Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (10): 5498-5516.DOI: 10.16085/j.issn.1000-6613.2023-1705
• Industrial catalysis • Previous Articles
Research progress on catalysts for hydrogen production by methanol steam reforming
FENG Kai( ), MENG Hao(
), MENG Hao( ), YANG Yusen(
), YANG Yusen( ), WEI Min
), WEI Min
- State Key Laboratory of Chemical Resource Engineering, College of Chemistry, Beijing University of Chemical Technology, Beijing 100029, China
-
Received:2023-09-26Revised:2024-03-21Online:2024-10-29Published:2024-10-15 -
Contact:MENG Hao, YANG Yusen
甲醇水蒸气重整制氢催化剂的研究进展
- 北京化工大学化学学院,化工资源有效利用国家重点实验室,北京 100029
-
通讯作者:孟浩,杨宇森 -
作者简介:冯凯(2000—),男,硕士研究生,研究方向为甲醇重整制氢和丙烷脱氢。E-mail:2022201055@buct.edu.cn。 -
基金资助:国家重点研发计划(2021YFC2103500);国家自然科学基金(22172006)
CLC Number:
Cite this article
FENG Kai, MENG Hao, YANG Yusen, WEI Min. Research progress on catalysts for hydrogen production by methanol steam reforming[J]. Chemical Industry and Engineering Progress, 2024, 43(10): 5498-5516.
冯凯, 孟浩, 杨宇森, 卫敏. 甲醇水蒸气重整制氢催化剂的研究进展[J]. 化工进展, 2024, 43(10): 5498-5516.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-1705
| 甲醇重整产氢 | 反应示意图 | 反应方程 | 优势 | 劣势 |
|---|---|---|---|---|
| 甲醇水蒸气重整 | 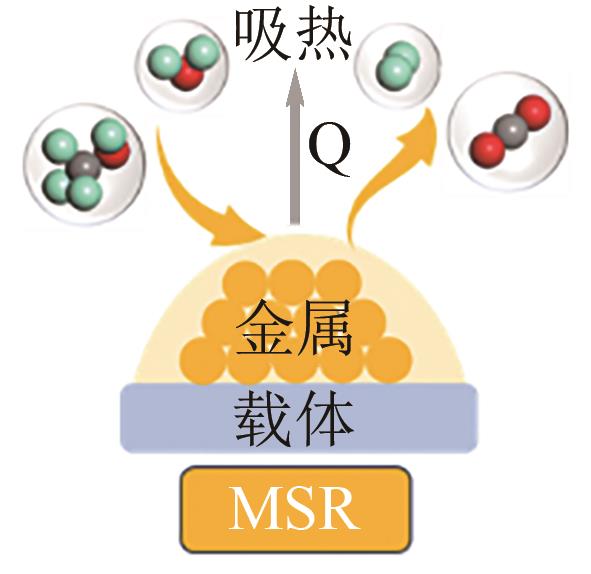 | H2产率最高;不需要提供氧气;CO含量较低 | 需要外部较高的能量供应 | |
| 甲醇部分氧化 | 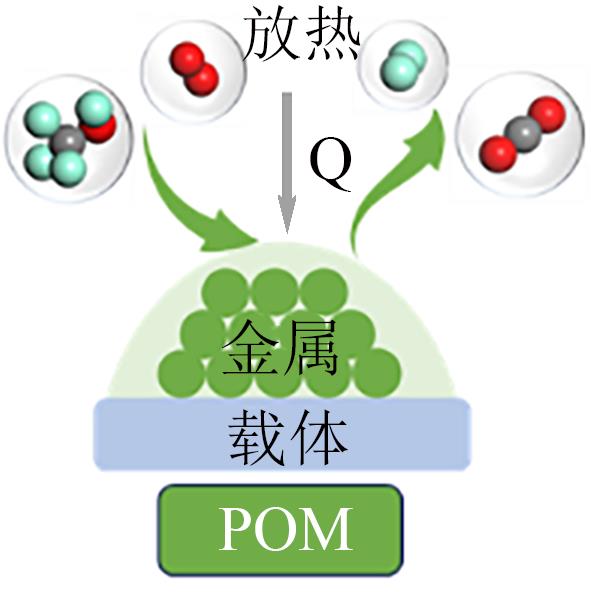 | 快速启动和响应时间;碳积累少;无需较高的热供应 | 较低的H2产率;H2容易过氧化;CO含量高 | |
| 甲醇自热重整 | 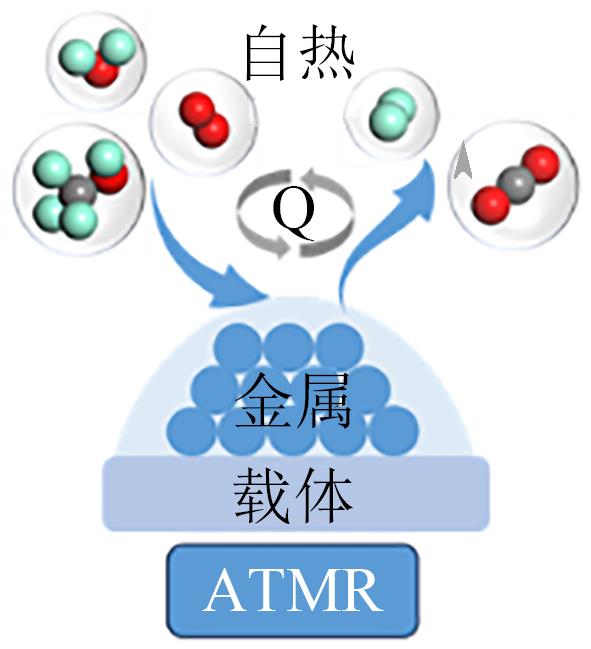 | 放热和吸热反应的耦合;简化热量管理;工作温度低;快速启动 | H2产率低;需要控制系统平衡放热和吸热过程;H2容易过氧化 | |
| 甲醇分解 |  | 反应简便;无需额外原料的引入 | H2产率最低;CO含量过高;催化剂容易积炭失活 |
| 甲醇重整产氢 | 反应示意图 | 反应方程 | 优势 | 劣势 |
|---|---|---|---|---|
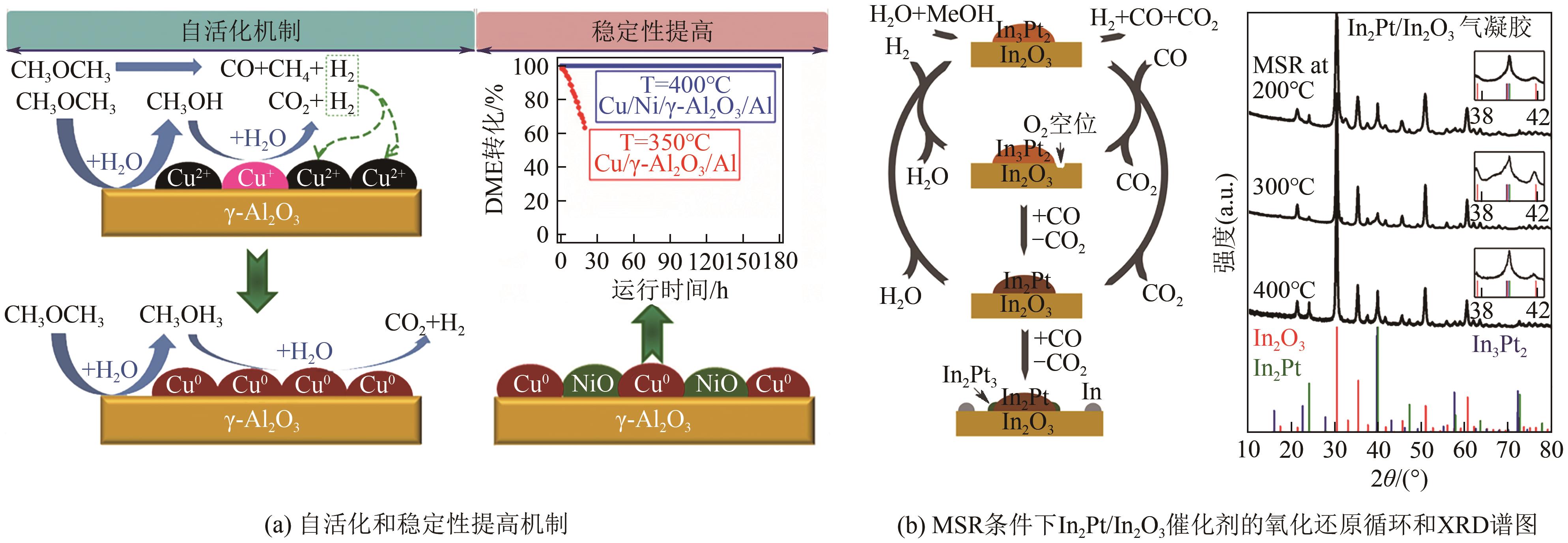
| 甲醇水蒸气重整 |  | H2产率最高;不需要提供氧气;CO含量较低 | 需要外部较高的能量供应 | |
| 甲醇部分氧化 |  | 快速启动和响应时间;碳积累少;无需较高的热供应 | 较低的H2产率;H2容易过氧化;CO含量高 | |
| 甲醇自热重整 |  | 放热和吸热反应的耦合;简化热量管理;工作温度低;快速启动 | H2产率低;需要控制系统平衡放热和吸热过程;H2容易过氧化 | |
| 甲醇分解 |  | 反应简便;无需额外原料的引入 | H2产率最低;CO含量过高;催化剂容易积炭失活 |
| 催化剂 | 反应温度/℃ | 水碳比 | 转化率/% | CO2选择性/% | CO选择性/% | H2生成速率 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 0.2%Pt/α-MoC | 190 | 1 | — | — | 0.14 | 18046 | [ |
| PdO/In2O3 | 260 | 1 | 50 | 93 | — | — | [ |
| CuPd/ZrO2 | 220 | 1.5 | 65 | — | 5 | 86.3mmol·h-1·gcat-1 | [ |
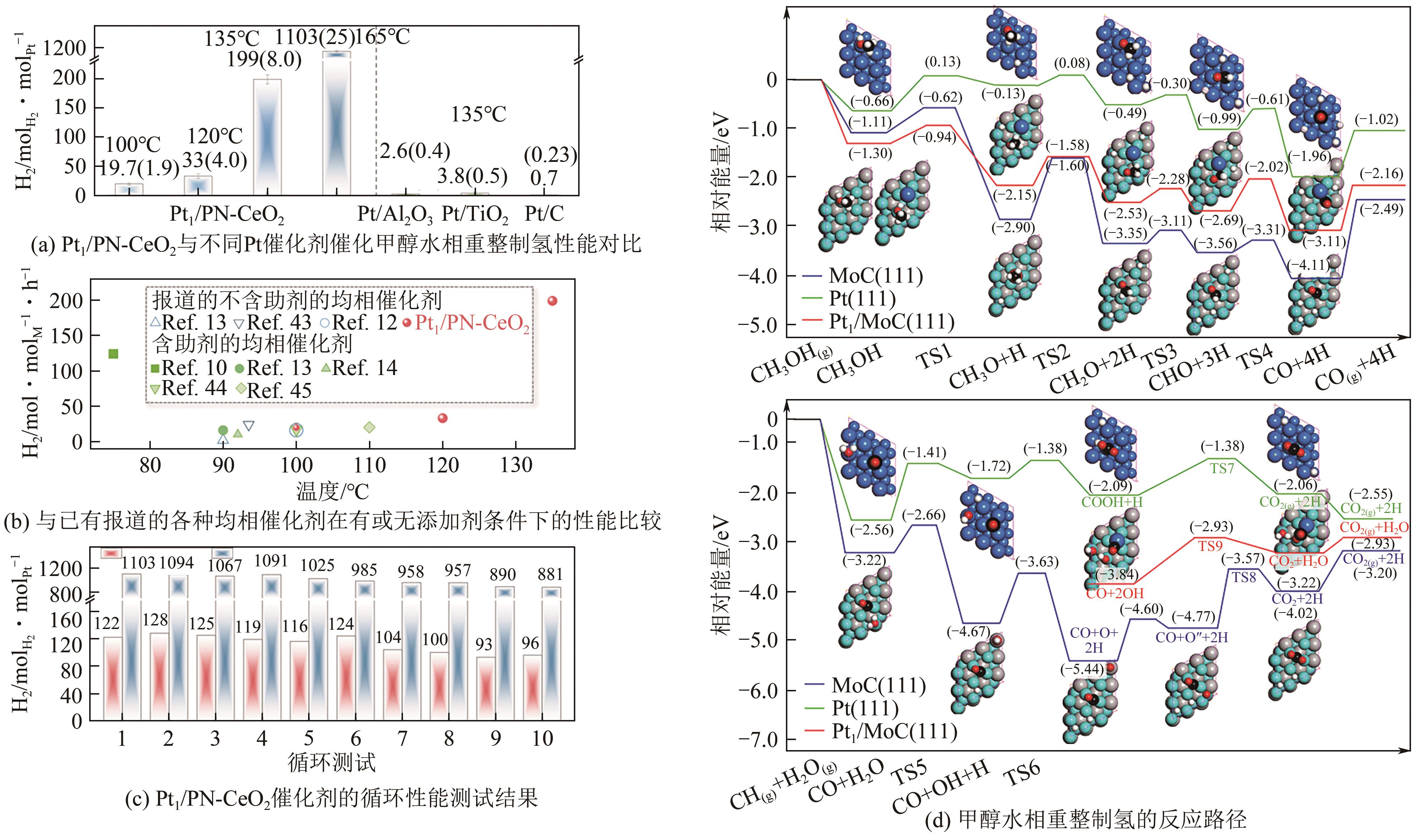
| Pt1/PN-CeO2 | 135 | 1 | — | — | 0.05 | 199mol | [ |
| 2% Ni/α-MoC | 240 | 1 | — | — | 0.7 | 1805mol | [ |
| Pt1/ZnO | 390 | 1.5 | 43 | — | — | — | [ |
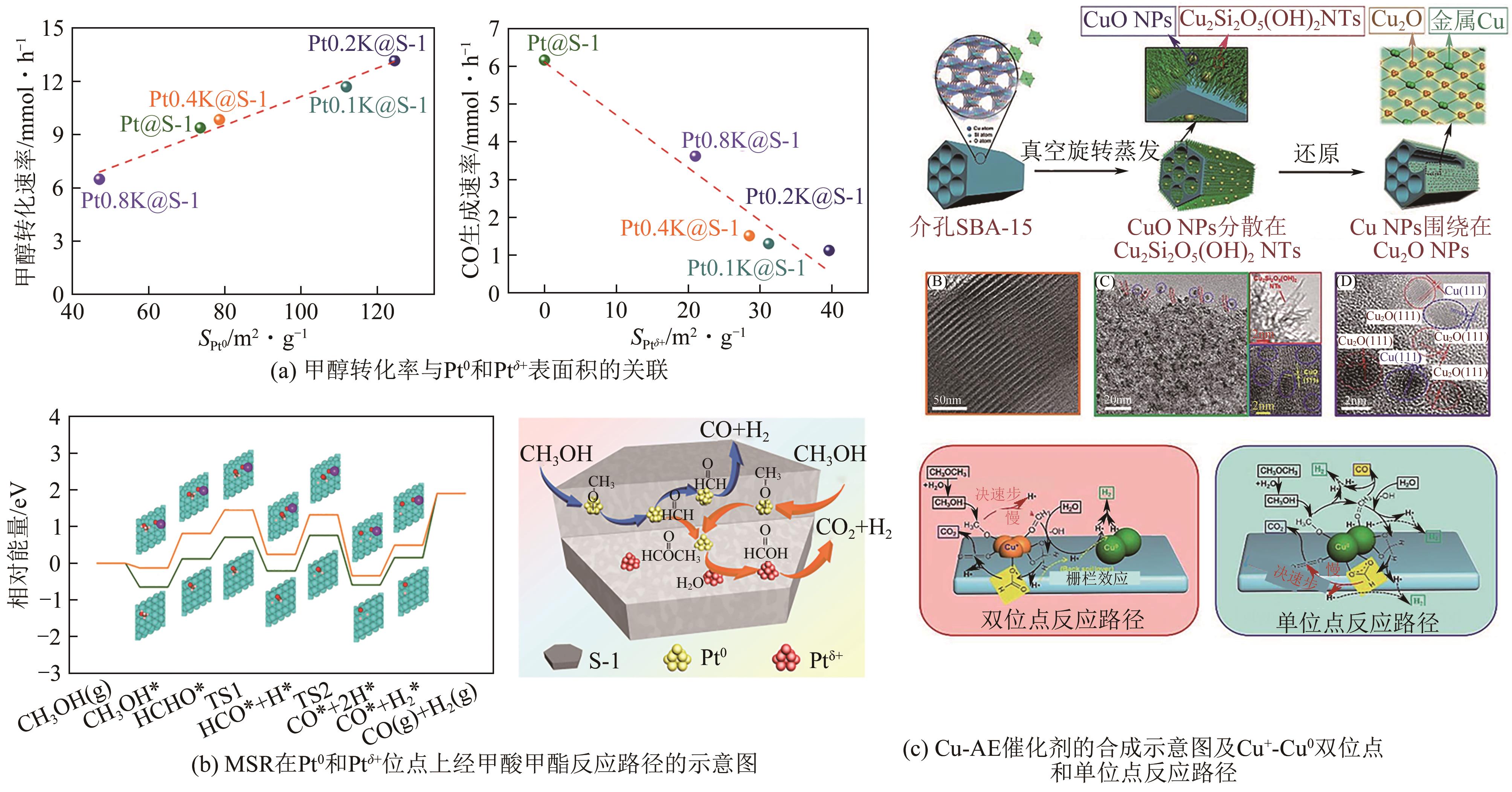
| Ru1/CeO2 | 350 | 3 | 26 | 98 | — | 139.6mL | [ |
| CuFe(50∶50) | 350 | 3 | 48 | 0 | 200mmol·kgcat-1·s-1 | [ | |
| Cu-Fe/硅酸盐 | 200 | — | 99 | — | 0 | 1.64μmol·s-1·gcat-1 | [ |
| Cu/Ni/γ-Al2O3 | 400 | 2 | 100 | 83.3 | 11 | — | [ |
| Ni0.2Cu0.8/BN | 320 | 1 | 100 | — | — | 1.8mol | [ |
| PtCo/MoS2 | 220 | 3 | — | — | — | 37142mol | [ |
| Cu-Zn/CeAlO3 | 320 | 6 | 98.9 | 96.1 | — | — | [ |
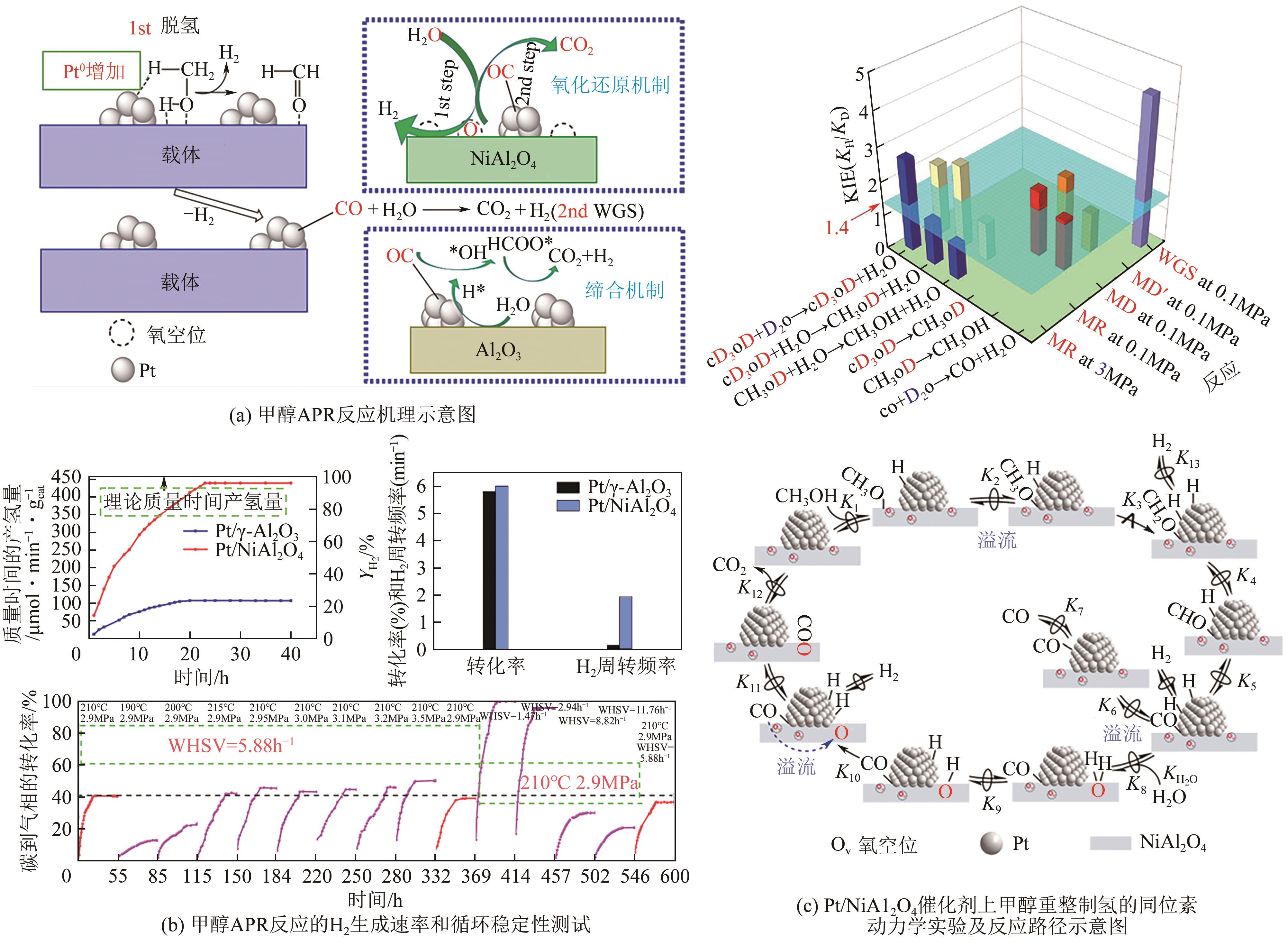
| Cu-In2O3 | 350 | 2 | 84 | — | 5 | 3.8µmol·gCu-1·s-1 | [ |
| Pt/In2O3/Al2O3 | 350 | 4 | 100 | 96.1 | 3.2 | 0.6mol·h-1·gcat-1 | [ |
| Pt/In2O3 | 300 | 1 | — | 99.5 | — | 1500mol | [ |
| In7Pt3 | 400 | 1 | — | 99.2 | — | 6mol·molpt-1·h-1 | [ |
| InPd/In2O3 | 300 | 1 | 26 | 99 | — | 50mmol | [ |
| Pt0.2K@S-1 | 400 | 3 | 45 | — | 1.9 | 61.12mmol | [ |
| 25Cu-AE | 400 | 2 | 100 | 89.3 | 10.4 | 1145mol·kgcat-1·h-1 | [ |
| CuZnO/γ-Al2O3/Al | 225 | 2 | 60 | 90 | — | — | [ |
| Pt/NiAl2O4 | 210 | 16 | 100 | 99.7 | 0.05 | 439.2μmol·min-1·gcat-1 | [ |
| Pd/ZnAl2O4 | 250 | 1.1 | 60 | 97 | 3 | 11.4μmol | [ |
| 30Cu/CeO2 | 200 | 1.3 | 4.5 | 99.5 | — | 0.21mol | [ |
| Cu/Sc2O3-ZnO | 400 | 1.5 | 100 | — | 11 | 140μmol·g-1·s-1 | [ |
| CuZrAl0.4 | 220 | 1.5 | 70 | — | 0.1 | 460.1mmol·gmet-1·h-1 | [ |
| 10Pr-NA | 300 | 1.5 | 95 | — | — | — | [ |
| 18GaCuMg | 200 | 1.5 | 90 | — | 0.4 | — | [ |
| Cu/ZrO2 | 260 | 1.3 | 87 | 100 | — | 260mmol·gcat-1·h-1 | [ |
| Cu/ZrO2 | 270 | 2 | 40 | 99 | — | 14μmol·gCu-1·s-1 | [ |
| Cu/ZnO/Al2O3 | 225 | 1.3 | 67 | — | — | — | [ |
| CuAl2O4 | 320 | 1.5 | 30 | 98.95 | 1.05 | 52mol·min-1·molCu-1 | [ |
| CuHAl-Ac-950 | 255 | 2.3 | 80 | — | 0.4 | — | [ |
| CuNi0.05/Al2O3 | 255 | 2.3 | 90 | — | 0.8 | — | [ |
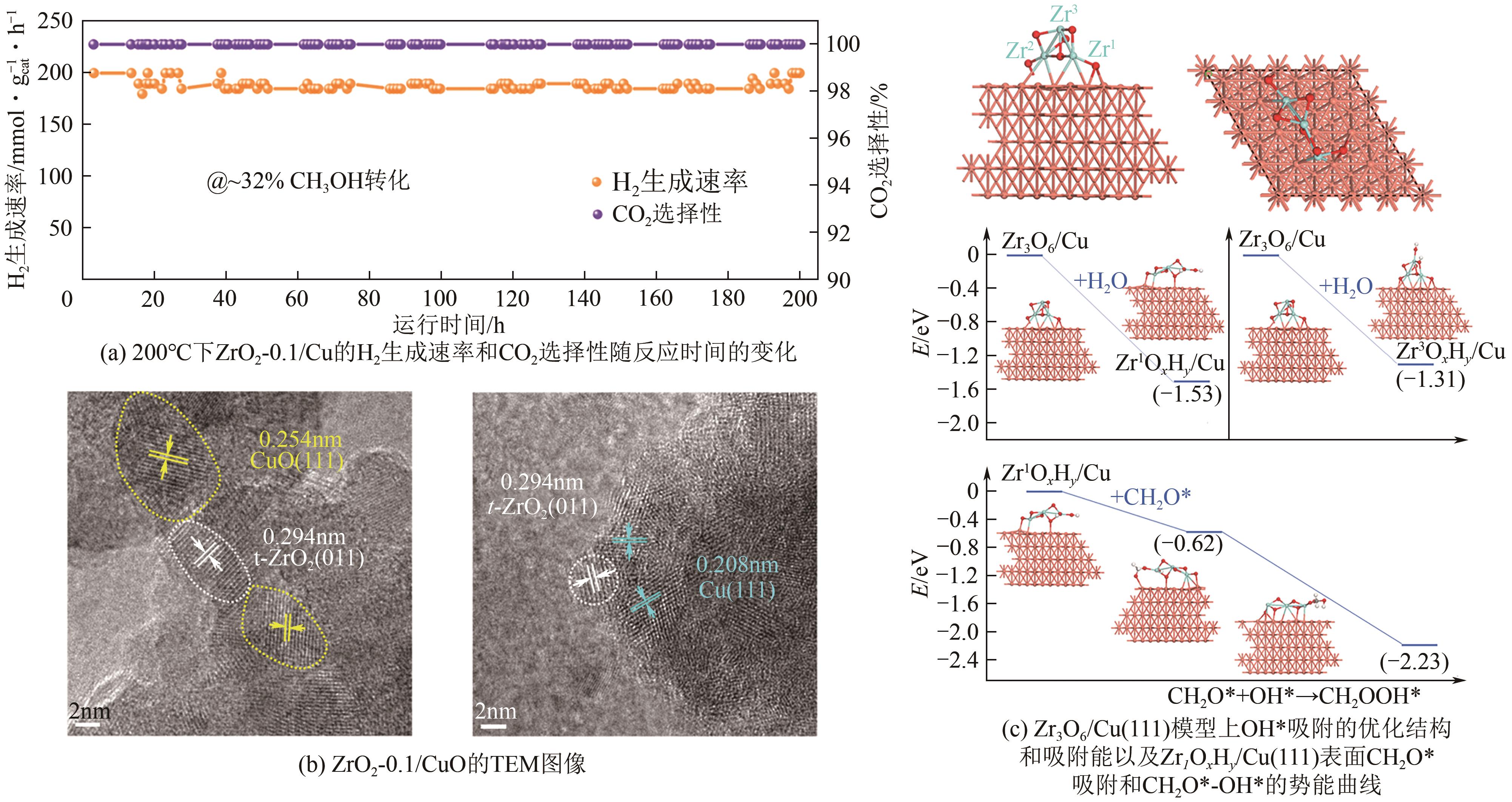
| 1.7Mg/Cu/Al2O3 | 255 | 2.3 | 96.5 | 96.2 | 3.8 | — | [ |
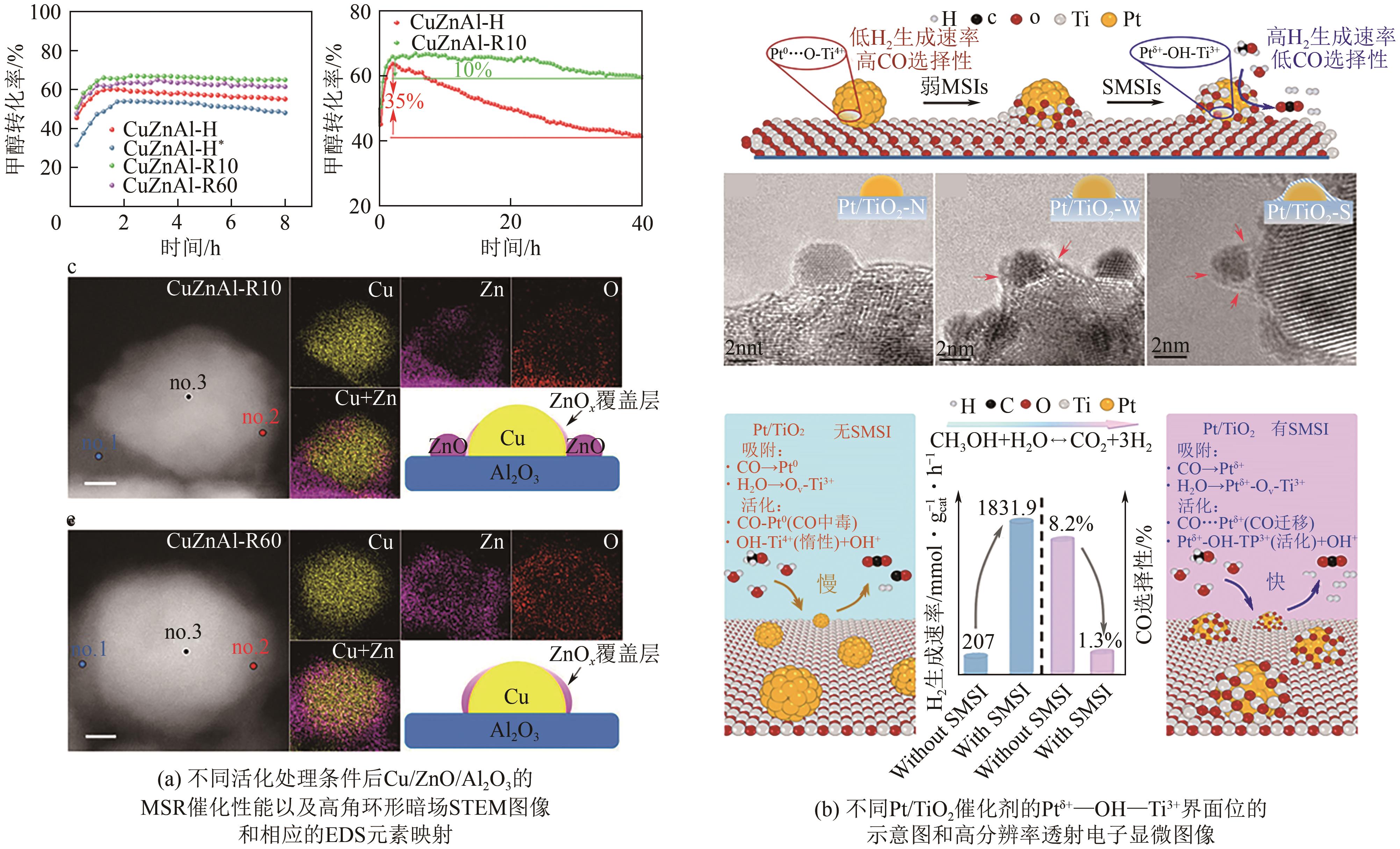
| CuAl2O4 | 300 | 2.3 | 95 | — | 1.0 | — | [ |
| 催化剂 | 反应温度/℃ | 水碳比 | 转化率/% | CO2选择性/% | CO选择性/% | H2生成速率 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 0.2%Pt/α-MoC | 190 | 1 | — | — | 0.14 | 18046 | [ |
| PdO/In2O3 | 260 | 1 | 50 | 93 | — | — | [ |
| CuPd/ZrO2 | 220 | 1.5 | 65 | — | 5 | 86.3mmol·h-1·gcat-1 | [ |
| Pt1/PN-CeO2 | 135 | 1 | — | — | 0.05 | 199mol | [ |
| 2% Ni/α-MoC | 240 | 1 | — | — | 0.7 | 1805mol | [ |
| Pt1/ZnO | 390 | 1.5 | 43 | — | — | — | [ |
| Ru1/CeO2 | 350 | 3 | 26 | 98 | — | 139.6mL | [ |
| CuFe(50∶50) | 350 | 3 | 48 | 0 | 200mmol·kgcat-1·s-1 | [ | |
| Cu-Fe/硅酸盐 | 200 | — | 99 | — | 0 | 1.64μmol·s-1·gcat-1 | [ |
| Cu/Ni/γ-Al2O3 | 400 | 2 | 100 | 83.3 | 11 | — | [ |
| Ni0.2Cu0.8/BN | 320 | 1 | 100 | — | — | 1.8mol | [ |
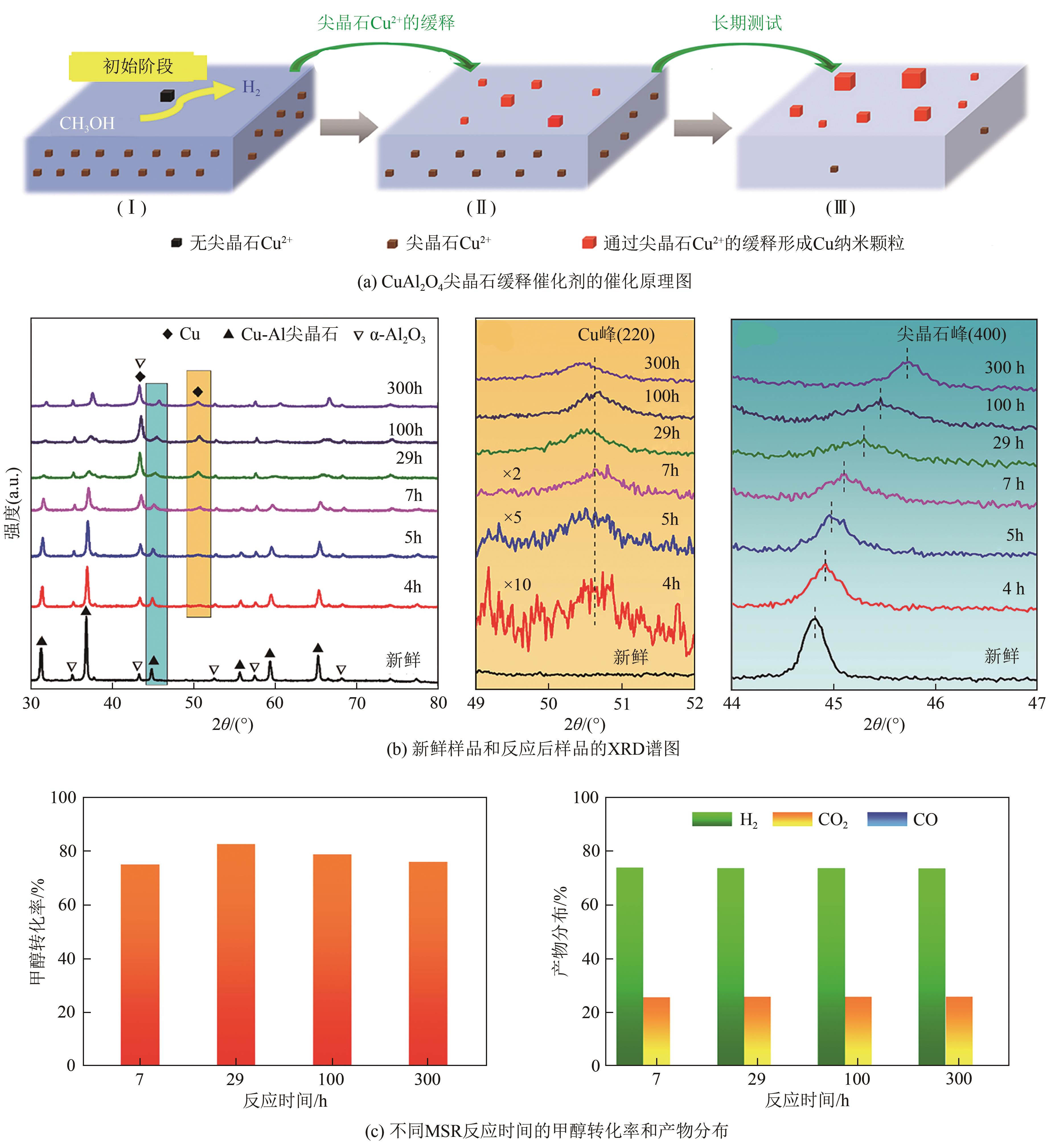
| PtCo/MoS2 | 220 | 3 | — | — | — | 37142mol | [ |
| Cu-Zn/CeAlO3 | 320 | 6 | 98.9 | 96.1 | — | — | [ |
| Cu-In2O3 | 350 | 2 | 84 | — | 5 | 3.8µmol·gCu-1·s-1 | [ |
| Pt/In2O3/Al2O3 | 350 | 4 | 100 | 96.1 | 3.2 | 0.6mol·h-1·gcat-1 | [ |
| Pt/In2O3 | 300 | 1 | — | 99.5 | — | 1500mol | [ |
| In7Pt3 | 400 | 1 | — | 99.2 | — | 6mol·molpt-1·h-1 | [ |
| InPd/In2O3 | 300 | 1 | 26 | 99 | — | 50mmol | [ |
| Pt0.2K@S-1 | 400 | 3 | 45 | — | 1.9 | 61.12mmol | [ |
| 25Cu-AE | 400 | 2 | 100 | 89.3 | 10.4 | 1145mol·kgcat-1·h-1 | [ |
| CuZnO/γ-Al2O3/Al | 225 | 2 | 60 | 90 | — | — | [ |
| Pt/NiAl2O4 | 210 | 16 | 100 | 99.7 | 0.05 | 439.2μmol·min-1·gcat-1 | [ |
| Pd/ZnAl2O4 | 250 | 1.1 | 60 | 97 | 3 | 11.4μmol | [ |
| 30Cu/CeO2 | 200 | 1.3 | 4.5 | 99.5 | — | 0.21mol | [ |
| Cu/Sc2O3-ZnO | 400 | 1.5 | 100 | — | 11 | 140μmol·g-1·s-1 | [ |
| CuZrAl0.4 | 220 | 1.5 | 70 | — | 0.1 | 460.1mmol·gmet-1·h-1 | [ |
| 10Pr-NA | 300 | 1.5 | 95 | — | — | — | [ |
| 18GaCuMg | 200 | 1.5 | 90 | — | 0.4 | — | [ |
| Cu/ZrO2 | 260 | 1.3 | 87 | 100 | — | 260mmol·gcat-1·h-1 | [ |
| Cu/ZrO2 | 270 | 2 | 40 | 99 | — | 14μmol·gCu-1·s-1 | [ |
| Cu/ZnO/Al2O3 | 225 | 1.3 | 67 | — | — | — | [ |
| CuAl2O4 | 320 | 1.5 | 30 | 98.95 | 1.05 | 52mol·min-1·molCu-1 | [ |
| CuHAl-Ac-950 | 255 | 2.3 | 80 | — | 0.4 | — | [ |
| CuNi0.05/Al2O3 | 255 | 2.3 | 90 | — | 0.8 | — | [ |
| 1.7Mg/Cu/Al2O3 | 255 | 2.3 | 96.5 | 96.2 | 3.8 | — | [ |
| CuAl2O4 | 300 | 2.3 | 95 | — | 1.0 | — | [ |
| 1 | FALCONE Pasquale Marcello, HIETE Michael, SAPIO Alessandro. Hydrogen economy and sustainable development goals: Review and policy insights[J]. Current Opinion in Green and Sustainable Chemistry, 2021, 31: 100506. |
| 2 | HOSSEINI Seyed Ehsan, WAHID Mazlan Abdul. Hydrogen from solar energy, a clean energy carrier from a sustainable source of energy[J]. International Journal of Energy Research, 2020, 44(6): 4110-4131. |
| 3 | WANG Qianru, GUO Jianping, CHEN Ping. Recent progress towards mild-condition ammonia synthesis[J]. Journal of Energy Chemistry, 2019, 36: 25-36. |
| 4 | YANG Bing, DING Weilu, ZHANG Honghua, et al. Recent progress in electrochemical synthesis of ammonia from nitrogen: Strategies to improve the catalytic activity and selectivity[J]. Energy & Environmental Science, 2021, 14(2): 672-687. |
| 5 | BEPARI Sujoy, KUILA Debasish. Steam reforming of methanol, ethanol and glycerol over nickel-based catalysts—A review[J]. International Journal of Hydrogen Energy, 2020, 45(36): 18090-18113. |
| 6 | 迟军, 俞红梅. 基于可再生能源的水电解制氢技术[J]. 催化学报, 2018, 39(3): 390-394. |
| CHI Jun, YU Hongmei. Water electrolysis based on renewable energy for hydrogen production[J]. Chinese Journal of Catalysis, 2018, 39(3): 390-394. | |
| 7 | EBERLE Ulrich, FELDERHOFF Michael, Ferdi SCHÜTH. Chemical and physical solutions for hydrogen storage[J]. Angewandte Chemie (International Ed in English), 2009, 48(36): 6608-6630. |
| 8 | VON HELMOLT Rittmar, EBERLE Ulrich. Fuel cell vehicles: Status 2007[J]. Journal of Power Sources, 2007, 165(2): 833-843. |
| 9 | CHEN Luning, HOU Kaipeng, LIU Yisheng, et al. Efficient hydrogen production from methanol using a single-site Pt1/CeO2 catalyst[J]. Journal of the American Chemical Society, 2019, 141(45): 17995-17999. |
| 10 | LIN Lili, ZHOU Wu, GAO Rui, et al. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts[J]. Nature, 2017, 544(7648): 80-83. |
| 11 | NEUMANN Matthias, TESCHNER Detre, Axel KNOP-GERICKE, et al. Controlled synthesis and catalytic properties of supported In-Pd intermetallic compounds[J]. Journal of Catalysis, 2016, 340: 49-59. |
| 12 | Cátia AZENHA, LAGARTEIRA Tiago, Cecilia MATEOS-PEDRERO, et al. Production of hydrogen from methanol steam reforming using CuPd/ZrO2 catalysts-Influence of the catalytic surface on methanol conversion and CO selectivity[J]. International Journal of Hydrogen Energy, 2021, 46(33): 17490-17499. |
| 13 | RANJEKAR Apoorva M, YADAV Ganapati D. Steam reforming of methanol for hydrogen production: A critical analysis of catalysis, processes, and scope[J]. Industrial & Engineering Chemistry Research, 2021, 60(1): 89-113. |
| 14 | SCHUBERT Teresa. Production routes of advanced renewable C1 to C4 alcohols as biofuel components-a review[J]. Biofuels, Bioproducts and Biorefining, 2020, 14(4): 845-878. |
| 15 | MEI Deqing, QIU Xingye, LIU Haiyu, et al. Progress on methanol reforming technologies for highly efficient hydrogen production and applications[J]. International Journal of Hydrogen Energy, 2022, 47(84): 35757-35777. |
| 16 | XU Xinhai, SHUAI Kaipeng, XU Ben. Review on copper and palladium based catalysts for methanol steam reforming to produce hydrogen[J]. Catalysts, 2017, 7(6): 183. |
| 17 | ZHANG Sai, LIU Yuxuan, ZHANG Mingkai, et al. Sustainable production of hydrogen with high purity from methanol and water at low temperatures[J]. Nature Communications, 2022, 13(1): 5527. |
| 18 | LIN Lili, YU Qiaolin, PENG Mi, et al. Atomically dispersed Ni/α-MoC catalyst for hydrogen production from methanol/water[J]. Journal of the American Chemical Society, 2021, 143(1): 309-317. |
| 19 | KANG Jeongmee, SONG Youjung, KIM Taejun, et al. Recent trends in the development of reactor systems for hydrogen production via methanol steam reforming[J]. International Journal of Hydrogen Energy, 2022, 47(6): 3587-3610. |
| 20 | LYTKINA A A, OREKHOVA N V, YAROSLAVTSEV A B. Catalysts for the steam reforming and electrochemical oxidation of methanol[J]. Inorganic Materials, 2018, 54(13): 1315-1329. |
| 21 | LUO Hui, BARRIO Jesús, SUNNY Nixon, et al. Progress and perspectives in photo- and electrochemical-oxidation of biomass for sustainable chemicals and hydrogen production[J]. Advanced Energy Materials, 2021, 11(43): 2101180. |
| 22 | ACURIO CERDA Karen, KATHOL Mark, PUROHIT Gunjan, et al. Cationic lignin as an efficient and biorenewable antimicrobial material[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(28): 10364-10379. |
| 23 | YE Runping, XIAO Shuwei, LAI Qinghua, et al. Advances in enhancing the stability of Cu-based catalysts for methanol reforming[J]. Catalysts, 2022, 12(7): 747. |
| 24 | GU Xiangkui, QIAO Botao, HUANG Chuanqi, et al. Supported single Pt1/Au1 atoms for methanol steam reforming[J]. ACS Catalysis, 2014, 4(11): 3886-3890. |
| 25 | QI Zhiyuan, CHEN Luning, ZHANG Shuchen, et al. Mechanism of methanol decomposition over single-site Pt1/CeO2 catalyst: A DRIFTS study[J]. Journal of the American Chemical Society, 2021, 143(1): 60-64. |
| 26 | CHEN Luning, QI Zhiyuan, PENG Xinxing, et al. Insights into the mechanism of methanol steam reforming tandem reaction over CeO2 supported single-site catalysts[J]. Journal of the American Chemical Society, 2021, 143(31): 12074-12081. |
| 27 | LIU Lichen, CORMA Avelino. Metal catalysts for heterogeneous catalysis: From single atoms to nanoclusters and nanoparticles[J]. Chemical Reviews, 2018, 118(10): 4981-5079. |
| 28 | ZHANG Leilei, ZHOU Maoxiang, WANG Aiqin, et al. Selective hydrogenation over supported metal catalysts: From nanoparticles to single atoms[J]. Chemical Reviews, 2020, 120(2): 683-733. |
| 29 | DONG Chunyang, LI Yinlong, CHENG Danyang, et al. Supported metal clusters: Fabrication and application in heterogeneous catalysis[J]. ACS Catalysis, 2020, 10(19): 11011-11045. |
| 30 | SHARMA Richa, KUMAR Amit, UPADHYAY Rajesh K. Bimetallic Fe-promoted catalyst for CO-free hydrogen production in high-temperature-methanol steam reforming[J]. ChemCatChem, 2019, 11(18): 4568-4580. |
| 31 | KUO Meite, CHEN Yunying, HUNG Weiying, et al. Synthesis of mesoporous Cu-Fe/silicates catalyst for methanol steam reforming[J]. International Journal of Hydrogen Energy, 2019, 44(28): 14416-14423. |
| 32 | FAN Feiyue, ZHANG Qi, WANG Xing, et al. A structured Cu-based/γ-Al2O3/Al plate-type catalyst for steam reforming of dimethyl ether: Self-activation behavior investigation and stability improvement[J]. Fuel, 2016, 186: 11-19. |
| 33 | KOVALSKII Andrey M, MATVEEV Andrei T, POPOV Zakhar I, et al. (Ni, Cu)/hexagonal BN nanohybrids—New efficient catalysts for methanol steam reforming and carbon monoxide oxidation[J]. Chemical Engineering Journal, 2020, 395: 125109. |
| 34 | WANG Ruiyi, LIU Huan, ZHENG Zhanfeng. Low temperature light-assisted hydrogen production from aqueous reforming ethylene glycol over Pt/Al2O3 and Pd/Al2O3 catalysts[J]. Journal of Fuel Chemistry and Technology, 2019, 47(12):1486-1494. |
| 35 | RANJEKAR Apoorva M, YADAV Ganapati D. Hydrogen production by steam reforming of methanol by Cu-Zn/CeAlO3 perovskite[J]. New Journal of Chemistry, 2023, 47(10): 4860-4870. |
| 36 | PLONER Kevin, SCHLICKER Lukas, GILI Albert, et al. Reactive metal-support interaction in the Cu-In2O3 system: Intermetallic compound formation and its consequences for CO2-selective methanol steam reforming[J]. Science and Technology of Advanced Materials, 2019, 20(1): 356-366. |
| 37 | LIU Di, Yong MEN, WANG Jinguo, et al. Highly active and durable Pt/In2O3/Al2O3 catalysts in methanol steam reforming[J]. International Journal of Hydrogen Energy, 2016, 41(47): 21990-21999. |
| 38 | HODGSON A, HAQ S. Water adsorption and the wetting of metal surfaces[J]. Surface Science Reports, 2009, 64(9): 381-451. |
| 39 | PHATAK Abhijit A, Nicholas DELGASS W, RIBEIRO Fabio H, et al. Density functional theory comparison of water dissociation steps on Cu, Au, Ni, Pd, and Pt[J]. The Journal of Physical Chemistry C, 2009, 113(17): 7269-7276. |
| 40 | Nicolas KÖWITSCH, THONI Lukas, KLEMMED Benjamin, et al. Unprecedented catalytic activity and selectivity in methanol steam reforming by reactive transformation of intermetallic In-Pt compounds[J]. The Journal of Physical Chemistry C, 2021, 125(18): 9809-9817. |
| 41 | Nicolas KÖWITSCH, BARTH Stefan, PLONER Kevin, et al. Properties of bulk In-Pt intermetallic compounds in methanol steam reforming[J]. ChemPhysChem, 2022(8):23. |
| 42 | RAMESHAN Christoph, LORENZ Harald, MAYR Lukas, et al. CO2-selective methanol steam reforming on In-doped Pd studied by in situ X-ray photoelectron spectroscopy[J]. Journal of Catalysis, 2012, 295(2/3): 186-194. |
| 43 | HAGHOFER Andreas, FERRI Davide, Karin FÖTTINGER, et al. Who is doing the job? Unraveling the role of Ga2O3 in methanol steam reforming on Pd2Ga/Ga2O3 [J]. ACS Catalysis, 2012, 2(11): 2305-2315. |
| 44 | SHAO Zilong, ZHANG Shunan, LIU Xiaofang, et al. Maximizing the synergistic effect between Pt0 and Pt δ + in a confined Pt-based catalyst for durable hydrogen production[J]. Applied Catalysis B: Environmental, 2022, 316: 121669. |
| 45 | YANG Huanhuan, CHEN Yanyan, CUI Xiaojing, et al. A highly stable copper-based catalyst for clarifying the catalytic roles of Cu0 and Cu+ species in methanol dehydrogenation[J]. Angewandte Chemie, 2018, 130(7): 1854-1858. |
| 46 | MA Kui, TIAN Ye, ZHAO Zhijian, et al. Achieving efficient and robust catalytic reforming on dual-sites of Cu species[J]. Chemical Science, 2019, 10(9): 2578-2584. |
| 47 | ZHANG Guiru, ZHAO Jiali, YANG Taotao, et al. In-situ self-assembled Cu2O/ZnO core-shell catalysts synergistically enhance the durability of methanol steam reforming[J]. Applied Catalysis A: General, 2021, 616: 118072. |
| 48 | CUI Zhonghui, SONG Song, LIU Huibin, et al. Synergistic effect of Cu+ single atoms and Cu nanoparticles supported on alumina boosting water-gas shift reaction[J]. Applied Catalysis B: Environmental, 2022, 313: 121468. |
| 49 | RUANO Daniel, CORED Jorge, Cátia AZENHA, et al. Dynamic structure and subsurface oxygen formation of a working copper catalyst under methanol steam reforming conditions: An in situ time-resolved spectroscopic study[J]. ACS Catalysis, 2019, 9(4): 2922-2930. |
| 50 | MAYR Lukas, Bernhard KlÖTZER, ZEMLYANOV Dmitry, et al. Steering of methanol reforming selectivity by zirconia-copper interaction[J]. Journal of Catalysis, 2015, 321: 123-132. |
| 51 | LI Didi, LI Yi, LIU Xiaohui, et al. NiAl2O4 spinel supported Pt catalyst: High performance and origin in aqueous-phase reforming of methanol[J]. ACS Catalysis, 2019, 9(10): 9671-9682. |
| 52 | WANG Xiuyi, LI Didi, GAO Zirui, et al. The nature of interfacial catalysis over Pt/NiAl2O4 for hydrogen production from methanol reforming reaction[J]. Journal of the American Chemical Society, 2023, 145(2): 905-918. |
| 53 | LIU Liang, LIN Yangjian, HU Yanru, et al. ZnAl2O4 spinel-supported PdZnβ catalyst with parts per million Pd for methanol steam reforming[J]. ACS Catalysis, 2022, 12(4): 2714-2721. |
| 54 | JIN Shiqing, LI Didi, WANG Zhen, et al. Dynamics of the Cu/CeO2 catalyst during methanol steam reforming[J]. Catalysis Science & Technology, 2022, 12(23): 7003-7009. |
| 55 | NING Jing, ZHOU Yan, SHEN Wenjie. Atomically dispersed copper species on ceria for the low-temperature water-gas shift reaction[J]. Science China Chemistry, 2021, 64(7): 1103-1110. |
| 56 | LYKHACH Yaroslava, KOZLOV Sergey M, Tomáš SKÁLA, et al. Counting electrons on supported nanoparticles[J]. Nature Materials, 2016, 15(3): 284-288. |
| 57 | ARAIZA Daniel G, Antonio GÓMEZ-CORTÉS, Gabriela DÍAZ. Reactivity of methanol over copper supported on well-shaped CeO2: A TPD-DRIFTS study[J]. Catalysis Science & Technology, 2017, 7(22): 5224-5235. |
| 58 | CHEN Fei, ZHANG Peipei, ZENG Yan, et al. Vapor-phase low-temperature methanol synthesis from CO2-containing syngas via self-catalysis of methanol and Cu/ZnO catalysts prepared by solid-state method[J]. Applied Catalysis B: Environmental, 2020, 279: 119382. |
| 59 | PLONER Kevin, WATSCHINGER Maximilian, NEZHAD Parastoo Delir Kheyrollahi, et al. Mechanistic insights into the catalytic methanol steam reforming performance of Cu/ZrO2 catalysts by in situ and operando studies[J]. Journal of Catalysis, 2020, 391: 497-512. |
| 60 | PU Yunchuan, LI Shuirong, YAN Shuai, et al. An improved Cu/ZnO catalyst promoted by Sc2O3 for hydrogen production from methanol reforming[J]. Fuel, 2019, 241: 607-615. |
| 61 | MATEOS-PEDRERO C, AZENHA C, D-A Pacheco Tanaka, et al. The influence of the support composition on the physicochemical and catalytic properties of Cu catalysts supported on Zirconia-Alumina for methanol steam reforming[J]. Applied Catalysis B: Environmental, 2020, 277: 119243. |
| 62 | LU Jichang, LEI Yanqiu, WAN Gengping, et al. Weakening the metal-support strong interaction to enhance catalytic performances of alumina supported Ni-based catalysts for producing hydrogen[J]. Applied Catalysis B: Environmental, 2020, 263: 118177. |
| 63 | SUN Zhao, LIU Junpeng, ZHANG Rongjun, et al. Fabricating Ga doped and MgO embedded nanomaterials for sorption-enhanced steam reforming of methanol[J]. Journal of Materials Chemistry A, 2022, 10(13): 7300-7313. |
| 64 | WANG Lucun, LIU Qian, CHEN Miao, et al. Structural evolution and catalytic properties of nanostructured Cu/ZrO2 catalysts prepared by oxalate gel-coprecipitation technique[J]. The Journal of Physical Chemistry C, 2007, 111(44): 16549-16557. |
| 65 | RHODES Michael D, Bell Alexis T. The effects of zirconia morphology on methanol synthesis from CO and H2 over Cu/ZrO2 catalysts: Part I. Steady-state studies[J]. Journal of Catalysis, 2005, 233(1): 198-209. |
| 66 | RHODEs Michael D, Pokrovski Konstantin A, Bell Alexis T. The effects of zirconia morphology on methanol synthesis from CO and H2 over Cu/ZrO2 catalysts: Part Ⅱ. Transient-response infrared studies[J]. Journal of Catalysis, 2005, 233(1): 210-220. |
| 67 | PLONER Kevin, NEZHAD Parastoo Delir Kheyrollahi, GILI Albert, et al. The sol-gel autocombustion as a route towards highly CO2-selective, active and long-term stable Cu/ZrO2 methanol steam reforming catalysts[J]. Materials Chemistry Frontiers, 2021, 5(13): 5093-5105. |
| 68 | Eva-Maria KÖCK, KOGLER Michaela, BIELZ Thomas, et al. In situ FT-IR spectroscopic study of CO2 and CO adsorption on Y2O3, ZrO2, and yttria-stabilized ZrO2 [J]. The Journal of Physical Chemistry C, Nanomaterials and Interfaces, 2013, 117(34): 17666-17673. |
| 69 | Eva-Maria KÖCK, KOGLER Michaela, Bernhard KLÖTZER, et al. Structural and electrochemical properties of physisorbed and chemisorbed water layers on the ceramic oxides Y2O3, YSZ, and ZrO2 [J]. ACS Applied Materials & Interfaces, 2016, 8(25): 16428-16443. |
| 70 | Eva-Maria KÖCK, KOGLER Michaela, Thomas GÖTSCH, et al. Surface chemistry of pure tetragonal ZrO2 and gas-phase dependence of the tetragonal-to-monoclinic ZrO2 transformation[J]. Dalton Transactions, 2017, 46(14): 4554-4570. |
| 71 | HENDERSON Michael A. Complexity in the decomposition of formic acid on the TiO2(110) surface[J]. The Journal of Physical Chemistry B, 1997, 101(2): 221-229. |
| 72 | XU Xinyi, LAN Tian, ZHAO Guofeng, et al. Interface-hydroxyl enabling methanol steam reforming toward CO-free hydrogen production over inverse ZrO2/Cu catalyst[J]. Applied Catalysis B: Environmental, 2023, 334: 122839. |
| 73 | LI Didi, XU Fang, TANG Xuan, et al. Induced activation of the commercial Cu/ZnO/Al2O3 catalyst for the steam reforming of methanol[J]. Nature Catalysis, 2022, 5(2): 99-108. |
| 74 | JIN Shiqing, ZHANG Zekai, LI Didi, et al. Alcohol-induced strong metal-support interactions in a supported copper/ZnO catalyst[J]. Angewandte Chemie International Edition, 2023, 62(21): e202301563. |
| 75 | WANG Hai, HUI Yu, NIU Yiming, et al. Construction of Ptδ+-O(H)-Ti3+ species for efficient catalytic production of hydrogen[J]. ACS Catalysis, 2023, 13(15): 10500-10510. |
| 76 | TABAKOVA T, IDAKIEV V, AVGOUROPOULOS G, et al. Highly active copper catalyst for low-temperature water-gas shift reaction prepared via a Cu-Mn spinel oxide precursor[J]. Applied Catalysis A: General, 2013, 451: 184-191. |
| 77 | KIM Nam Dong, PARK Jae Ryul, PARK Dae Sung, et al. Promoter effect of Pd in CuCr2O4 catalysts on the hydrogenolysis of glycerol to 1, 2-propanediol[J]. Green Chemistry, 2012, 14(9): 2638-2646. |
| 78 | 李光俊, 郗宏娟, 张素红, 等. 尖晶石CuM2O4(M=Al、Fe、Cr)催化甲醇重整反应的特性[J]. 燃料化学学报, 2012, 40(12): 1466-1471. |
| LI Guangjun, XI Hongjuan, ZHANG Suhong, et al. Catalytic characteristics of spinel CuM2O4(M=Al, Fe, Cr) for the steam reforming of methanol[J]. Journal of Fuel Chemistry and Technology, 2012, 40(12): 1466-1471. | |
| 79 | HUANG Yung-Han, WANG Sea-Fue, TSAI An-Pang, et al. Reduction behaviors and catalytic properties for methanol steam reforming of Cu-based spinel compounds CuX2O4 (X=Fe, Mn, Al, La)[J]. Ceramics International, 2014, 40(3): 4541-4551. |
| 80 | LIU Yajie, QIN Fajie, HOU Xiaoning, et al. Effects of ball milling medium on Cu-Al spinel sustained release catalyst for H2 generation from methanol steam reforming[J]. Journal of Fuel Chemistry and Technology, 2023, 51(5): 665-671. |
| 81 | XI Hongjuan, HOU Xiaoning, LIU Yajie, et al. Cu-Al spinel oxide as an efficient catalyst for methanol steam reforming[J]. Angewandte Chemie International Edition, 2014, 53(44): 11886-11889. |
| 82 | LIU Yajie, QING Shaojun, HOU Xiaoning, et al. Temperature dependence of Cu-Al spinel formation and its catalytic performance in methanol steam reforming[J]. Catalysis Science & Technology, 2017, 7(21): 5069-5078. |
| 83 | QING Shaojun, HOU Xiaoning, LIU Yajie, et al. Strategic use of CuAlO2 as a sustained release catalyst for production of hydrogen from methanol steam reforming[J]. Chemical Communications, 2018, 54(86): 12242-12245. |
| 84 | LIU Yajie, QING Shaojun, HOU Xiaoning, et al. Cu-Ni-Al spinel oxide as an efficient durable catalyst for methanol steam reforming[J]. ChemCatChem, 2018, 10(24): 5698-5706. |
| 85 | HOU Xiaoning, QING Shaojun, LIU Yajie, et al. Cu1- x Mg x Al3 spinel solid solution as a sustained release catalyst: One-pot green synthesis and catalytic performance in methanol steam reforming[J]. Fuel, 2021, 284: 119041. |
| 86 | HOU Xiaoning, QING Shaojun, LIU Yajie, et al. Enhancing effect of MgO modification of Cu-Al spinel oxide catalyst for methanol steam reforming[J]. International Journal of Hydrogen Energy, 2020, 45(1): 477-489. |
| 87 | LIU Yajie, KANG Hefei, HOU Xiaoning, et al. Sustained release catalysis: Dynamic copper releasing from stoichiometric spinel CuAl2O4 during methanol steam reforming[J]. Applied Catalysis B: Environmental, 2023, 323: 122043. |
| [1] | LIU Zhentao, MEI Jinlin, WANG Chunya, DUAN Aijun, GONG Yanjun, XU Chunming, WANG Xilong. Development in catalysts for one-step hydrogenation of bio-jet fuels [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 4909-4924. |
| [2] | XIANG Haoyin, CHEN Liangyong. Evaluation of Ni, Ce, Zn and Cu modified Fe2O3/Al2O3 oxygen carriers for methane-fueled chemical looping hydrogen generation process [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4320-4332. |
| [3] | WU Zeliang, GUAN Qihui, CHEN Shixia, WANG Jun. Advances in selective hydrogenation of alkynes to alkenes [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4366-4381. |
| [4] | WANG Yufei, JIA Yu, ZHANG Yisheng, XUE Wei, LI Fang, WANG Yanji. Synthesis of p-aminophenol by transfer hydrogenation of nitrobenzene using formic acid as hydrogen source [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4421-4431. |
| [5] | GONG Decheng, SHEN Qian, ZHU Xianqing, HUANG Yun, XIA Ao, ZHANG Jingmiao, ZHU Xun, LIAO Qiang. Recent progress in the production of hydrogen-rich syngas via supercritical water gasification of microalgae [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3709-3728. |
| [6] | CHEN Liang, LUO Dongmei, WANG Zhenghao, ZHONG Shan, TANG Siyang, LIANG Bin. Research progress of industrial by-product gas-fueled chemical looping hydrogen generation technology [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3729-3746. |
| [7] | WANG Yingjie, ZHU Xinli. Highly dispersed Ni-Cu/SiO2 synthesized by sol-gel method for prompting direct deoxygenation of m-cresol to toluene [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3824-3833. |
| [8] | JIANG Huizhen, LUO Kai, WANG Yan, FEI Hua, WU Dengke, YE Zhuocheng, CAO Xiongjin. Construction and application of waste biomass composite phase change materials [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3934-3945. |
| [9] | SHI Jiating, WANG Hui, PU Kaikai, ZHAO Ting, NIE Lijun, ZHENG Na, GAO Yuhang, XUE Kunkun, SHI Jianhui. Enhanced hydrogen peroxide production performance in visible light from ultra-thin g-C3N4 nanosheets with carbon vacancies [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 4148-4154. |
| [10] | ZHANG Zhen, ZHANG Fan, YUN Zhiting. Carbon reduction and techno-economic analysis of using green hydrogen in chemical and petrochemical industry [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3021-3028. |
| [11] | ZENG Zhuang, LI Kezhi, YUAN Zhiwei, DU Jintao, LI Zhuoshi, WANG Yue. Advances in modified Fischer-Tropsch synthesis catalysts for CO/CO2 hydrogenation to higher alcohols [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3061-3079. |
| [12] | WAN Chengfeng, LI Zhida, ZHANG Chunyue, LU Lu. Highly efficient electrocatalytic water splitting by MXene supported CoP nanorods [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3232-3239. |
| [13] | ZHOU Yuntao, WANG Hongxing, LI Xingang, CUI Lifeng. Application and research progress of CeO2 support in CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2723-2738. |
| [14] | ZHOU Qiuming, NIU Congcong, LYU Shuaishuai, LI Hongwei, WEN Fuli, XU Run, LI Mingfeng. Promoting CO2 hydrogenation to methanol through product transformation and separation [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2776-2785. |
| [15] | LU Xinxin, CAI Dongren, ZHAN Guowu. Research progress in the construction of integrated catalysts based on solid precursors and their application in CO2 hydrogenation [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2786-2802. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||