Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (10): 5369-5380.DOI: 10.16085/j.issn.1000-6613.2023-1527
• Chemical processes and equipment • Previous Articles
Energy integration and carbon flow analysis of process of CO2 chemical transformation to dimethyl carbonate and ethylene glycol
ZONG Huajian1( ), LI Ying1(
), LI Ying1( ), ZHANG Xiangping2
), ZHANG Xiangping2
- 1.School of Environmental and Chemical Engineering, Dalian Jiaotong University, Dalian 116028, Liaoning, China
2.Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China
-
Received:2023-09-01Revised:2023-11-22Online:2024-10-29Published:2024-10-15 -
Contact:LI Ying
CO2化学转化碳酸二甲酯/乙二醇的能量集成和碳流分析
- 1.大连交通大学环境与化学工程学院,辽宁 大连 116028
2.中国科学院过程工程研究所,北京 100190
-
通讯作者:李英 -
作者简介:纵华健(2000—),男,硕士研究生,研究方向为化工过程模拟。E-mail:2422379654@qq.com。 -
基金资助:国家自然科学基金(U22A20416);大连理工大学精细化工国家重点实验室开放课题基金(KF2116)
CLC Number:
Cite this article
ZONG Huajian, LI Ying, ZHANG Xiangping. Energy integration and carbon flow analysis of process of CO2 chemical transformation to dimethyl carbonate and ethylene glycol[J]. Chemical Industry and Engineering Progress, 2024, 43(10): 5369-5380.
纵华健, 李英, 张香平. CO2化学转化碳酸二甲酯/乙二醇的能量集成和碳流分析[J]. 化工进展, 2024, 43(10): 5369-5380.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-1527
| 组分i | 组分j | aij | aji | bij | bji | cij | cji |
|---|---|---|---|---|---|---|---|
| 甲醇 | DMC | -2.0637 | 2.6639 | 642.56 | -1104.8 | 0 | 0 |
| 甲醇 | EC | -0.5409 | 15.892 | 4589.3 | -1278.9 | -2.2771 | -2.0488 |
| 甲醇 | EG | -32.587 | 2.2712 | 10000 | -599.11 | 0 | 0 |
| DMC | EC | 2.5273 | -6.7598 | 167.34 | 442.34 | -0.7321 | 1.0398 |
| DMC | EG | 0 | 0 | -236.13 | -146.88 | 0 | 0 |
| EC | EG | 0 | 0 | -275.66 | -27.96 | 0 | 0 |
| 组分i | 组分j | aij | aji | bij | bji | cij | cji |
|---|---|---|---|---|---|---|---|
| 甲醇 | DMC | -2.0637 | 2.6639 | 642.56 | -1104.8 | 0 | 0 |
| 甲醇 | EC | -0.5409 | 15.892 | 4589.3 | -1278.9 | -2.2771 | -2.0488 |
| 甲醇 | EG | -32.587 | 2.2712 | 10000 | -599.11 | 0 | 0 |
| DMC | EC | 2.5273 | -6.7598 | 167.34 | 442.34 | -0.7321 | 1.0398 |
| DMC | EG | 0 | 0 | -236.13 | -146.88 | 0 | 0 |
| EC | EG | 0 | 0 | -275.66 | -27.96 | 0 | 0 |
| 上下限 | 反应精馏塔 | 高压塔 | 低压塔 | |||
|---|---|---|---|---|---|---|
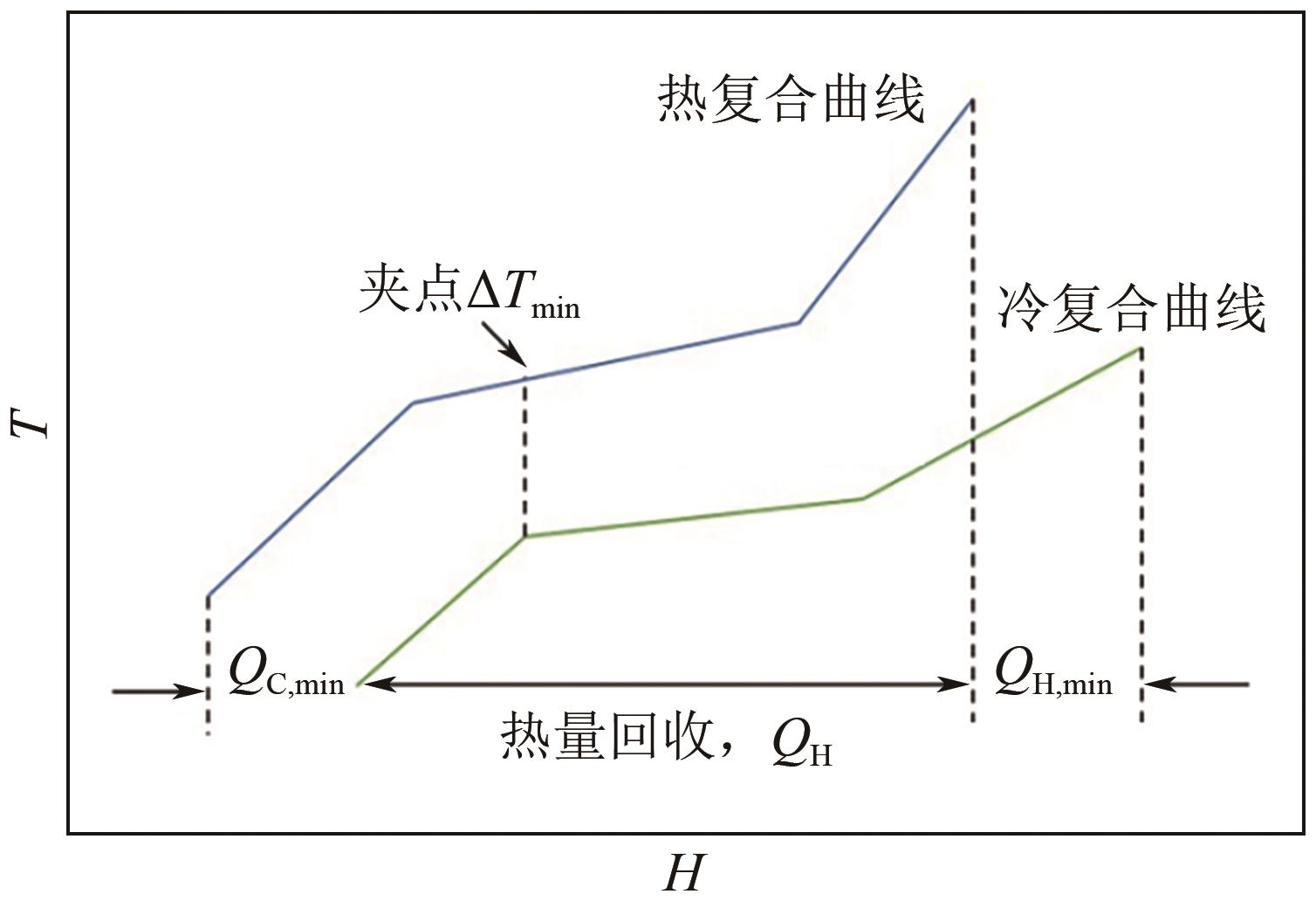
| 压力/bar | 回流比 | 压力/bar | 回流比 | 压力/bar | 回流比 | |
| 上限 | 0.1 | 0.2 | 10 | 0.2 | 1 | 3 |
| 下限 | 0.3 | 1.0 | 20 | 1.2 | 5 | 9 |
| 上下限 | 反应精馏塔 | 高压塔 | 低压塔 | |||
|---|---|---|---|---|---|---|
| 压力/bar | 回流比 | 压力/bar | 回流比 | 压力/bar | 回流比 | |
| 上限 | 0.1 | 0.2 | 10 | 0.2 | 1 | 3 |
| 下限 | 0.3 | 1.0 | 20 | 1.2 | 5 | 9 |
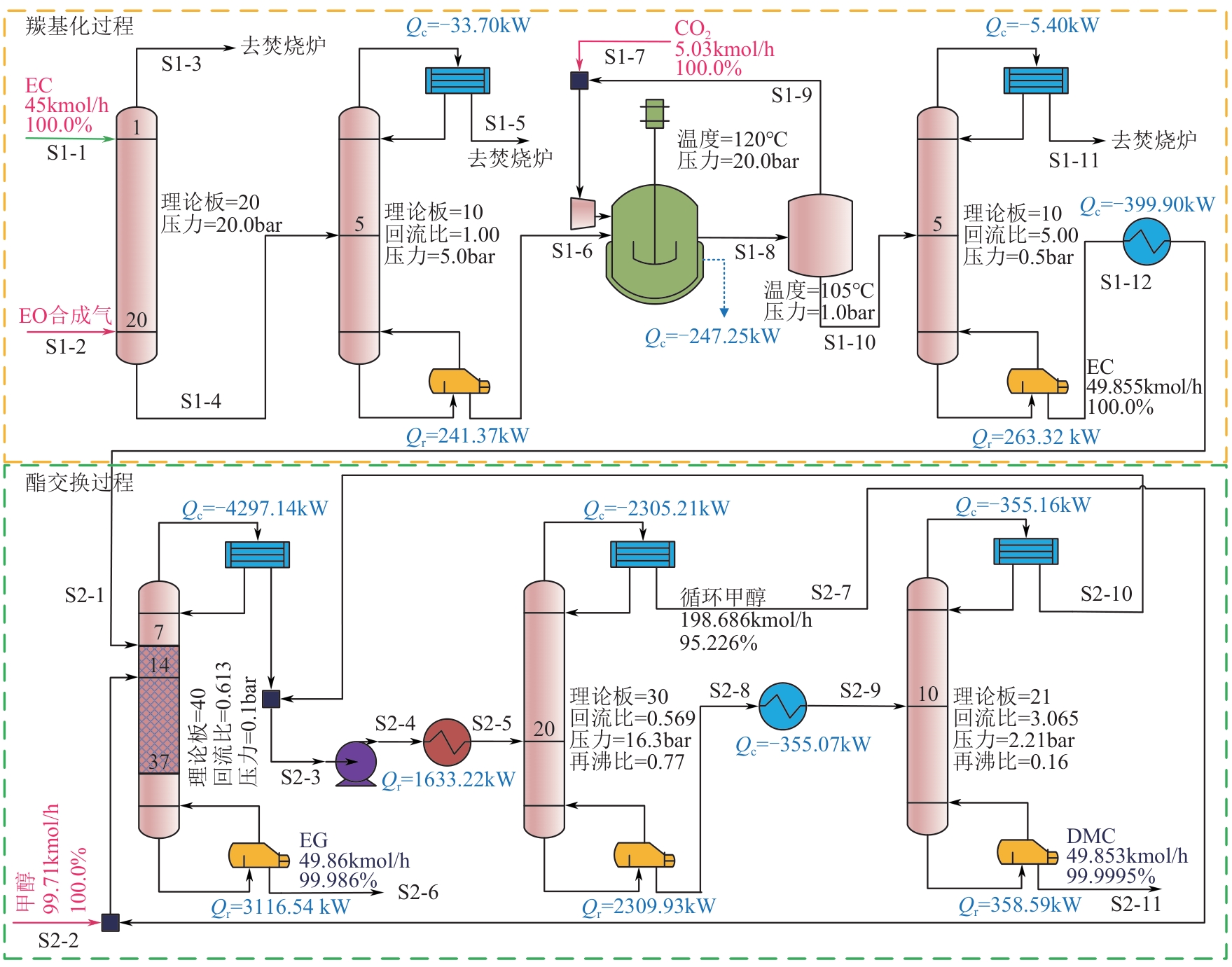
| 流股 | 温度 /℃ | 压力 /bar | 摩尔流量 /kmol·h-1 | 质量流量/kg·h-1 | ||||
|---|---|---|---|---|---|---|---|---|
| EO | CO2 | CH4 | C2H4 | EC | ||||
| S1-1 | 25 | 20 | 45 | 0 | 0 | 0 | 0 | 3962.8 |
| S1-2 | 50 | 20 | 169.5 | 220.3 | 126.1 | 1603.7 | 1729.8 | 0 |
| S1-3 | 39.5 | 20 | 160 | 6.26 | 119.7 | 1584 | 1639.2 | 0.06 |
| S1-4 | 58.1 | 20.2 | 54.47 | 214 | 6.38 | 19.76 | 90.67 | 3962.7 |
| S1-5 | -111.4 | 5 | 4.61 | 0.05 | 6.38 | 19.76 | 90.67 | 0 |
| S1-6 | 171 | 5 | 49.86 | 213.9 | 0 | 0 | 0 | 3962.8 |
| S1-7 | 120 | 20 | 5.03 | 0 | 221.4 | 0 | 0 | 0 |
| S1-8 | 120 | 20 | 54.72 | 0.15 | 212.3 | 0 | 0 | 4393.5 |
| S1-9 | 105 | 0.98 | 4.69 | 0.09 | 204.65 | 0 | 0 | 3.15 |
| S1-10 | 105 | 0.98 | 50.03 | 0.06 | 7.66 | 0 | 0 | 4390.4 |
| S1-11 | -99.5 | 0.5 | 0.17 | 0.06 | 7.66 | 0 | 0 | 0 |
| S1-12 | 219.5 | 0.5 | 49.855 | 0 | 0 | 0 | 0 | 4390.4 |
| 流股 | 温度 /℃ | 压力 /bar | 摩尔流量 /kmol·h-1 | 质量流量/kg·h-1 | ||||
|---|---|---|---|---|---|---|---|---|
| EO | CO2 | CH4 | C2H4 | EC | ||||
| S1-1 | 25 | 20 | 45 | 0 | 0 | 0 | 0 | 3962.8 |
| S1-2 | 50 | 20 | 169.5 | 220.3 | 126.1 | 1603.7 | 1729.8 | 0 |
| S1-3 | 39.5 | 20 | 160 | 6.26 | 119.7 | 1584 | 1639.2 | 0.06 |
| S1-4 | 58.1 | 20.2 | 54.47 | 214 | 6.38 | 19.76 | 90.67 | 3962.7 |
| S1-5 | -111.4 | 5 | 4.61 | 0.05 | 6.38 | 19.76 | 90.67 | 0 |
| S1-6 | 171 | 5 | 49.86 | 213.9 | 0 | 0 | 0 | 3962.8 |
| S1-7 | 120 | 20 | 5.03 | 0 | 221.4 | 0 | 0 | 0 |
| S1-8 | 120 | 20 | 54.72 | 0.15 | 212.3 | 0 | 0 | 4393.5 |
| S1-9 | 105 | 0.98 | 4.69 | 0.09 | 204.65 | 0 | 0 | 3.15 |
| S1-10 | 105 | 0.98 | 50.03 | 0.06 | 7.66 | 0 | 0 | 4390.4 |
| S1-11 | -99.5 | 0.5 | 0.17 | 0.06 | 7.66 | 0 | 0 | 0 |
| S1-12 | 219.5 | 0.5 | 49.855 | 0 | 0 | 0 | 0 | 4390.4 |
| 流股 | 温度 /℃ | 压力 /bar | 摩尔流量 /kmol·h-1 | 质量流量/kg·h-1 | |||
|---|---|---|---|---|---|---|---|
| 甲醇 | EC | DMC | EG | ||||
| S2-1 | 25 | 0.4 | 49.855 | 0 | 4390.4 | 0 | 0 |
| S2-2 | 25 | 0.1 | 99.71 | 3194.9 | 0 | 0 | 0 |
| S2-3 | 19.2 | 1 | 258.04 | 6131.3 | 0 | 6007.1 | 0 |
| S2-4 | 19.9 | 16.4 | 258.04 | 6131.3 | 0 | 6007.1 | 0 |
| S2-5 | 160.6 | 16.3 | 258.04 | 6131.3 | 0 | 6007.1 | 0 |
| S2-6 | 132.2 | 0.1 | 49.86 | 0.16 | 0.18 | 0 | 3094.3 |
| S2-7 | 157.2 | 16.3 | 198.69 | 6062.4 | 0 | 854.5 | 0 |
| S2-8 | 206.1 | 16.3 | 59.35 | 68.8 | 0 | 5152.6 | 0 |
| S2-9 | 112.4 | 2.2 | 59.35 | 68.8 | 0 | 5152.6 | 0 |
| S2-10 | 97.3 | 2.2 | 9.50 | 68.8 | 0 | 662.0 | 0 |
| S2-11 | 116.8 | 2.2 | 49.853 | 0.008 | 0 | 4490.69 | 0 |
| 流股 | 温度 /℃ | 压力 /bar | 摩尔流量 /kmol·h-1 | 质量流量/kg·h-1 | |||
|---|---|---|---|---|---|---|---|
| 甲醇 | EC | DMC | EG | ||||
| S2-1 | 25 | 0.4 | 49.855 | 0 | 4390.4 | 0 | 0 |
| S2-2 | 25 | 0.1 | 99.71 | 3194.9 | 0 | 0 | 0 |
| S2-3 | 19.2 | 1 | 258.04 | 6131.3 | 0 | 6007.1 | 0 |
| S2-4 | 19.9 | 16.4 | 258.04 | 6131.3 | 0 | 6007.1 | 0 |
| S2-5 | 160.6 | 16.3 | 258.04 | 6131.3 | 0 | 6007.1 | 0 |
| S2-6 | 132.2 | 0.1 | 49.86 | 0.16 | 0.18 | 0 | 3094.3 |
| S2-7 | 157.2 | 16.3 | 198.69 | 6062.4 | 0 | 854.5 | 0 |
| S2-8 | 206.1 | 16.3 | 59.35 | 68.8 | 0 | 5152.6 | 0 |
| S2-9 | 112.4 | 2.2 | 59.35 | 68.8 | 0 | 5152.6 | 0 |
| S2-10 | 97.3 | 2.2 | 9.50 | 68.8 | 0 | 662.0 | 0 |
| S2-11 | 116.8 | 2.2 | 49.853 | 0.008 | 0 | 4490.69 | 0 |
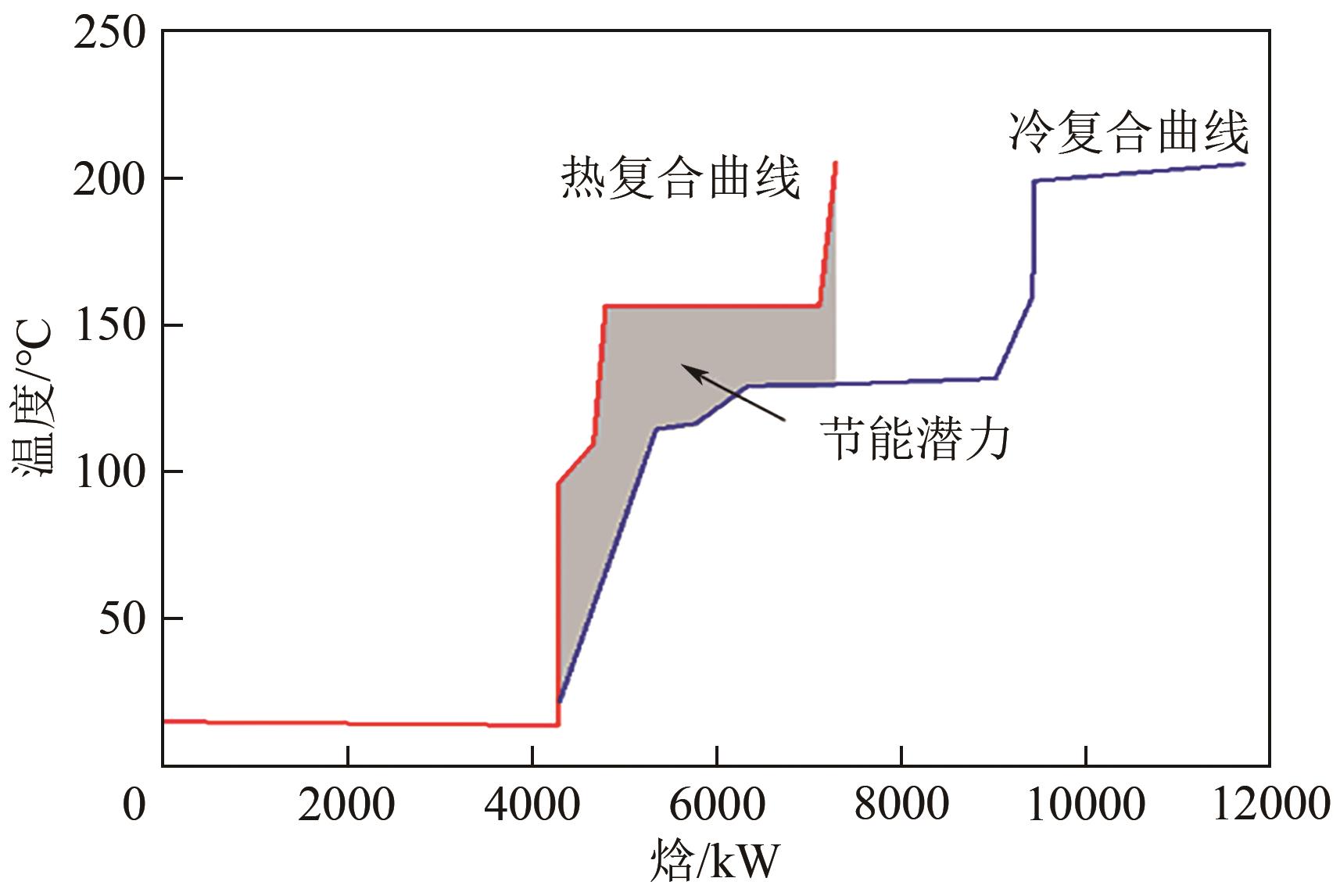
| 项目 | 能量集成前 | 能量集成后 | 对比/% |
|---|---|---|---|
| 热公用工程/kW | 7418.4 | 4425.9 | -40.34 |
| 冷公用工程/kW | 7292.7 | 4297.2 | -41.07 |
| 总换热面积/m2 | 268.74 | 322.08 | 19.85 |
| 总投资成本/103USD | 177.07 | 226.05 | 27.66 |
| 加热操作费/103USD·a-1 | 536.89 | 329.77 | -38.58 |
| 冷却操作费/103USD·a-1 | 562.47 | 542.51 | -3.54 |
| 年度总成本/103USD·a-1 | 1146.10 | 927.29 | -19.09 |
| 项目 | 能量集成前 | 能量集成后 | 对比/% |
|---|---|---|---|
| 热公用工程/kW | 7418.4 | 4425.9 | -40.34 |
| 冷公用工程/kW | 7292.7 | 4297.2 | -41.07 |
| 总换热面积/m2 | 268.74 | 322.08 | 19.85 |
| 总投资成本/103USD | 177.07 | 226.05 | 27.66 |
| 加热操作费/103USD·a-1 | 536.89 | 329.77 | -38.58 |
| 冷却操作费/103USD·a-1 | 562.47 | 542.51 | -3.54 |
| 年度总成本/103USD·a-1 | 1146.10 | 927.29 | -19.09 |
| 工艺 | DMC纯度/% | EG纯度/% | 热量消耗/kW·h·(kg DMC)-1 | 参考文献 | |
|---|---|---|---|---|---|
| 集成前 | 集成后 | ||||
| EC路线 | 99.16 (99.2) | 99.99 (99.9) | 2.54 | — | [ |
| 尿素路线 | 99.74 (99.7) | — | 16.49 | — | [ |
| BAYER法 | 100.0 (100.0) | — | 2.93 | — | [ |
| 酯交换-变压精馏 | 100.0 | 99.82 | 2.44 | 1.25 | [ |
| 酯交换-变压精馏 | 99.99 | 99.80 | 11.08 | — | [ |
| 酯交换-苯酚萃取精馏 | 99.80 | 99.80 | 4.34 | — | [ |
| 酯交换-苯胺萃取精馏 | 99.50 | 99.50 | 2.78 | — | [ |
| 酯交换-苯胺萃取精馏 | 99.50 | 99.50 | — | 1.85 | [ |
| 本文 | 99.999 | 99.986 | 1.76 | 1.10 | 模拟 |
| 工艺 | DMC纯度/% | EG纯度/% | 热量消耗/kW·h·(kg DMC)-1 | 参考文献 | |
|---|---|---|---|---|---|
| 集成前 | 集成后 | ||||
| EC路线 | 99.16 (99.2) | 99.99 (99.9) | 2.54 | — | [ |
| 尿素路线 | 99.74 (99.7) | — | 16.49 | — | [ |
| BAYER法 | 100.0 (100.0) | — | 2.93 | — | [ |
| 酯交换-变压精馏 | 100.0 | 99.82 | 2.44 | 1.25 | [ |
| 酯交换-变压精馏 | 99.99 | 99.80 | 11.08 | — | [ |
| 酯交换-苯酚萃取精馏 | 99.80 | 99.80 | 4.34 | — | [ |
| 酯交换-苯胺萃取精馏 | 99.50 | 99.50 | 2.78 | — | [ |
| 酯交换-苯胺萃取精馏 | 99.50 | 99.50 | — | 1.85 | [ |
| 本文 | 99.999 | 99.986 | 1.76 | 1.10 | 模拟 |
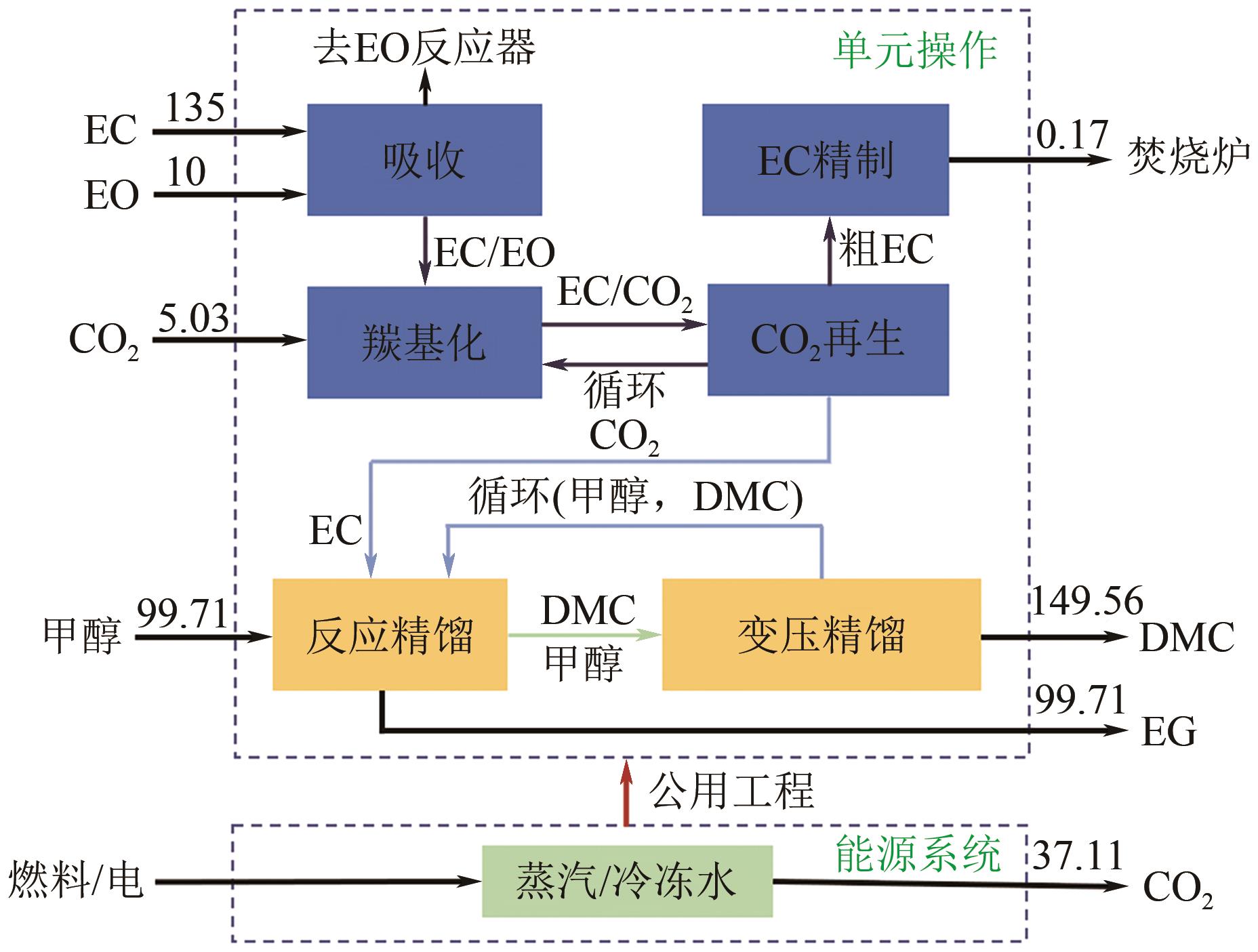
| 工艺 | 净CO2排放/kg CO2·(kg DMC)-1 | 参考文献 |
|---|---|---|
| EC路线 | 0.45 | [ |
| 尿素路线 | 1.28 | [ |
| 膜生物反应器 | 0.52 | [ |
| 渗透汽化 | 0.67 | [ |
| 脱水反应精馏 | 0.71 | [ |
| CO2与MeOH直接合成 | 0.34 | [ |
| 本文 | 0.31 | 模拟 |
| 工艺 | 净CO2排放/kg CO2·(kg DMC)-1 | 参考文献 |
|---|---|---|
| EC路线 | 0.45 | [ |
| 尿素路线 | 1.28 | [ |
| 膜生物反应器 | 0.52 | [ |
| 渗透汽化 | 0.67 | [ |
| 脱水反应精馏 | 0.71 | [ |
| CO2与MeOH直接合成 | 0.34 | [ |
| 本文 | 0.31 | 模拟 |
| 1 | 张凡, 王树众, 李艳辉, 等. 中国制造业碳排放问题分析与减排对策建议[J]. 化工进展, 2022, 41(3): 1645-1653. |
| ZHANG Fan, WANG Shuzhong, LI Yanhui, et al. Analysis of CO2 emission and countermeasures and suggestions for emission reduction in Chinese manufacturing[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1645-1653. | |
| 2 | 巩金龙. CO2化学转化研究进展概述[J]. 化工学报, 2017, 68(4): 1282-1285. |
| GONG Jinlong. A brief overview on recent progress on chemical conversion of CO2 [J]. CIESC Journal, 2017, 68(4): 1282-1285. | |
| 3 | Hrvoje MIKULČIĆ, RIDJAN SKOV Iva, DOMINKOVIĆ Dominik Franjo, et al. Flexible Carbon Capture and Utilization technologies in future energy systems and the utilization pathways of captured CO2 [J]. Renewable and Sustainable Energy Reviews, 2019, 114: 109338. |
| 4 | 姚向阳, 张彦, 高嵩, 等. 面向可持续发展的二氧化碳化学研究进展[J]. 华中师范大学学报(自然科学版), 2019, 53(6): 834-846. |
| YAO Xiangyang, ZHANG Yan, GAO Song, et al. Progress on carbon dioxide chemistry towards sustainable development[J]. Journal of Central China Normal University (Natural Sciences), 2019, 53(6): 834-846. | |
| 5 | YANG Na, WANG Rui. Sustainable technologies for the reclamation of greenhouse gas CO2 [J]. Journal of Cleaner Production, 2015, 103: 784-792. |
| 6 | FIORANI G, PEROSA A, SELVA M. Dimethyl carbonate: A versatile reagent for a sustainable valorization of renewables[J]. Green Chemistry, 2018, 20(2): 288-322. |
| 7 | XU Baohua, WANG Jinquan, SUN Jian, et al. Fixation of CO2 into cyclic carbonates catalyzed by ionic liquids: A multi-scale approach[J]. Green Chemistry, 2015, 17(1): 108-122. |
| 8 | TUNDO P, MUSOLINO M, ARICÒ F. The reactions of dimethyl carbonate and its derivatives[J]. Green Chemistry, 2018, 20(1): 28-85. |
| 9 | SONG Qingwen, ZHOU Zhihua, HE Liangnian. Efficient, selective and sustainable catalysis of carbon dioxide[J]. Green Chemistry, 2017, 19(16): 3707-3728. |
| 10 | ZHANG Suojiang, SUN Jian, ZHANG Xiaochun, et al. Ionic liquid-based green processes for energy production[J]. Chemical Society Reviews, 2014, 43(22): 7838-7869. |
| 11 | PETER Goodrich, NIMAL Gunaratne H Q, JOHAN Jacquemin, et al. Sustainable cyclic carbonate production, utilizing carbon dioxide and azolate ionic liquids[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(7): 5635-5641. |
| 12 | YANG Chaokun, LIU Mengshuai, ZHANG Jiaxu, et al. Facile synthesis of DBU-based ionic liquids cooperated with ZnI2 as catalysts for efficient cycloaddition of CO2 to epoxides under mild and solvent-free conditions[J]. Molecular Catalysis, 2018, 450: 39-45. |
| 13 | LIU Mengshuai, LI Xin, LIANG Lin, et al. Protonated triethanolamine as multi-hydrogen bond donors catalyst for efficient cycloaddition of CO2 to epoxides under mild and cocatalyst-free conditions[J]. Journal of CO2 Utilization, 2016, 16: 384-390. |
| 14 | WANG Lin, ZHANG Guangyou, KODAMA Koichi, et al. An efficient metal- and solvent-free organocatalytic system for chemical fixation of CO2 into cyclic carbonates under mild conditions[J]. Green Chemistry, 2016, 18(5): 1229-1233. |
| 15 | 郭艳东, 佟佳欢, 刘晓敏, 等. 负载型离子液体的研究进展及发展趋势[J]. 中国科学(化学), 2016, 46(12): 1305-1316. |
| GUO Yandong, TONG Jiahuan, LIU Xiaomin, et al. Recent advances and development of supported ionic liquids[J]. SCIENTIA SINICA Chimica, 2016, 46(12): 1305-1316. | |
| 16 | CHENG Weiguo, CHEN Xi, SUN Jian, et al. SBA-15 supported triazolium-based ionic liquids as highly efficient and recyclable catalysts for fixation of CO2 with epoxides[J]. Catalysis Today, 2013, 200: 117-124. |
| 17 | 贺玥玥, 顾晓华, 潘子鹤, 等. KF/MgO催化醇解制备碳酸二甲酯的超声强化研究[J]. 石油化工, 2019, 48(10): 996-1000. |
| HE Yueyue, GU Xiaohua, PAN Zihe, et al. Enhancement of ultrasonic on preparation of dimethyl carbonate through alcoholysis catalyzed by KF/MgO[J]. Petrochemical Technology, 2019, 48(10): 996-1000. | |
| 18 | WANG San-Jang, YU Cheng-Ching, HUANG Hsiao-Ping. Plant-wide design and control of DMC synthesis process via reactive distillation and thermally coupled extractive distillation[J]. Computers & Chemical Engineering, 2010, 34(3): 361-373. |
| 19 | 董营, 肖颖, 黄耀东, 等. 萃取精馏分离碳酸二甲酯-乙醇二元共沸物[J]. 化工进展, 2013, 32(4): 750-756, 768. |
| DONG Ying, XIAO Ying, HUANG Yaodong, et al. Separation of binary azeotrope ethanol-dimethyl carbonate by extractive distillation[J]. Chemical Industry and Engineering Progress, 2013, 32(4): 750-756, 768. | |
| 20 | 李文秀, 连利燕, 张志刚, 等. 萃取精馏分离碳酸二甲酯和甲醇共沸物[J]. 化学工程, 2012, 40(7): 14-17, 25. |
| LI Wenxiu, LIAN Liyan, ZHANG Zhigang, et al. Separation of dimethyl carbonate-methanol mixture by extractive distillation[J]. Chemical Engineering, 2012, 40(7): 14-17, 25. | |
| 21 | HU Chi-Chih, CHENG Shueh-Hen. Development of alternative methanol/dimethyl carbonate separation systems by extractive distillation—A holistic approach[J]. Chemical Engineering Research and Design, 2017, 127: 189-214. |
| 22 | HSU Kai-Yi, HSIAO Yuan-Chang, I-Lung CHIEN. Design and control of dimethyl carbonate-methanol separation via extractive distillation in the dimethyl carbonate reactive-distillation process[J]. Industrial & Engineering Chemistry Research, 2010, 49(2): 735-749. |
| 23 | SHI Li, WANG San-Jang, WONG David Shan-Hill, et al. Novel process design of synthesizing propylene carbonate for dimethyl carbonate production by indirect alcoholysis of urea[J]. Industrial & Engineering Chemistry Research, 2017, 56(40): 11531-11544. |
| 24 | ZHANG Qingrui, PENG Jiayao, ZHANG Kai. Separation of an azeotropic mixture of dimethyl carbonate and methanol via partial heat integration pressure swing distillation[J]. Asia-Pacific Journal of Chemical Engineering, 2017, 12(1): 50-64. |
| 25 | GU Xincheng, ZHANG Xiaochun, YANG Zifeng, et al. Technical-environmental assessment of CO2 conversion process to dimethyl carbonate/ethylene glycol[J]. Journal of Cleaner Production, 2021, 288: 125598. |
| 26 | Danielle BALLIVET-TKATCHENKO, CHAMBREY Stéphane, KEISKI Riitta, et al. Direct synthesis of dimethyl carbonate with supercritical carbon dioxide: Characterization of a key organotin oxide intermediate[J]. Catalysis Today, 2006, 115(1/2/3/4): 80-87. |
| 27 | Ondřej VOPIČKA, Kryštof PILNÁČEK, FRIESS Karel. Separation of methanol-dimethyl carbonate vapour mixtures with PDMS and PTMSP membranes[J]. Separation and Purification Technology, 2017, 174: 1-11. |
| 28 | LI Qing, KISS Anton A. Novel pervaporation-assisted pressure swing reactive distillation process for intensified synthesis of dimethyl carbonate[J]. Chemical Engineering and Processing: Process Intensification, 2021, 162: 108358. |
| 29 | SOUZA Lorena F S, FERREIRA Priscila R R, DE MEDEIROS José Luiz, et al. Production of DMC from CO2 via indirect route: Technical-economical-environmental assessment and analysis[J]. ACS Sustainable Chemistry & Engineering, 2014, 2(1): 62-69. |
| 30 | KONGPANNA Pichayapan, PAVARAJARN Varong, GANI Rafiqul, et al. Techno-economic evaluation of different CO2-based processes for dimethyl carbonate production[J]. Chemical Engineering Research and Design, 2015, 93: 496-510. |
| 31 | YU Bor-Yih, CHEN Mengkai, I-Lung CHIEN. Assessment on CO2 utilization through rigorous simulation: Converting CO2 to dimethyl carbonate[J]. Industrial & Engineering Chemistry Research, 2018, 57(2): 639-652. |
| 32 | 吴青海, 任天瑞. 固载化离子液体催化环氧乙烷和二氧化碳合成碳酸乙烯酯[J]. 过程工程学报, 2012, 12(2): 302-309. |
| WU Qinghai, REN Tianrui. Synthesis of ethylene carbonate via carbon dioxide and ethylene oxide catalyzed by immobilized ionic liquid[J]. The Chinese Journal of Process Engineering, 2012, 12(2): 302-309. | |
| 33 | HE Yueyue, CHENG Huaigang, PAN Zihe, et al. Ultrasonic process intensification during the preparation of dimethyl carbonate based on the alcoholysis of ethylene carbonate and the kinetic behavior of dimethyl carbonate[J]. Reaction Chemistry & Engineering, 2021, 6(11): 2170-2180. |
| 34 | FANG Yunjin, XIAO Wende. Experimental and modeling studies on a homogeneous reactive distillation system for dimethyl carbonate synthesis by transesterification[J]. Separation and Purification Technology, 2004, 34(1/2/3): 255-263. |
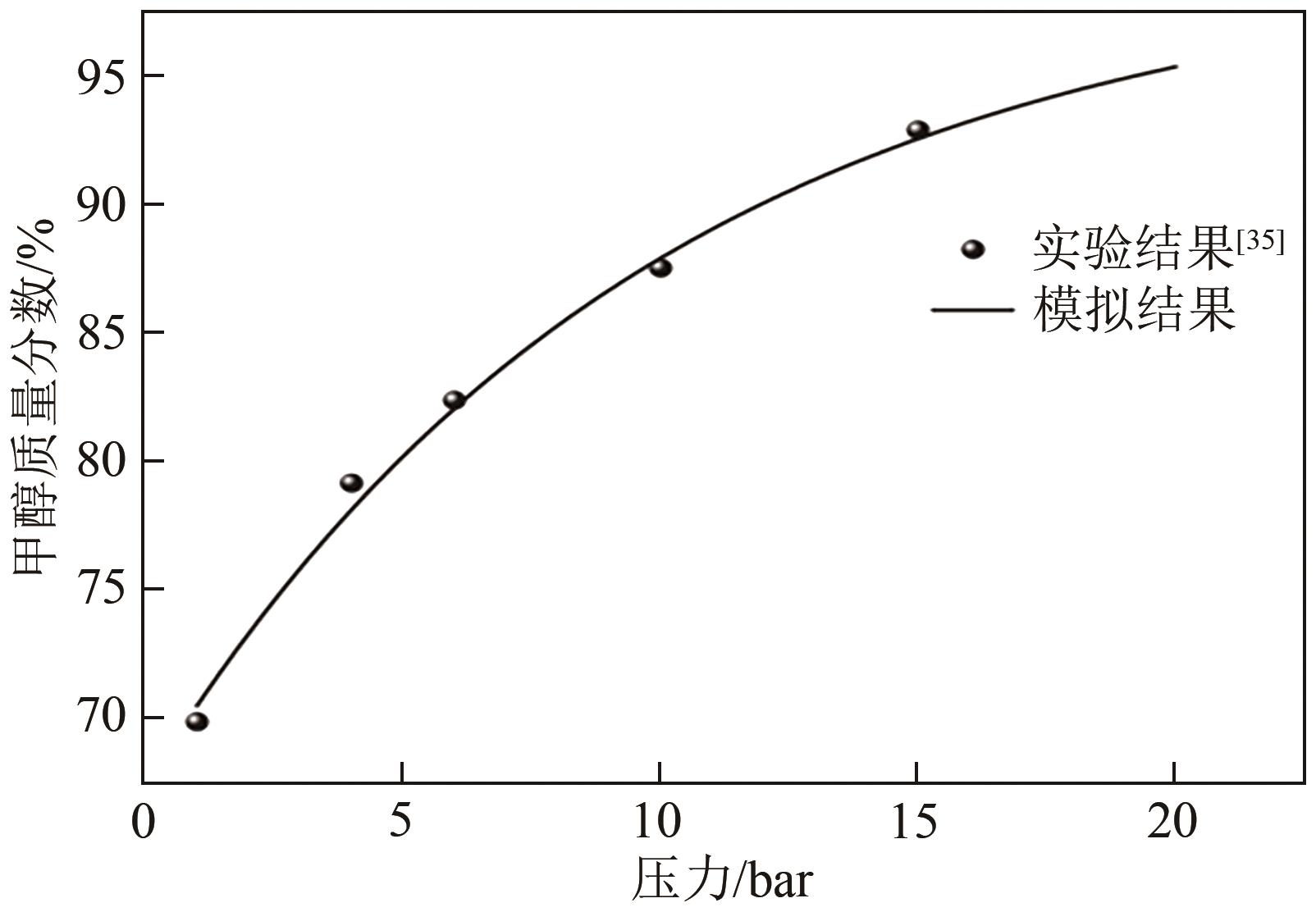
| 35 | 陈嵩嵩, 董丽, 张军平, 等. 酯交换法制备碳酸二甲酯过程模拟与系统㶲分析[J]. 过程工程学报, 2018, 18(6): 1307-1314. |
| CHEN Songsong, DONG Li, ZHANG Junping, et al. Process simulation and system exergy analysis for dimethyl carbonate production with transesterification[J]. The Chinese Journal of Process Engineering, 2018, 18(6): 1307-1314. | |
| 36 | 张莘, 高伟, 齐鸣, 等. 基于多目标优化精馏系统综述[J]. 化工进展, 2019, 38(S1): 1-9. |
| ZHANG Shen, GAO Wei, QI Ming, et al. A review of optimization rectification systems based on multi-objective[J]. Chemical Industry and Engineering Progress, 2019, 38(S1): 1-9. | |
| 37 | 翟建, 刘育良, 李鲁闽, 等. 萃取精馏分离苯/环己烷共沸体系模拟与优化[J]. 化工学报, 2015, 66(9): 3570-3579. |
| ZHAI Jian, LIU Yuliang, LI Lumin, et al. Simulation and optimization of extractive distillation for separation of azeotropic benzene/cyclohexane system[J]. CIESC Journal, 2015, 66(9): 3570-3579. | |
| 38 | 李帅. 基于NSGA-Ⅱ的蒸汽动力系统经济性与环境影响优化[D]. 大连: 大连理工大学, 2019. |
| LI Shuai. Optimization of economic and environmental impact for steam power plant based on NSGA-Ⅱ[D]. Dalian: Dalian University of Technology, 2019. | |
| 39 | 葛玉林. 常减压蒸馏流程模拟与优化及换热网络综合[D]. 大连: 大连理工大学, 2007. |
| GE Yulin. Flow simulation, optimization, and heat exchangers network synthesis of atmosphere-vacuum distillation[D]. Dalian: Dalian University of Technology, 2007. | |
| 40 | GADALLA M, OLUJIĆ Ž, DE RIJKE A, et al. Reducing CO2 emissions of internally heat-integrated distillation columns for separation of close boiling mixtures[J]. Energy, 2005, 31(13): 2409-2417. |
| 41 | LEE Tzong-Shing, LU Wanchen. An evaluation of empirically-based models for predicting energy performance of vapor-compression water chillers[J]. Applied Energy, 2010, 87(11): 3486-3493. |
| 42 | SEIDER W, SEADER J, LEWIN D. Product & process design principles-synthesis, analysis, and evaluation[M]. USA: John Wiley and Sons, Inc., 2006: 565-567. |
| 43 | MRAYED Sabri, SHAMS Mohamed BIN, Mohammed AL-KHAYYAT, et al. Application of pinch analysis to improve the heat integration efficiency in a crude distillation unit[J]. Cleaner Engineering and Technology, 2021, 4: 100168. |
| 44 | KONGPANNA Pichayapan, BABI Deenesh K, PAVARAJARN Varong, et al. Systematic methods and tools for design of sustainable chemical processes for CO2 utilization[J]. Computers & Chemical Engineering, 2016, 87: 125-144. |
| 45 | WU Tsai-Wei, I-Lung CHIEN. CO2 utilization feasibility study: Dimethyl carbonate direct synthesis process with dehydration reactive distillation[J]. Industrial & Engineering Chemistry Research, 2020, 59(3): 1234-1248. |
| 46 | OHNO Hajime, IKHLAYEL Mahdi, TAMURA Masazumi, et al. Direct dimethyl carbonate synthesis from CO2 and methanol catalyzed by CeO2 and assisted by 2-cyanopyridine: A cradle-to-gate greenhouse gas emission study[J]. Green Chemistry, 2021, 23(1): 457-469. |
| [1] | WANG Yanan, LIU Linlin, ZHUANG Yu, DU Jian. Synchronous optimization and heat integration of the production process from EO to EG based on surrogate model [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5234-5241. |
| [2] | HAN Wei, HAN Hengwen, CHENG Wei, TANG Weijian. Research progress of biomass fuels technology driven by carbon neutrality [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2463-2474. |
| [3] | LI Ping, CHEN Xiule, ZHANG Qiang, NIAN Tengfei, WANG Yuxing, WANG Meng. Optimization of compounding ratio of fume-suppressing asphalt and evaluation of its effect of fume suppression [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1923-1933. |
| [4] | ZHANG Shuming, LIU Huazhang. Optimization of Fe1-x O ammonia synthesis catalyst by BP neural network model [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1302-1308. |
| [5] | SUN Yuyu, CAI Xinlei, TANG Jihai, HUANG Jingjing, HUANG Yiping, LIU Jie. Optimization and energy-saving of a reactive distillation process for the synthesis of methyl methacrylate [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 56-63. |
| [6] | WANG Junjie, PAN Yanqiu, NIU Yabin, YU Lu. Molecular level catalytic reforming model construction and application [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3404-3412. |
| [7] | LIN Hai, WANG Yufei. Distributed wind farm layout optimization considering noise constraint [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3394-3403. |
| [8] | LING Shan, LIU Juming, ZHANG Qiancheng, LI Yan. Research progress on simulated moving bed separation process and its optimization methods [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2233-2244. |
| [9] | DAI Min, YANG Fusheng, ZHANG Zaoxiao, LIU Guilian, FENG Xiao. 3E Multi-objective optimization of hexane oil distillation process based on multi-strategy ensemble optimization algorithm [J]. Chemical Industry and Engineering Progress, 2022, 41(6): 2852-2863. |
| [10] | HAN Wentao, HAN Zhenwei, LI Hong, GAO Xin, LI Xingang. Simulation of reactive distillation for the synthesis of ethyl levulinate and energy saving optimization of dividing wall column [J]. Chemical Industry and Engineering Progress, 2022, 41(4): 1759-1769. |
| [11] | CHEN Danyang, ZHU Jianyu, WU Qin, WANG Ziqing, ZHANG Jinli. KF/MgO catalyzed transesterification of glycerol and dimethyl carbonate to glycerol carbonate [J]. Chemical Industry and Engineering Progress, 2022, 41(4): 2082-2089. |
| [12] | ZHU Changhui, ZHU Wenchao, LUO Jia, TIAN Baohe, SUN Jialin, ZOU Zhiyun. Recent advances in microwave-intensified transesterification for biodiesel preparation [J]. Chemical Industry and Engineering Progress, 2022, 41(10): 5145-5154. |
| [13] | LI Dan, YANG Siyu, QIAN Yu. Syngas cryogenic separation process combined with lithium bromide absorption refrigeration and organic Rankine cycle [J]. Chemical Industry and Engineering Progress, 2022, 41(10): 5236-5246. |
| [14] | YUE Qianqian, GAO Lijing, XIAO Guomin, WEI Ruiping, LEI Yan. Process of the reactor and progress of biodiesel continuous production [J]. Chemical Industry and Engineering Progress, 2021, 40(S2): 81-88. |
| [15] | LAI Jianing, GAO Xin, CONG Haifeng, LI Hong, LI Xingang. Simulation and optimization of synthesizing solketal by reactive distillation process [J]. Chemical Industry and Engineering Progress, 2021, 40(7): 3584-3590. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||