Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (9): 5122-5131.DOI: 10.16085/j.issn.1000-6613.2021-2435
• Resources and environmental engineering • Previous Articles Next Articles
Comprehensive recovery of cerium and manganese from waste CeO x -MnO x -based SCR denitrification catalysts by reductive acid leaching
YU Zhengwei1,2,3( ), ZHANG Xiaoxia2, LEI Jie2, LI Ao2, WANG Guangying4, DING Xiang1, LONG Hongming1,2(
), ZHANG Xiaoxia2, LEI Jie2, LI Ao2, WANG Guangying4, DING Xiang1, LONG Hongming1,2( )
)
- 1.Key Laboratory of Metallurgical Emission Reduction & Resources Recycling (Anhui University of Technology), Ministry of Education, Maanshan 243002, Anhui, China
2.School of Metallurgical Engineering, Anhui University of Technology, Maanshan 243002, Anhui, China
3.Energy Saving and Pollution Control in Metallurgical Process Engineering Technology Research Center of Education Department of Anhui Province, Maanshan 243002, Anhui, China
4.Anhui Yuanchen Environmental Protection Technology Company Limited, Hefei 230012, Anhui, China
-
Received:2021-11-26Revised:2022-04-12Online:2022-09-27Published:2022-09-25 -
Contact:LONG Hongming
废CeO x -MnO x 基SCR脱硝催化剂还原酸浸综合回收铈锰
余正伟1,2,3( ), 张晓霞2, 雷杰2, 李澳2, 王光应4, 丁祥1, 龙红明1,2(
), 张晓霞2, 雷杰2, 李澳2, 王光应4, 丁祥1, 龙红明1,2( )
)
- 1.冶金减排与资源综合利用教育部重点实验室(安徽工业大学),安徽 马鞍山 243002
2.安徽工业大学冶金工程学院,安徽 马鞍山 243002
3.冶金过程节能与污染控制安徽省教育厅工程技术研究中心,安徽 马鞍山 243002
4.安徽元琛环保科技股份有限公司,安徽 合肥 230012
-
通讯作者:龙红明 -
作者简介:余正伟(1984—),男,博士,讲师,研究方向为固废资源化利用。E-mail:yuzhengwei@ahut.edu.cn。 -
基金资助:国家自然科学基金(51704009);冶金减排与资源综合利用教育部重点实验室开放基金(JKF21-07);冶金过程节能与污染控制安徽省教育厅工程技术研究中心开放基金(GKF20-2)
CLC Number:
Cite this article
YU Zhengwei, ZHANG Xiaoxia, LEI Jie, LI Ao, WANG Guangying, DING Xiang, LONG Hongming. Comprehensive recovery of cerium and manganese from waste CeO x -MnO x -based SCR denitrification catalysts by reductive acid leaching[J]. Chemical Industry and Engineering Progress, 2022, 41(9): 5122-5131.
余正伟, 张晓霞, 雷杰, 李澳, 王光应, 丁祥, 龙红明. 废CeO x -MnO x 基SCR脱硝催化剂还原酸浸综合回收铈锰[J]. 化工进展, 2022, 41(9): 5122-5131.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2021-2435
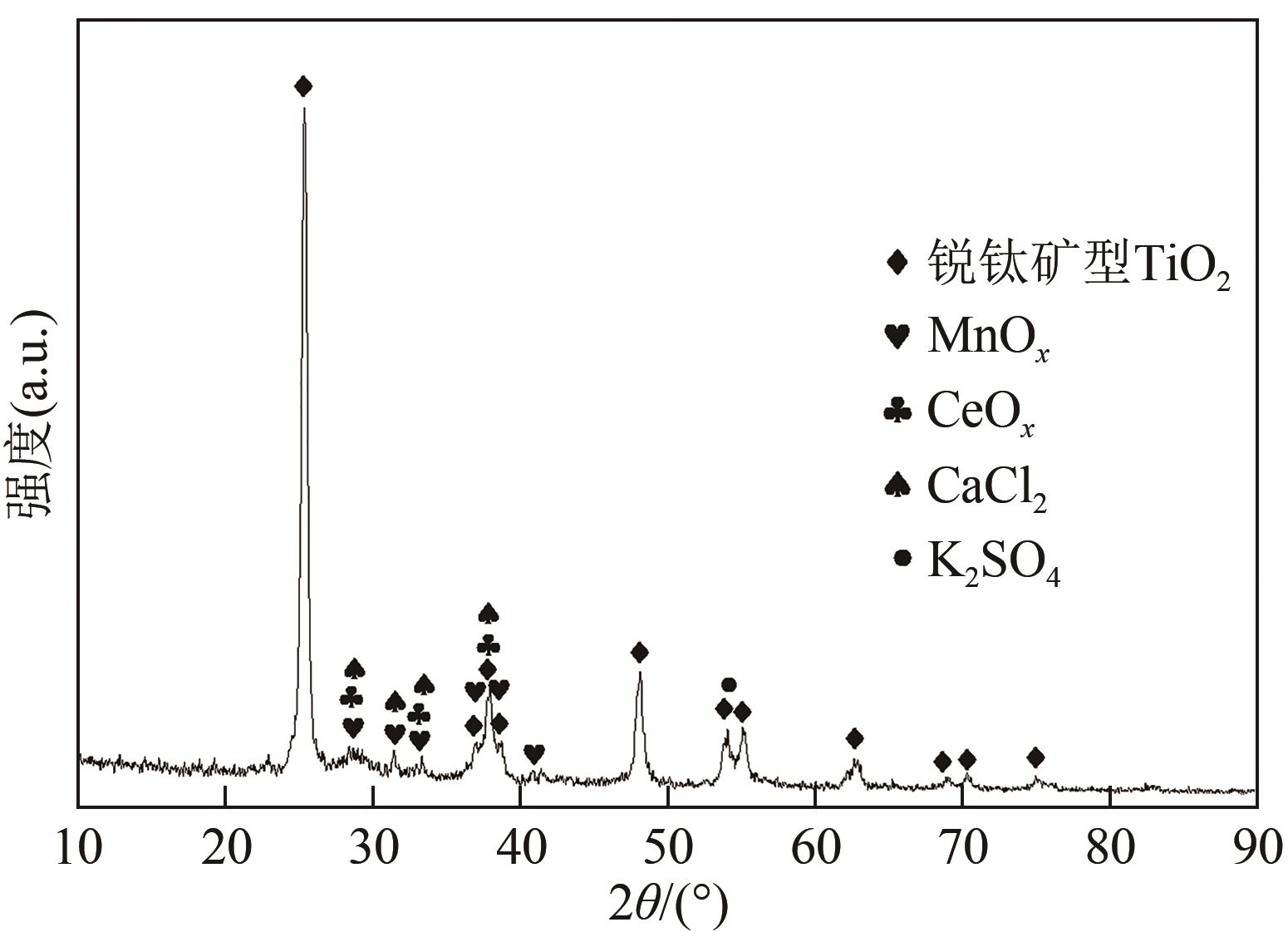
| TiO2 | MnO | CeO2 | SO3 | CaO | MgO | Na2O | K2O | 其他 |
|---|---|---|---|---|---|---|---|---|
| 72.53 | 11.20 | 2.57 | 3.80 | 2.57 | 1.14 | 0.48 | 0.60 | 5.11 |
| TiO2 | MnO | CeO2 | SO3 | CaO | MgO | Na2O | K2O | 其他 |
|---|---|---|---|---|---|---|---|---|
| 72.53 | 11.20 | 2.57 | 3.80 | 2.57 | 1.14 | 0.48 | 0.60 | 5.11 |
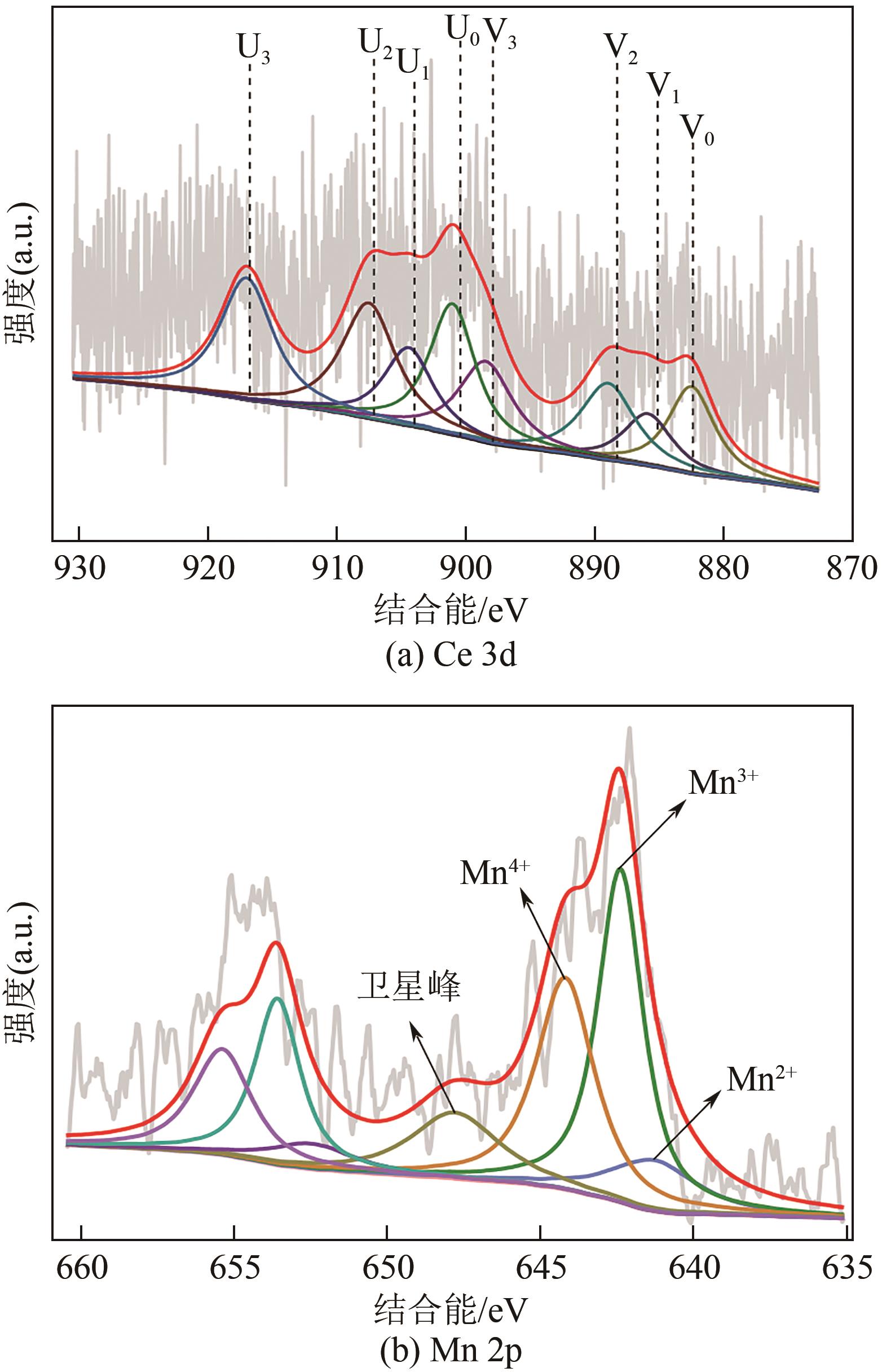
| 样品 | 化合价比例/% |
|---|---|
| Ce3+/Ce | 22.67 |
| Ce4+/Ce | 77.33 |
| Mn2+/Mn | 23.22 |
| Mn3+/Mn | 41.88 |
| Mn4+/Mn | 34.49 |
| 样品 | 化合价比例/% |
|---|---|
| Ce3+/Ce | 22.67 |
| Ce4+/Ce | 77.33 |
| Mn2+/Mn | 23.22 |
| Mn3+/Mn | 41.88 |
| Mn4+/Mn | 34.49 |
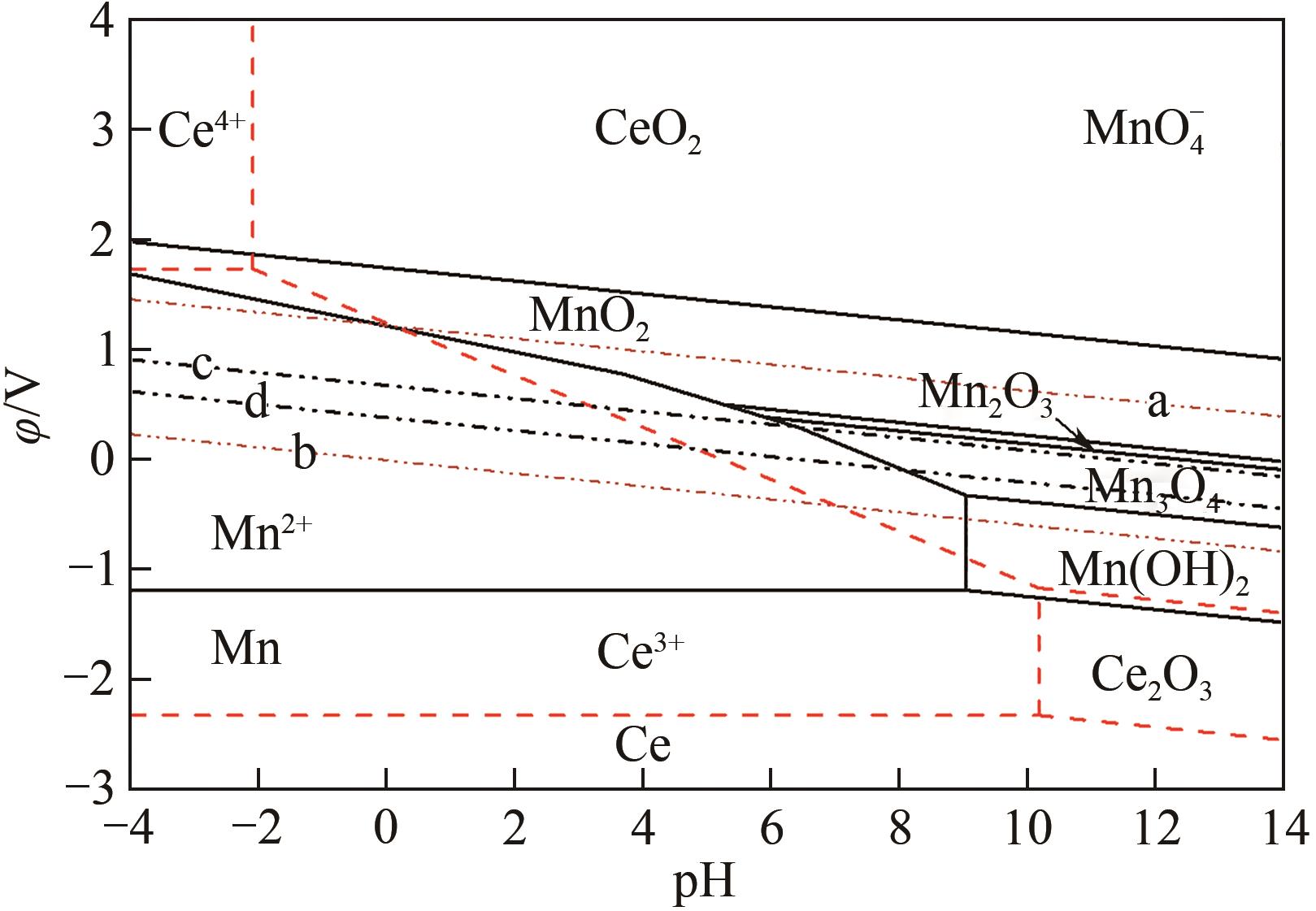
| 序号 | 平衡反应 | φ-pH关系式 |
|---|---|---|
| a | O2+4H++4e- | φ=1.2290-0.0592pH |
| b | H++2e- | φ=-0.0592pH |
| c | O2+2H++2e- | φ=0.682-0.0592pH |
| d | C6H6O6+2H++2e- | φ=0.39-0.0592pH |
| (1) | Ce3++3e- | φ=-2.3239+0.0197lg |
| (2) | Ce4++e- | φ=1.7432+0.0592lg( |
| (3) | Ce2O3+6H+ | pH=10.1936-3lg |
| (4) | CeO2+4H+ | pH=-2.096-1/4lg |
| (5) | 2CeO2+2H++2e- | φ=-0.5606-0.0592pH |
| (6) | CeO2+4H++e- | φ=1.2485-0.2366pH-0.0592lg |
| (7) | Ce2O3+6H++6e- | φ=-1.7209-0.0592pH |
| 序号 | 平衡反应 | φ-pH关系式 |
|---|---|---|
| a | O2+4H++4e- | φ=1.2290-0.0592pH |
| b | H++2e- | φ=-0.0592pH |
| c | O2+2H++2e- | φ=0.682-0.0592pH |
| d | C6H6O6+2H++2e- | φ=0.39-0.0592pH |
| (1) | Ce3++3e- | φ=-2.3239+0.0197lg |
| (2) | Ce4++e- | φ=1.7432+0.0592lg( |
| (3) | Ce2O3+6H+ | pH=10.1936-3lg |
| (4) | CeO2+4H+ | pH=-2.096-1/4lg |
| (5) | 2CeO2+2H++2e- | φ=-0.5606-0.0592pH |
| (6) | CeO2+4H++e- | φ=1.2485-0.2366pH-0.0592lg |
| (7) | Ce2O3+6H++6e- | φ=-1.7209-0.0592pH |
| 序号 | 平衡反应 | φ-pH关系式 |
|---|---|---|
| a | O2+4H++4e- | φ=1.2290-0.0592pH |
| b | H++2e- | φ=-0.0592pH |
| c | O2+2H++2e- | φ=0.682-0.0592pH |
| d | C6H6O6+2H++2e- | φ=0.39-0.0592pH |
| (8) | Mn2++2e- | φ=-1.185+0.0296lg |
| (9) | Mn(OH)2+2H+ | pH=9.06-0.0592lg |
| (10) | Mn(OH)2+2H++2e- | φ=-0.649-0.0592pH |
| (11) | Mn3O4+2H++2e- | φ=0.216-0.0592pH |
| (12) | Mn3O4+8H++2e- | φ=1.824-0.237pH-0.0887lg |
| (13) | 3Mn2O3+2H++2e- | φ=0.743-0.0592pH |
| (14) | Mn2O3+6H++2e- | φ=1.443-0.177pH-0.0592lg |
| (15) | 2MnO2+2H++2e- | φ=0.819-0.0592pH |
| (16) | φ=1.754-0.1789pH+0.01972lg | |
| (17) | MnO2+4H++2e- | φ=1.224-0.1183pH-0.02956lg |
| 序号 | 平衡反应 | φ-pH关系式 |
|---|---|---|
| a | O2+4H++4e- | φ=1.2290-0.0592pH |
| b | H++2e- | φ=-0.0592pH |
| c | O2+2H++2e- | φ=0.682-0.0592pH |
| d | C6H6O6+2H++2e- | φ=0.39-0.0592pH |
| (8) | Mn2++2e- | φ=-1.185+0.0296lg |
| (9) | Mn(OH)2+2H+ | pH=9.06-0.0592lg |
| (10) | Mn(OH)2+2H++2e- | φ=-0.649-0.0592pH |
| (11) | Mn3O4+2H++2e- | φ=0.216-0.0592pH |
| (12) | Mn3O4+8H++2e- | φ=1.824-0.237pH-0.0887lg |
| (13) | 3Mn2O3+2H++2e- | φ=0.743-0.0592pH |
| (14) | Mn2O3+6H++2e- | φ=1.443-0.177pH-0.0592lg |
| (15) | 2MnO2+2H++2e- | φ=0.819-0.0592pH |
| (16) | φ=1.754-0.1789pH+0.01972lg | |
| (17) | MnO2+4H++2e- | φ=1.224-0.1183pH-0.02956lg |
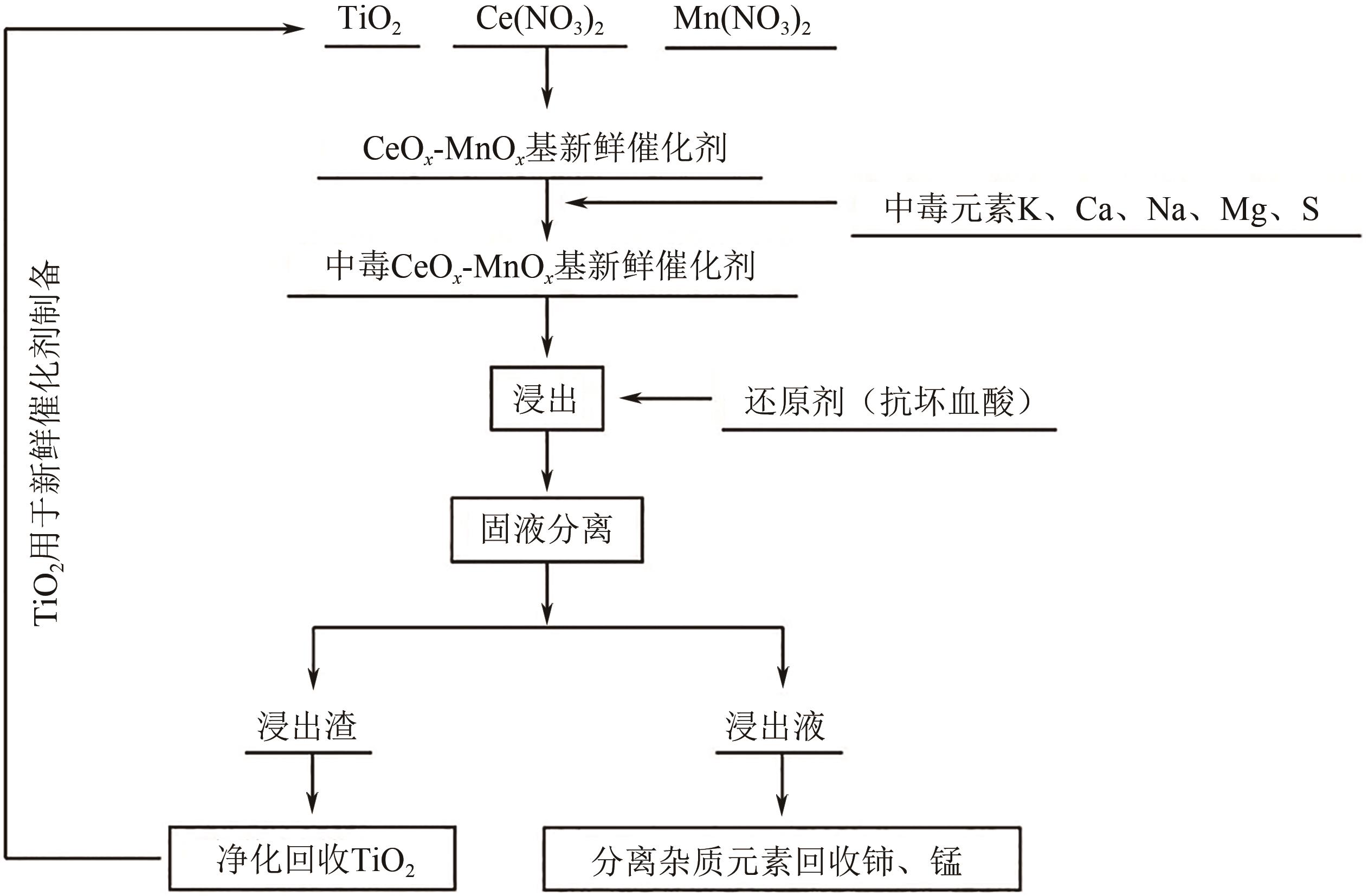
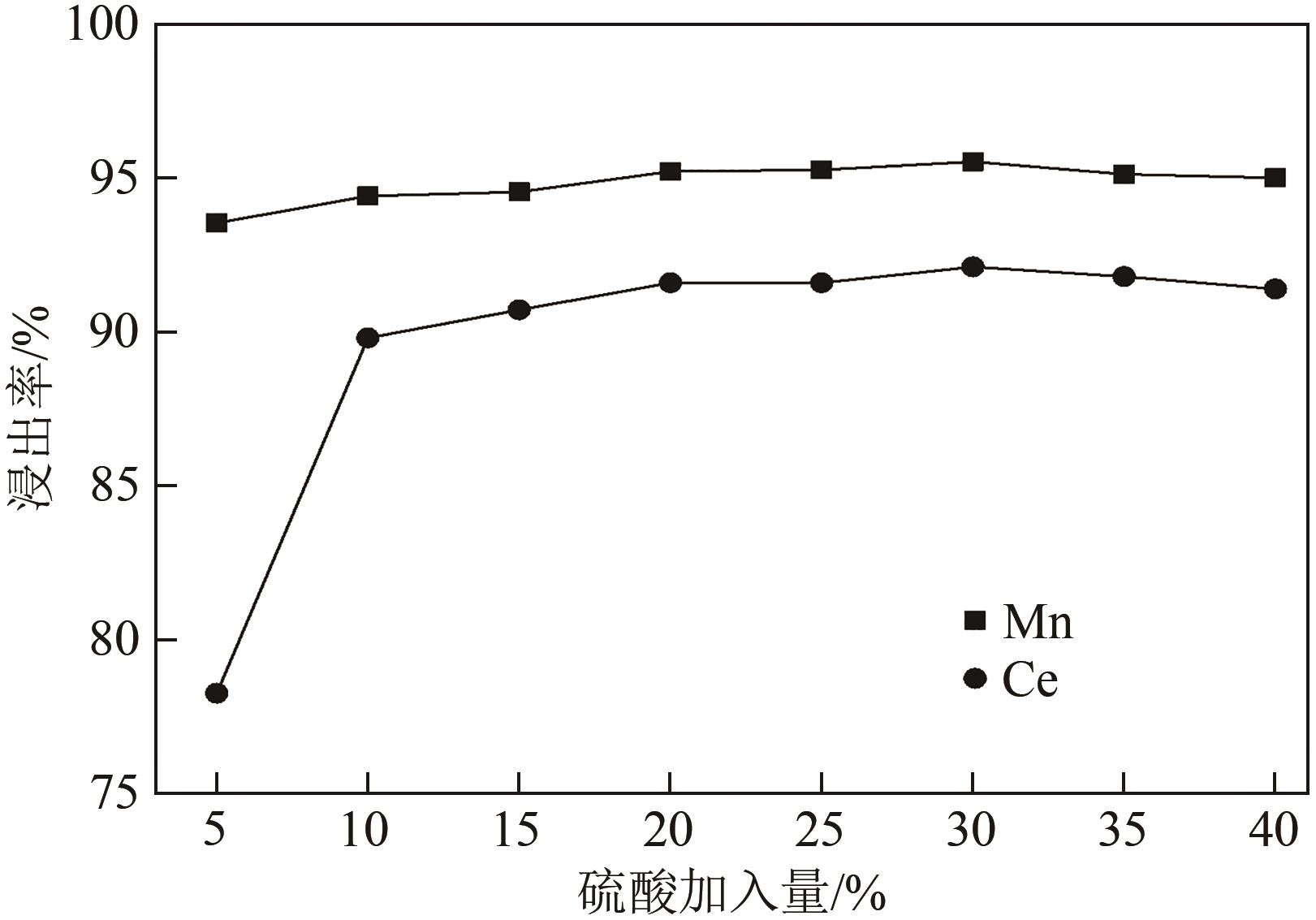
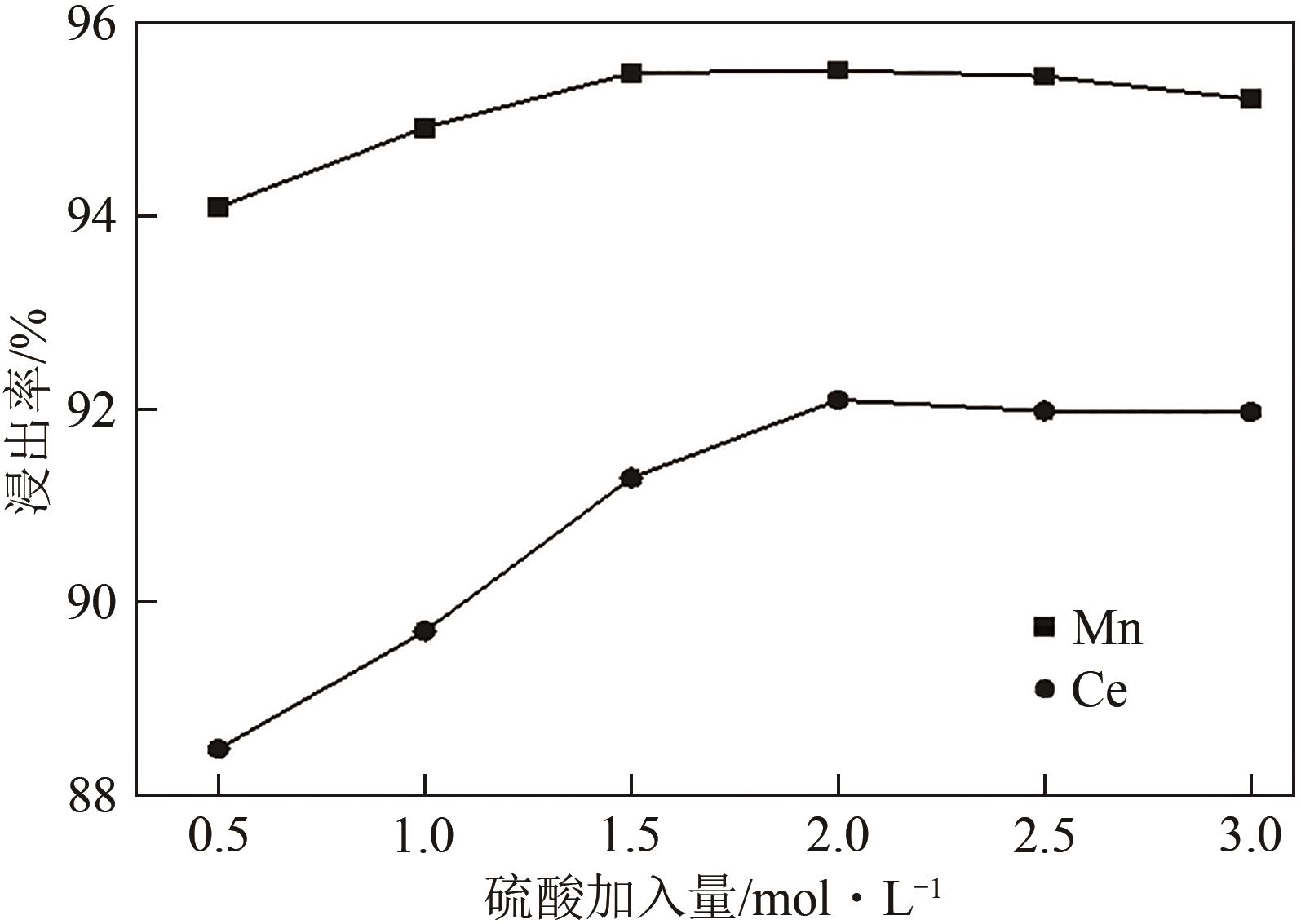
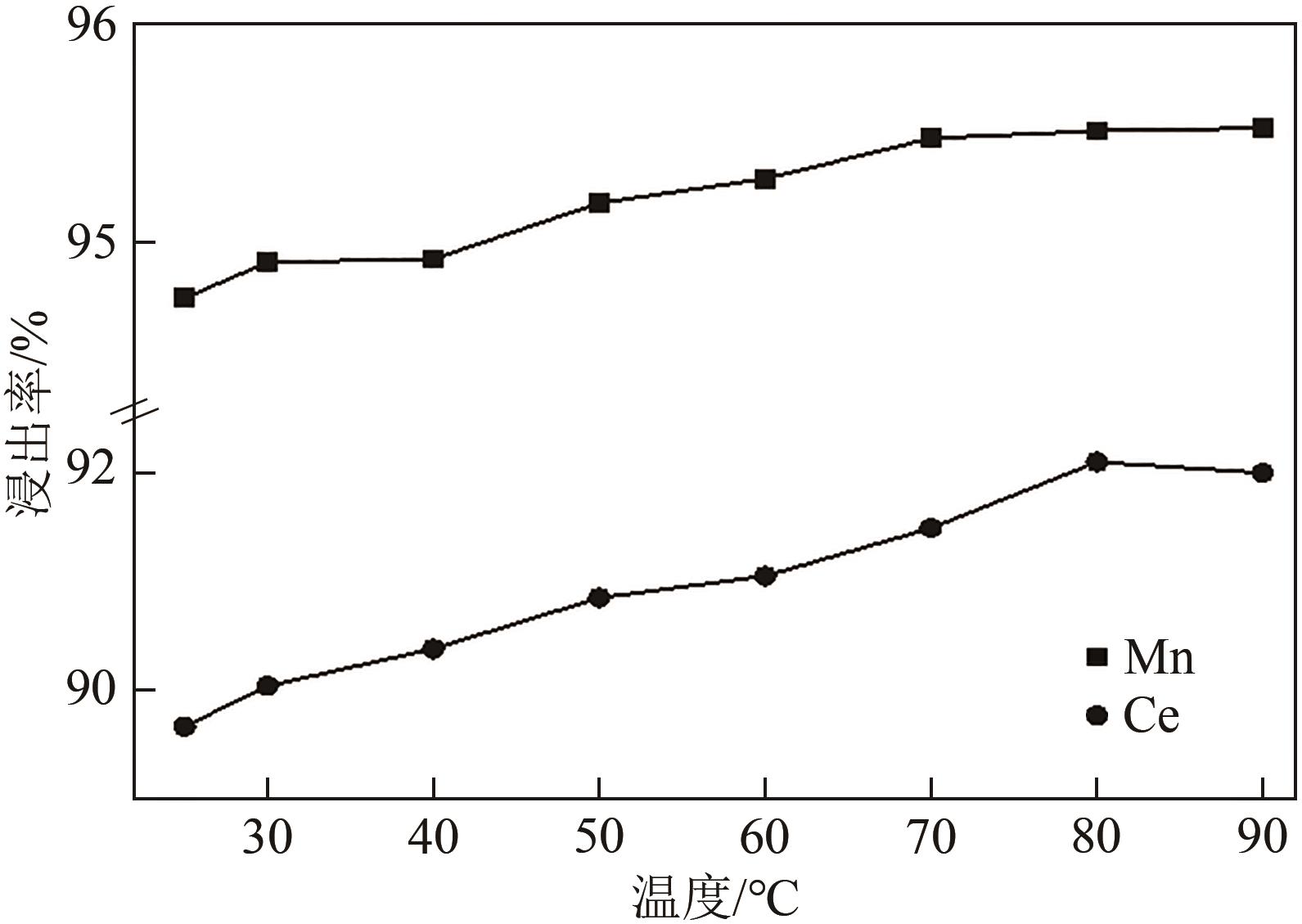
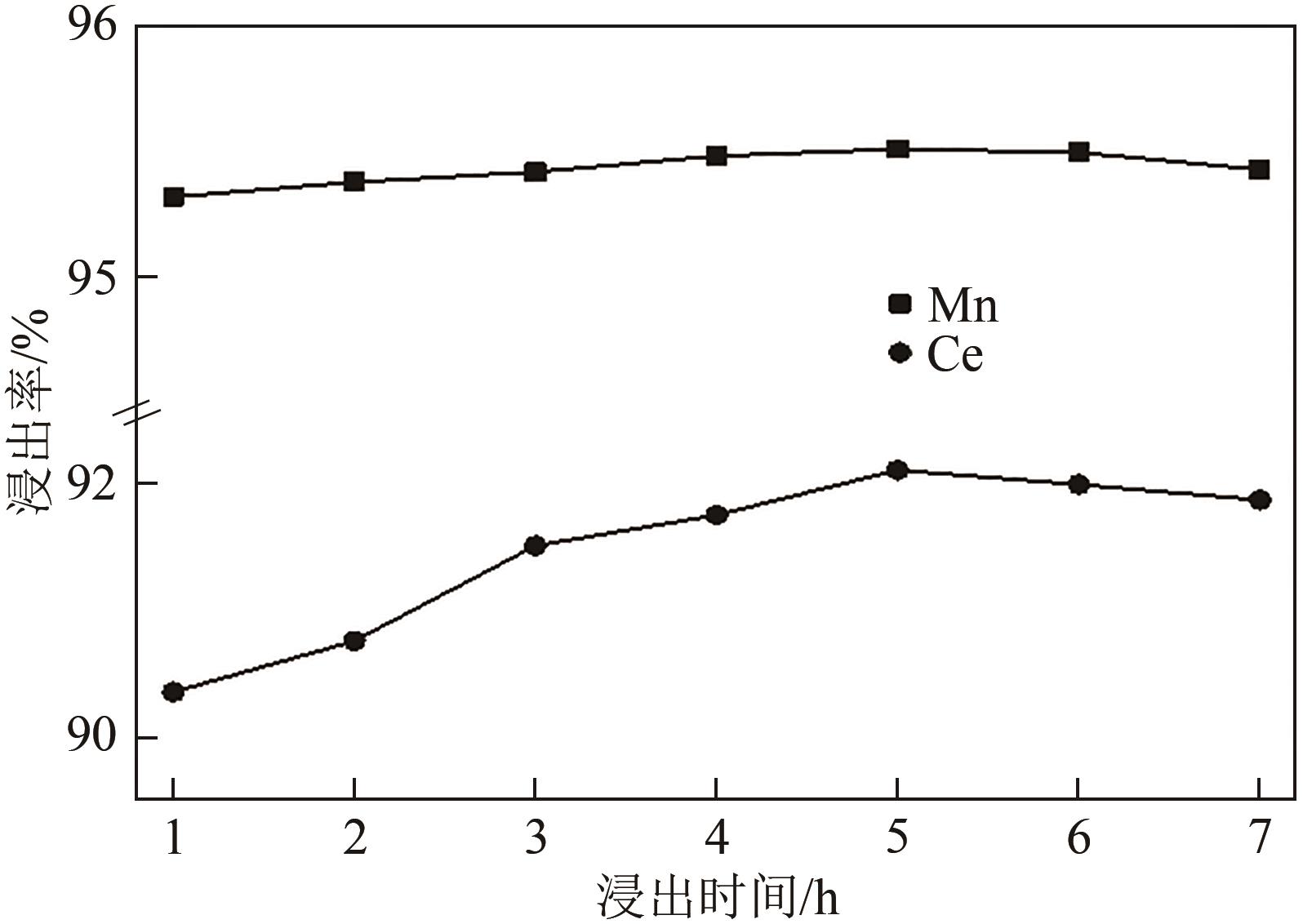
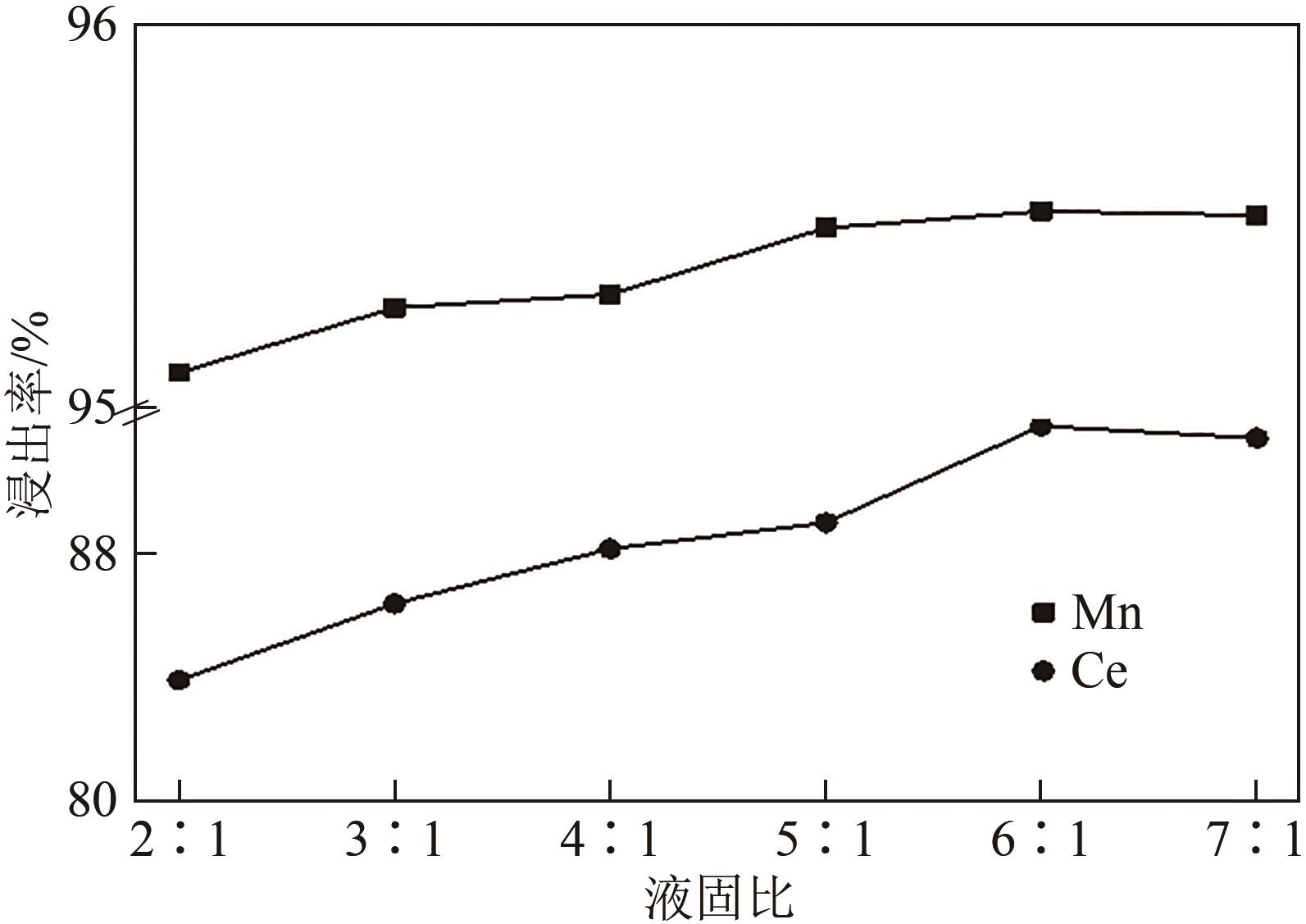
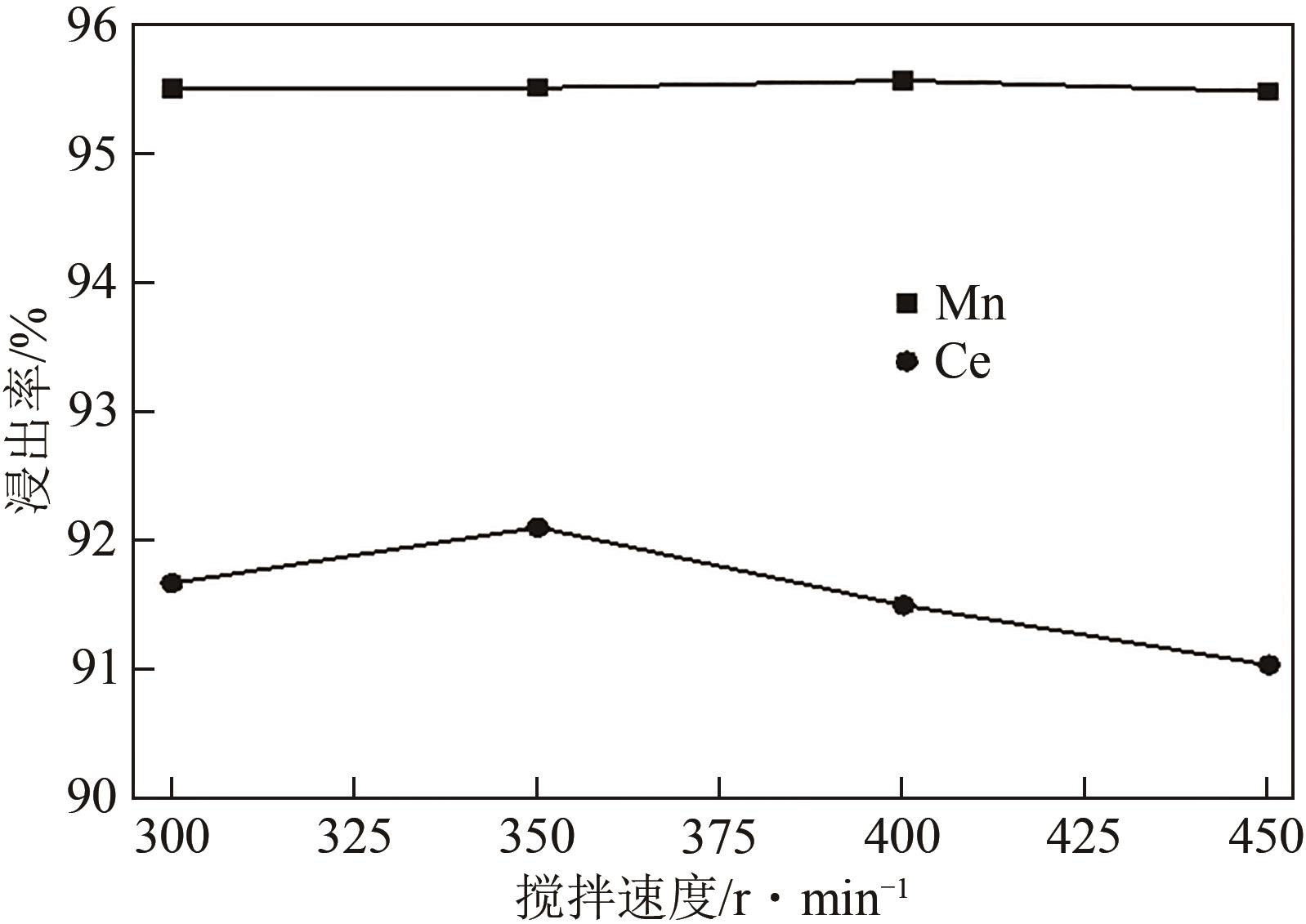
| 还原剂 | 温度/℃ | 浸出率/% | 残留率/% | |||
|---|---|---|---|---|---|---|
| Ce | Mn | Ti | 浸出渣 | |||
| 无 | 25 | 29.34 | 32.02 | 87.74 | 86.80 | |
| 无 | 80 | 59.72 | 72.55 | 87.83 | 81.70 | |
| 抗坏血酸 | 80 | 92.08 | 95.51 | 91.20 | 77.90 | |
| Na2SO3 | 80 | 57.63 | 92.48 | 89.50 | 78.10 | |
| H2O2 | 80 | 88.49 | 94.10 | 84.73 | 71.10 | |
| 还原剂 | 温度/℃ | 浸出率/% | 残留率/% | |||
|---|---|---|---|---|---|---|
| Ce | Mn | Ti | 浸出渣 | |||
| 无 | 25 | 29.34 | 32.02 | 87.74 | 86.80 | |
| 无 | 80 | 59.72 | 72.55 | 87.83 | 81.70 | |
| 抗坏血酸 | 80 | 92.08 | 95.51 | 91.20 | 77.90 | |
| Na2SO3 | 80 | 57.63 | 92.48 | 89.50 | 78.10 | |
| H2O2 | 80 | 88.49 | 94.10 | 84.73 | 71.10 | |
| TiO2 | MnO | CeO2 | SO3 | CaO | MgO | 其他 |
|---|---|---|---|---|---|---|
| 93.58 | 0.78 | 0.33 | 0.78 | 0.04 | 0.12 | 4.37 |
| TiO2 | MnO | CeO2 | SO3 | CaO | MgO | 其他 |
|---|---|---|---|---|---|---|
| 93.58 | 0.78 | 0.33 | 0.78 | 0.04 | 0.12 | 4.37 |
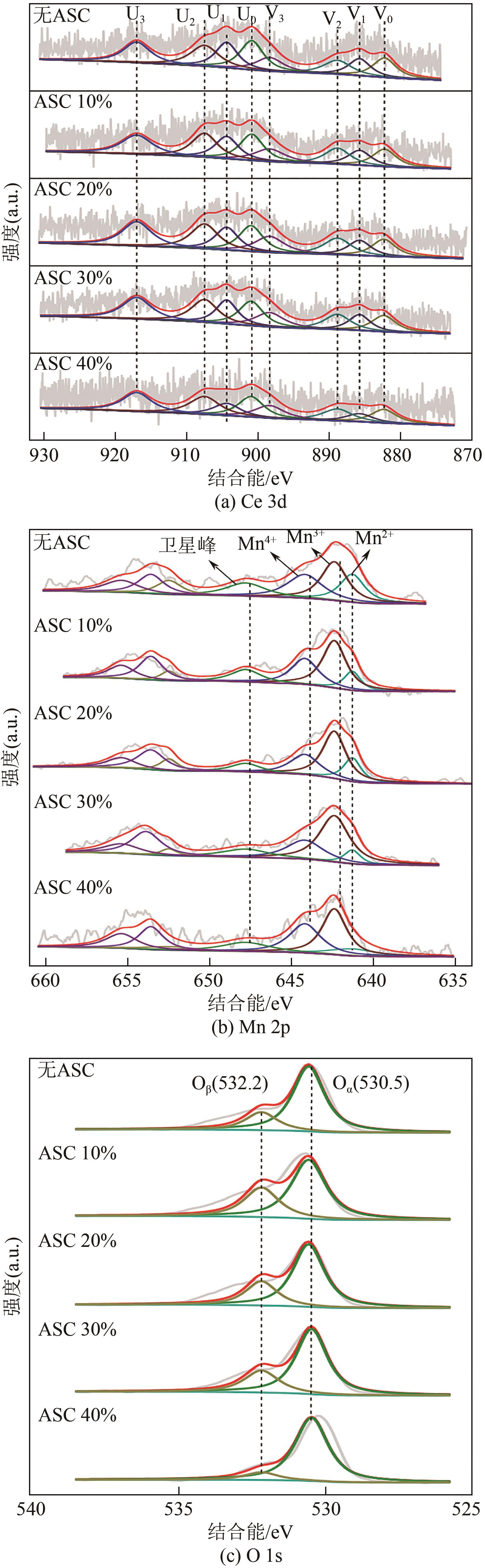
| 样品 | Ce3+/Ce | Ce4+/Ce | Mn2+/Mn | Mn4+/Mn | Oβ/(Oα+Oβ) | Oα/(Oα+Oβ) |
|---|---|---|---|---|---|---|
| 原料 | 22.67 | 77.33 | 23.22 | 34.49 | 10.88 | 89.12 |
| 抗坏血酸 加入量10% | 24.18 | 75.82 | 23.82 | 29.39 | 27.16 | 72.84 |
| 抗坏血酸 加入量20% | 25.27 | 74.73 | 25.89 | 28.93 | 27.97 | 72.03 |
| 抗坏血酸 加入量30% | 28.38 | 71.62 | 36.35 | 27.17 | 33.47 | 66.53 |
| 抗坏血酸 加入量40% | 28.14 | 71.86 | 36.03 | 26.89 | 21.70 | 78.30 |
| 样品 | Ce3+/Ce | Ce4+/Ce | Mn2+/Mn | Mn4+/Mn | Oβ/(Oα+Oβ) | Oα/(Oα+Oβ) |
|---|---|---|---|---|---|---|
| 原料 | 22.67 | 77.33 | 23.22 | 34.49 | 10.88 | 89.12 |
| 抗坏血酸 加入量10% | 24.18 | 75.82 | 23.82 | 29.39 | 27.16 | 72.84 |
| 抗坏血酸 加入量20% | 25.27 | 74.73 | 25.89 | 28.93 | 27.97 | 72.03 |
| 抗坏血酸 加入量30% | 28.38 | 71.62 | 36.35 | 27.17 | 33.47 | 66.53 |
| 抗坏血酸 加入量40% | 28.14 | 71.86 | 36.03 | 26.89 | 21.70 | 78.30 |
| 1 | MOHAN S, DINESHA P, KUMAR S. NO x reduction behaviour in copper zeolite catalysts for ammonia SCR systems: a review[J]. Chemical Engineering Journal, 2020, 384: 123253. |
| 2 | TANG Changjin, ZHANG Hongliang, DONG Lin. Ceria-based catalysts for low-temperature selective catalytic reduction of NO with NH3 [J]. Catalysis Science & Technology, 2016, 6(5): 1248-1264. |
| 3 | 邢奕, 张文伯, 苏伟, 等. 中国钢铁行业超低排放之路[J]. 工程科学学报, 2021, 43(1): 1-9. |
| XING Yi, ZHANG Wenbo, SU Wei, et al. Research of ultra-low emission technologies of the iron and steel industry in China[J]. Chinese Journal of Engineering, 2021, 43(1): 1-9. | |
| 4 | 闫晓淼, 李玉然, 朱廷钰, 等. 钢铁烧结烟气多污染物排放及协同控制概述[J]. 环境工程技术学报, 2015, 5(2): 85-90. |
| YAN Xiaomiao, LI Yuran, ZHU Tingyu, et al. Review of emission and simultaneous control of multiple pollutants from iron-steel sintering flue gas[J]. Journal of Environmental Engineering Technology, 2015, 5(2): 85-90. | |
| 5 | 张洪亮, 施琦, 龙红明, 等. 烧结烟气中氮氧化物脱除工艺分析[J]. 钢铁, 2017, 52(5): 100-106. |
| ZHANG Hongliang, SHI Qi, LONG Hongming, et al. Analysis of NO x removal process in sintering flue gas[J]. Iron & Steel, 2017, 52(5): 100-106. | |
| 6 | 苏玉栋, 李咸伟, 范晓慧. 烧结过程中NO x 减排技术研究进展[J]. 烧结球团, 2013, 38(6): 41-44, 54. |
| SU Yudong, LI Xianwei, FAN Xiaohui. Research progress of NO x reduction technology in sintering process[J]. Sintering and Pelletizing, 2013, 38(6): 41-44, 54. | |
| 7 | LING Shaohua, JING Changyong, ZHANG Lijuan. Analysis denitration technology for iron-steel sintering flue gas[C]//Proceedings of the 2015 International Symposium on Material, Energy and Environment Engineering. November 28-29, 2015. Changsha, China. Paris, France: Atlantis Press, 2015. |
| 8 | 王淑勤, 刘丽凤, 程伟良. 低温SCR脱硝催化技术的应用进展[J]. 能源与环境, 2021(2): 65-69. |
| WANG Shuqin, LIU Lifeng, CHENG Weiliang. Application progress of low-temperature SCR denitrification catalytic technology[J]. Energy and Environment, 2021(2): 65-69. | |
| 9 | 刘福东, 单文坡, 石晓燕, 等. 用于NH3选择性催化还原NO x 的钒基催化剂[J]. 化学进展, 2012, 24(4): 445-455. |
| LIU Fudong, SHAN Wenpo, SHI Xiaoyan, et al. Vanadium-based catalysts for the selective catalytic reduction of NO x with NH3 [J]. Progress in Chemistry, 2012, 24(4): 445-455. | |
| 10 |
丁龙, 钱立新, 杨涛, 等. 烧结烟气中Zn对V2O5-WO3/TiO2催化剂脱除NO x 和二 英性能的影响[J]. 工程科学学报, 2021, 43(8): 1125-1135. 英性能的影响[J]. 工程科学学报, 2021, 43(8): 1125-1135.
|
| DING Long, QIAN Lixin, YANG Tao, et al. Influence of Zn in the iron ore sintering flue gas on the removal of NO x and dioxins by V2O5-WO3/TiO2 catalyst[J]. Chinese Journal of Engineering, 2021, 43(8): 1125-1135. | |
| 11 | ZHANG Qijun, WU Yufeng, ZUO Tieyong. Titanium extraction from spent selective catalytic reduction catalysts in a NaOH molten-salt system: thermodynamic, experimental, and kinetic studies[J]. Metallurgical and Materials Transactions B, 2019, 50(1): 471-479. |
| 12 | 侯学军, 章小明, 程文博, 等. 废钒钛基SCR催化剂的处置方法研究进展[J]. 化工进展, 2021, 40(10): 5313-5324. |
| HOU Xuejun, ZHANG Xiaoming, CHENG Wenbo, et al. Research on disposal methods of spent vanadium-titanium-based catalysts[J]. Chemical Industry and Engineering Progress, 2021, 40(10): 5313-5324. | |
| 13 | CHOI In Hyeok, MOON Gyeonghye, LEE Jin Young, et al. Extraction of tungsten and vanadium from spent selective catalytic reduction catalyst for stationary application by pressure leaching process[J]. Journal of Cleaner Production, 2018, 197: 163-169. |
| 14 | MOON Gyeonghye, KIM Jin Hyeong, LEE Jin Young, et al. Leaching of spent selective catalytic reduction catalyst using alkaline melting for recovery of titanium, tungsten, and vanadium[J]. Hydrometallurgy, 2019, 189: 105132. |
| 15 | LI Qichao, LIU Zhenyu, LIU Qingya. Kinetics of vanadium leaching from a spent industrial V2O5/TiO2 catalyst by sulfuric acid[J]. Industrial & Engineering Chemistry Research, 2014, 53(8): 2956-2962. |
| 16 | ZHANG Qijun, WU Yufeng, LI Lili, et al. Sustainable approach for spent V2O5-WO3/TiO2 catalysts management: selective recovery of heavy metal vanadium and production of value-added WO3-TiO2 photocatalysts[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(9): 12502-12510. |
| 17 | TANG Xingfu, LI Yonggang, HUANG Xiumin, et al. MnO x -CeO2 mixed oxide catalysts for complete oxidation of formaldehyde: effect of preparation method and calcination temperature[J]. Applied Catalysis B: Environmental, 2006, 62(3/4): 265-273. |
| 18 | KLIMCZAK M, KERN P, HEINZELMANN T, et al. High-throughput study of the effects of inorganic additives and poisons on NH3-SCR catalysts—Part Ⅰ: V2O5-WO3/TiO2 catalysts[J]. Applied Catalysis B: Environmental, 2010, 95(1/2): 39-47. |
| 19 | 吴维昌. 标准电极电位数据手册[M]. 北京: 科学出版社, 1991. |
| WU Weichang. Manual of standard electrode potentials data[M]. Beijing: Science Press, 1991. | |
| 20 | 梁英教. 无机物热力学数据手册[M]. 沈阳: 东北大学出版社, 1993. |
| LIANG Yingjiao. Manual of inorganic thermodynamics data[M]. Shenyang: Northeast University Press, 1993. | |
| 21 | 叶大伦, 胡建华. 实用无机物热力学数据手册[M]. 2版. 北京: 冶金工业出版社, 2002. |
| YE Dalun, HU Jianhua. Manual of practical inorganic thermodynamics data[M]. 2nd ed. Beijing: Metallurgical Industry Press, 2002. | |
| 22 | 李照刚, 陈为亮, 张建军, 等. 响应曲面法优化软锰矿还原浸出的工艺[J]. 化学工程, 2018, 46(2): 72-78. |
| LI Zhaogang, CHEN Weiliang, ZHANG Jianjun, et al. Reductive leaching technology of pyrolusite optimized by response surface methodology[J]. Chemical Engineering (China), 2018, 46(2): 72-78. | |
| 23 | LI Qian, RAO Xuefei, XU Bin, et al. Extraction of manganese and zinc from their compound ore by reductive acid leaching[J]. Transactions of Nonferrous Metals Society of China, 2017, 27(5): 1172-1179. |
| 24 | 张兆雪. 稀土荧光粉废料碱熔—水浸—还原酸浸回收稀土工艺研究[D]. 赣州: 江西理工大学, 2016. |
| ZHANG Zhaoxue. Process research on recovery of rare earth of waste rare earth fluorescent powders by alkali fusion-washing-reduction acid leaching[D]. Ganzhou: Jiangxi University of Science and Technology, 2016. | |
| 25 | LUO Jian, ZHANG Qiuhua, Javier GARCIA-MARTINEZ, et al. Adsorptive and acidic properties, reversible lattice oxygen evolution, and catalytic mechanism of cryptomelane-type manganese oxides as oxidation catalysts[J]. Journal of the American Chemical Society, 2008, 130(10): 3198-3207. |
| 26 | YE Bora, LEE Minwoo, JEONG Bora, et al. Partially reduced graphene oxide as a support of Mn-Ce/TiO2 catalyst for selective catalytic reduction of NO x with NH3 [J]. Catalysis Today, 2019, 328: 300-306. |
| 27 | LI Lulu, WU Yaohui, HOU Xueyan, et al. Investigation of two-phase intergrowth and coexistence in Mn-Ce-Ti-O catalysts for the selective catalytic reduction of NO with NH3: structure-activity relationship and reaction mechanism[J]. Industrial & Engineering Chemistry Research, 2019, 58(2): 849-862. |
| [1] | MA Yi, CAO Shiwei, WANG Jiajun, LIN Liqun, XING Yan, CAO Tengliang, LU Feng, ZHAO Zhenlun, ZHANG Zhijun. Research progress in recovery of spent cathode materials for lithium-ion batteries using deep eutectic solvents [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 219-232. |
| [2] | ZHAO Wei, ZHAO Deyin, LI Shihan, LIU Hongda, SUN Jin, GUO Yanqiu. Synthesis and application of triazine drag reducing agent for nature gas pipeline [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 391-399. |
| [3] | ZHANG Jie, WANG Fangfang, XIA Zhonglin, ZHAO Guangjin, MA Shuangchen. Current SF6 emission, emission reduction and future prospects under “carbon peaking and carbon neutrality” [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 447-460. |
| [4] | WANG Lele, YANG Wanrong, YAO Yan, LIU Tao, HE Chuan, LIU Xiao, SU Sheng, KONG Fanhai, ZHU Canghai, XIANG Jun. Influence of spent SCR catalyst blending on the characteristics and deNO x performance for new SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 489-497. |
| [5] | ZHAO Jingchao, TAN Ming. Effect of surfactants on the reduction of industrial saline wastewater by electrodialysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 529-535. |
| [6] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [7] | SHU Bin, CHEN Jianhong, XIONG Jian, WU Qirong, YU Jiangtao, YANG Ping. Necessity analysis of promoting the development of green methanol under the goal of carbon neutrality [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4471-4478. |
| [8] | ZHANG Qi, ZHAO Hong, RONG Junfeng. Research progress of anti-toxicity electrocatalysts for oxygen reduction reaction in PEMFC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4677-4691. |
| [9] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| [10] | ZHU Chuanqiang, RU Jinbo, SUN Tingting, XIE Xingwang, LI Changming, GAO Shiqiu. Characteristics of selective non-catalytic reduction of NO x with solid polymer denitration agent [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4939-4946. |
| [11] | ZHANG Lihong, JIN Yaoru, CHENG Fangqin. Resource utilization of coal gasification slag [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4447-4457. |
| [12] | WANG Baoying, WANG Huangying, YAN Junying, WANG Yaoming, XU Tongwen. Research progress of polymer inclusion membrane in metal separation and recovery [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3990-4004. |
| [13] | WANG Yaogang, HAN Zishan, GAO Jiachen, WANG Xinyu, LI Siqi, YANG Quanhong, WENG Zhe. Strategies for regulating product selectivity of copper-based catalysts in electrochemical CO2 reduction [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4043-4057. |
| [14] | LIU Yi, FANG Qiang, ZHONG Dazhong, ZHAO Qiang, LI Jinping. Cu facets regulation of Ag/Cu coupled catalysts for electrocatalytic reduction of carbon dioxide [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4136-4142. |
| [15] | LYU Jie, HUANG Chong, FENG Ziping, HU Yafei, SONG Wenji. Performance and control system of gas engine heat pump based on waste heat recovery [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4182-4192. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||