA Hiroki, et al. SnO2-modified Pt electrocatalysts for ammonia-fueled anion exchange membrane fuel cells[J]. Electrochimica Acta, 2015, 173: 364-369. |
| [58] | KATAYAMA Yu, OKANISHI Takeou, MUROYAMA Hiroki, et al. Electrochemical oxidation of ammonia over rare earth oxide modified platinum catalysts[J]. The Journal of Physical Chemistry C, 2015, 119(17): 9134-9141. |
| [59] | LI Yi, PILLAI Hemanth Somarajan, WANG Teng, et al. High-performance ammonia oxidation catalysts for anion-exchange membrane direct ammonia fuel cells[J]. Energy & Environmental Science, 2021, 14(3): 1449-1460. |
| [60] | LIU Zhenzhong, LI Yi, ZHANG Xiangsong, et al. Surface structure engineering of PtPd nanoparticles for boosting ammonia oxidation electrocatalysis[J]. ACS Applied Materials & Interfaces, 2022, 14(25): 28816-28825. |
| [61] | MORITA Seitaro, KUDO Eiji, SHIRASAKA Ryo, et al. Electrochemical oxidation of ammonia by multi-wall-carbon-nanotube-supported Pt shell-Ir core nanoparticles synthesized by an improved Cu short circuit deposition method[J]. Journal of Electroanalytical Chemistry, 2016, 762: 29-36. |
| [62] | LIU Jie, CHEN Bin, KOU Yue, et al. Pt-Decorated highly porous flower-like Ni particles with high mass activity for ammonia electro-oxidation[J]. Journal of Materials Chemistry A, 2016, 4(28): 11060-11068. |
| [63] | JIANG Zexing, YU Tianqi, CHEN Jinli, et al. Regulating competitive adsorption on Pt nanoparticles by introducing Pb to expedite hydrogen production via ammonia oxidation[J]. ACS Applied Nano Materials, 2023, 6(3): 1889-1897. |
| [64] | WANG Jun, Jaeyoung HEO, CHEN Changqiang, et al. Ammonia oxidation enhanced by photopotential generated by plasmonic excitation of a bimetallic electrocatalyst[J]. Angewandte Chemie International Edition, 2020, 59(42): 18430-18434. |
| [65] | MYERS Clifford E, FRANZEN Hugo F, ANDEREGG James W. X-ray photoelectron spectra and bonding in transition-metal phosphides[J]. Inorganic Chemistry, 1985, 24(12): 1822-1824. |
| [66] | WANG Renyu, LIU Huijuan, ZHANG Kai, et al. Ni(Ⅱ)/Ni(Ⅲ) redox couple endows Ni foam-supported Ni2P with excellent capability for direct ammonia oxidation[J]. Chemical Engineering Journal, 2021, 404: 126795. |
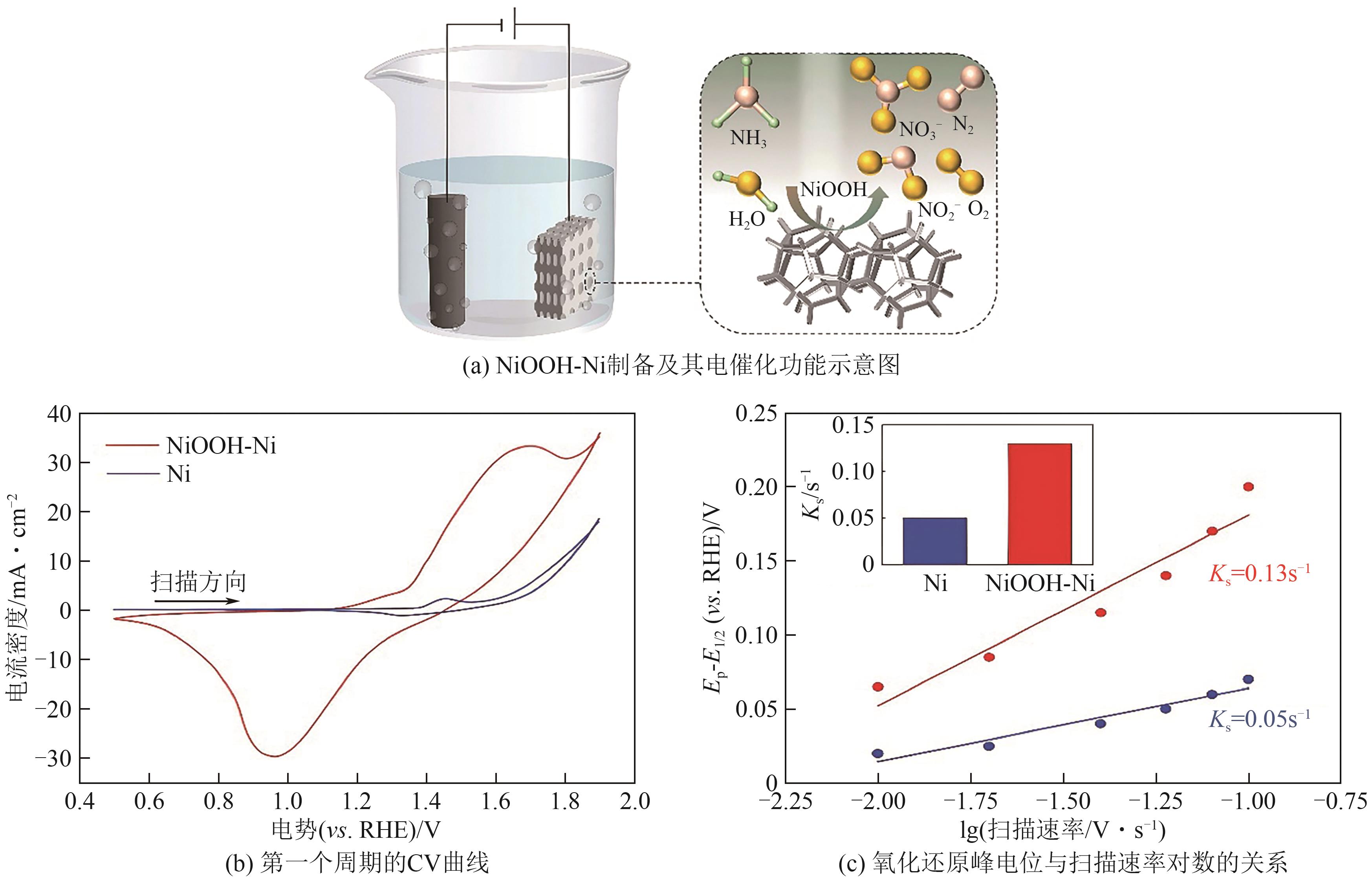
| [67] | LIU Hanwen, YANG Chengjie, DONG Chungli, et al. Electrocatalytic ammonia oxidation to nitrite and nitrate with NiOOH-Ni[J]. Advanced Energy Materials, 2024, 14(42): 2401675. |
| [68] | WANG Jiong, GAN Liyong, ZHANG Wenyu, et al. In situ formation of molecular Ni-Fe active sites on heteroatom-doped graphene as a heterogeneous electrocatalyst toward oxygen evolution[J]. Science Advances, 2018, 4(3): eaap7970. |
| [69] | MEDFORD Andrew J, VOJVODIC Aleksandra, HUMMELSHØJ Jens S, et al. From the Sabatier principle to a predictive theory of transition-metal heterogeneous catalysis[J]. Journal of Catalysis, 2015, 328: 36-42. |
| [70] | XU Wei, LAN Rong, DU Dongwei, et al. Directly growing hierarchical nickel-copper hydroxide nanowires on carbon fibre cloth for efficient electrooxidation of ammonia[J]. Applied Catalysis B: Environmental, 2017, 218: 470-479. |
| [71] | XU Wei, DU Dongwei, LAN Rong, et al. Electrodeposited NiCu bimetal on carbon paper as stable non-noble anode for efficient electrooxidation of ammonia[J]. Applied Catalysis B: Environmental, 2018, 237: 1101-1109. |
| [72] | ZHANG Huimin, WANG Hailong, ZHOU Luanqi, et al. Efficient and highly selective direct electrochemical oxidation of ammonia to dinitrogen facilitated by NiCu diatomic site catalysts[J]. Applied Catalysis B: Environmental, 2023, 328: 122544. |
| [73] | ZHU Mingke, YANG Yi, XI Shibo, et al. Deciphering NH3 adsorption kinetics in ternary Ni-Cu-Fe oxyhydroxide toward efficient ammonia oxidation reaction[J]. Small, 2021, 17(7): 2005616. |
| [74] | NAGITA Kenji, YUHARA Yoshiki, FUJII Kenta, et al. Ni- and Cu-co-intercalated layered manganese oxide for highly efficient electro-oxidation of ammonia selective to nitrogen[J]. ACS Applied Materials & Interfaces, 2021, 13(24): 28098-28107. |
| [75] | SHIH Yu-Jen, HSU Ching-Hsiang. Kinetics and highly selective N2 conversion of direct electrochemical ammonia oxidation in an undivided cell using NiCo oxide nanoparticle as the anode and metallic Cu/Ni foam as the cathode[J]. Chemical Engineering Journal, 2021, 409: 128024. |
| [76] | ALMOMANI Fares, SALAH SAAD Mohammed ALI H. Electrochemical oxidation of ammonia (NH4 +/NH3) on synthesized nickel-cobalt oxide catalyst[J]. International Journal of Hydrogen Energy, 2021, 46(6): 4678-4690. |
| [77] | HE Shi, CHEN Yufeng, WANG Mengdi, et al. Metal nitride nanosheets enable highly efficient electrochemical oxidation of ammonia[J]. Nano Energy, 2021, 80: 105528. |
| [78] | JIN Yongzhen, CHEN Xin, WANG Jianhui. From inert to active: A cocktail-like mediation of an Ag/Ni mixture for electrocatalytic ammonia oxidation reaction[J]. Chemical Communications, 2022, 58(76): 10631-10634. |
| [79] | HUANG Jingjing, CAI Jinmeng, WANG Jianhui. Nanostructured wire-in-plate electrocatalyst for high-durability production of hydrogen and nitrogen from alkaline ammonia solution[J]. ACS Applied Energy Materials, 2020, 3(5): 4108-4113. |
| [80] | SONG Jingjin, YANG Yinhai, JIA Yingna, et al. Improved NH3-N conversion efficiency to N2 activated by BDD substrate on NiCu electrocatalysis process[J]. Separation and Purification Technology, 2021, 276: 119350. |
| [81] | WANG Hailong, TONG Xing, ZHOU Luanqi, et al. Unique three-dimensional nanoflower-like NiCu electrodes constructed by Co, S co-doping for efficient ammonia oxidation reaction[J]. Separation and Purification Technology, 2022, 303: 122293. |
| [82] | SHIH Yu-Jen, HUANG Yaohui, HUANG C P. Electrocatalytic ammonia oxidation over a nickel foam electrode: Role of Ni(OH)2(s)-NiOOH(s) nanocatalysts[J]. Electrochimica Acta, 2018, 263: 261-271. |
| [83] | SHIH Yu-Jen, HUANG Yaohui, HUANG C P. In-situ electrochemical formation of nickel oxyhydroxide (NiOOH) on metallic nickel foam electrode for the direct oxidation of ammonia in aqueous solution[J]. Electrochimica Acta, 2018, 281: 410-419. |
| [84] | YANG Anzhou, WANG Jingchun, SU Keying, et al. Modulating hydroxyl-rich interfaces on nickel-copper double hydroxide nanotyres to pre-activate alkaline ammonia oxidation reactivity[J]. Chemistry—A European Journal, 2021, 27(15): 4869-4875. |
| [85] | JIANG Xuan, YING Diwen, LIU Xi, et al. Identification of the role of Cu site in Ni-Cu hydroxide for robust and high selective electrochemical ammonia oxidation to nitrite[J]. Electrochimica Acta, 2020, 345: 136157. |
| [86] | MEDVEDEV Jury J, TOBOLOVSKAYA Yulia, MEDVEDEVA Xenia V, et al. Pathways of ammonia electrooxidation on nickel hydroxide anodes and an alternative route towards recycled fertilizers[J]. Green Chemistry, 2022, 24(4): 1578-1589. |
| [87] | ZHANG Huimin, WANG Hailong, TONG Xing, et al. Sulfur induced surface reconfiguration of Ni1Cu3-S-T/CP anode for high-efficiency ammonia electro-oxidation[J]. Chemical Engineering Journal, 2023, 452: 139582. |
| [88] | HOU Jing, CHENG Yingying, PAN Hui, et al. Tailored bimetallic Ni-Sn catalyst for electrochemical ammonia oxidation to dinitrogen with high selectivity[J]. Inorganic Chemistry, 2023, 62(9): 3986-3992. |
| [89] | Eglė LATVYTĖ, ZHU Xuanheng, WU Liang, et al. A low-temperature ammonia electrolyser for wastewater treatment and hydrogen production[J]. International Journal of Hydrogen Energy, 2024, 52: 265-282. |
| [90] | ZHANG Shuo, ZHAO Yanchao, YAN Liting, et al. Electrochemical ammonia oxidation reaction on defect-rich TiO nanofibers: Experimental and theoretical studies[J]. International Journal of Hydrogen Energy, 2021, 46(79): 39208-39215. |
| [91] | HUANG Jingjing, CHEN Zhe, CAI Jinmeng, et al. Activating copper oxide for stable electrocatalytic ammonia oxidation reaction via in situ introducing oxygen vacancies[J]. Nano Research, 2022, 15(7): 5987-5994. |
| [92] | UDACHYAN Iranna, BHANUSHALI Jayesh T, MIZRAHI Amir, et al. Manganese carbonate as an efficient electrocatalyst for the conversion of ammonia (NH4 +/NH3) to dinitrogen[J]. Sustainable Energy & Fuels, 2023, 7(17): 4088-4093. |
| [93] | CLEETUS Annie, TELLER Hanan, SCHECHTER Alex. CuCr bimetallic catalyst for selective electrooxidation of ammonia at room temperature[J]. ChemCatChem, 2023, 15(7): e202300035. |
| [1] | QIN Fei, ZHANG Zhi, SONG Guangchun, WANG Wuchang, LI Yuxing, WANG Shixin, HE Sicheng, WANG Jiangyan. Advances in research on the molecular dynamics behaviors of hydrate-based hydrogen storage [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 112-123. |
| [2] | LIU Zhe, ZHOU Shunli, LI Yongxiang, ZHANG Chengxi, LIU Yipeng. Research progress on alkyl naphthalene synthesis catalysts [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 144-158. |
| [3] | LIN Yijie, QIAO Peng, LI Xinrui, ZHANG Hongbin, WANG Xueqin. Construction and application of heterostructures of photocatalyst TiO2 nanomaterials [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 159-177. |
| [4] | WANG Tao, ZHANG Xuebing, ZHANG Qi, CHEN Qiang, ZHANG Kui, MEN Zhuowu. Effects of reduction-carburization temperature and inlet CO concentration on industrial precipitated iron-based catalyst for Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 178-184. |
| [5] | BAO Xinde, LIU Biye, HUANG Renwei, HONG Yuhao, GUAN Xin, LIN Jinguo. Preparation of biomass-based@CuNiOS composite catalysts for the reduction of organic dye [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 185-196. |
| [6] | ZHAO Siyang, LI Chenran, LIU Yang. Process optimization for regulating diene selectivity of MTO regenerated catalyst through pre-carbon deposition using C4 by-product [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 205-212. |
| [7] | ZHAO Yulong, CAI Kai, YU Shanqing. Influence of pore structure of alumina on the adsorption, diffusion and reactivity of hydrocarbon molecules in catalytic cracking [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 213-221. |
| [8] | LI Junliang, LI Yue, SUN Daolai. Hydrodeoxygenation of 1,2-butanediol to 1-butanol over Cu/SiO2-Al2O3 catalyst [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 222-231. |
| [9] | LIU Chao, DING Chengao, WU Baoshun, LEI Xinyu, WANG Guangying, YU Zhengwei. Effect of TiO2 support particle size on the denitrification and water/sulfur poisoning resistance of RuO x -V2O5-WO3/TiO2 catalyst [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 232-242. |
| [10] | ZHANG Hanlin, YUE Xuehai, LIU Junxi, YIN Fengjun. Fabrication of high stability electrocatalyst for oxygen evolution reaction by Ru-Sr-Ir electrodeposition [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 243-251. |
| [11] | WANG Lu, HE Yangdong, LI Yaxin, FAN Rui, CHENG Shijin, ZHANG Jie. Structural design and performance optimization of high-performance polymeric membranes for He/CH4 and He/N2 separation [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 261-276. |
| [12] | HONG Kang, ZHANG Chong, MA Hongli, SUN Yongrong, JIANG Liqun, BAO Guirong. Research progress of biomass hard charcoal as an anode material for sodium-ion batteries [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 340-349. |
| [13] | ZHU Ying, LI Yilin, LIU Jianguo, CAO Yingnan, HUO Yaoqiang, LIU Wei, WANG Juan, LI Yiting, ZHANG Ximei, LI Bin. Membrane fouling composition and mechanism of coking wastewater membrane treatment process [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 518-527. |
| [14] | WANG Wenjun, LIU Ruixin, WANG Jun, ZHANG Qinglei, HOU Li’an. Research progress of visible light degradation of indoor VOCs by titanium dioxide materials [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5351-5362. |
| [15] | CAO Jiangfei, LEI Xiaotong, HUANG Zhiyi, HUANG Jiankai, CHEN Fan, YANG Pianpian, XIE Chunsheng. Preparation of iron-nitrogen doped carbon microspheres and their activation for PS degradation of rhodamine B [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5406-5415. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||