Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (10): 5612-5632.DOI: 10.16085/j.issn.1000-6613.2023-1691
• Materials science and technology • Previous Articles
Advances of adsorption materials for hydrogen purification
SU Shikun1( ), LIU Tang1,2, JIN Ye1, ZHENG Jinyu1(
), LIU Tang1,2, JIN Ye1, ZHENG Jinyu1( )
)
- 1.New Energy Research Institute, RIPP, SINOPEC, Beijing 100083, China
2.School of Chemical and Environmental Engineering, China University of Petroleum (Beijing), Beijing 102249, China
-
Received:2023-09-25Revised:2023-11-27Online:2024-10-29Published:2024-10-15 -
Contact:ZHENG Jinyu
氢气纯化吸附材料研究进展
- 1.中石化石油化工科学研究院有限公司新能源研究所,北京 100083
2.中国石油大学(北京)化学工程与环境学院,北京 102249
-
通讯作者:郑金玉 -
作者简介:苏士焜(1991—),男,博士,研究方向为气体纯化分离、多孔催化材料等。E-mail:tmacssk@163.com。
CLC Number:
Cite this article
SU Shikun, LIU Tang, JIN Ye, ZHENG Jinyu. Advances of adsorption materials for hydrogen purification[J]. Chemical Industry and Engineering Progress, 2024, 43(10): 5612-5632.
苏士焜, 刘唐, 金也, 郑金玉. 氢气纯化吸附材料研究进展[J]. 化工进展, 2024, 43(10): 5612-5632.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-1691
| 气源 | H2 | CO | CO2 | CH4 | N2 | H2O | 其他 |
|---|---|---|---|---|---|---|---|
| 煤气化气 | 25~35 | 35~45 | 15~25 | 0.1~0.3 | 0.5~1 | 15~20 | 0.2~1 |
| 甲烷重整气 | 70~75 | 10~15 | 10~15 | 1~3 | 0.1~0.5 | — | — |
| 甲醇重整气 | 75~80 | 0.5~2 | 20~25 | — | — | — | — |
| 焦炉煤气 | 45~60 | 5~10 | 2~5 | 25~30 | 2~5 | — | 2.5~5 |
| 合成氨尾气 | 60~75 | — | — | — | 15~20 | 1~3 | 11~18 |
| 生物质气 | 25~35 | 30~40 | 10~15 | 10~20 | 1 | — | 0.5~2 |
| 气源 | H2 | CO | CO2 | CH4 | N2 | H2O | 其他 |
|---|---|---|---|---|---|---|---|
| 煤气化气 | 25~35 | 35~45 | 15~25 | 0.1~0.3 | 0.5~1 | 15~20 | 0.2~1 |
| 甲烷重整气 | 70~75 | 10~15 | 10~15 | 1~3 | 0.1~0.5 | — | — |
| 甲醇重整气 | 75~80 | 0.5~2 | 20~25 | — | — | — | — |
| 焦炉煤气 | 45~60 | 5~10 | 2~5 | 25~30 | 2~5 | — | 2.5~5 |
| 合成氨尾气 | 60~75 | — | — | — | 15~20 | 1~3 | 11~18 |
| 生物质气 | 25~35 | 30~40 | 10~15 | 10~20 | 1 | — | 0.5~2 |
| 方法 | 原理 | 典型进料气 | 产品氢纯度/% | 技术难点 |
|---|---|---|---|---|
| 低温分离 | 相对挥发度的差别 | 石化废气,含氢在30%~80%内 | 90~98 | 不易得到高纯度氢气 |
| 聚合物膜分离 | 穿过膜的扩散速率差别 | 石化废气和氨吹扫气 | 92~98 | He、CO2、H2O也可能会穿过膜 |
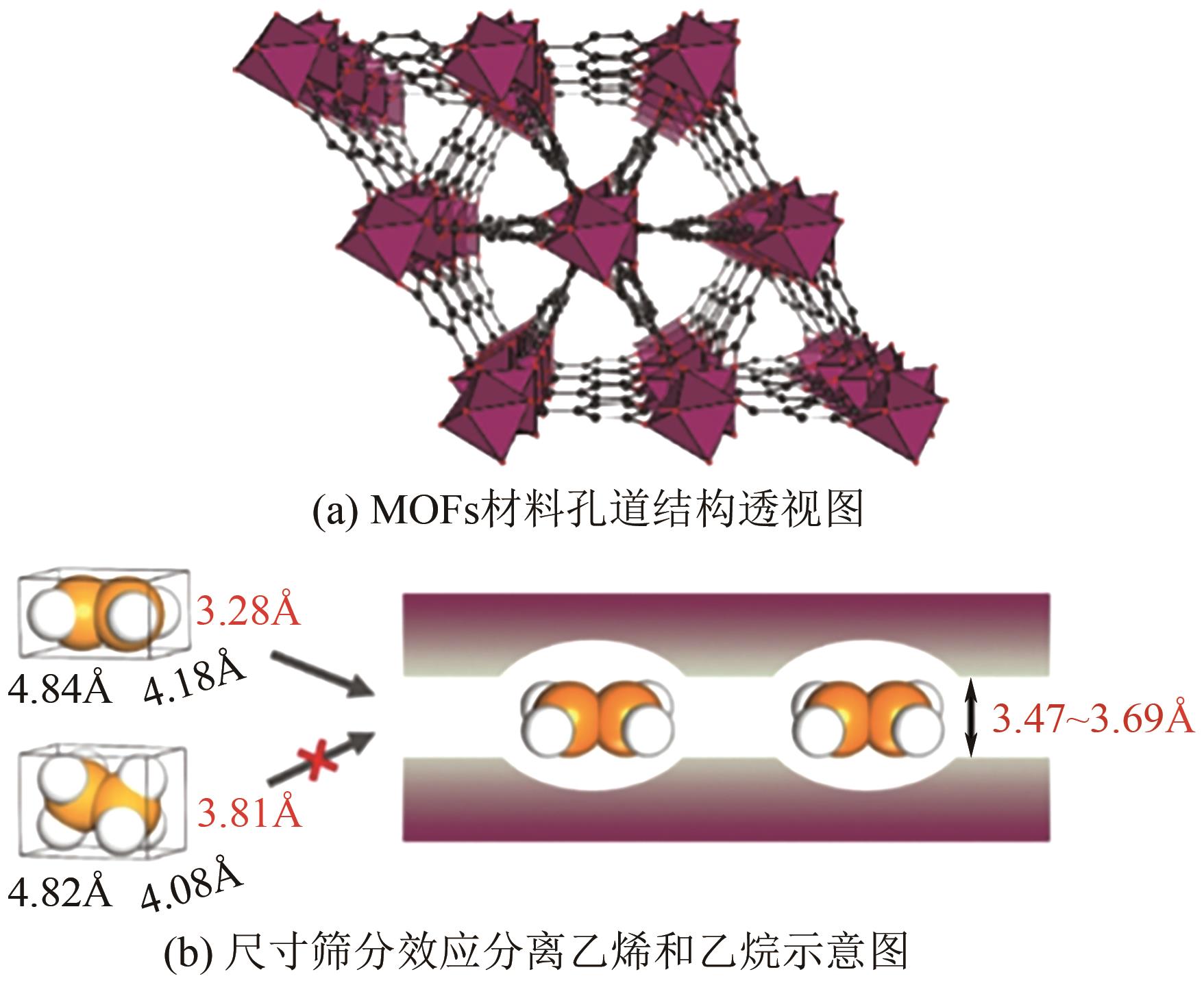
| 钯膜分离 | 氢气选择性渗透 | 任何含氢气体 | ≥99.999 | 硫化物和不饱和烃会削弱膜的渗透性 |
| 金属氢化物分离 | 氢与金属形成金属氢化物的可逆反应 | 氨吹扫气 | 99.999 | O2、CO、硫化物会使材料中毒 |
| 变压吸附 | 吸附剂选择性吸附杂质 | 任何富氢气体 | 99.999 | 吹扫气阶段有氢气损失,回收率相对较低 |
| 方法 | 原理 | 典型进料气 | 产品氢纯度/% | 技术难点 |
|---|---|---|---|---|
| 低温分离 | 相对挥发度的差别 | 石化废气,含氢在30%~80%内 | 90~98 | 不易得到高纯度氢气 |
| 聚合物膜分离 | 穿过膜的扩散速率差别 | 石化废气和氨吹扫气 | 92~98 | He、CO2、H2O也可能会穿过膜 |
| 钯膜分离 | 氢气选择性渗透 | 任何含氢气体 | ≥99.999 | 硫化物和不饱和烃会削弱膜的渗透性 |
| 金属氢化物分离 | 氢与金属形成金属氢化物的可逆反应 | 氨吹扫气 | 99.999 | O2、CO、硫化物会使材料中毒 |
| 变压吸附 | 吸附剂选择性吸附杂质 | 任何富氢气体 | 99.999 | 吹扫气阶段有氢气损失,回收率相对较低 |
| 气体名称 | 动力学直径/Å | 极化率/Å3 | 偶极矩/Å | 四极矩/Å2 |
|---|---|---|---|---|
| H2 | 2.83~2.90 | 0.8042 | 0 | 0.662 |
| CH4 | 3.76 | 2.593 | 0 | 0 |
| CO2 | 3.30 | 2.911 | 0 | 4.30 |
| CO | 3.69~3.76 | 1.950 | 0.11 | 2.50 |
| N2 | 3.64 | 1.740 | 0 | 1.52 |
| 气体名称 | 动力学直径/Å | 极化率/Å3 | 偶极矩/Å | 四极矩/Å2 |
|---|---|---|---|---|
| H2 | 2.83~2.90 | 0.8042 | 0 | 0.662 |
| CH4 | 3.76 | 2.593 | 0 | 0 |
| CO2 | 3.30 | 2.911 | 0 | 4.30 |
| CO | 3.69~3.76 | 1.950 | 0.11 | 2.50 |
| N2 | 3.64 | 1.740 | 0 | 1.52 |
| 吸附模型 | 模型表达式 | 参数及意义 |
|---|---|---|
| Langmuir | q为吸附量,qm为最大吸附量,b为Langmuir平衡常数,p为压力 | |
| 扩展Langmuir | qi 和pi 为气体混合物吸附量和分压,qmi 、bi 为纯组的对应方程拟合参数,j为混合物中各纯组分,n为混合物中的气体种类数 | |
| Toth | q为吸附量,qm为最大吸附量,b为Toth平衡常数,n是和吸附剂不均匀性相关的量纲为1参数,p为压力 | |
| 扩展Toth | qi 和pi 为气体混合物吸附量和分压,qmi 、bi 、ti 为纯组分i的对应方程拟合参数,j为混合物中各纯组分,n为混合物中的气体种类数 | |
| Virial | p为压力,q为吸附量,KH为Henry常数,S为吸附剂比表面积,A、B为Virial系数 |
| 吸附模型 | 模型表达式 | 参数及意义 |
|---|---|---|
| Langmuir | q为吸附量,qm为最大吸附量,b为Langmuir平衡常数,p为压力 | |
| 扩展Langmuir | qi 和pi 为气体混合物吸附量和分压,qmi 、bi 为纯组的对应方程拟合参数,j为混合物中各纯组分,n为混合物中的气体种类数 | |
| Toth | q为吸附量,qm为最大吸附量,b为Toth平衡常数,n是和吸附剂不均匀性相关的量纲为1参数,p为压力 | |
| 扩展Toth | qi 和pi 为气体混合物吸附量和分压,qmi 、bi 、ti 为纯组分i的对应方程拟合参数,j为混合物中各纯组分,n为混合物中的气体种类数 | |
| Virial | p为压力,q为吸附量,KH为Henry常数,S为吸附剂比表面积,A、B为Virial系数 |
| 交换离子 | 分子筛 | 交换条件 | 吸附气体 | 参考文献 |
|---|---|---|---|---|
| 碱金属离子 | 13X | 0.5mol/L盐溶液,固液比1∶80,353K下反应4h | CO、CH4、N2 | [ |
| 碱金属离子和H+ | RHO | 1mol/L盐溶液,固液比1∶10,353K下反应4h | CO2 | [ |
| Na+、K+、Cs+、NH4+、Ca2+、Mg2+、Ba2+ | 天然斜发沸石分子筛 | 2mol/L盐溶液,固液比1∶10,微沸下反应84h | CO、CH4、O2、N2 | [ |
| 碱金属离子 | 13X、NaY | 1mol/L盐溶液,固液比1∶10,350K下反应5h | CO2 | [ |
| Ca2+、Mg2+ | 13X | 1mol/L盐溶液,固液比1∶10,353K微波下反应0.5h | CO、CH4、CO2、H2 | [ |
| NH4+、Li+、Cu2+ | 13X | 1mol/L盐溶液,固液比1∶5,353K下反应4h | CH4、CO2、N2 | [ |
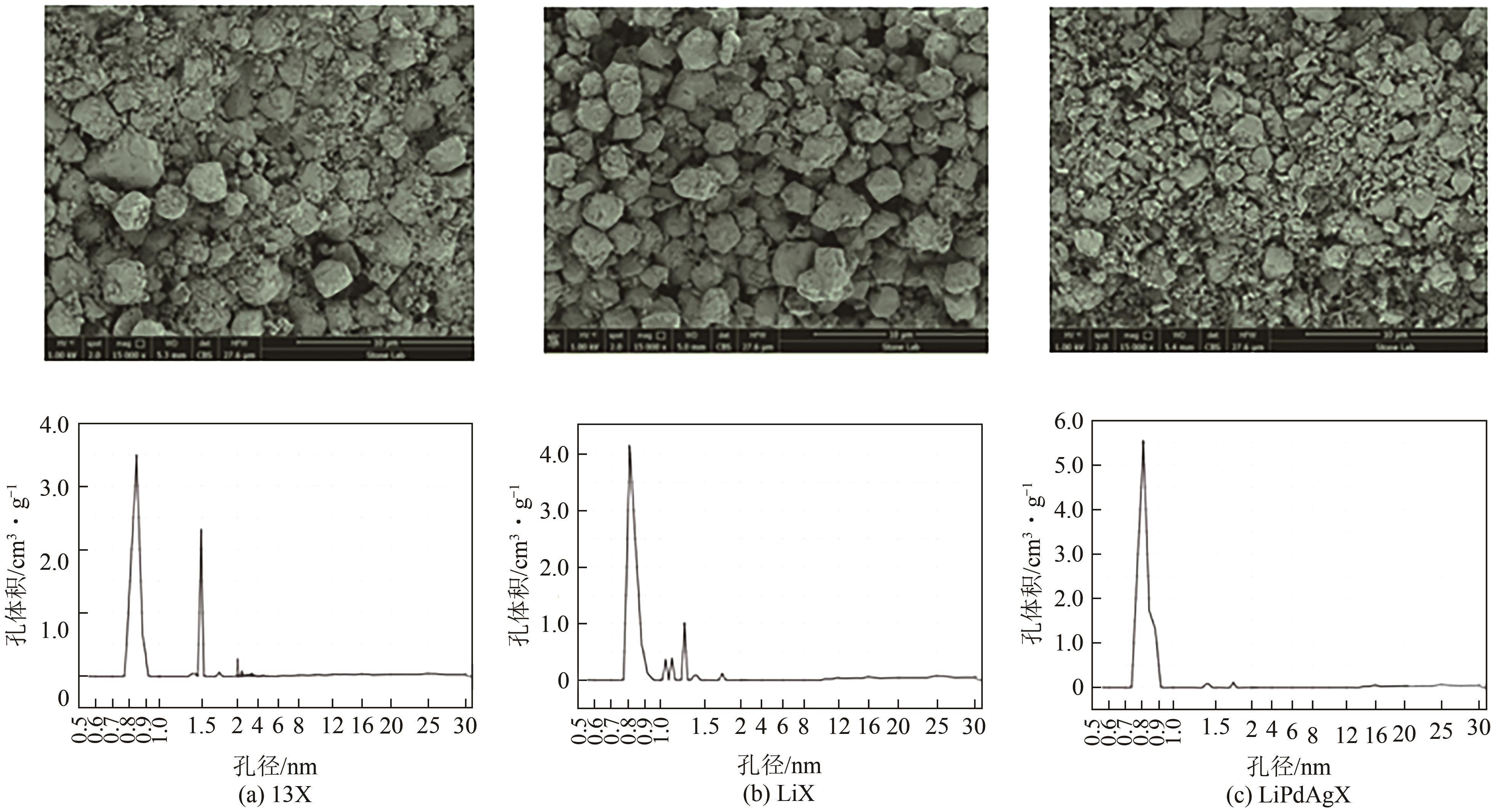
| Li+、Pd2+、Ag+ | 13X | 0.4mol/L的Li+溶液,343K下反应3h得到LiX;依次于 PdCl2、AgNO3、LiCl2中交换得到LiPdAgX | CO2 | [ |
| 交换离子 | 分子筛 | 交换条件 | 吸附气体 | 参考文献 |
|---|---|---|---|---|
| 碱金属离子 | 13X | 0.5mol/L盐溶液,固液比1∶80,353K下反应4h | CO、CH4、N2 | [ |
| 碱金属离子和H+ | RHO | 1mol/L盐溶液,固液比1∶10,353K下反应4h | CO2 | [ |
| Na+、K+、Cs+、NH4+、Ca2+、Mg2+、Ba2+ | 天然斜发沸石分子筛 | 2mol/L盐溶液,固液比1∶10,微沸下反应84h | CO、CH4、O2、N2 | [ |
| 碱金属离子 | 13X、NaY | 1mol/L盐溶液,固液比1∶10,350K下反应5h | CO2 | [ |
| Ca2+、Mg2+ | 13X | 1mol/L盐溶液,固液比1∶10,353K微波下反应0.5h | CO、CH4、CO2、H2 | [ |
| NH4+、Li+、Cu2+ | 13X | 1mol/L盐溶液,固液比1∶5,353K下反应4h | CH4、CO2、N2 | [ |
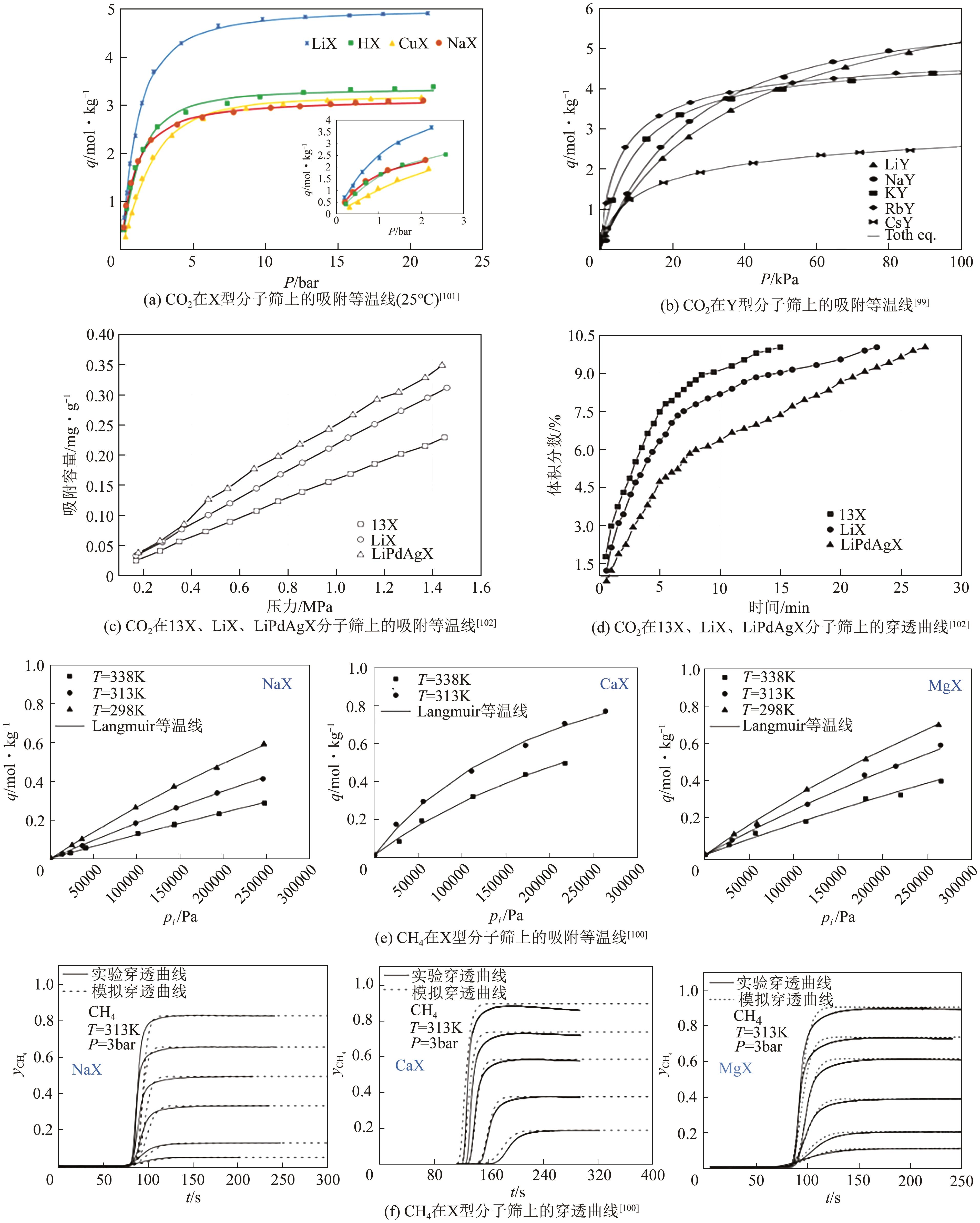
| Li+、Pd2+、Ag+ | 13X | 0.4mol/L的Li+溶液,343K下反应3h得到LiX;依次于 PdCl2、AgNO3、LiCl2中交换得到LiPdAgX | CO2 | [ |
| MOFs | 吸附气体 | 吸附条件 | 吸附量/mmol·g-1 | 选择性 | 参考文献 |
|---|---|---|---|---|---|
| MOF-5 | CO2 CH4 H2 | 298K、4MPa | 22.5 10 0.8 | — | [ |
| MOF-5 | CH4 H2 | 300K、3MPa | 9 0.58 | — | [ |
| MOF-74 | CO2 H2 CO2∶H2=1∶4 | 313K、4MPa | 13 2 — | — 380 | [ |
| MOF-74 | CO2∶H2=1∶4 CH4∶H2=1∶1 CO2∶CH4∶H2=4∶1∶20 | 313K、4MPa | — | 380 15 300 | [ |
| Cu-BTC | CO2 CH4 CO H2 | 308K、0.6MPa 308K、0.6MPa 303K、0.08MPa 303K、0.5MPa | 9.2 3.1 0.65 0.41 | — | [ |
| Cu-TDPAT | CO2∶H2= 1:4 CH4∶H2= 1:1 | 298K、4MPa | 12.5 8 | 80 — | [ |
| UiO-66 | CO2;CO2∶H2= 3:7 CH4;CH4∶H2=3:7 CO;CO∶H2=3:7 H2 | 298K、4MPa | 8.2 6.7 5 1.4 | 100 18 12 — | [ |
| UiO-66-Br | CO2;CO2∶H2=3∶7 CH4;CH4∶H2=3∶7 CO;CO∶H2=3∶7 H2 | 298K、4MPa | 7 5 4.5 1.2 | 130 21 15 — | [ |
| UTSA-16 | CO2 CH4 CO H2 | 298K、4MPa 298K、4MPa 298K、0.5MPa 298K、4MPa | 4.9 2.4 0.9 0.5 | — — — | [ |
| MOFs | 吸附气体 | 吸附条件 | 吸附量/mmol·g-1 | 选择性 | 参考文献 |
|---|---|---|---|---|---|
| MOF-5 | CO2 CH4 H2 | 298K、4MPa | 22.5 10 0.8 | — | [ |
| MOF-5 | CH4 H2 | 300K、3MPa | 9 0.58 | — | [ |
| MOF-74 | CO2 H2 CO2∶H2=1∶4 | 313K、4MPa | 13 2 — | — 380 | [ |
| MOF-74 | CO2∶H2=1∶4 CH4∶H2=1∶1 CO2∶CH4∶H2=4∶1∶20 | 313K、4MPa | — | 380 15 300 | [ |
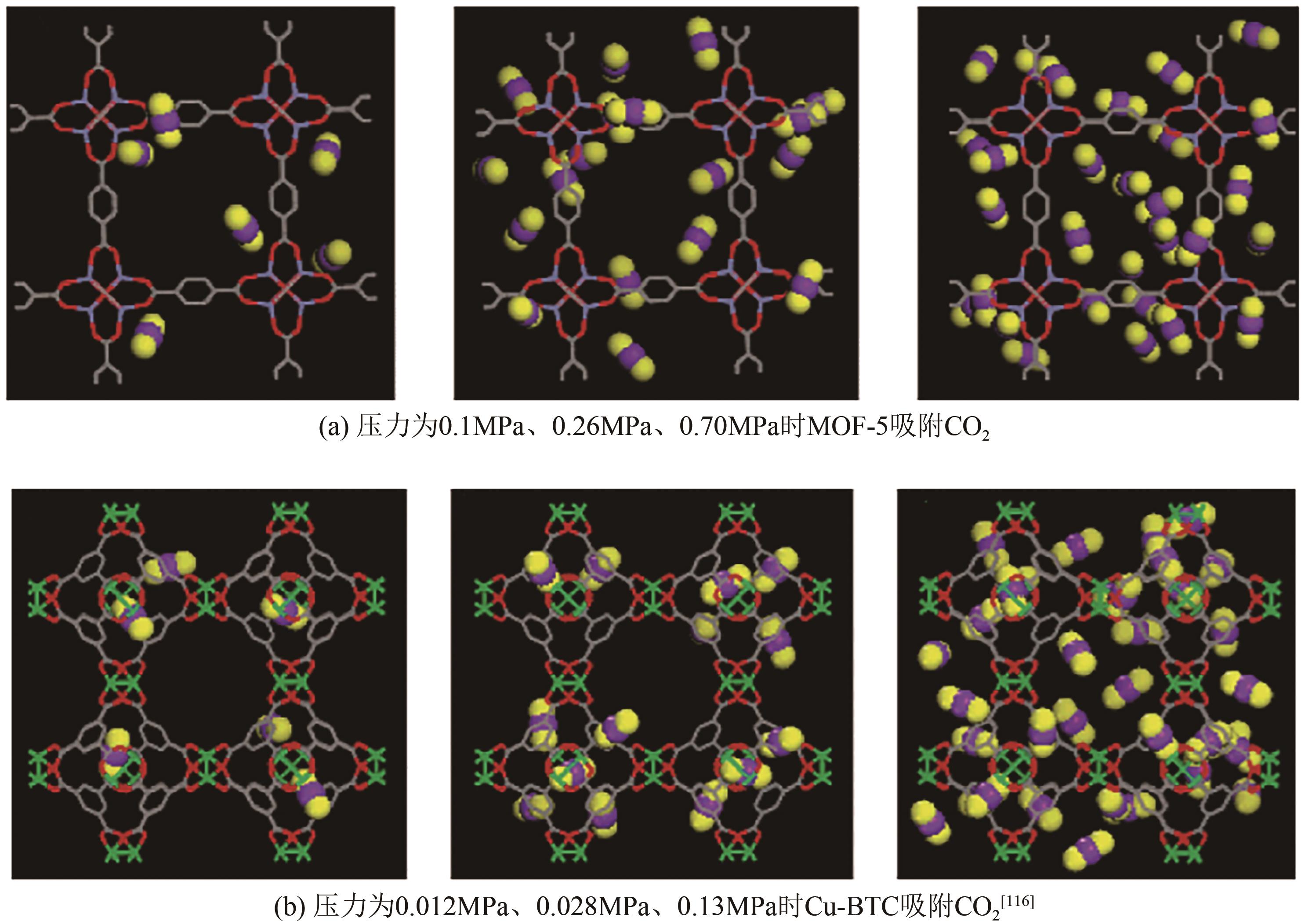
| Cu-BTC | CO2 CH4 CO H2 | 308K、0.6MPa 308K、0.6MPa 303K、0.08MPa 303K、0.5MPa | 9.2 3.1 0.65 0.41 | — | [ |
| Cu-TDPAT | CO2∶H2= 1:4 CH4∶H2= 1:1 | 298K、4MPa | 12.5 8 | 80 — | [ |
| UiO-66 | CO2;CO2∶H2= 3:7 CH4;CH4∶H2=3:7 CO;CO∶H2=3:7 H2 | 298K、4MPa | 8.2 6.7 5 1.4 | 100 18 12 — | [ |
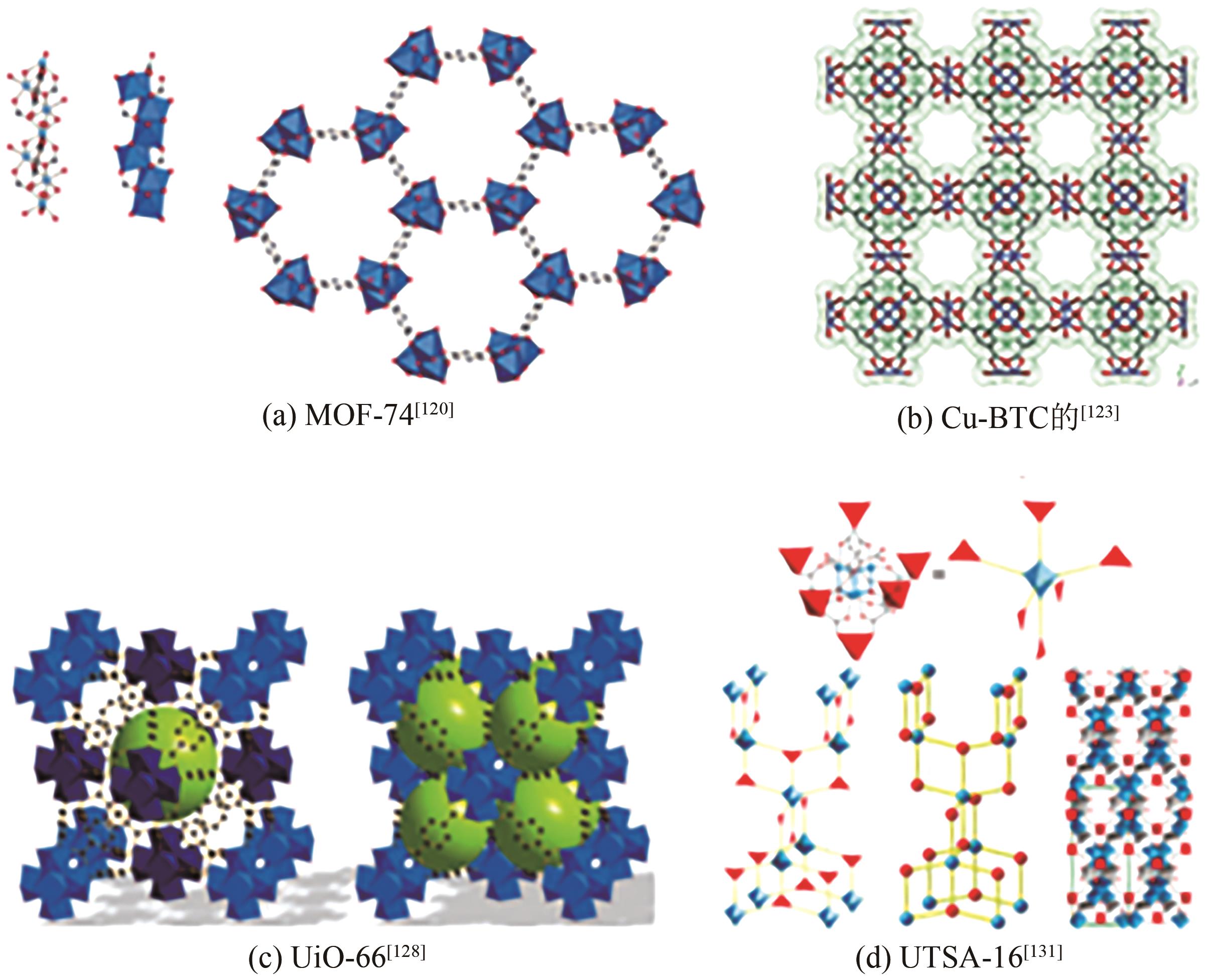
| UiO-66-Br | CO2;CO2∶H2=3∶7 CH4;CH4∶H2=3∶7 CO;CO∶H2=3∶7 H2 | 298K、4MPa | 7 5 4.5 1.2 | 130 21 15 — | [ |
| UTSA-16 | CO2 CH4 CO H2 | 298K、4MPa 298K、4MPa 298K、0.5MPa 298K、4MPa | 4.9 2.4 0.9 0.5 | — — — | [ |
| 1 | 刘美琴, 李奠础, 乔建芬, 等. 氢能利用与碳质材料吸附储氢技术[J]. 化工时刊, 2013, 27(11): 35-38. |
| LIU Meiqin, LI Dianchu, QIAO Jianfen, et al. The use of hydrogen energy and hydrogen adsorption storage technology of carbonaceous materials[J]. Chemical Industry Times, 2013, 27(11): 35-38. | |
| 2 | 王欣, 王苏礼, 刘伟, 等. 日本和德国的氢能产业发展现状综述[J]. 现代商业, 2019(24): 26-27. |
| WANG Xin, WANG Suli, LIU Wei, et al. Summary of the development status of hydrogen energy industry in Japan and Germany[J]. Modern Business, 2019(24): 26-27. | |
| 3 | 吴善略, 张丽娟. 世界主要国家氢能发展规划综述[J]. 科技中国, 2019(7): 91-97. |
| WU Shanlue, ZHANG Lijuan. Summary of hydrogen energy development planning in major countries in the world[J]. China Scitechnology Business, 2019(7): 91-97. | |
| 4 | DU Zhemin, LIU Congmin, ZHAI Junxiang, et al. A review of hydrogen purification technologies for fuel cell vehicles[J]. Catalysts, 2021, 11(3): 393. |
| 5 | DAWOOD Furat, ANDA Martin, SHAFIULLAH G M. Hydrogen production for energy: An overview[J]. International Journal of Hydrogen Energy, 2020, 45(7): 3847-3869. |
| 6 | 孙强, 贺玉刚, 严大洲, 等. 高纯氢制备工艺研究进展[J]. 精细石油化工进展, 2020, 21(3): 42-44, 57. |
| SUN Qiang, HE Yugang, YAN Dazhou, et al. Progress of research on study of high purity hydrogen production technology[J]. Advances in Fine Petrochemicals, 2020, 21(3): 42-44, 57. | |
| 7 | DECOSTE Jared B, PETERSON Gregory W, SCHINDLER Bryan J, et al. The effect of water adsorption on the structure of the carboxylate containing metal-organic frameworks Cu-BTC, Mg-MOF-74, and UiO-66[J]. Journal of Materials Chemistry A, 2013, 1(38): 11922-11932. |
| 8 | Pia KÜSGENS, ROSE Marcus, SENKOVSKA Irena, et al. Characterization of metal-organic frameworks by water adsorption[J]. Microporous and Mesoporous Materials, 2009, 120(3): 325-330. |
| 9 | AASADNIA Majid, MEHRPOOYA Mehdi, GHORBANI Bahram. A novel integrated structure for hydrogen purification using the cryogenic method[J]. Journal of Cleaner Production, 2021, 278: 123872. |
| 10 | 陈绍华, 邢丕峰, 陈文梅. 稀贵金属在氢气纯化中的应用[J]. 稀有金属, 2003, 27(1): 8-17. |
| CHEN Shaohua, XING Pifeng, CHEN Wenmei. The application of rare-noble metals to the purification of hydrogen[J]. Chinese Journal of Rare Metals, 2003, 27(1): 8-17. | |
| 11 | SONG Chunfeng, LIU Qingling, DENG Shuai, et al. Cryogenic-based CO2 capture technologies: State-of-the-art developments and current challenges[J]. Renewable and Sustainable Energy Reviews, 2019, 101: 265-278. |
| 12 | LI Panyuan, WANG Zhi, QIAO Zhihua, et al. Recent developments in membranes for efficient hydrogen purification[J]. Journal of Membrane Science, 2015, 495: 130-168. |
| 13 | 李忠于, 黄伟, 张楚璠. 燃料电池用高纯氢纯化技术研究进展[J]. 能源化工, 2020, 41(5): 1-7. |
| LI Zhongyu, HUANG Wei, ZHANG Chufan. Research progress on high purity hydrogen purification technology for fuel cell[J]. Energy Chemical Industry, 2020, 41(5): 1-7. | |
| 14 | 栾永超, 熊亚林, 何广利, 等. 氢气分离膜研究进展[J]. 中国工程科学, 2022, 24(3): 140-152. |
| LUAN Yongchao, XIONG Yalin, HE Guangli, et al. Research progress of hydrogen separation membrane[J]. Strategic Study of CAE, 2022, 24(3): 140-152. | |
| 15 | YUN Samhun, OYAMA S TED. Correlations in palladium membranes for hydrogen separation: A review[J]. Journal of Membrane Science, 2011, 375(1/2): 28-45. |
| 16 | WARD Timothy L, Tien DAO. Model of hydrogen permeation behavior in palladium membranes[J]. Journal of Membrane Science, 1999, 153(2): 211-231. |
| 17 | TSUCHIMOTO K, YUKAWA H, NAMBU T, et al. Design of Nb-W-Mo alloy membrane for hydrogen separation and purification[J]. Journal of Alloys and Compounds, 2013, 580: S391-S396. |
| 18 | RAHIMPOUR M R, SAMIMI F, BABAPOOR A, et al. Palladium membranes applications in reaction systems for hydrogen separation and purification: A review[J]. Chemical Engineering and Processing: Process Intensification, 2017, 121: 24-49. |
| 19 | NAYEBOSSADRI S, SPEIGHT J, BOOK David. University of Birmingham Hydrogen separation from blended natural gas and hydrogen by Pd-based membranes[J]. International Journal Hydrogen Energy, 2019, 44: 29092-29099. |
| 20 | ZHAO Chenyang, SUN Bing, JIANG Jie, et al. H2 purification process with double layer BCC-PdCu alloy membrane at ambient temperature[J]. International Journal of Hydrogen Energy, 2020, 45(35): 17540-17547. |
| 21 | NAVARRO R M, GUIL R, FIERRO J L G. Introduction to hydrogen production[M]//Compendium of Hydrogen Energy. Amsterdam: Elsevier, 2015: 21-61. |
| 22 | KONG Seong Young, KIM Da Hye, HENKENSMEIER Dirk, et al. Ultrathin layered Pd/PBI-HFA composite membranes for hydrogen separation[J]. Separation and Purification Technology, 2017, 179: 486-493. |
| 23 | KIADEHI Afshin Dehghani, TAGHIZADEH Majid. Fabrication, characterization, and application of palladium composite membrane on porous stainless steel substrate with NaY zeolite as an intermediate layer for hydrogen purification[J]. International Journal of Hydrogen Energy, 2019, 44(5): 2889-2904. |
| 24 | IULIANELLI A, GHASEMZADEH K, MARELLI M, et al. A supported Pd-Cu/Al2O3 membrane from solvated metal atoms for hydrogen separation/purification[J]. Fuel Processing Technology, 2019, 195: 106141. |
| 25 | María YÁÑEZ, ORTIZ Alfredo, GORRI Daniel, et al. Comparative performance of commercial polymeric membranes in the recovery of industrial hydrogen waste gas streams[J]. International Journal of Hydrogen Energy, 2021, 46(33): 17507-17521. |
| 26 | Neha PAL, AGARWAL Madhu, MAHESHWARI Karishma, et al. A review on types, fabrication and support material of hydrogen separation membrane[J]. Materials Today: Proceedings, 2020, 28: 1386-1391. |
| 27 | STRUGOVA D V, M Yu ZADOROZHNYY, BERDONOSOVA E A, et al. Novel process for preparation of metal-polymer composite membranes for hydrogen separation[J]. International Journal of Hydrogen Energy, 2018, 43(27): 12146-12152. |
| 28 | REZAKAZEMI Mashallah, SHAHIDI Kazem, MOHAMMADI Toraj. Hydrogen separation and purification using crosslinkable PDMS/zeolite A nanoparticles mixed matrix membranes[J]. International Journal of Hydrogen Energy, 2012, 37(19): 14576-14589. |
| 29 | PEYDAYESH Mohammad, MOHAMMADI Toraj, BAKHTIARI Omid. Effective hydrogen purification from methane via polyimide Matrimid® 5218- Deca-dodecasil 3R type zeolite mixed matrix membrane[J]. Energy, 2017, 141: 2100-2107. |
| 30 | XIAO Jinsheng, TONG Liang, YANG Tianqi, et al. Lumped parameter simulation of hydrogen storage and purification systems using metal hydrides[J]. International Journal of Hydrogen Energy, 2017, 42(6): 3698-3707. |
| 31 | DUNIKOV D, BORZENKO V, BLINOV D, et al. Biohydrogen purification using metal hydride technologies[J]. International Journal of Hydrogen Energy, 2016, 41(46): 21787-21794. |
| 32 | YANG F S, CHEN X Y, WU Z, et al. Experimental studies on the poisoning properties of a low-plateau hydrogen storage alloy LaNi4.3Al0.7 against CO impurities[J]. International Journal of Hydrogen Energy, 2017, 42(25): 16225-16234. |
| 33 | HANADA Nobuko, ASADA Hirotaka, NAKAGAWA Tessui, et al. Effect of CO2 on hydrogen absorption in Ti-Zr-Mn-Cr based AB2 type alloys[J]. Journal of Alloys and Compounds, 2017, 705: 507-516. |
| 34 | 王春燕, 杨莉娜, 王念榕, 等. 变压吸附技术在天然气脱除CO2上的应用探讨[J]. 石油规划设计, 2013, 24(1): 52-55. |
| WANG Chunyan, YANG Lina, WANG Nianrong, et al. Discussion on application of pressure swing adsorption technology in CO2 removal from natural gas[J]. Petroleum Planning & Engineering, 2013, 24(1): 52-55. | |
| 35 | SONG C.Hydrogen and syngas production and purification technologies[M]. New York: John Wiley & Sons, Inc. 2009. |
| 36 | 寇丹. 变压吸附制氢装置改进及工艺优化研究[D]. 北京: 北京理工大学, 2016. |
| KOU Dan. PSA hydrogen plant improved and research of the process optimization[D]. Beijing: Beijing Institute of Technology, 2016. | |
| 37 | SIRCAR S, GOLDEN T C. Purification of hydrogen by pressure swing adsorption[J]. Separation Science and Technology, 2000, 35(5): 667-687. |
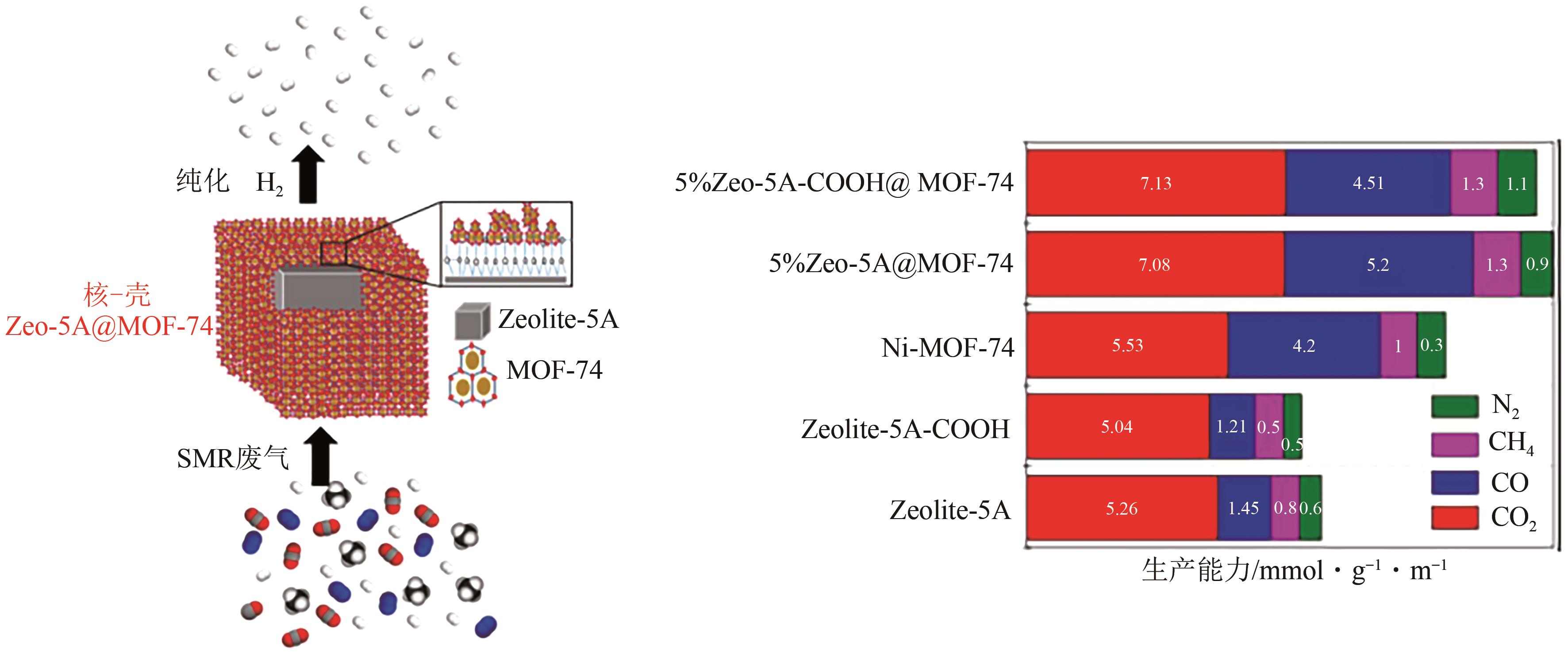
| 38 | Qasim AL-NADDAF, ROWNAGHI Ali A, REZAEI Fateme. Multicomponent adsorptive separation of CO2, CO, CH4, N2, and H2 over core-shell zeolite-5A@MOF-74 composite adsorbents[J]. Chemical Engineering Journal, 2020, 384: 123251. |
| 39 | 刘珊珊, 柴玉超, 关乃佳, 等. 分子筛材料在小分子吸附分离中的应用[J]. 高等学校化学学报, 2021, 42(1): 268-288. |
| LIU Shanshan, CHAI Yuchao, GUAN Naijia, et al. Small molecule adsorption and separation on zeolites[J]. Chemical Journal of Chinese Universities, 2021, 42(1): 268-288. | |
| 40 | KELLER J U, STAUDT R, Gas adsorption equilibria : experimental methods and adsorptive isotherms[M]. Springer Science & Business Media, 2005 |
| 41 | LIN Jerry Y S. Molecular sieves for gas separation[J]. Science, 2016, 353(6295): 121-122. |
| 42 | KUZNICKI Steven M, BELL Valerie A, NAIR Sankar, et al. A titanosilicate molecular sieve with adjutsable pores for size-selective adsorption of molecules[J]. Nature, 2001, 412(6848): 720-724. |
| 43 | 赵振国. 吸附作用应用原理[M]. 北京: 化学工业出版社, 2005: 123-134. |
| ZHAO Zhenguo. Application principle of adsorption[M]. Beijing: Chemical Industry Press, 2005: 123-134. | |
| 44 | BAO Zongbi, WANG Jiawei, ZHANG Zhiguo, et al. Molecular sieving of ethane from ethylene through the molecular cross-section size differentiation in gallate-based metal-organic frameworks[J]. Angewandte Chemie International Edition, 2018, 57(49): 16020-16025. |
| 45 | LI Kunhao, OLSON David H, SEIDEL Jonathan, et al. Zeolitic imidazolate frameworks for kinetic separation of propane and propene[J]. Journal of the American Chemical Society, 2009, 131(30): 10368-10369. |
| 46 | LI Jianrong, KUPPLER Ryan J, ZHOU Hongcai. Selective gas adsorption and separation in metal-organic frameworks[J]. Chemical Society Reviews, 2009, 38(5): 1477-1504. |
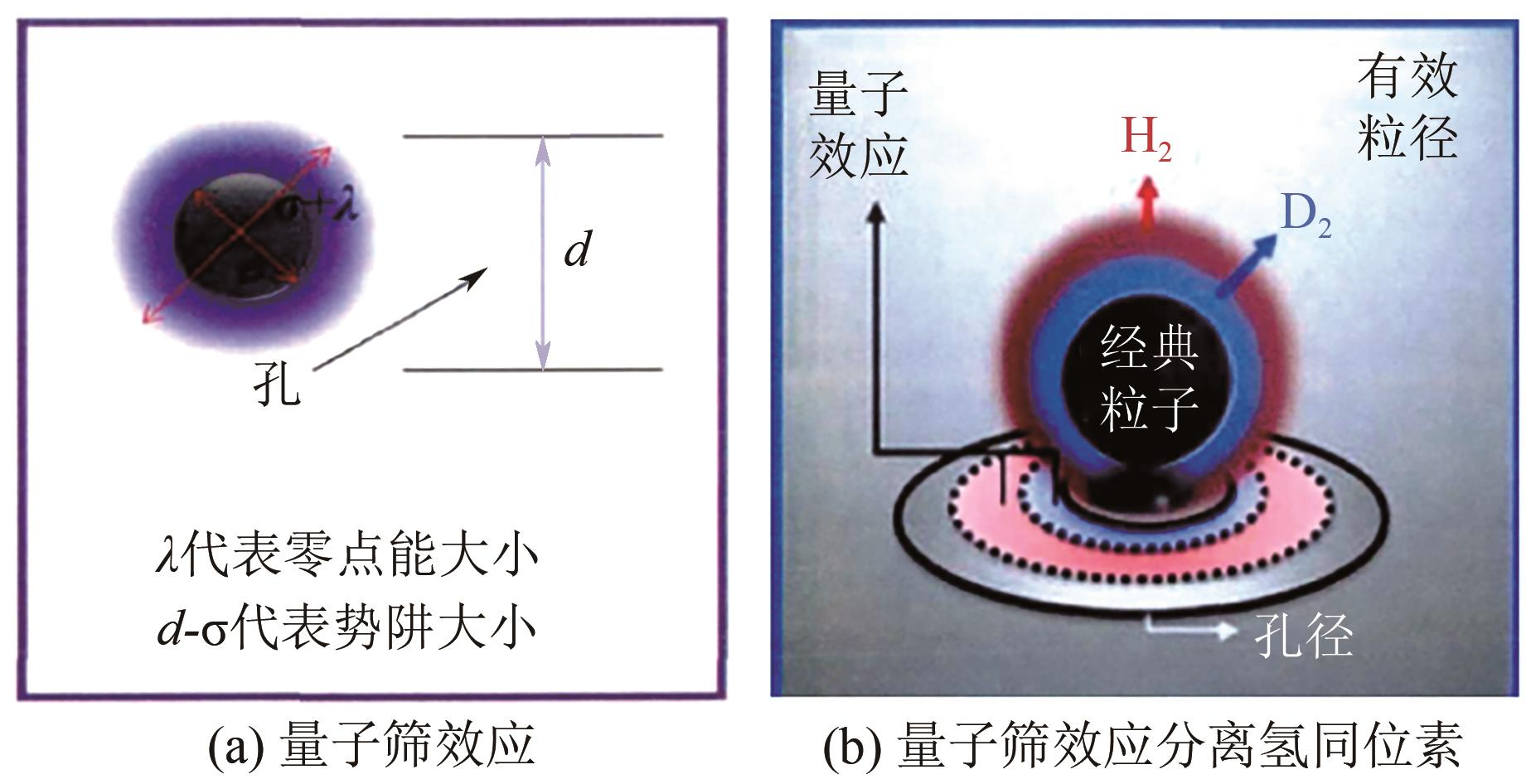
| 47 | BEENAKKER J J M, BORMAN V D, S Yu KRYLOV. Molecular transport in subnanometer pores: Zero-point energy, reduced dimensionality and quantum sieving[J]. Chemical Physics Letters, 1995, 232(4): 379-382. |
| 48 | TEUFEL Julia, Hyunchul OH, HIRSCHER Michael, et al. MFU-4—A metal-organic framework for highly effective H2/D2 separation[J]. Advanced Materials, 2013, 25(4): 635-639. |
| 49 | 曹大伟. 微孔金属有机框架材料氢同位素热力学量子筛效应研究[D]. 绵阳: 中国工程物理研究院, 2017. |
| CAO Dawei. Study on quantum screen effect of hydrogen isotope thermodynamics in porous metal-organic frame materials[D].Mianyang: China Academy of Engineering Physics, 2017. | |
| 50 | 张延鹏, 张胜中, 范得权, 等. 用于甲烷/氮气分离的活性炭吸附剂研究进展[J]. 当代化工, 2021, 50(10): 2466-2470, 2474. |
| ZHANG Yanpeng, ZHANG Shengzhong, FAN Dequan, et al. Research progress of activated carbon for CH4/N2 separation[J]. Contemporary Chemical Industry, 2021, 50(10): 2466-2470, 2474. | |
| 51 | 彭育志, 明越, 肖金生, 等. 活性炭/沸石层状床氢气纯化穿透曲线热效应[J]. 工程热物理学报, 2016, 37(7): 1511-1518. |
| PENG Yuzhi, MING Yue, XIAO Jinsheng, et al. Thermal effects on hydrogen purification breakthrough curves of activated carbon/zeolite layered bed[J]. Journal of Engineering Thermophysics, 2016, 37(7): 1511-1518. | |
| 52 | RIBEIRO Ana M, GRANDE Carlos A, LOPES Filipe V S, et al. A parametric study of layered bed PSA for hydrogen purification[J]. Chemical Engineering Science, 2008, 63(21): 5258-5273. |
| 53 | YANG Se-Il, CHOI Do-Young, JANG Seong-Cheol, et al. Hydrogen separation by multi-bed pressure swing adsorption of synthesis gas[J]. Adsorption, 2008, 14(4): 583-590. |
| 54 | CHO Soon Haeng, BHAT Sodankoor Garadi Thirumaleshwara, HAN Sang Sup, et al. Pressure swing adsorption apparatus and method for hydrogen purification using the same: US8298319[P]. 2012-10-30. |
| 55 | Sol AHN, YOU Young-Woo, LEE Dong-Geun, et al. Layered two- and four-bed PSA processes for H2 recovery from coal gas[J]. Chemical Engineering Science, 2012, 68(1): 413-423. |
| 56 | DELGADO José A, ÁGUEDA V I, UGUINA M A, et al. Adsorption and diffusion of H2, CO, CH4, and CO2 in BPL activated carbon and 13X zeolite: Evaluation of performance in pressure swing adsorption hydrogen purification by simulation[J]. Industrial & Engineering Chemistry Research, 2014, 53(40): 15414-15426. |
| 57 | BAILEY Susan E, OLIN Trudy J, Mark BRICKA R, et al. A review of potentially low-cost sorbents for heavy metals[J]. Water Research, 1999, 33(11): 2469-2479. |
| 58 | GUO Nannan, ZHANG Su, WANG Luxiang, et al. Application of plant-based porous carbon for supercapacitors[J]. Acta Physico-Chimica Sinica, 2020, 36(2): 1903055-. |
| 59 | YAVARY Milad, ALE EBRAHIM Habib, FALAMAKI Cavus. Competitive adsorption equilibrium isotherms of CO, CO2, CH4, and H2 on activated carbon and zeolite 5A for hydrogen purification[J]. Journal of Chemical & Engineering Data, 2016, 61(10): 3420-3427. |
| 60 | YI Honghong, LI Fenrong, NING Ping, et al. Adsorption separation of CO2, CH4, and N2 on microwave activated carbon[J]. Chemical Engineering Journal, 2013, 215/216: 635-642. |
| 61 | LOPES Filipe V S, GRANDE Carlos A, RIBEIRO Ana M, et al. Enhancing capacity of activated carbons for hydrogen purification[J]. Industrial & Engineering Chemistry Research, 2009, 48(8): 3978-3990. |
| 62 | LOPES Filipe V S, GRANDE Carlos A, RODRIGUES Alírio E. Activated carbon for hydrogen purification by pressure swing adsorption: Multicomponent breakthrough curves and PSA performance[J]. Chemical Engineering Science, 2011, 66(3): 303-317. |
| 63 | HE Bojun, LIU Jinglei, ZHANG Ying, et al. Comparison of structured activated carbon and traditional adsorbents for purification of H2 [J]. Separation and Purification Technology, 2020, 239: 116529. |
| 64 | PAN Hongyan, ZHAO Jingyun, LIN Qian, et al. Preparation and characterization of activated carbons from bamboo sawdust and its application for CH4 selectivity adsorption from a CH4/N2 system[J]. Energy & Fuels, 2016, 30(12): 10730-10738. |
| 65 | LI Dawei, WANG Yu, ZHANG Xiaoxiao, et al. Effects of compacting activated carbons on their volumetric CO2 adsorption performance[J]. Fuel, 2020, 262: 116540. |
| 66 | 袁翠翠. CO2活化法制备煤基微孔活性炭的研究[D]. 徐州: 中国矿业大学, 2016. |
| YUAN Cuicui. Research on preparation of coal-based microporous activated carbon with CO2 as activating agent[D]. Xuzhou: China University of Mining and Technology, 2016. | |
| 67 | 赵敏, 潘红艳, 郑蓓蕾, 等. 碱改性活性炭富集低浓度含氧煤层气甲烷的研究[J]. 天然气化工(C1化学与化工), 2016, 41(3): 37-42. |
| ZHAO Min, PAN Hongyan, ZHENG Beilei, et al. Methane enrichment from low concentration of coal mine methane by alkali-modified activated carbons[J]. Natural Gas Chemical Industry, 2016, 41(3): 37-42. | |
| 68 | 张梦竹, 李琳, 刘俊新, 等. 碱改性活性炭表面特征及其吸附甲烷的研究[J]. 环境科学, 2013, 34(1): 39-44. |
| ZHANG Mengzhu, LI Lin, LIU Junxin, et al. Surface characteristics of alkali modified activated carbon and the adsorption capacity of methane[J]. Environmental Science, 2013, 34(1): 39-44. | |
| 69 | ZHAO Guofeng, BAI Peng, ZHU Hongmei, et al. The modification of activated carbons and the pore structure effect on enrichment of coal-bed methane[J]. Asia-Pacific Journal of Chemical Engineering, 2008, 3(3): 284-291. |
| 70 | PAN Hongyan, YI Yun, LIN Qian, et al. Effect of surface chemistry and textural properties of activated carbons for CH4 selective adsorption through low-concentration coal bed methane[J]. Journal of Chemical & Engineering Data, 2016, 61(6): 2120-2127. |
| 71 | SHEN Wenzhong, LI Zhijie, LIU Yihong. Surface chemical functional groups modification of porous carbon[J]. Recent Patents on Chemical Engineeringe, 2008, 1(1): 27-40. |
| 72 | SHAMSUDIN I K, ABDULLAH A, IDRIS I, et al. Hydrogen purification from binary syngas by PSA with pressure equalization using microporous palm kernel shell activated carbon[J]. Fuel, 2019, 253: 722-730. |
| 73 | KWON Sunil, YOU Youngwoo, Hyungseob LIM, et al. Selective CO adsorption using sulfur-doped Ni supported by petroleum-based activated carbon[J]. Journal of Industrial and Engineering Chemistry, 2020, 83: 289-296. |
| 74 | HAROUN M F, MOUSSOUNDA P S, LÉGARÉ P. Theoretical study of methane adsorption on perfect and defective Ni(111) surfaces[J]. Catalysis Today, 2008, 138(1/2): 77-83. |
| 75 | Silvia GONZÁLEZ, Francesc VIÑES, GARCÍA Juan Fernando, et al. A DF-vdW study of the CH4 adsorption on different Ni surfaces[J]. Surface Science, 2014, 625: 64-68. |
| 76 | 郑蓓蕾, 林倩, 潘红艳, 等. 响应面法优化甲烷吸附用活性炭的镍改性工艺[J]. 过程工程学报, 2016, 16(3): 431-437. |
| ZHENG Beilei, LIN Qian, PAN Hongyan, et al. Optimization of the nickel modified active carbon used for adsorption of methane by response surface method[J]. The Chinese Journal of Process Engineering, 2016, 16(3): 431-437. | |
| 77 | Frank ABILD-PEDERSEN, LYTKEN Ole, Jakob ENGBÆK, et al. Methane activation on Ni(111): Effects of poisons and step defects[J]. Surface Science, 2005, 590(2/3): 127-137. |
| 78 | LIU Hongyan, TENG Botao, FAN Maohong, et al. CH4 dissociation on the perfect and defective MgO(001) supported Ni4 [J]. Fuel, 2014, 123: 285-292. |
| 79 | LANZINI A, GUERRA C, LEONE P, et al. Influence of the microstructure on the catalytic properties of SOFC anodes under dry reforming of methane[J]. Materials Letters, 2016, 164: 312-315. |
| 80 | ABDELJAOUED Amna, RELVAS Frederico, MENDES Adélio, et al. Simulation and experimental results of a PSA process for production of hydrogen used in fuel cells[J]. Journal of Environmental Chemical Engineering, 2018, 6: 338-355. |
| 81 | GOLMAKANI Ayub, FATEMI Shohreh, TAMNANLOO Javad. Investigating PSA, VSA, and TSA methods in SMR unit of refineries for hydrogen production with fuel cell specification[J]. Separation and Purification Technology, 2017, 176: 73-91. |
| 82 | KHAN Nazmul Abedin, JHUNG Sung Hwa. Adsorptive removal and separation of chemicals with metal-organic frameworks: Contribution of π-complexation[J]. Journal of Hazardous Materials, 2017, 325: 198-213. |
| 83 | YANG R T, Adsorbents fundamentals and applications[M]. Hoboken: John Wiley & Sons, 2004: 3-4. |
| 84 | HUANG Helen Y, PADIN Joel, YANG Ralph T. Comparison of π-complexations of ethylene and carbon monoxide with Cu+ and Ag+ [J]. Industrial & Engineering Chemistry Research, 1999, 38(7): 2720-2725. |
| 85 | The Ky VO, Youn-Sang BAE, CHANG Bong-Jun, et al. Highly CO selective Cu(Ⅰ)-doped MIL-100(Fe) adsorbent with high CO/CO2 selectivity due to π complexation: Effects of Cu(Ⅰ) loading and activation temperature[J]. Microporous and Mesoporous Materials, 2019, 274: 17-24. |
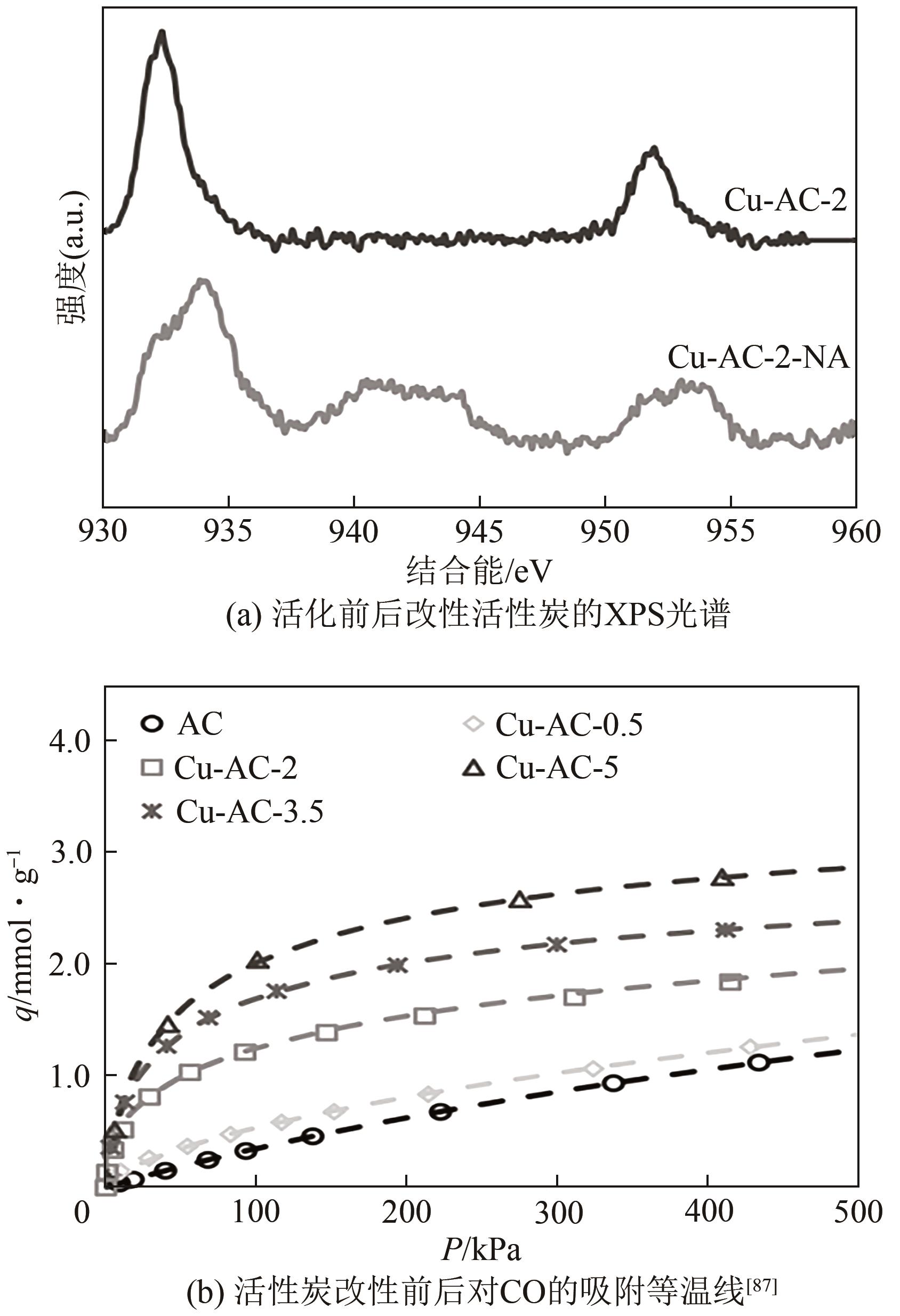
| 86 | 陈鸿雁, 涂晋林, 施亚钧. 载铜活性炭吸附分离氢气中的微量CO[J]. 华东理工大学学报(自然科学版), 1995, 21(1): 1-6. |
| CHEN Hongyan, TU Jinlin, SHI Yajun. Removal of trace CO from hydrogen by adsorption on active carbon supported copper[J]. Journal of East China University of Science and Technology, 1995, 21(1): 1-6. | |
| 87 | RELVAS Frederico, WHITLEY Roger D, SILVA Carlos, et al. Single-stage pressure swing adsorption for producing fuel cell grade hydrogen[J]. Industrial & Engineering Chemistry Research, 2018, 57(14): 5106-5118. |
| 88 | GAO Fei, WANG Yaquan, WANG Xiao, et al. Selective CO adsorbent CuCl/AC prepared using CuCl2 as a precursor by a facile method[J]. RSC Advances, 2016, 6(41): 34439-34446. |
| 89 | XUE Cailong, HAO Wenming, CHENG Wenping, et al. CO adsorption performance of CuCl/activated carbon by simultaneous Reduction-Dispersion of mixed Cu(Ⅱ) salts[J]. Materials, 2019, 12(10): 1605. |
| 90 | MA Jinghong, LI Li, REN Jin, et al. CO adsorption on activated carbon-supported Cu-based adsorbent prepared by a facile route[J]. Separation and Purification Technology, 2010, 76(1): 89-93. |
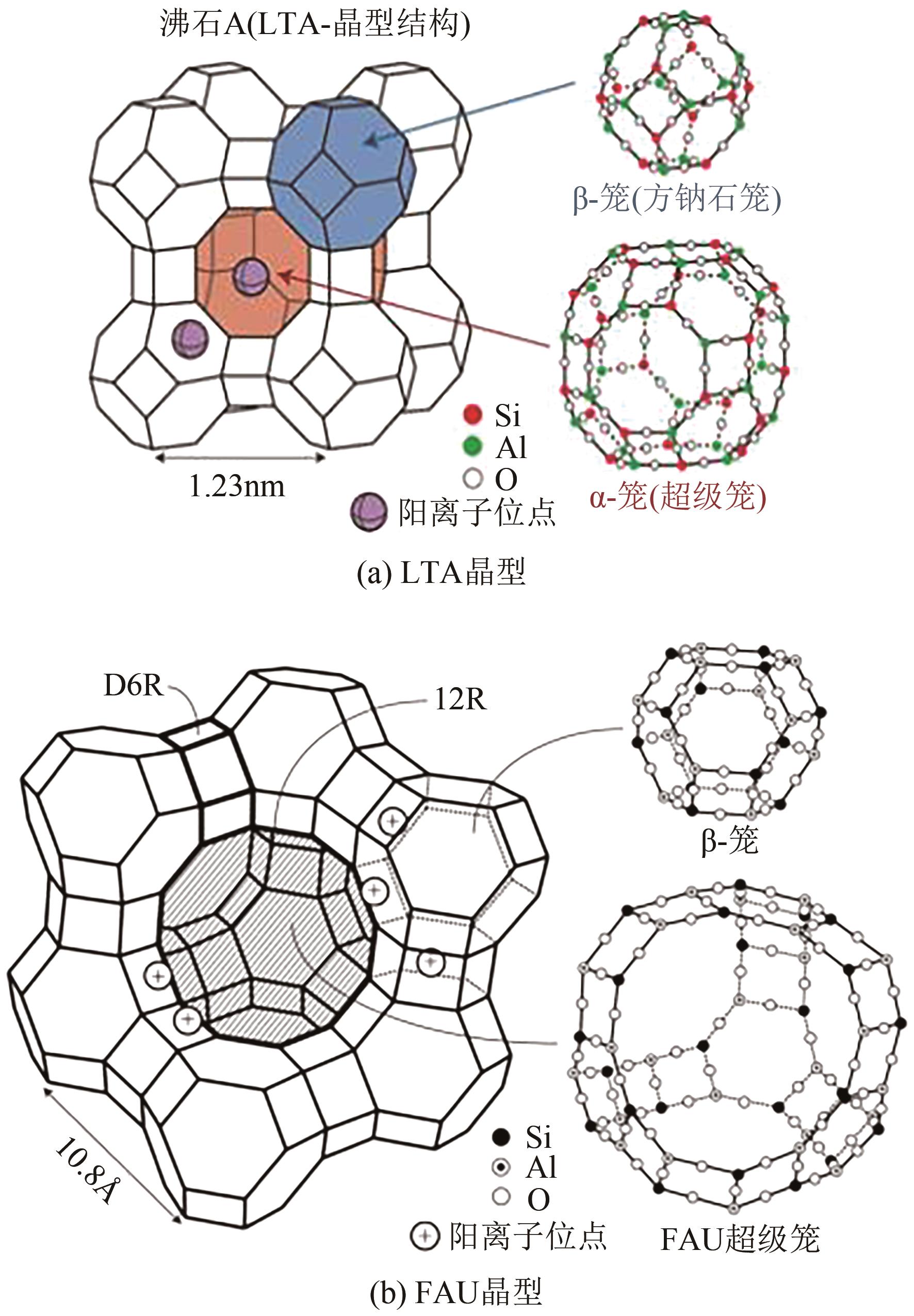
| 91 | 徐如人, 庞文琴. 分子筛与多孔材料化学[M]. 北京: 科学出版社, 2004: 69-70, 420-436. |
| XU Ruren, PANG Wenqin. Molecular sieves and porous materials chemistry[M]. Beijing: Science Press, 2004: 69-70, 420-436. | |
| 92 | PETROV I, MICHALEV Todor. Synthesis of zeolite A: A review[J]. НАУЧНИ ТРУДОВЕ НА РУСЕНСКИЯ УНИВЕРСИТЕТ, 2012, 51: 31-35. |
| 93 | NOZUE Yasuo, AMAKO Yusaku, KAWANO Ryoko, et al. Insulating state and metallic phase transition of heavily sodium-doped low-silica X (LSX) zeolites[J]. Journal of Physics and Chemistry of Solids, 2012, 73(12): 1538-1541. |
| 94 | ZHANG Junfang, BURKE N, ZHANG Shuichang, et al. Thermodynamic analysis of molecular simulations of CO2 and CH4 adsorption in FAU zeolites[J]. Chemical Engineering Science, 2014, 113: 54-61. |
| 95 | MONTANARI Tania, SALLA Isabel, BUSCA Guido. Adsorption of CO on LTA zeolite adsorbents: An IR investigation[J]. Microporous and Mesoporous Materials, 2008, 109(1/2/3): 216-222. |
| 96 | PILLAI Renjith S, SETHIA Govind, JASRA Raksh V. Sorption of CO, CH4, and N2 in alkali metal ion exchanged zeolite-X: Grand canonical Monte Carlo simulation and volumetric measurements[J]. Industrial & Engineering Chemistry Research, 2010, 49(12): 5816-5825. |
| 97 | LOZINSKA Magdalena M, MANGANO Enzo, MOWAT John P S, et al. Understanding carbon dioxide adsorption on univalent cation forms of the flexible zeolite Rho at conditions relevant to carbon capture from flue gases[J]. Journal of the American Chemical Society, 2012, 134(42): 17628-17642. |
| 98 | ARCOYA A, GONZÁLEZ J A, LLABRE G, et al. Role of the countercations on the molecular sieve properties of a clinoptilolite[J]. Microporous Materials, 1996, 7(1): 1-13. |
| 99 | WALTON Krista S, ABNEY Morgan B, DOUGLAS LEVAN M. CO2 adsorption in Y and X zeolites modified by alkali metal cation exchange[J]. Microporous and Mesoporous Materials, 2006, 91(1/2/3): 78-84. |
| 100 | BREA P, DELGADO J A, ÁGUEDA Vicente I, et al. Multicomponent adsorption of H2, CH4, CO and CO2 in zeolites NaX, CaX and MgX. Evaluation of performance in PSA cycles for hydrogen purification[J]. Microporous and Mesoporous Materials, 2019, 286: 187-198. |
| 101 | GOLIPOUR Haleh, MOKHTARANI Babak, MAFI Morteza, et al. Systematic measurements of CH4 and CO2 adsorption isotherms on cation-exchanged zeolites 13X[J]. Journal of Chemical & Engineering Data, 2019, 64(10): 4412-4423. |
| 102 | CHEN S J, ZHU M, FU Y, et al. Using 13X, LiX, and LiPdAgX zeolites for CO2 capture from post-combustion flue gas[J]. Applied Energy, 2017, 191: 87-98. |
| 103 | XIE Youchang, ZHANG Jiaping, QIU Jianguo, et al. Zeolites modified by CuCl for separating CO from gas mixtures containing CO2 [J]. Adsorption, 1997, 3(1): 27-32. |
| 104 | GAO Fei, WANG Yaquan, WANG Shuhai. Selective adsorption of CO on CuCl/Y adsorbent prepared using CuCl2 as precursor: Equilibrium and thermodynamics[J]. Chemical Engineering Journal, 2016, 290: 418-427. |
| 105 | 李淑娜, 张东辉, 鲁东东. CO吸附剂制备及其吸附脱除微量CO的性能[J]. 化学工业与工程, 2014, 31(5): 1-7. |
| LI Shuna, ZHANG Donghui, LU Dongdong. Preparation of carbon monoxide adsorbent and its adsorption performance for removing trace carbon monoxide[J]. Chemical Industry and Engineering, 2014, 31(5): 1-7. | |
| 106 | HERNÁNDEZ-MALDONADO Arturo J, YANG Ralph T. Desulfurization of commercial liquid fuels by selective adsorption via π-complexation with Cu(Ⅰ)-Y zeolite[J]. Industrial & Engineering Chemistry Research, 2003, 42(13): 3103-3110. |
| 107 | 张子宝. Cu-π络合吸附剂及其脱除CO研究[D]. 大连: 大连理工大学, 2021. |
| ZHANG Zibao. Study on Cu π-complexed adsorbent and its removal of CO[D]. Dalian: Dalian University of Technology, 2021. | |
| 108 | QIN Juxiang, WANG Zhimin, LIU Xiaoqin, et al. Low-temperature fabrication of Cu(Ⅰ) sites in zeolites by using a vapor-induced reduction strategy[J]. Journal of Materials Chemistry A, 2015, 3(23): 12247-12251. |
| 109 | LI Hailian, EDDAOUDI Mohamed, GROY Thomas L, et al. Establishing microporosity in open metal-organic frameworks: Gas sorption isotherms for Zn(BDC) (BDC=1,4-benzenedicarboxylate)[J]. Journal of the American Chemical Society, 1998, 120(33): 8571-8572. |
| 110 | GHANBARI Taravat, ABNISA Faisal, WAN DAUD Wan Mohd Ashri. A review on production of metal organic frameworks (MOF) for CO2 adsorption[J]. Science of the Total Environment, 2020, 707: 135090. |
| 111 | 边青敏, 忻睦迪, 邹亢, 等. 分子筛和金属有机骨架材料用于乙烯/乙烷分离的研究进展[J]. 石油学报(石油加工), 2020, 36(4): 866-877. |
| BIAN Qingmin, XIN Mudi, ZOU Kang, et al. Advances in separation of ethylene and ethane with molecular sieve and metal-organic frameworks[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2020, 36(4): 866-877. | |
| 112 | GUO Ya, HU Jiangliang, LIU Xiaowei, et al. Scalable solvent-free preparation of [Ni3(HCOO)6] frameworks for highly efficient separation of CH4 from N2 [J]. Chemical Engineering Journal, 2017, 327: 564-572. |
| 113 | GALLO Marco, Daniel GLOSSMAN-MITNIK. Fuel gas storage and separations by metal-organic frameworks: Simulated adsorption isotherms for H2 and CH4 and their equimolar mixture[J]. The Journal of Physical Chemistry C, 2009, 113(16): 6634-6642. |
| 114 | TYLIANAKIS E, FROUDAKIS G E. Grand canonical Monte Carlo method for gas adsorption and separation[J]. Journal of Computational and Theoretical Nanoscience, 2009, 6(2): 335-348. |
| 115 | GUO Haichao, SHI Fan, MA Zhengfei, et al. Molecular simulation for adsorption and separation of CH4/H2 in zeolitic imidazolate frameworks[J]. The Journal of Physical Chemistry C, 2010, 114(28): 12158-12165. |
| 116 | YANG Qingyuan, ZHONG Chongli. Molecular simulation of carbon dioxide/methane/hydrogen mixture adsorption in metal-organic frameworks[J]. The Journal of Physical Chemistry B, 2006, 110(36): 17776-17783. |
| 117 | FISCHER Michael, HOFFMANN Frank, Michael FRÖBA. Metal-organic frameworks and related materials for hydrogen purification: Interplay of pore size and pore wall polarity[J]. RSC Advances, 2012, 2(10): 4382-4396. |
| 118 | 刘秀英, 袁俊鹏, 李晓东, 等. 甲烷/氢气在超微孔金属有机骨架中的吸附与分离性能研究[J]. 化工新型材料, 2021, 49(2): 178-181, 186. |
| LIU Xiuying, YUAN Junpeng, LI Xiaodong, et al. Study on adsorption and separation of UM-MOFs for CH4/H2 [J]. New Chemical Materials, 2021, 49(2): 178-181, 186. | |
| 119 | MENG Zhaoshun, LU Ruifeng, RAO Dewei, et al. Catenated metal-organic frameworks: Promising hydrogen purification materials and high hydrogen storage medium with further lithium doping[J]. International Journal of Hydrogen Energy, 2013, 38(23): 9811-9818. |
| 120 | ROSI Nathaniel L, KIM Jaheon, EDDAOUDI Mohamed, et al. Rod packings and metal-organic frameworks constructed from rod-shaped secondary building units[J]. Journal of the American Chemical Society, 2005, 127(5): 1504-1518. |
| 121 | HERM Zoey R, SWISHER Joseph A, SMIT Berend, et al. Metal-organic frameworks as adsorbents for hydrogen purification and precombustion carbon dioxide capture[J]. Journal of the American Chemical Society, 2011, 133(15): 5664-5667. |
| 122 | HERM Zoey R, KRISHNA Rajamani, LONG Jeffrey R. CO2/CH4, CH4/H2 and CO2/CH4/H2 separations at high pressures using Mg2(dobdc)[J]. Microporous and Mesoporous Materials, 2012, 151: 481-487. |
| 123 | CHUI Stephen S-Y, LO Samuel M-F, CHARMANT Jonathan P H, et al. A chemically functionalizable nanoporous material[Cu3(TMA)2(H2O)3] n [J]. Science, 1999, 283(5405): 1148-1150. |
| 124 | SILVA Bruna, SOLOMON Ioan, RIBEIRO Ana M, et al. H2 purification by pressure swing adsorption using CuBTC[J]. Separation and Purification Technology, 2013, 118: 744-756. |
| 125 | JAMALI Sadegh, MOFARAHI Masoud, RODRIGUES Alirio E. Investigation of a novel combination of adsorbents for hydrogen purification using Cu-BTC and conventional adsorbents in pressure swing adsorption[J]. Adsorption, 2018, 24(5): 481-498. |
| 126 | WU Haohan, YAO Kexin, ZHU Yihan, et al. Cu-TDPAT, an Rht-type dual-functional metal-organic framework offering significant potential for use in H2 and natural gas purification processes operating at high pressures[J]. The Journal of Physical Chemistry C, 2012, 116(31): 16609-16618. |
| 127 | ASGARI Mehrdad, STREB Anne, VAN DER SPEK Mijndert, et al. Synergistic material and process development: Application of a metal-organic framework, Cu-TDPAT, in single-cycle hydrogen purification and CO2 capture from synthesis gas[J]. Chemical Engineering Journal, 2021, 414: 128778. |
| 128 | WIERSUM Andrew D, Estelle SOUBEYRAND-LENOIR, YANG Qingyuan, et al. An evaluation of UiO-66 for gas-based applications[J]. Chemistry: an Asian Journal, 2011, 6(12): 3270-3280. |
| 129 | RAMSAHYE, N A, GAO, J, JOBIC, H, et al. Adsorption and diffusion of light hydrocarbons in UiO-66(Zr): A combination of experimental and modeling tools[J]. The Journal of Physical Chemistry C: Nanomaterials and Interfaces, 2014, 118(47): 27470-27482. |
| 130 | BANU Ana-Maria, FRIEDRICH Daniel, BRANDANI Stefano, et al. A multiscale study of MOFs as adsorbents in H2 PSA purification[J]. Industrial & Engineering Chemistry Research, 2013, 52(29): 9946-9957. |
| 131 | XIANG Shengchang, WU Xintao, ZHANG Jianjun, et al. A 3D canted antiferromagnetic porous metal-organic framework with anatase topology through assembly of an analogue of polyoxometalate[J]. Journal of the American Chemical Society, 2005, 127(47): 16352-16353. |
| 132 | XIANG Shengchang, HE Yabing, ZHANG Zhangjing, et al. Microporous metal-organic framework with potential for carbon dioxide capture at ambient conditions[J]. Nature Communications, 2012, 3: 954. |
| 133 | AGUEDA Vicente I, DELGADO José A, UGUINA María A, et al. Adsorption and diffusion of H2, N2, CO, CH4 and CO2 in UTSA-16 metal-organic framework extrudates[J]. Chemical Engineering Science, 2015, 124: 159-169. |
| 134 | BREA P, DELGADO J A, ÁGUEDA Vicente I, et al. Comparison between MOF UTSA-16 and BPL activated carbon in hydrogen purification by PSA[J]. Chemical Engineering Journal, 2019, 355: 279-289. |
| 135 | DELGADO José A, ÁGUEDA Vicente I, UGUINA María A, et al. Comparison and evaluation of agglomerated MOFs in biohydrogen purification by means of pressure swing adsorption (PSA)[J]. Chemical Engineering Journal, 2017, 326: 117-129. |
| 136 | BOSCH Mathieu, ZHANG Muwei, ZHOU Hongcai. Increasing the stability of metal-organic frameworks[J]. Advances in Chemistry, 2014, 2014: 182327. |
| 137 | Mircea DINCǍ, YU Anta F, LONG Jeffrey R. Microporous metal-organic frameworks incorporating 1,4-benzeneditetrazolate: Syntheses, structures, and hydrogen storage properties[J]. Journal of the American Chemical Society, 2006, 128(27): 8904-8913. |
| 138 | NANDI Shyamapada, DE LUNA Phil, DAFF Thomas D, et al. A single-ligand ultra-microporous MOF for precombustion CO2 capture and hydrogen purification[J]. Science Advances, 2015, 1(11): e1500421. |
| 139 | Qasim AL-NADDAF, THAKKAR Harshul, REZAEI Fateme. Novel zeolite-5A@MOF-74 compositeadsorbents with core-shell structure for H2 purification[J]. ACS Applied Materials & Interfaces, 2018, 10(35): 29656-29666. |
| 140 | 董秀婷, 张文, 赵颂, 等. 金属有机骨架材料的复合成型[J]. 化学进展, 2021, 33(12): 2173-2187. |
| DONG Xiuting, ZHANG Wen, ZHAO Song, et al. Shaping methods for metal-organic framework composites[J]. Progress in Chemistry, 2021, 33(12): 2173-2187. | |
| 141 | MA Qinglang, ZHANG Teng, WANG Bo. Shaping of metal-organic frameworks, a critical step toward industrial applications[J]. Matter, 2022, 5(4): 1070-1091. |
| [1] | BIAN Weibai, ZHANG Ruixuan, PAN Jianming. Research progress on preparation methods of inorganic metal lithium ion sieve materials [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4173-4186. |
| [2] | ZHANG Yesu, QUAN Yanhong, DING Xinxin, REN Jun. Synthesis and application of chainlike MFI type zeolites [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4382-4392. |
| [3] | HUANG Kun, XU Ming, WU Xiujuan, PEI Sijia, LIU Dawei, MA Xiaoxun, XU Long. Research progress on preparation and microstructural characteristics regulation of biomass activated carbon [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2475-2493. |
| [4] | LIANG Yanyan, ZHANG Junliang, GUO Yunya, ZHANG Yanting. The role of seed in the synthesis of molecular sieves [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1275-1292. |
| [5] | ZHOU Yihuan, XIE Qiang, ZHOU Hongyang, LIANG Dingcheng, LIU Jinchang. Modeling of porous carbon materials based on molecular simulation: State-of-the art [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1535-1551. |
| [6] | GUO Yingchun, LIANG Xiaoyi. Effect of citric acid modification on the spherical activated carbon's ammonia adsorption performance [J]. Chemical Industry and Engineering Progress, 2024, 43(2): 1082-1088. |
| [7] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [8] | ZHANG Lihong, JIN Yaoru, CHENG Fangqin. Resource utilization of coal gasification slag [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4447-4457. |
| [9] | ZHANG Yaojie, ZHANG Chuanxiang, SUN Yue, ZENG Huihui, JIA Jianbo, JIANG Zhendong. Application of coal-based graphene quantum dots in supercapacitors [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4340-4350. |
| [10] | XING Xianjun, LUO Tian, BU Yuzheng, MA Peiyong. Preparation of biochar from walnut shells activated by H3PO4 and its application in Cr(Ⅵ) adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1527-1539. |
| [11] | ZHOU Hongyang, ZHOU Yihuan, ZHANG Lianxiu, LIANG Dingcheng, XIE Qiang. Heel of VOCs in activated carbon: Formation mechanism and influencing factors [J]. Chemical Industry and Engineering Progress, 2023, 42(11): 5969-5980. |
| [12] | LIU Yajuan. Research status of membrane fouling mitigation by PAC in submerged PAC-AMBRs [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 457-468. |
| [13] | QI Yuan, XU Xinrong, RUAN Wei, WU Hao, WU Ke, ZHOU Yaming, YANG Hongmin. Characterization of aniline adsorption by modified activated carbon fiber [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 622-630. |
| [14] | SUN Xianhang, REN Zhu, ZHANG Guojun, SUN Yuan, FAN Kaifeng, HUANG Weiqiu. Study on the desorption mechanism of toluene in activated carbon under supercritical CO2 [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 631-636. |
| [15] | LIU Nan, HU Yiming, YANG Ying, LI Hongjin, GAO Zhuqing, HAO Xiuli. Microwave assisted co-pyrolysis of waste polypropylene /activated carbon to produce combustible pyrolysis gas and light pyrolysis oil [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 150-159. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||